Abstract
Background
Eosinophils are hallmark cells of allergic Th2 respiratory inflammation. However, the relative importance of eosinophil activation and the induction of effector functions such as the expression of IL-13 to allergic Th2 pulmonary disease remain to be defined.
Methods
Wild type or cytokine deficient (IL-13−/− or IL-4−/−) eosinophils treated with cytokines (GM-CSF, IL-4, IL-33) were adoptively transferred into eosinophil-deficient recipient mice subjected to allergen provocation using established models of respiratory inflammation. Allergen-induced pulmonary changes were assessed.
Results
In contrast to the transfer of untreated blood eosinophils to the lungs of recipient eosinophildeficient mice, which induced no immune/inflammatory changes either in the lung or lung draining lymph nodes (LDLNs), pretreatment of blood eosinophils with GM-CSF prior to transfer elicited trafficking of these eosinophils to LDLNs. In turn, these LDLN eosinophils elicited the accumulation of dendritic cells and CD4+ T cells to these same LDLNs without inducing pulmonary inflammation. However, exposure of eosinophils to GM-CSF, IL-4 and IL-33 prior to transfer induced not only immune events in the LDLN, but also allergen-mediated increases in airway Th2 cytokine/chemokine levels, the subsequent accumulation of CD4+ T cells as well as alternatively activated (M2) macrophages, and the induction of pulmonary histopathologies. Significantly, this allergic respiratory inflammation was dependent on eosinophil-derived IL-13 whereas IL-4 expression by eosinophils had no significant role.
Conclusion
The data demonstrate the differential activation of eosinophils as a function of cytokine exposure and suggest that eosinophil-specific IL-13 expression by activated cells is a necessary component of the subsequent allergic Th2 pulmonary pathologies.
Keywords: Asthma, Eosinophil-deficient, IL-13, IL-33, T cells
INTRODUCTION
Allergic respiratory inflammation is commonly associated with an induced airway eosinophilia that is often considered a characteristic feature of the Th2 polarized pulmonary immune microenvironment in these patients. This polarization of immune responses in the lung is highlighted by the accumulation of Th2 lymphocytes, increased levels of Th2 cytokines (e.g., IL-4, -5, and IL-13)) and Th2 chemokines (e.g., MDC and TARC), antibody switching leading the production of allergen-specific IgE and IgG1, and the transition of macrophages from the classical inflammatory (M1) phenotype to the alternatively activated (M2) phenotypes (reviewed in (1)). The historical role of eosinophils as part of these Th2 responses is almost universally associated with the dogmatic perspective of eosinophils as downstream mediators of destructive activities (e.g., toxic cationic granule protein release (2)). However, an increasing number of studies have highlighted roles for eosinophils that are far more complex than that of exclusively end-stage destructive cells (reviewed in (3, 4)). For example, specific eosinophilmediated events occurring in the lung now include contributions to remodeling/repair (5, 6) and the resolution of inflammation through release of mediators such TGF-β (7), IL-10 (8) and 12/15- lipoxygenase mediators (e.g., protectin D1) (9), respectively. Eosinophils have also been demonstrated to induce T cell proliferation (10, 11), increase the pulmonary levels of TARC and MDC (12), and promote the recruitment of effector T cells (12–14) to the lung in response to allergen provocation. More importantly, a deficiency in eosinophils results in ablation of these activities and in corresponding reductions in the Th2 immune responses occurring in mouse models of respiratory inflammation (reviewed in (15)). Thus, accumulating pulmonary eosinophils are capable of modulating the Th2 immune microenvironment and may contribute to important negative and positive regulatory loops modulating Th2 immune/inflammatory responses following allergen provocation.
In this report, we performed a series of eosinophil adoptive cell transfer studies into the lungs of allergen-challenged eosinophil-deficient mice (i.e., PHIL (16)). Our goal was to define mechanisms by which pulmonary eosinophils elicit the recruitment of allergen-specific effector T cells and the subsequent establishment of a Th2-polarized inflammatory milieu and the development of allergic pulmonary inflammation. These studies showed that peripheral blood eosinophils recruited to the lung likely undergo activation events stratifying these eosinophils into functional groups that mediate unique effector functions, including an ability to traffic to the LDLNs, promote T cell proliferation, and also the induction of IL-13 expression. This eosinophil-derived IL-13 was shown to be critical for lung expression of the Th2 chemokines MDC and TARC, the recruitment of pulmonary effector T cells, accumulation of M2 macrophage, and the development of allergic respiratory inflammation (17, 18). Significantly, the data presented establish the importance of this eosinophil-derived IL-13 expression and suggest that eosinophils accumulating in the lungs differentially mediate activities as immune responses evolve following allergen provocation.
MATERIALS AND METHODS
Mice
All studies were performed with 8–16 week old male and female mice on the C57BL/6 background. Eosinophil-deficient PHIL (16) and IL-5 transgenic NJ.1638 (19) mice were generated from established institutional colonies. IL-4−/− mice (C57BL/6-Il4tm1Nnt/J) were purchased from the Jackson Laboratories (Jackson Research Laboratories, Bar Harbor, ME). IL-13−/− mice were a gift of Andrew McKenzie (20). Mice were maintained in ventilated micro-isolator cages housed in the specific pathogen-free animal facility at the Mayo Clinic Arizona. Protocols and studies involving animals were performed in accordance with National Institutes of Health and Mayo Foundation institutional guidelines.
OVA sensitization/challenge protocols
Mice were sensitized with 100µl intraperitoneal (i.p.) injections of 400µg/mL OVA grade VI (Sigma- Aldrich) and 2.25mg Imject Alum (Thermo Scientific) on days 0 and 14 of the protocol and challenged with aerosolized OVA (1% (w/v) in saline) for 30 minutes on days 24, 25, 26 (i.e., OVA-treated). Mice were assessed on day 28 as described previously (12). In some groups of mice, eosinophils (0.5–1×107 eosinophils in 25µl of PBS) were adoptively transferred via the trachea (i.t.), as described previously (21) and Figure 1(A), on days 24, 25, 26, and 27 of the protocol. Controls include saline challenged mice and mice receiving saline transfers rather than cell transfers.
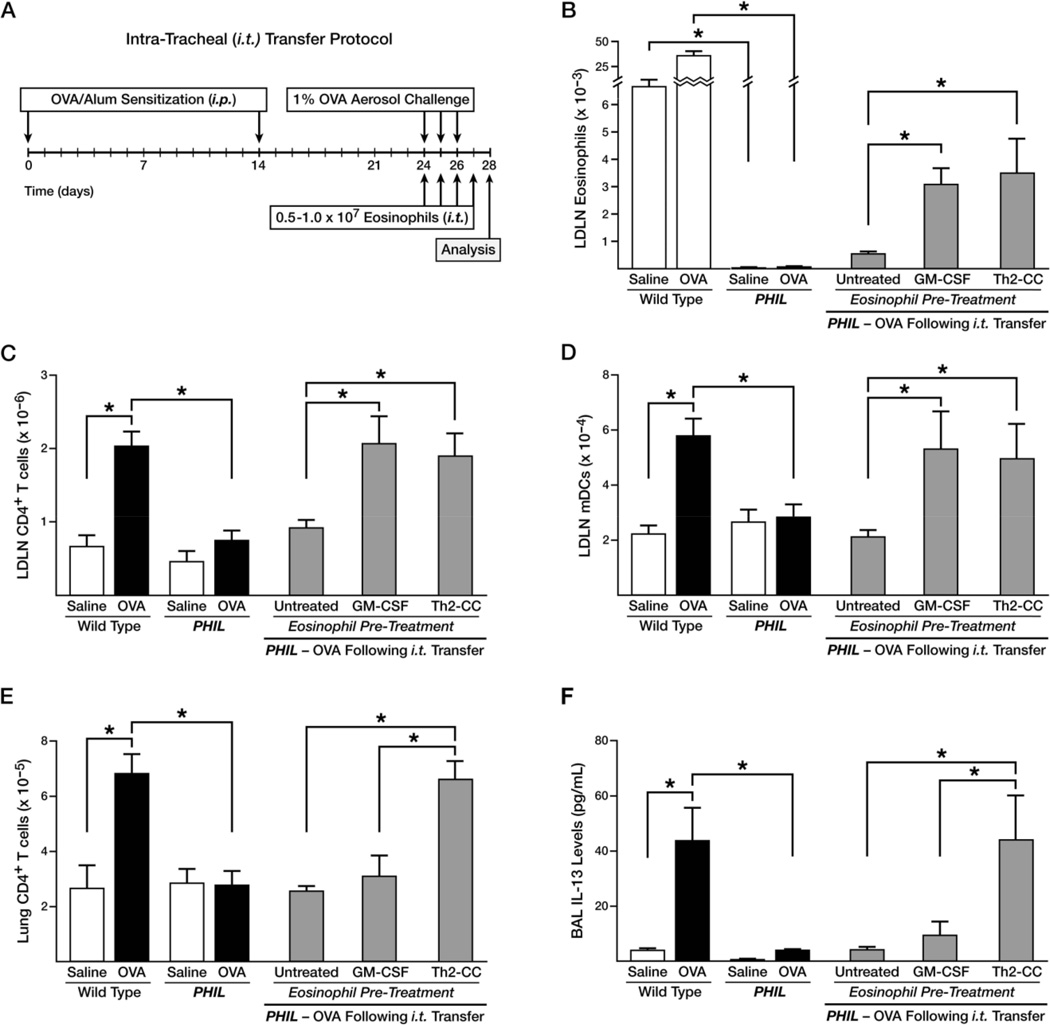
Figure 1. Differentially activated airway eosinophils induce unique immune responses in the pulmonary compartments of OVA challenged PHIL mice following eosinophil adoptive transfer.
(A) Schematic diagram of the allergic respiratory model and the eosinophil adoptive transfer strategy. Wild type and PHIL mice were subjected to OVA sensitization on day 0 and 14, acute OVA challenge on days 24–26 (OVAtreated), and assessed on day 28. Control animals were treated with saline alone. PHIL mice received eosinophils that were untreated (no cytokines), pre-treated with GM-CSF, or pre-treated with a Th2-cc cytokine cocktail (i.e., GM-CSF, IL-4, IL-33) for 24–48 hours prior to adoptive transfer (i.t.) into the lungs of mice on days 24–27 of the protocol. The resulting accumulations in the LDLNs of (B) eosinophils (Gr1+/CCR3+/Siglec-F+), (C) CD4+ T cells (CD4+/TCR-β+), and (D) myeloid DCs (F4/80−/Gr1−/CD11c+) as well as the accumulation in the lungs of (E) CD4+ T cells (CD4+/TCR-β+) were determined by flow cytometry. (F) BAL levels of IL-13 were measured from each group of mice. IL-17 and FN-γ levels were not detectable in the BALs of these mice. All data are derived from at least three independent experiments each with a total of least 5 mice per group (Mean ± SEM.). *p<0.05.
Isolation and culture of mouse peripheral blood eosinophils
Eosinophils were isolated from IL-5 expressing transgenic mice (NJ.1638/+/+), NJ.1638/IL-4−/−, or NJ.1638/IL-13−/− as described previously (10). In brief, eosinophils were separated by density gradient centrifugation using Histopaque 1119 (Sigma-Aldrich), followed by water lysis of contaminating red blood cells, washes with MACS buffer (PBS, 0.5% (w/v) BSA, 2mM EDTA), and cell depletion with magnetic beads (Miltenyi Biotech) conjugated with antibodies to CD45R/B220 (i.e., B cells) and CD90.2/Thy1.2 (i.e., T cells and ILC2s). As shown in Supplementary Figure 1, these isolated peripheral blood eosinophil populations were >98% pure by both examination of Diff-Quick stained (Siemens Healthcare Diagnostics) cytospin preparations and flow cytometry assessment (Gr1+/CCR3+/Siglec-F+). Eosinophils were transferred to recipient mice following pre-treatment with various cytokines in culture prior to adoptive transfer; control eosinophils were simply cultured in the absence of cytokines (Untreated). Specifically, eosinophils were cultured (5×106/mL) in complete RPMI-1640 media [RPMI-1640 (supplemented with GlutaMax and 25mM HEPES (72400-120; Life Technologies)), 10% FBS (HyClone Technologies), 50µM beta-mercaptoethanol, 10µg/ml penicillin, 10µg/ml streptomycin, and 2mM L-glutamine (Life Technologies)] for 24–48 hours. Cytokine pretreated eosinophils were generated by either culturing cells with GM-CSF (10ng/mL (Peprotech)) alone or with a cytokine cocktail (Th2-cc) that included GMCSF (10ng/mL) + IL-4 (10ng/mL) + IL-33 (30ng/mL) (Peprotech) (22). The viability of eosinophils following ex vivo culture was assessed by Trypan blue exclusion and revealed that untreated blood eosinophils displayed 90–95% viability following 24 hours of culture; this decreased only slightly (85–90% viability) after 48 hours of culture. Ex vivo cultures of eosinophils pre-treated with either GM-CSF alone or the Th2-cc cytokine cocktail displayed ≥95% viability following culture for 24 and 48 hours. Eosinophils derived from IL-4−/− or IL-13−/− knockout mice, respectively, did not display any Th2-cc cocktail-induced changes in cytokine expression (Eve Technologies, Alberta, Canada) relative to wild type eosinophils (other than the specific loss of either IL-4 or IL-13 expression, data not shown). Cells were washed with PBS/0.5% (w/v) BSA three times prior to suspending in PBS (2–4 × 108 cells/mL) for intratracheal transfer (i.t.).
Airway inflammation measurements
Lung, LDLN, and BAL flow cytometry and cell number determinations and cytokine levels are detailed in this article’s Supplementary Methods and Data.
Histology and immunohistochemistry
Immunohistochemistry methods are detailed in this article’s Supplementary Methods and Data.
Statistics
All data are derived from a minimum of three independent experiments that included one to six mice per experiment. Data were analyzed using GraphPad Prism 5 statistics program. Statistical analysis was performed using Student’s t-tests with error bars representing the mean ± SEM. Differences between means were considered significant when p<0.05.
RESULTS
Cytokine treatment of eosinophils leads to a differentially activated phenotype that elicits unique pulmonary immune responses upon transfer into the lungs of OVA-treated PHIL mice
The contributory role of eosinophils to allergen-induced Th2 pulmonary inflammation was assessed using eosinophil-deficient mice and adoptive transfer techniques. In particular, eosinophils were either untreated (i.e., cultured without cytokines) or pre-treated with various cytokines to induce differential states of activation prior to transfer into the lungs of eosinophil-deficient PHIL mice. Blood-derived eosinophils (>98% pure) from IL-5 transgenic mice were specifically chosen to avoid complications associated with peritoneal cavity-derived or splenic eosinophils (i.e., partial activation occurring with peritoneal-derived eosinophils (23) or the contamination of purified splenic eosinophils with immature metamyelocytes as well as macrophages/DCs(19)). Moreover, the use of blood-derived eosinophils avoids the potential artefacts of cultured bone marrow-derived eosinophils (24, 25). OVA-treated PHIL mice were given 0.5–1 ×107 eosinophils by intratracheal administration (i.t.) each day of allergen challenge (days 24–26) as well as day 27 of the protocol (Figure 1(A)). Significantly, i.t. adoptive transfer of untreated eosinophils into the lungs of OVA-treated PHIL mice was insufficient to induce immune responses in the LDLN relative to wild type levels. This included a failure of eosinophils to migrate to the LDLN (Supplementary Figure 2) and a failure to induce accumulation of CD4+ T cells (Figure 1(B)) and myeloid DCs (Figure 1(C)) in the LDLN of these mice. We have shown previously that transfer of eosinophils to the periphery via intraperitoneal injection (i.p.) resulted in the migration of eosinophils to the LDLN and the subsequent accumulation of DCs as well as the T cell activation occurring in the LDLN of allergen challenged PHIL mice (10). Thus, the failure of blood eosinophils to elicit any of these LDLN cellularity changes suggested that the pulmonary microenvironment of OVAtreated PHIL mice was not sufficient to activate i.t. transferred blood eosinophils; particularly in the absence of circulating eosinophils (21) and effector Th2 cells (12).
The hypothesis that eosinophils required ‘priming’ or activation in the lung prior to executing effector functions leading to Th2 pulmonary immune responses was tested by exposing blood-derived eosinophils to pulmonary innate cytokines (26) prior to adoptive transfer to the lungs of OVA-treated PHIL mice. Blood-derived eosinophils were cultured in the absence of exogenous cytokines (i.e., Untreated), cultured with GM-CSF (10ng/ml), or cultured with a cytokine cocktail (Th2-cc) that included GM-CSF (10ng/ml), IL-4 (10ng/ml) and IL-33 (30ng/ml) for 24–48 hours prior to i.t. transfer. As shown in Figures 1(B) and (C), culture with GM-CSF alone or with Th2-cc was sufficient to induce T cell and DC accumulation in the LDLN of OVA-treated PHIL mice at levels similar to OVA-treated wild type mice. These data demonstrated that unlike untreated eosinophils, culture with GM-CSF promoted eosinophil recruitment to the LDLN (Figure 1(D)) as well as T cell and DC activation within the lymph nodes. The number of eosinophils recruited to the LDLN in these PHIL mice was comparable to the number of eosinophils found in the LDLN of OVA-treated IL-5−/− mice (Supplementary Figure 3(A)) that have less than 10% of the number of eosinophils as wild type mice. This low number of eosinophils was sufficient to induce T cell activation in the LDLN of these mice (Supplementary Figure 3(B) and (10)). Despite the ability of GM-CSF treated eosinophils to induce T cell activation in the LDLN, T cells were not found to accumulate in the lungs of OVA-treated PHIL mice (Figure 1(E)). This suggested GM-CSF treatment of eosinophils failed to fully activate these cells at a level sufficient to induce Th2 pathways within the lung microenvironment, including the recruitment of T cells and an increase of IL-13 in the BAL from the lungs of these mice (Figure 1(F)). However, treating eosinophils with the Th2-cc cytokine cocktail (GM-CSF, IL-4, and IL-33) increased both T cell recruitment to the lung as well as IL-13 expression to wild type levels. Cytokine measurements of cultured eosinophils demonstrated that untreated cells were found to constitutively expressed IL-4 and IL-5 after 48 hours in culture (234.0 ± 25.6pg/mL and 212 ± 7.0pg/mL, respectively). The addition of GM-CSF significantly enhanced production of IL-5 (953 ± 9.9pg/mL) but not IL-4 (187 ± 27.1pg/mL) and did not induce expression of IL-13 (which remained undetectable). As reported previously (22, 27, 28), the addition of IL-33 (i.e., Th2-cc culture conditions) led to an increase of IL-13 from these cells (Untreated: ND, GM-CSF: ND, Th2-cc 894.5 ± 28.8pg/mL). Thus, exposure of eosinophils to this more complex array of cytokines differentially activates these granulocytes, expanding their potential effector functions.
Eosinophils activated with an IL-33 containing cytokine cocktail are primed to induce Th2 histopathological inflammation in the lungs of OVA-treated PHIL mice
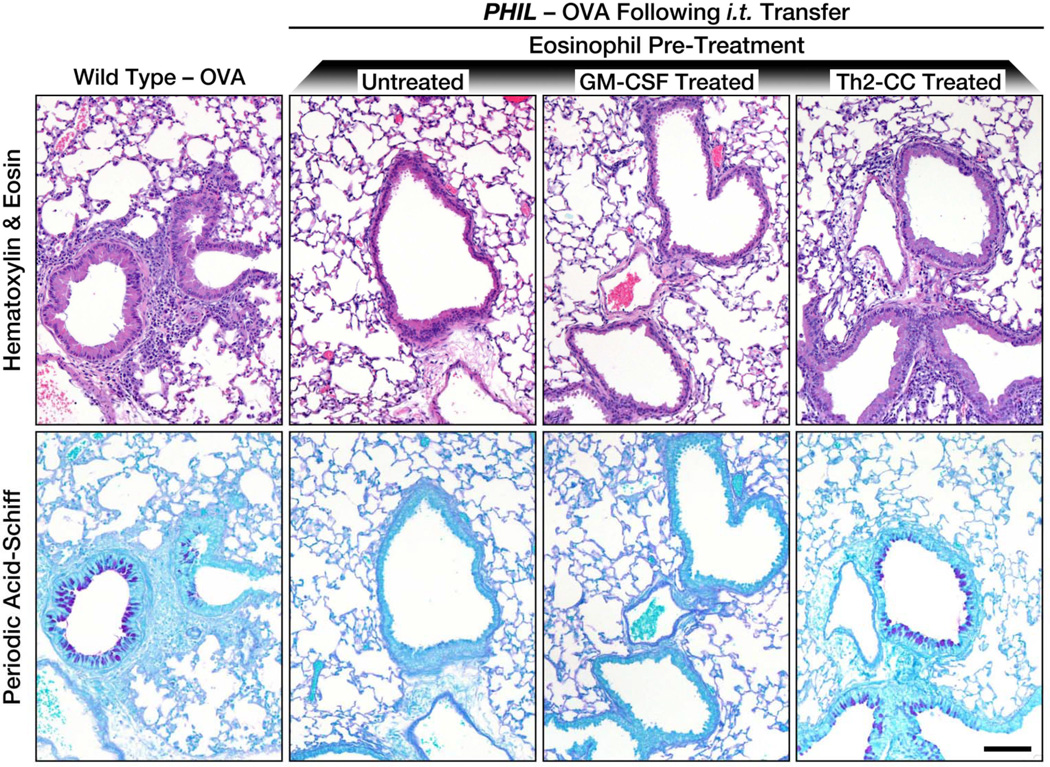
Th2-cc treatment of eosinophils prior to adoptive transfer (i.t.) of eosinophils into OVA-treated PHIL mice induced significant pulmonary histopathologies (Figure 2), including cellular infiltration of the central airways and distal airspaces (H & E staining) as well as increased goblet cell metaplasia/airway epithelial mucin accumulation (PAS staining). As expected based on the lack of induced lung immune responses (i.e., T cell recruitment and IL-13 expression), GM-CSF treated eosinophils had histopathologies that were undistinguishable from PHIL mice receiving (i.t.) untreated blood-derived eosinophils during OVA challenge, and are comparable to previously reported responses of OVA-treated PHIL mice (16). These data demonstrate that different levels of eosinophil activation induce different eosinophil effector functions in allergen models of allergic respiratory inflammation. One level of activation (i.e., GM-CSF exposure) promoted eosinophil recruitment to LDLN and eosinophildependent T cell activation in the LDLN while another level of activation (Th2-cc) was able to induce eosinophil effector phenotypes that induced both Th2 immune pathologies as well as lung histopathologies.
Figure 2. Differentially activated eosinophils induce Th2-associated histopathologies in the lungs of OVA challenged PHIL mice following eosinophil adoptive transfer.
Histopathologies in OVA-treated PHIL mice were determined following adoptive transfer (i.t.) of untreated eosinophils (i.e., no cytokine pre-treatments) as well as eosinophils pre-treated with GM-CSF or a Th2-cc cytokine cocktail (i.e., GM-CSF, IL-4, IL-33). Representative images of lung sections (compared to OVA-treated wild type mice) are shown for assessment of cellular inflammation (hematoxylin-eosin staining) and goblet cell metaplasia/airway epithelial cell mucin accumulation (PAS staining - dark purple cells). Scale bar = 100µm.
Eosinophil-derived IL-13, and not IL-4, is required to induce Th2 pulmonary immune responses during allergen challenge
The unique ability of adoptively transferred Th2-cc treated eosinophils to induce significant BAL IL-13 production and Th2 pulmonary inflammation in OVA-treated PHIL mice suggested a possible link between eosinophils and IL-13 expression in the lung. This was confirmed by flow cytometry (Supplementary Figure 4) demonstrating that eosinophils represented the largest cell population of IL- 13 expressing lung leukocytes in OVA-treated wild type mice (~10–15% of total lung leukocytes). In addition, these data also showed that the majority of lung eosinophils (~70%) stain positive for IL-13 expression.
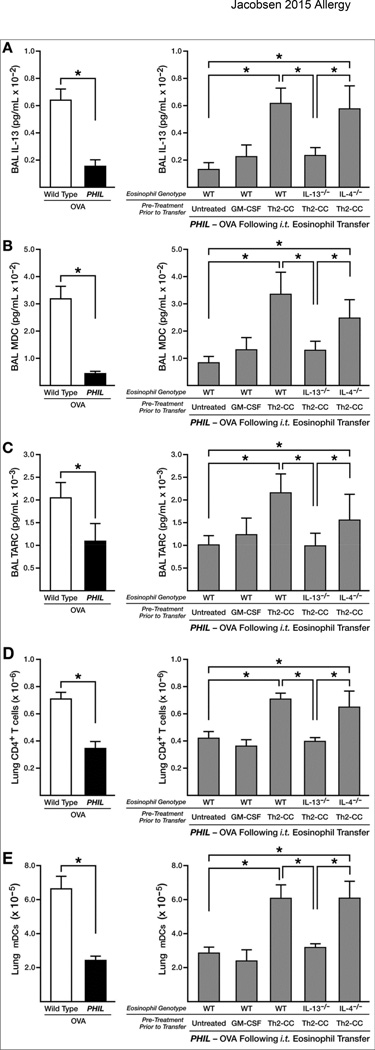
Eosinophils deficient in IL-13−/− or IL-4−/− were pre-treated with a Th2-cc cytokine cocktail (i.e., GM-CSF, IL-4, IL-33) and transferred i.t. into OVA-treated PHIL mice at the time of allergen challenge to test the unique significance of these cytokines. These data showed that IL-13-deficient eosinophils were unable to induce wild type levels of IL-13 in the BAL (Figure 3(A)), suggesting eosinophils were a potentially significant source of airway IL-13. The Th2 cell chemokines MDC (CCL22) and TARC (CCL17), which are targets of IL-13(29), also increased to similar levels upon transfer (i.t.) of either Th2-cc treated wild type and IL-4−/− eosinophils into OVA-treated PHIL mice (Figures 3(B) and (C)). However, no increases in either chemokine were observed following the transfer of IL-13-deficient eosinophils. As anticipated, OVA-treated PHIL mice that receive IL-13-deficient eosinophils were unable to recruit CD4+ T cells to the lung compartment (Figure 3(D)), despite the proliferation of T effector cells in the LDLN of these mice (Supplementary Figure 5(A)). These findings were confirmed in OVA-treated IL-5−/− mice, whereby eosinophil-derived IL-13 from adoptively transferred eosinophils (i.t.) was necessary to induce the recruitment of CD4+ T cells into the lungs of OVA-treated IL-5−/− mice to wild type levels (Supplementary Figure 6). Significantly, IL-5−/− mice do not require Th2-cc cytokine treatment for this IL-13-dependent Th2 induced pulmonary inflammation, suggesting that the partial ablation of eosinophils in these cytokine knockout mice (~8% of wild type levels following OVA provocation (21)) was sufficient to induce activation of “resting” eosinophils following adoptive transfer. In contrast to IL- 13, eosinophil-derived IL-4 did not play an apparent role in the recruitment of T cells to the lungs of OVA-treated PHIL mice or in the accumulation of myeloid DCs in the lung or LDLN (Figures 3(E) and Supplementary Figure 5(B)).
Figure 3. Eosinophil-derived IL-13, and not IL-4, contributes to the larger immune responses occurring in the lungs of OVA challenged PHIL mice following eosinophil adoptive transfer.
Immune responses in OVA-treated wild type and PHIL mice were determined relative to responses occurring OVA-treated PHIL mice following adoptive transfer (i.t.) of wild type eosinophils that were untreated (no cytokines), pre-treated with GM-CSF, or pre-treated with a Th2-cc cytokine cocktail (i.e., GM-CSF, IL-4, IL-33) for 24–48 hours prior to adoptive transfer (i.t.) into the lungs of mice on days 24–27 of the protocol. Additional groups of OVA-treated PHIL mice were adoptively transferred (i.t.) with IL-13−/− or IL-4−/− eosinophils pre-treated with a Th2-cc cytokine cocktail (i.e., GM-CSF, IL-4, and IL-33). BAL levels of (A) IL-13 and the Th2-associated chemokines (B) MDC and (C) TARC were determined by ELISA. IL-17 and IFN-γ were not detectable in the BAL of these mice. The accumulation of CD4+ T cells (CD4+/TCR-β+) and myeloid DCs (F4/80−/Gr1−/CD11c+) in the lungs (panels (D) and (E), respectively) were determined by flow cytometry. All data are derived from at least three independent experiments each with cohort sizes of 2–6 mice (Mean ± SEM). *p<0.05
Eosinophil-derived IL-13, and not IL-4, contributes to the Th2 histopathological inflammation in the lungs of OVA-treated PHIL mice
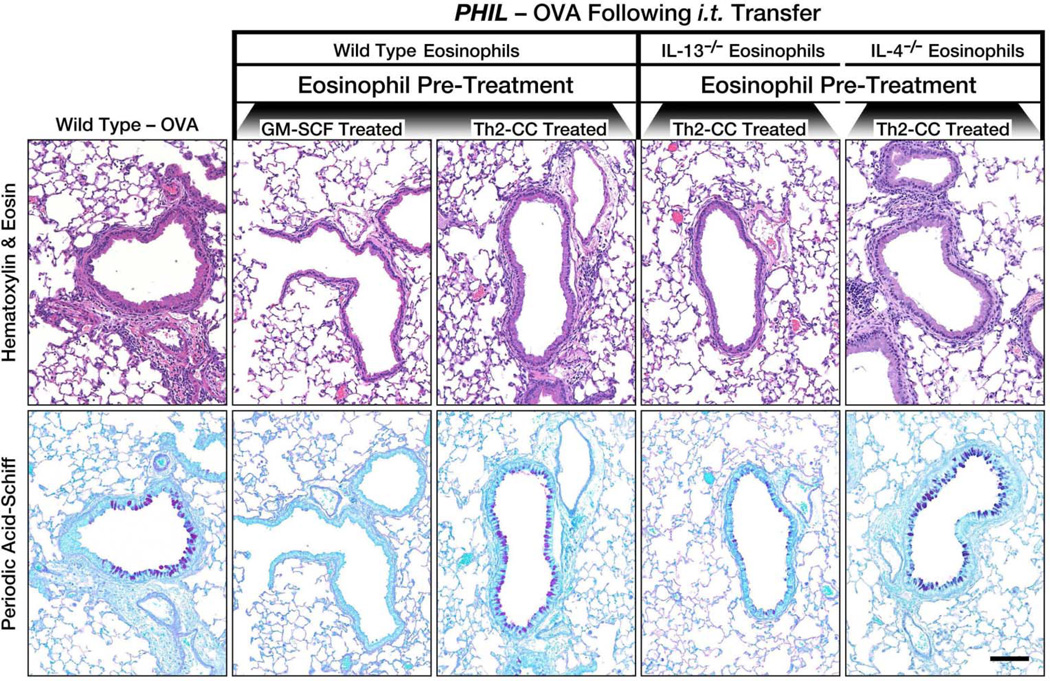
Eosinophil-derived IL-13 expression induced specific effects on the histopathological responses to allergen challenge. The induced cellular infiltration of the airways and distal airspaces (H and E stain) by Th2-cc treated eosinophils in OVA-treated PHIL mice was attenuated in OVA-treated PHIL mice receiving (i.t.) IL-13-deficient Th2-cc treated eosinophils (Figure 4). Moreover, the goblet cell metaplasia (PAS staining) in the airways of OVA-treated PHIL mice receiving IL-13-deficient Th2-cc treated eosinophils was attenuated as compared to mice receiving IL-13-proficient Th2-cc treated eosinophils during allergen challenge. The histopathologies occurring in OVA-treated PHIL mice receiving (i.t.) IL-4-deficient Th2-cc treated eosinophils were comparable to those occurring in wild type and PHIL mice following the transfer (i.t.) of wild type Th2-cc treated eosinophils, indicating a role for eosinophil-derived IL-13, but not IL-4, for the induction of histopathologies in this model of allergic asthma.
Figure 4. Eosinophil-derived IL-13 uniquely contributes to the Th2-associated pulmonary histopathologies occurring in the lungs of OVA challenged PHIL mice following eosinophil adoptive transfer.
Th2-associated pulmonary histopathologies in OVA-treated PHIL mice were determined following adoptive transfer (i.t.) of wild type, IL-13−/− or IL-4−/− eosinophils pre-treated with GM-CSF [wild type eosinophils] or a Th2-cc cytokine cocktail (i.e., GM-CSF, IL-4, IL-33) [wild type, IL-13−/− or IL-4−/− eosinophils]. Representative images of lung sections (compared to OVA-treated wild type mice) are shown for assessment of cellular inflammation (hematoxylin-eosin staining) and goblet cell metaplasia/airway epithelial cell mucin accumulation (PAS staining - dark purple cells). Scale bar = 100µm.
Eosinophil-derived IL-13 is a mediator of alternatively activated (M2) macrophage accumulation in the lungs of OVA-treated PHIL mice
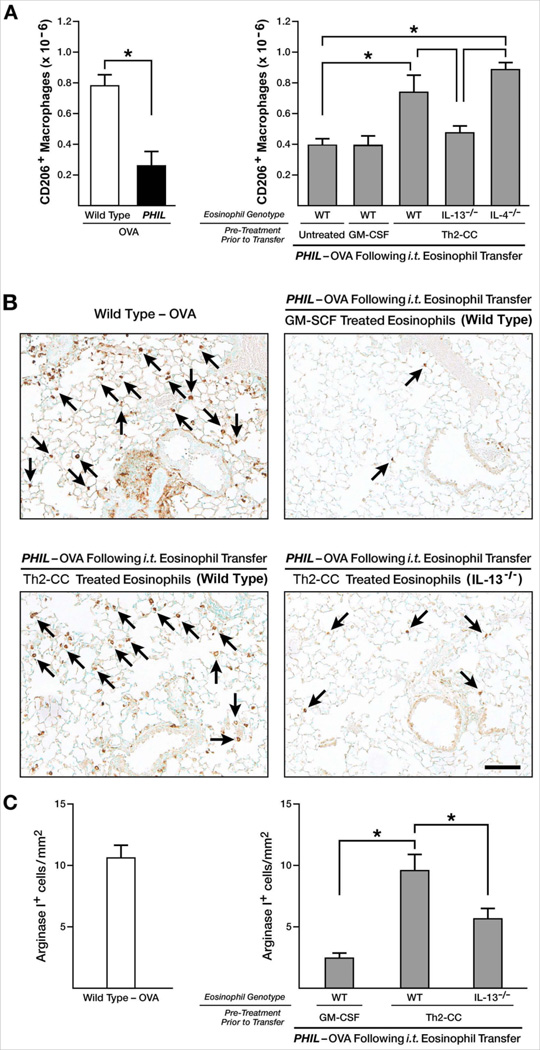
The dependence of allergen-mediated M2 macrophage accumulation on eosinophils and eosinophil-derived IL-13 was determined via two strategies. As shown in Figure 5(A), Th2-cc treated eosinophils were uniquely capable of inducing accumulation of M2 macrophages in the lungs of OVA-treated PHIL mice to levels similar to that of OVA-treated wild type mice as determined by flow cytometry identifying CD206+ macrophage (i.e, M2 (18)). A second method of detection was used to visualize the presence of arginase I-positive (i.e., M2 macrophages (18)) in the lungs of OVA-treated mice by immunohistochemistry (Figure 5(B)). Arginase I+ cells that were morphologically identified as macrophages (mononuclear cells with large cytoplasm to nucleus ratio) were counted per unit area of distal airspaces (mm2) in wild type and PHIL mice (Figure 5(C)). In agreement with the flow cytometry data, total cell counts per unit area of lung sections showed a significant increase in the numbers of M2 macrophages in mice receiving Th2-cc treated eosinophils. By using both methods, M2 macrophage accumulation in the pulmonary compartment was shown to be attenuated in OVA-treated PHIL mice receiving (i.t.) IL-13-deficient Th2-cc treated eosinophils. The role of eosinophil-derived IL-13 in this process was confirmed by demonstrating similar changes in CD206+ macrophages and arginase I+ cells in IL-5−/− mice (Supplementary Figure 7). IL-4 from eosinophils did not contribute to the accumulation of pulmonary M2 macrophages (data not shown and Supplementary Figure 7), thus demonstrating a unique role for eosinophil-derived IL-13 in inducing the accumulation of M2 polarized macrophage upon allergen challenge.
Figure 5. Eosinophil-derived IL-13, but not IL-4, contributes to accumulation of alternatively activated (M2) macrophages in the lungs of OVA challenged PHIL mice following eosinophil adoptive transfer.
(A) M2 macrophage (CD206+/F4/80+/Gr1−/CD11b+) accumulation in the lung of OVA-treated wild type and PHIL mice was determined by flow cytometry relative to OVA-treated PHIL mice following adoptive transfer (i.t.) of wild type eosinophils that were untreated (no cytokines), pretreated with GM-CSF, or pretreated with a Th2-cc cytokine cocktail (i.e., GM-CSF, IL-4, IL-33) for 24–48 hours prior to adoptive transfer (i.t.) into the lungs of mice on days 24–27 of the protocol. Additional groups of OVA-treated PHIL mice were adoptively transferred (i.t.) with IL-13−/− or IL-4−/− eosinophils pre-treated with a Th2-cc cytokine cocktail (i.e., GMCSF, IL-4, and IL-33). These data are derived from at least three independent experiments each with cohort sizes of 2–6 mice (Mean ± SEM). *p<0.05
(B) Representative images of lungs sections stained for Arginase I by immunohistochemistry. Arginase I+ macrophages (arrows) were identified as positive staining (brown color) cells with a unique morphology (large mononuclear cells with a high cytoplasm to nucleus ratio).
(C) Arginase I+ macrophages were counted in the airspaces of entire mid-coronal lung sections, counting sections derived from >3 mice per cohort in three independent experiments. The histogram shows the number of Arginase I+ cells per mm2 of distal air space. Scale bar = 100µm.
DISCUSSION
The adoptive transfer of eosinophils into allergen-provoked recipient mice congenitally deficient of eosinophils described here and elsewhere (e.g., (10, 12–14)) highlight the utility of this reductionist approach to test pathways and events that promote allergic pulmonary inflammation. The strategy employed here using this approach to identify eosinophils displaying different levels of activation represents a unique conceptual tactic worth noting: Instead of attempting to stratify eosinophils on the basis of physical properties (i.e., unique cell surface phenotype (30), sedimentary density (i.e., hypodense activated vs resting normodense eosinophils (31)), or a unique event (i.e., degranulation (32)), activation states were identified based on functionality. Specifically, the activities mediated by resting eosinophils (and therefore the identifiable functions) are inherently distinctive as compared to differentially activated eosinophils. We suspect that these differential activities mediate a transition from localized innate inflammatory/immune responses to allergen-specific T2 polarized acquired immunity that culminates in the accumulation in the lung of eosinophils, allergen-specific CD4+ effector T cells, and the development of characteristic histopathologies and lung dysfunction.
This study demonstrated that exposure to two early innate cytokine candidates associated with Th2 inflammation, GM-CSF and IL-33, were likely to have significant effects on the eosinophils accumulating in the lungs following allergen provocation. (Table 1) For example, the demonstration that GM-CSF treated eosinophils migrated to the LDLN correlated with findings by others that GM-CSF, and the up-regulation of IL-5, induced adhesion molecule expression on eosinophils (33). Moreover, increases in adhesion and motility did not appear to require exposure to IL-4/13 (34) demonstrating a unique immune function for eosinophils that are “primed” to migrate in the absence of significant pulmonary Th2 signals. The induced lymphatic trafficking ability of GM-CSF “primed” eosinophils is potentially significant given earlier studies that demonstrated LDLN eosinophils promoted T cell polarization in response to allergen challenge, even in the absence of pulmonary eosinophilia (10). Collectively, the ability of GM-CSF treated eosinophils to migrate from the lung to the LDLN and induce accumulation of mDCs and stimulate T cell proliferation suggests a unique immune regulatory function for these “primed” granulocytes. In contrast, eosinophils treated with a cocktail of GM-CSF, IL-33 and IL-4 (i.e., Th2-cc) promoted the induction of local pulmonary innate pathways that are representative of allergen provocation. These Th2-cc “activated” eosinophils were capable of both contributing to the expansion of allergen-specific T cells but also to the recruitment of effector T cells to the lung. This T cell accumulation was associated with induced lung pathologies, providing a mechanistic link among eosinophils, T cells, and allergic pulmonary pathologies. Thus, in a larger perspective this stratification suggests that eosinophil activities are not just present or absent, but instead represent a continuum of specific effector functions that have independent modulatory roles in the evolving allergic immune responses associated with asthma.
Table 1.
Cytokine-dependent Activation of Pulmonary Eosinophil Effector Functions and Their Role Upon Transfer (i.t.) into OVA-treated PHIL mice
| Eosinophil Activation | Cytokine Trigger | Defining Functional Activity(ies) |
|---|---|---|
| Resting | Untreated |
|
| Primed | GM-CSF |
|
| Activated | GM-CSF, IL-4, IL-33 |
|
Lung draining lymph node (LDLN), alternatively activated macrophage (M2), ovalbumin (OVA), myeloid dendritic cells (mDC)
Recent studies have implicated eosinophil induced IL-4 and/or IL-13 expression as key regulatory events mediated by these granulocytes in an expanding array of inflammatory/disease settings (15, 35–37). In particular, airway allergen studies using eosinophil-deficient mouse models (e.g., ΔdblGATA-1 (38), PHIL (12), and IL-5−/−eotaxin-1−/− (39)) highlight defects in IL-13 and IL-4 production upon allergen challenge that correlate with attenuated allergic inflammatory responses that occur in IL-13 and IL-4 deficient mice (17). Interestingly, in the current studies eosinophil deficiencies in either IL-4 or IL-13 expression had no role in the expansion of T cells in LDLN following allergen provocation (including the recruitment of mDC to these lymph nodes), suggesting the link among IL-13/IL-4, eosinophils, T cells, and allergic inflammation is restricted to events in the lung. We have shown in a previous report that eosinophil-dependent up-regulation of the chemokines TARC and MDC was likely responsible for the recruitment of effector Th2 T cells to the lungs of allergen challenged mice (12). The present studies provide a specific mechanism for this earlier observation. TARC and MDC are downstream targets of IL-13 (29), suggesting that “activated” pulmonary eosinophils likely lead to the recruitment of allergenspecific lung effector T cells through this IL-13 expression. The importance of eosinophil-derived IL-13 in T cell recruitment has now been demonstrated in three different strains of mice (PHIL, IL-5−/−, and ΔdblGATA-1 (14)), indicating it is a not an artifactual observation of either the allergen protocol used or the specific strain of eosinophil-deficient understudy. Our studies also highlight a role for eosinophilderived IL-13, and not IL-4, in the accumulation of M2 macrophages in the airways upon allergen provocation. This observation is noteworthy because M2 macrophages not only amplify immune responses by up-regulating expression of eotaxin-2, TARC, and MDC(40), (18), but are also intermediate cells that contribute to resolution of inflammation (41).
Significantly, a recent report (43) demonstrated that many of the IL-13 expressing cells in asthma patients are also major basic protein positive, suggesting that these cells in humans are eosinophils; a contention supported by the observations that human eosinophils have the ability to produce IL-13 (44). These studies suggest eosinophils are potentially primary modulators of allergen-induced immune responses and not simply downstream end-stage effectors unilaterally regulated by other cells (e.g., innate type 2 lymphoid cells (ILC2s) or CD4+ T cells (reviewed in (45)). Indeed, based on the studies presented here, we suggest that eosinophils themselves are an important source of IL-13 that contributes directly to the Th2 polarized immune microenvironment of the lung following allergen provocation. The self-promoting character of this proposed hypothesis and its implications on the development of allergic respiratory inflammation have not escaped us. Namely, by linking eosinophil mediated activities with mechanisms of allergen-specific T cell recruitment to the lung, a sustaining positive regulatory loop between eosinophils and acquired/adaptive immune responses is created. Moreover, if left unchecked this regulatory loop would be able to promote and sustain the pulmonary histopathologies and lung dysfunction associated with an allergic provocation. Thus, along with CD4+ T cells, ILC2s, dendritic cells, alveolar macrophages, and an equally long list of other immunity-modulating leukocytes found in the lung following allergen provocation, eosinophils are important contributors to pathways promoting the development of Th2 immune/inflammatory responses linked with allergen provocation. That is, eosinophils likely represent one of multiple immune cells in the lung that synergistically mediate immune responses leading to, and perhaps sustaining, allergic respiratory inflammation in respiratory patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank the Mayo Clinic Core Facility resources (i.e., the Animal, Flow Cytometric, and Histology Core Facilities) for their attention to detail and support of these studies. We also wish to thank members of Lee Laboratories who provided assistance in data collection, the necessary organization/infrastructure needed to complete the studies presented, and the review of various drafts of this manuscript. We also wish to acknowledge the invaluable assistance of the Mayo Clinic Arizona medical graphic artist, Marv Ruona, and the excellent administrative support provided to Lee Laboratories by Linda Mardel and Shirley (“Charlie”) Kern.
This work is supported by Mayo Foundation for Medical Education and Research, Mayo Clinic Sidney Luckman Family Predoctoral Fellowship, ADD and grants from National Institutes of Health [NAL (HL058723), JJL (HL065228), JJL (HL124165), and ADD (HL124959)] and American Heart Association [EAJ (11SDG7510043)]. Funding sources did not have a role in these activities.
Footnotes
Copyright transfer is subject to applicable Mayo terms located on the following page: http://www.mayo.edu/copyright/
AUTHOR CONTRIBUTIONS
The authors alone are responsible for study design, collection, analysis and interpretation of data, writing of the report and the decision to submit the article for publication.
The authors have no competing interests to disclose.
REFERENCES
- 1.Holgate ST. Innate and adaptive immune responses in asthma. Nature medicine. 2012;18(5):673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 2.Gleich GJ, Loegering DA. Immunobiology of eosinophils. Annu Rev Immunol. 1984;2:429–459. doi: 10.1146/annurev.iy.02.040184.002241. [DOI] [PubMed] [Google Scholar]
- 3.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in Health and Disease: The LIAR Hypothesis. Clinical and Experimental Allergy. 2010;40(4):563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am J Pathol. 2006;169(6):2117–2126. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305(5691):1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 7.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Shiraishi Y, Ashino S, Han J, Jia Y, Wang M, et al. Eosinophils Contribute to the Resolution of Lung Allergic Responses Following Repeated Allergen Challenge. Journal of Allergy & Clinical Immunology. 2014 doi: 10.1016/j.jaci.2014.08.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187(11):6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H-B, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. Journal of Immunology. 2007;179(11):7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic Pulmonary Inflammation in Mice is Dependent on Eosinophil-induced Recruitment of Effector T Cells. Journal of Experimental Medicine. 2008;205(3):699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, et al. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J. Exp. Med. 2008;205(6):1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh ER, Thakar J, Stokes K, Huang F, Albert R, August A. Computational and experimental analysis reveals a requirement for eosinophil-derived IL-13 for the development of allergic airway responses in C57BL/6 mice. J Immunol. 2011;186(5):2936–2949. doi: 10.4049/jimmunol.1001148. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen EA, Lee NA, Lee JJ. Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clinical & Experimental Allergy. 2014;44(9):1119–1136. doi: 10.1111/cea.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 17.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184(4):1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annual review of immunology. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158(3):1332–1344. [PubMed] [Google Scholar]
- 20.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9(3):423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 21.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 22.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 Exacerbates Eosinophil- Mediated Airway Inflammation. J Immunol. 2010;185(6):3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 23.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81(6):1434–1444. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 24.Wen T, Besse JA, Mingler MK, Fulkerson PC, Rothenberg ME. Eosinophil adoptive transfer system to directly evaluate pulmonary eosinophil trafficking in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):6067–6072. doi: 10.1073/pnas.1220572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181(6):4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: Gateway to allergic sensitization. The Journal of allergy and clinical immunology. 2014;134(3):499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183(10):6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 28.Bouffi C, Rochman M, Zust CB, Stucke EM, Kartashov A, Fulkerson PC, et al. IL-33 Markedly Activates Murine Eosinophils by an NF-kappaB-Dependent Mechanism Differentially Dependent upon an IL-4-Driven Autoinflammatory Loop. J Immunol. 2013;191(8):4317–4325. doi: 10.4049/jimmunol.1301465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Curr Allergy Asthma Rep. 2004;4(2):123–131. doi: 10.1007/s11882-004-0057-6. [DOI] [PubMed] [Google Scholar]
- 30.Johansson MW. Activation states of blood eosinophils in asthma. Clinical and experimental allergy : Journal of the British Society for Allergy and Clinical Immunology. 2014 doi: 10.1111/cea.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuda T, Dunnette SL, Reed CE, Ackerman SJ, Peters MS, Gleich GJ. Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. American Review of Respiratory Disease. 1985;132(5):981–985. doi: 10.1164/arrd.1985.132.5.981. [DOI] [PubMed] [Google Scholar]
- 32.Kim CK, Callaway Z, Kim DW, Kita H. Eosinophil degranulation is more important than eosinophilia in identifying asthma in chronic cough. J Asthma. 2011;48(10):994–1000. doi: 10.3109/02770903.2011.623335. [DOI] [PubMed] [Google Scholar]
- 33.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51(2):213–340. [PubMed] [Google Scholar]
- 34.Johansson MW, Annis DS, Mosher DF. alpha(M)beta(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. American journal of respiratory cell and molecular biology. 2013;48(4):503–510. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153(2):376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110(24):9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103(44):16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195(11):1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez AC, Coyle AJ, et al. CC chemokine receptor (CCR)3/Eotaxin is followed by CCR4/Monocyte- derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge In vivo. J Exp Med. 2000;191(2):265–274. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ariel A, Serhan CN. New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation. Frontiers in immunology. 2012;3:4. doi: 10.3389/fimmu.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science. 2011;332(6026):243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, et al. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. The Journal of allergy and clinical immunology. 2004;114(5):1106–1109. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Schmid-Grendelmeier P, Altznauer F, Fischer B, Bizer C, Straumann A, Menz G, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169(2):1021–1027. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 45.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nature Immunology. 2013;14(6):536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.