Abstract
Objective
Angiotensin II (AngII) infusion causes aortic medial thickening via stimulation of AT1a receptors. The purpose of this study was to determine the cellular loci of AT1a receptors that mediate this AngII-induced aortic pathology.
Approach and Results
Saline or AngII was infused into AT1a receptor floxed mice expressing Cre under control of cell-specific promoters. Initially, AT1a receptors were depleted in aortic smooth muscle cell and endothelium by expressing Cre under control of SM22 and Tie2 promoters, respectively. Deletion of AT1a receptors in either cell type had no effect on AngII-induced medial thickening. To determine whether this effect was related to neural stimulation, AT1a receptors were depleted using an Eno2 driven Cre. Depletion of AT1a receptors in neural cells attenuated AngII-induced medial thickening of the ascending, but not descending aorta. Lineage tracking studies, using ROSA26-LacZ, demonstrated that Eno2 was also expressed in adventitial cells adjacent to the region of attenuated thickening. To determine whether adventitial fibroblasts contributed to this attenuation, AT1a receptors in fibroblasts were depleted using S100A4 driven Cre. Similar to Eno2-Cre, AngII-induced medial thickening was attenuated in the ascending, but not the descending aorta. Lineage tracking demonstrated an increase of S100A4-LacZ positive cells in the media of the ascending region during AngII infusion.
Conclusion
AT1a receptor depletion in fibroblasts attenuates AngII-induced medial hyperplasia in the ascending aorta.
Keywords: Angiotensin II, Endothelium, Smooth muscle cell, Fibroblasts, Angiotensin II type 1a receptor, Medial thickening
INTRODUCTION
Angiotensin II (AngII) infusion into rodents promotes aortic pathology including pronounced medial thickening that has been attributed to smooth muscle cell (SMC) hypertrophy and hyperplasia.1–3 AngII infusion promotes a uniform degree of medial thickening throughout the aorta due to region-specific mechanisms, with hyperplasia in the ascending aorta and hypertrophy in the rest of the aorta.3 While AngII infusion increases blood pressure, equivalent increases during norephinephrine infusion does not cause medial thickening. This is consistent with AngII-induced arterial medial thickening being independent of blood pressure in mice.3,4
Both pharmacological and genetic manipulations have demonstrated that AngII-induced medial thickening is attributable to stimulation of AT1 receptors, or AT1a receptors in rodents.3,5 The role of AT1 receptors in promoting medial thickening has been inferred from cell culture studies that have demonstrated AngII promotes SMC hypertrophy and hyperplasia.6–8 AngII-induced aortic medial thickening is inhibited by co-administration of losartan to antagonize AT1 receptors.9 Furthermore, AT1a receptor deletion prevents AngII-induced medial expansion in mice.3 Deletion of AT1b receptors had no effect on aortic changes during AngII infusion, despite its mediation of aortic contractile responses.10,11
While there has been uniformity in defining AT1a receptors as regulators of AngII-induced aortic changes in vivo, the cellular loci of this effect has not been determined. Development of AT1a receptor floxed mice provides an approach to examine cell-specific effects of AngII stimulation.12,13 Since medial thickening is entirely attributable to hypertrophic and hyperplastic responses in SMCs, this seems the most likely effector cell during AngII infusion. However, AT1a receptor deletion in a SMC-specific manner had no effect on aortic medial expansion.5 Therefore, the cell type activated by AngII to promote aortic thickening remains unknown. The present study initially determined the effects of AT1a receptor deletion in SMCs and confirmed a previous study in finding no effect on AngII-induced aortic medial thickening.5 Subsequently, we examined a series of cell types to identify those responsible for AngII-induced medial expansion. These studies identified AT1a receptor stimulation on fibroblasts as the basis for AngII-induced aortic medial expansion in the ascending region.
MATERIALS AND METHODS
Detailed description of Materials and Methods are available in the online only Data Supplement.
RESULTS
SMC-specific AT1aR deficiency had no effect on AngII-induced hypertension and aortic medial thickening
Since medial thickening is attributable to vascular SMC proliferation, this cell type is an obvious potential target for AngII. First, we generated mice with AT1a receptor deletion in SMCs by expressing Cre under control of the SM22 promoter. Deletion of AT1a receptor expression was validated by mRNA abundance as described previously.12 Male non-transgenic littermates (SM22-Cre 0/0) were used as wild type controls. Both SM22-Cre 0/0 and SMC-specific AT1a receptor deficient (SM22-Cre +/0) mice were infused with either saline or AngII for 28 days. SMC-specific deletion of AT1a receptors did not affect body weight during AngII infusion (Table I in the online-only Data Supplement). To examine the role of SMC-specific AT1a receptor deficiency in blood pressure responsiveness to AngII, we measured systolic blood pressure prior to and during the last week of AngII infusion. SMC-specific AT1a receptor deficiency had no effect on systolic blood pressure during AngII infusion (Table I in the online-only Data Supplement), in agreement with other studies.5
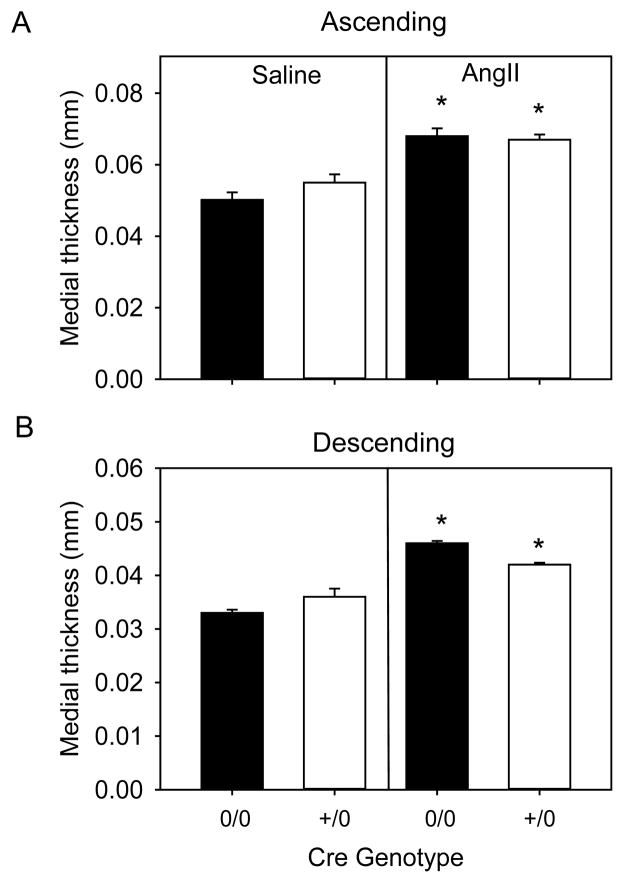
To quantify AngII-induced vascular changes, medial thickness and area were measured by morphometric analysis in the ascending and descending aorta. Medial thickness and area significantly increased in mice infused with AngII compared to saline groups in both ascending and descending regions (p<0.05, Figure 1A and 1B, Table II in the online-only Data Supplement). However, medial thickness in both ascending and descending aortic regions were not different between SM22-Cre 0/0 and SM22-Cre +/0 mice infused with AngII (Figure 1A and 1B, Table II in the online-only Data Supplement). Medial areas in both aortic regions were also similar between AngII groups (Table I in the online-only Data Supplement), demonstrating that deficiency of AT1a receptors in SMCs had no effect on AngII-induced medial thickening in either the ascending or descending aorta.
Figure 1. SMC-specific AT1a receptor deficiency had no effect on AngII-induced aortic medial thickening.
Medial thickness was measured in ascending (A) and descending (B) aortas from AT1a receptor SM22-Cre 0/0 and SM22-Cre +/0 mice infused with either saline (n=4 in each genotype) or AngII (n=6–10 in each genotype). Histobars with error bars are mean ± SEM. * denotes P<0.05 for comparisons of saline versus AngII within Cre genotype by two-way ANOVA with Holm-Sidak post hoc test.
Endothelial cell specific-AT1aR deficiency had no effect on AngII-induced hypertension and aortic medial thickening
Endothelial cells also represent a potential target since this cell type is juxapositioned to the aortic medial layer and secretes bioactive substances that influence SMC function. We generated endothelial cell-specific AT1a receptor deficient mice by breeding AT1a receptor floxed mice to mice expressing Cre controlled by the Tie2 promoter. Validation of receptor deletion was described previously.12 Similar to SMCs, male non-transgenic littermates (Tie2-Cre 0/0) were compared to endothelial cell-specific AT1a receptor deficient (Tie2-Cre +/0) mice and infused with either saline or AngII for 28 days. Endothelial cell-specific AT1a receptor deficiency had no effect on body weight or blood pressure during AngII infusion (Table I in the online-only Data Supplement).
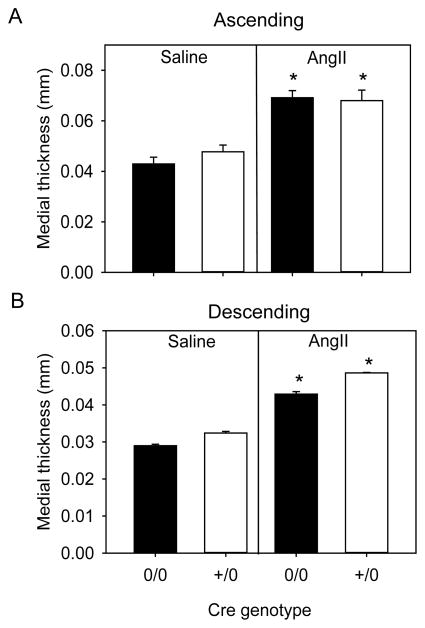
Medial thickness and area of ascending and descending aortas were measured to determine the role of endothelial cells in AngII-induced vascular pathology. As expected, medial thickness and medial area significantly increased in mice infused with AngII compared to saline in both ascending and descending regions of aortas (p<0.05, Figure 2A and 2B, Table I and III in the online-only Data Supplement). However, medial thickness and area were not different between AngII infused groups in both aortic regions examined in Tie2-Cre 0/0 and Tie2-Cre +/0 mice (Figure 2A and 2B and Tables I and III in the online-only Data Supplement).
Figure 2. Endothelial cell-specific AT1a receptor deficiency had no effect on AngII-induced aortic medial thickening.
Medial thickening was measured in ascending (A) and descending (B) aortas from AT1a receptor Tie2-Cre 0/0 and Tie2-Cre +/0 mice infused with either saline (n= 4 in each genotype) or AngII (n=6–10 in each genotype). Histobars with error bars are mean ± SEM. * denotes P<0.05 for comparisons of saline versus AngII within Cre genotype by two-way ANOVA with Holm-Sidak post hoc test.
Neuronal specific AT1aR deficiency had no effect on AngII-induced blood pressure changes, but decreased medial hyperplasia in the ascending aorta
To assess whether AngII stimulates a neuronal response that would promote medial changes, AT1a receptor floxed mice were bred to mice expressing Cre driven by the Eno2 promoter. AT1a receptor deficient mice with Cre expression driven by the Eno2 promoter (Eno2-Cre +/0) were compared to male non-transgenic littermates (Eno2-Cre 0/0). These mice were infused with either saline or AngII for 28 days. AT1a receptor depletion was validated by demonstrating a decrease of mRNA abundance of the AT1a receptor in the brain of Eno2-Cre +/0 mice compared to non-transgenic littermates (Figure IA in the online-only Data Supplement). There were no differences in body weight among all the study groups. Eno2-Cre +/0 and Eno2-Cre 0/0 mice had similar increases of blood pressure in response to AngII infusion (Table IV in the online-only Data Supplement).
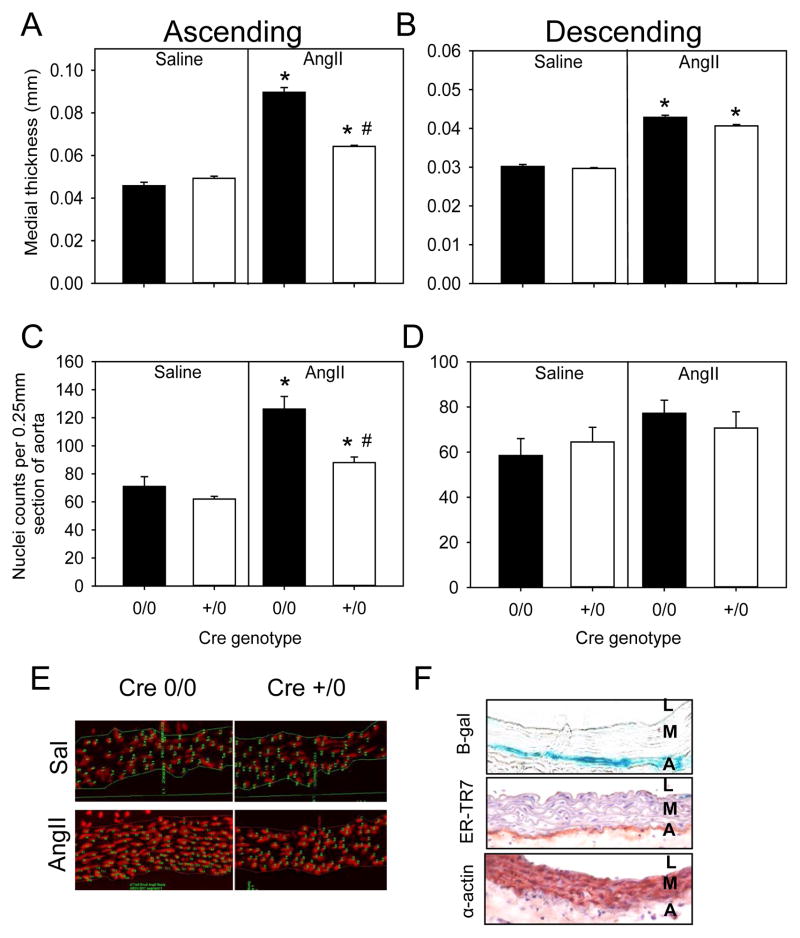
We measured aortic medial thickness to determine the effects of neuronal deletion of AT1a receptors during AngII infusion. As expected, there was a significant increase in medial thickness and area in mice infused with AngII compared to saline groups in Eno2-Cre 0/0 mice (p<0.05; Figure 3A and B, Tables IV and V in the online-only Data Supplement). Interestingly, there was a significant decrease in medial thickness in AT1a receptor depletion in Eno2-Cre +/0 compared to Eno2-Cre 0/0 mice in the ascending aorta with AngII infusion (p<0.05, Figure 3A). However, this reduction was not observed in the descending aorta during AngII infusion (Figure 3B).
Figure 3. Neuronal-specific AT1a receptor deficiency attenuated AngII-induced medial hyperplasia in the ascending aorta.
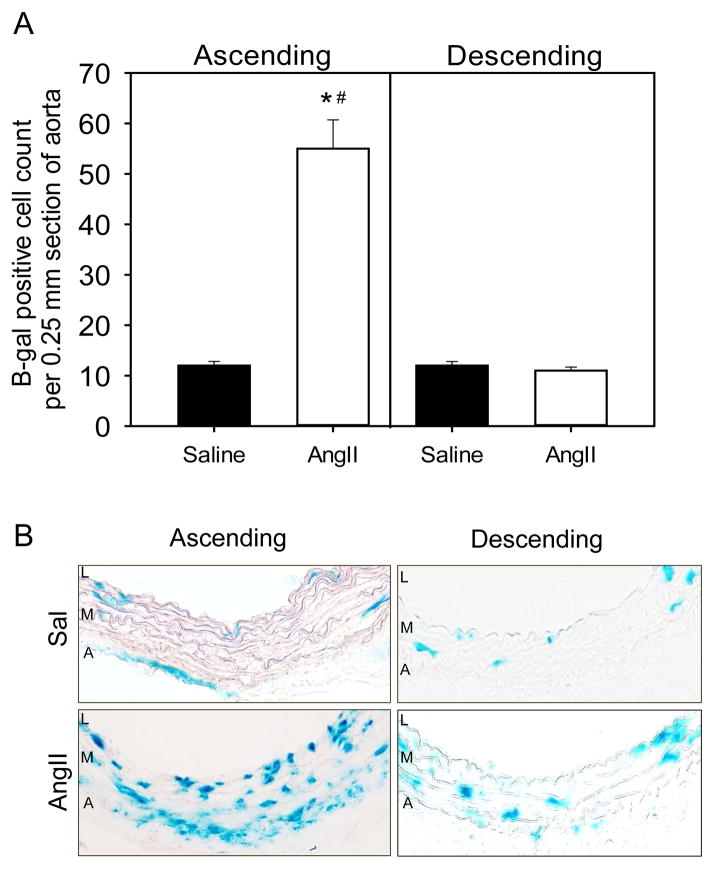
Medial thickening was measured in ascending (A) and descending (B) aortas from AT1a receptor Eno2-Cre 0/0 and Eno2-Cre +/0 mice infused with either saline (n= 4 in each genotype) or AngII (n=8–12 in each genotype). Histobars are mean ± SEM. * denotes P<0.05 for comparisons of saline versus AngII within Cre genotype, and # denotes Eno2-Cre 0/0 versus Eno2-Cre +/0 AngII infused groups within ascending aorta by two-way ANOVA with Holm-Sidak post hoc test. Nuclei density were normalized to aortic section length (0.25 mm) in ascending (C) and descending (D) aortic sections from AT1a receptor Eno2-Cre 0/0 and Eno2-Cre +/0 mice infused with either saline (n=3) or AngII (n=4). Histobars with error bars represent mean ± SEMs. * denotes P<0.05 for comparisons of saline versus AngII within Cre genotype and # denotes P<0.05 for comparing Eno2-Cre 0/0 versus Eno2-Cre +/0 within AngII infused groups by two-way ANOVA with Holm-Sidak post hoc test. (E) Representative ascending aortic sections stained with propidium iodide from AT1a receptor Eno2-Cre 0/0 and Eno2-Cre +/0 mice infused with either saline or AngII. Nuclei stain red. (F) Ascending aortic sections were stained for β-gal, reticular fibroblasts (ER-TR7) and SMCs (α-actin) in Eno2-LacZ mice infused with AngII. L=Lumen, M=Media and A=Adventitia.
AngII-induced medial thickening is attributable to an increase in space between intra-laminar layers of the aorta.3 Our previous studies have demonstrated that AngII-induced aortic medial expansion in the ascending aorta was due to hyperplasia.3 Therefore, we compared ascending and descending aortic tissue sections stained with propidium iodide.3 We found no difference in nuclei numbers in either ascending and descending aortas of saline-infused mice between genotypes (Figures 3C and 3D). However, AngII infusion into Eno2-Cre +/0 mice significantly decreased number of nuclei compared to Eno2-Cre 0/0 mice (p< 0.05, Figures 3C) in the ascending aorta. Therefore, these results demonstrated that AT1a receptor deficiency under control of the Eno2 promoter attenuated AngII-induced medial hyperplasia in the ascending aorta.
Many cell-specific promoters have promiscuous expression that lead to broader expression than defined by their nomenclature.14 To determine the specificity of the Eno2-driven Cre, we bred these transgenic mice to ROSA26-lacZ reporter mice. Whole brain tissue mounts showed presence of β-galactosidase (β-gal) activity in Eno2-Cre +/0 mice, whereas no positive activity was detected in non-transgenic littermates (Figure IB in the online-only Data Supplement). Interestingly, we also found β-gal activity restricted to the ascending aorta, with no expression in the descending aorta in Eno2-Cre expressing mice (Figure IC in the online-only Data Supplement). Furthermore, to determine expression of Eno2-lacZ in the aorta, we serially sectioned the ascending aorta and found that β-gal staining co-localized with reticular fibroblast immunostaining, but not with α-actin, a smooth muscle marker (Figure 3F). Our results delineated that in addition to neuronal tissues, Eno2-promoter driven Cre led to depletion of floxed genes in the adventitia of the ascending aorta.
Fibroblast cell-specific AT1aR deficiency had no effect on AngII-induced blood pressure but attenuated medial hyperplasia in the ascending aorta
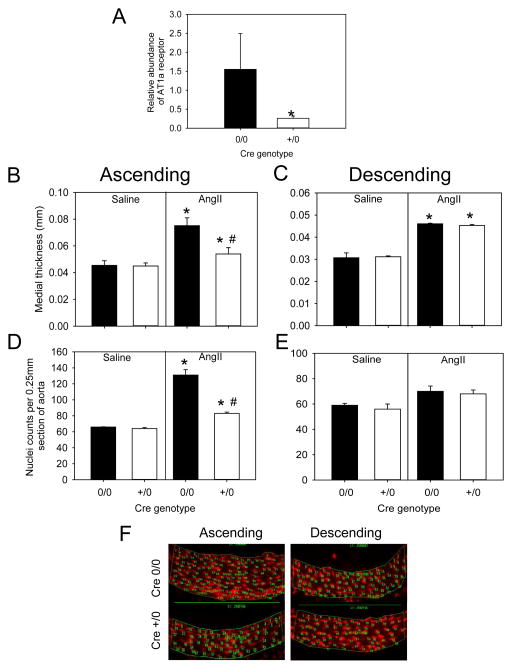
To define the role of fibroblasts, we generated fibroblast cell-specific AT1a receptor deficient mice by breeding AT1a receptor floxed mice to mice with the Cre transgene under control of the S100A4 promoter. Depletion of AT1a receptors was validated with significant reduction of receptor mRNA abundance in aortic adventitia in AT1a receptor cell-specific deficient mice (S100A4-Cre +/0) compared to non-transgenic littermates (S100A4-Cre 0/0; p< 0.05; Figure 4A). Male S100A4-Cre 0/0 and S100A4-Cre +/0 mice were infused with either saline or AngII for 28 days. Genotype and infusion had no effect on body weight. Blood pressure increased during AngII infusion to a similar rate in the two genotypes (Table VI in the online-only Data Supplement).
Figure 4. Fibroblast cell-specific AT1a receptor deficiency decreased AngII induced medial hyperplasia in the ascending aorta.
(A) Abundance of AT1a receptor mRNA in aortic adventitia of AT1a receptor floxed S100A4-Cre 0/0 (n=3) and S100A4-Cre +/0 (n=3) mice. Histobars with error bars represent mean ± SEM. * denotes P<0.05 in comparing S100A4-Cre 0/0 versus S100A4-Cre +/0 mice by Student’s t-test. Medial thickness was measured in ascending (B) and descending (C) aortas from AT1a receptor floxed S100A4-Cre 0/0 and S100A4-Cre +/0 mice infused with either saline (n=4–6 in each genotype) or AngII (n=8–10 in each genotype). Histobars with error bars are mean ± SEMs. * denotes P<0.05 when comparing saline versus AngII and # denotes P<0.05 when comparing S100A4-Cre 0/0 versus S100A4-Cre +/0 in AngII infused groups by two-way ANOVA with Holm-Sidak post hoc test. Nuclei density represented by normalization to aortic section length (0.25 mm) in ascending (D) and descending (E) sections from AT1a receptor floxed S100A4-Cre 0/0 and S100A4-Cre +/0 mice infused with either saline (n=3 in each genotype) or AngII (n=5 in each genotype). Histobars with error bars represent mean ± SEMs. * denotes P<0.05 when comparing saline versus AngII within Cre genotype and # denotes P<0.05 when comparing S100A4-Cre 0/0 versus S100A4-Cre +/0 in AngII infused groups of the ascending aortic by two-way ANOVA with Holm-Sidak post hoc test. (F) Representative photomicrographs of ascending and descending aortas stained with propidium iodide from AT1a receptor S100A4-Cre 0/0 and S100A4-Cre +/0 mice infused with AngII.
Medial thickness and area were measured in S100A4-Cre 0/0 and S100A4-Cre +/0 mice infused with either saline or AngII for 28 days. Consistent with both groups used in this study, AngII increased medial thickness and area (P<0.05; Figure 4B and 4C, Tables VI and VII in the online-only Data Supplement). However, AngII-infused S100A4-Cre +/0 mice had significantly decreased medial thickness compared to S100A4-Cre 0/0 mice in the ascending aorta (p<0.05, Figure 4B). In contrast, medial thickness or area were not different in descending aortas between the two genotypes infused with AngII (Figure 4C, Tables VI and VII in the online-only Data Supplement). Our results demonstrate that fibroblast cell-specific AT1a receptor deficiency attenuated AngII-induced medial thickening in the ascending aorta.
In a subsequent study, ascending and descending aortic regions of S100AA4-Cre 0/0 and +/0 mice infused with either saline or AngII were stained with propidium iodide. There was no difference in nuclei counts in ascending and descending aortas of saline-infused mice (Figure 4D and 4E). However, AT1a receptor S100A4-Cre +/0 aortas had fewer number of nuclei compared to 0/0 ascending aortas during AngII-infusion (P<0.05, Figure 4D and 4F). In contrast, there was no change in nuclei counts of descending aortas after AngII infusion between the two genotypes (Figure 4E and 4F). Therefore, deficiency of AT1a receptor under control of the S100A4 promoter attenuated AngII-induced medial hyperplasia in the ascending aorta.
Presence of lineage tracked S100A4 positive cells in adventitial and medial layers of the ascending aorta
Since AT1a receptor deficiency driven by the S100A4 promoter is involved in medial hyperplasia, we examined cell lineage tracking of S100A4 in aortic tissues. This involved breeding S100A4-Cre mice to ROSA26-lacZ mice and infusing the progeny with either saline or AngII for 28 days. At termination, ascending and descending aortas were harvested and serial sections were stained for β-gal. Nuclei positive for β-gal were counted in both ascending and descending aortic regions (Figure IIIA in the online-only Data Supplement). Nuclei counts increased significantly after AngII infusion compared to saline infusion in the ascending region (p<0.05, Figure 5A). In saline-infused mice, S100A4-lacZ expression was detected sparsely in the media and adventitia of ascending and descending aortas (Figure 5B). However, after 28 days of AngII infusion, S100A4-lacZ expression increased as more positive staining nuclei were detected in adventitial and medial layers of ascending aortas (Figure 5B). In contrast, there was no change in S100A4-lacZ positive cell numbers present in descending aortas during AngII infusion (Figure 5B).
Figure 5. S100A4 positive cells increase in media during AngII infusion in the ascending aorta.
(A) Nuclei density represented by normalization to aortic section length (0.25 mm) in ascending and descending aortic regions from S100A4-Cre ROSA26-lacZ mice infused with either saline or AngII. Histobars represent mean ± SEMs. * denotes P<0.05 when comparing saline versus AngII within aortic regions and # denotes P<0.05 when comparing in AngII infused groups between aortic regions by two way ANOVA with Holm-Sidak post hoc test. (B) Representative photomicrographs of ascending and descending aortic sections from S100A4-Cre ROSA26-lacZ mice stained for β-gal prior to and after AngII infusion. Blue staining cells are positive for β-gal. L=Lumen, M=Media and A=Adventitia.
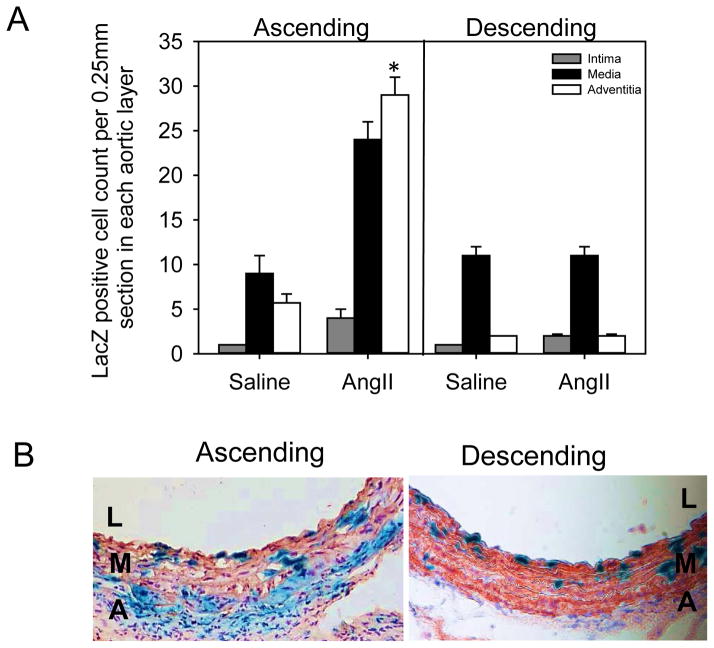
We further examined β-gal positive nuclei counts in the three layers of the aorta - intima, media and adventitia (Figure 6A). The results demonstrated that nuclei positive for β-gal were increased significantly in the adventitial and medial layers after 28 days of AngII infusion only in the ascending aortic region (Figure 6A and Figure III online-only Data Supplement). However, there was no change in the number of nuclei positive for β-gal in the descending region (Figure 6A). Throughout the aortic media, nuclei positive for β-gal were also positive for α-actin (Figure 6B).
Figure 6. Increase of S100A4-LacZ positive nuclei in adventitia and medial layers of the AngII-infused ascending aorta.
(A) Nuclei density represented by normalization to aortic section length (0.25 mm) in the three layers (intima, media and adventitia) of ascending and descending aortic regions from S100A4-Cre ROSA26-lacZ mice infused with either saline or AngII. Histobars with error bars represent mean ± SEMs. * denotes P<0.05 when comparing saline versus AngII in media and adventitial layers by two-way ANOVA with Holm-Sidak post hoc test. (B) Representative photomicrographs of ascending and descending aortic sections stained for β-gal (blue) and α-actin (red) in S100A4-Cre ROSA26-lacZ mice infused with AngII for 28 days. L=lumen, M=media and A=adventitia.
Serial sections of saline or AngII infused ascending aortas were examined for cell types and mesenchymal phenotype. As expected, medial SMCs stained positive for α-actin. Fibroblast immunostaining for reticular subtypes with ER-TR7, were more abundant in adventitia of AngII-infused aortas compared to saline-infused aortas. While immunostaining for S100A4 yielded diffuse positive areas across the AngII-infused aorta. Mesenchymal phenotypic staining for vimentin was predominant in all cell types of the ascending aorta of AngII infused mice (Figure IV in the online-only Data Supplement).
DISCUSSION
AngII infusion into mice promotes uniform aortic medial thickening attributable to expansion of either the number or size of SMCs in a region-specific manner.3 Several studies have demonstrated that AngII-induced medial expansion is due to stimulation of AT1 receptors.3,5 In rodents, the AT1a subtype mediates these effects.3,5 The AT1 receptor expressing cell type responsible for this hypertrophy has not been defined and was the basis for the present studies that generated AT1a receptor deficiencies in smooth muscle, endothelial, neural, and fibroblasts cells. Using these mice with cell-specific deletion of AT1a receptors, the present study demonstrated that AngII stimulation of AT1a receptors in fibroblasts plays a critical role in medial thickening of the ascending aorta.
AngII-induced medial thickening is due primarily to increased distance between elastin layers with cells that immunostain for SMC markers.3,5 Also, cultured SMCs respond to AngII with hypertrophic and hyperplastic responses via AT1 receptor stimulation.1,8 Therefore, deletion of AT1a receptors in SMCs would be expected to attenuate medial thickening in vivo. Despite this expectation, previous studies demonstrated that AT1a receptor deletion from SMCs had no effect on medial thickening or blood pressure changes in response to AngII infusion.5,13 This was confirmed in the present study. While AT1a receptor mRNA is present in mouse aortic SMCs, its function is unclear.3 Ex vivo, mouse aortic rings do not contract to AngII, except for the infra-renal region.12,15,16 Aortic tissue also expresses AT1b receptors, but deletion of this subtype had no effect on AngII-induced aortic pathology.11 Therefore, there has been consistent observations in the restricted responses of mouse aortic SMCs in vivo to AngII. The negative effects are also potentially attributable to incomplete deletion of AT1a receptors. We were unable to determine the deletion of the AT1 receptor protein due to limitations of available antibodies.12,17,18 However, previous studies have demonstrated the highly efficient deletion of genes including AT1a receptors3,5 using the SM22 Cre promoter.19 Hence, the collective data is consistent with aortic medial thickening not being attributable to a direct effect of AngII on SMCs.
Endothelial cells also express AT1a receptors and release potential mediators that can influence changes in SMC function, including adversely regulating nitric oxide and oxygen radical availability.20 However, depletion of AT1a receptors had no effect on blood pressure, as described previously.12,13 We have demonstrated previously that deletion of AT1a receptors in endothelial cells had a modest effect on reducing AngII-induced expansion of aortic arches and maximal medial thickness in hyper-cholesterolemic mice.12 The ability to discern effects of endothelial-specific AT1a receptor depletion in these earlier studies may be attributable to hypercholesterolemia-induced augmentation of AngII responses in AT1a receptor wild-type mice.21,22 However, in the present study, endothelial-specific depletion of AT1a receptors had no discernable effect on AngII-induced medial expansion.
Since deletion of AT1a receptors on two major cell types in aortic intimal and medial tissue showed no effect on AngII-induced blood pressure changes, other cell types were investigated. This included neuronal cells that exert a pivotal role in the renin angiotensin system and blood pressure regulation.23 A previous study showed that mice with AT1a receptor overexpression, under control of an Eno2 promoter, had enhanced responses to intracerebroventricularly injected AngII.24 Our studies used an Eno2 regulated Cre to delete AT1a receptors in neuronal cells and we were unable to discern an effect on blood pressure responses during peripheral administration of AngII. Despite this lack of effect on blood pressure, there was a pronounced reduction in AngII-induced medial thickening in the ascending aorta. One potential interpretation of this result is that the Eno2 promoter could delete AT1a receptors in cell types other than neurons. Expression of the Eno2 promoter has been demonstrated in many regions of the brain, but also in peripheral tissues.25 In our own studies, there was a striking presence of Eno2 lineage-tracked cells in the adventitia of the ascending aorta, that co-localized with markers of reticular fibroblasts. This spatial expression was coincident with the region in which medial AngII-induced thickening was attenuated in AT1a receptor floxed mice expressing Eno2 regulated Cre. These findings inferred that AT1a receptor expression in fibroblasts mediated some aspects of AngII-induced medial thickening.
There is increasing awareness that adventitial cells exert a profound effect on the arterial wall.26 This includes adventitial fibroblasts that are thought to contribute to aortic pathologies.27–29 To determine the role of AT1a receptor expression on fibroblasts, we used mice expressing Cre under control of the S100A4 promoter. There is an increasing complexity to fibroblast phenotypes,30 with S100A4 not being ubiquitously expressed in all subtypes.31 Conversely, S100A4 promoter expression may also occur in other cell types, such as SMC, endothelium, and hematopoietic cells.32–34 Therefore, the expression Cre under the control of the S100A4 promoter is unlikely to specifically delete AT1a receptor only in fibroblasts. However, this study failed to demonstrated any effect of deleting AT1a receptors in mice expressing Cre under the control of SM22, that expresses in SMCs,14 and Tie2 that expresses in endothelial and hematopoietic cells.35 Therefore, the use of multiple Cre transgenics enhances that likelihood that the observed phenotype was due to lack of ATa1 receptor stimulation in a fibroblast-specific manner.
The mechanisms by which deletion of AT1a receptors in S100A4 expressing cells attenuated medial thickening in the ascending aortic media remain to be determined. Lineage tracking studies demonstrated that AngII promoted a marked increase of S100A4 expressing cells in the media of the ascending aorta during AngII infusion. Immunostaining for LacZ positive medial cells co-localized with the SMC marker, alpha actin, while being unstained by markers for reticular fibroblasts. One potential basis for the presence of lineage tracked cells in the media is migration from the adventitia. Consistent with this proposal, several studies have provided evidence that fibroblasts migrate and acquire SMC-like phenotypes in the arterial wall in response to injury.36 Alternatively, the increased presence of LacZ positive cells in the media may be a consequence of AngII influencing phenotypic modulation that is a function of SMC plasticity.37 Future studies will need to incorporate a broader range of approaches for manipulating fibroblast phenotype to determine the function of this cell type.
In summary, this study used multiple lines of mice with different cell-specific deletion of AT1a receptors to garner insight into mechanisms of AngII-induced medial thickening. Results confirmed a previous study that delineated AT1a receptor expression on SMCs was not responsible for this pathological change. The pivotal finding that S100A4 expressing cells attenuated AngII-induced medial expansion in the ascending aorta provides a stimulus for further study of fibroblast phenotypes in arterial pathology.
Supplementary Material
Significance.
Angiotensin II (AngII) promotes smooth muscle cell (SMC) proliferation in the media of the aorta. The receptor responsible for this effect is the angiotensin II type 1a (AT1a) receptor in rodents. Surprisingly, deletion of the AT1a receptor in SMCs had no effect on AngII-mediated cellular proliferation. Deletion of AT1a receptors in endothelial cells also had no effect. Unexpectedly, deletion of AT1a receptors in neuronal cells using the Eno2 promoter significantly attenuated AngII-induced medial proliferation in the ascending aorta. Lineage tracking detected Eno2 positive cells in the adventitia of the ascending aorta that co-localized with fibroblasts. Deletion of the AT1a receptor in fibroblasts also significantly attenuated AngII-mediated cellular proliferation in the ascending aorta.
Acknowledgments
SOURCES OF FUNDING
These studies were supported by funding from the National Heart, Lung, and Blood Institute Grants HL107319 to AD and American Heart Association Great Rivers Affiliate Post Doctoral Fellowships: 10POST3140016 and 12POST12030363 to AP.
Abbreviations
- SMC
Smooth muscle cells
- Ang
Angiotensin
- β-gal
β-galactosidase
- Eno2
Enolase 2
Footnotes
DISCLOSURES: None.
References
- 1.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension. 2005;46:732–737. doi: 10.1161/01.HYP.0000182660.74266.6d. [DOI] [PubMed] [Google Scholar]
- 3.Owens AP, 3rd, Subramanian V, Moorleghen JJ, Guo Z, McNamara CA, Cassis LA, Daugherty A. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differentiation 3. Circ Res. 2010;106:611–619. doi: 10.1161/CIRCRESAHA.109.212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su EJ, Lombardi DM, Siegal J, Schwartz SM. Angiotensin II induces vascular smooth muscle cell replication independent of blood pressure. Hypertension. 1998;31:1331–1337. doi: 10.1161/01.hyp.31.6.1331. [DOI] [PubMed] [Google Scholar]
- 5.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1418. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 7.Stouffer GA, Owens GK. Angiotensin II-induced mitogenesis of spontaneously hypertensive rat-derived cultured smooth muscle cells is dependent on autocrine production of transforming growth factor-beta. Circ Res. 1992;70:820–828. doi: 10.1161/01.res.70.4.820. [DOI] [PubMed] [Google Scholar]
- 8.Touyz RM, He G, Deng LY, Schiffrin EL. Role of extracellular signal-regulated kinases in angiotensin II-stimulated contraction of smooth muscle cells from human resistance arteries. Circulation. 1999;99:392–399. doi: 10.1161/01.cir.99.3.392. [DOI] [PubMed] [Google Scholar]
- 9.Sabri A, Levy BI, Poitevin P, Caputo L, Faggin E, Marotte F, Rappaport L, Samuel JL. Differential roles of AT1 and AT2 receptor subtypes in vascular trophic and phenotypic changes in response to stimulation with angiotensin II. Arterio Thromb Vasc Biol. 1997;17:257–264. doi: 10.1161/01.atv.17.2.257. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Z, Zhang SH, Wagner C, Kurtz A, Maeda N, Coffman T, Arendshorst WJ. Angiotensin AT1B receptor mediates calcium signaling in vascular smooth muscle cells of AT1A receptor-deficient mice. Hypertension. 1998;31:1171–1177. doi: 10.1161/01.hyp.31.5.1171. [DOI] [PubMed] [Google Scholar]
- 11.Poduri A, Owens AP, 3rd, Howatt DA, Moorleghen JJ, Balakrishnan A, Cassis LA, Daugherty A. Regional variation in aortic AT1b receptor mRNA abundance is associated with contractility but unrelated to atherosclerosis and aortic aneurysms. PLoS One. 2012;7:e48462. doi: 10.1371/journal.pone.0048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, Daugherty A. Depletion of endothelial or smooth muscle cell-specific angiotensin II type 1a receptors does not influence aortic aneurysms or atherosclerosis in LDL receptor deficient mice. PLoS One. 2012;7:e51483. doi: 10.1371/journal.pone.0051483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JC, Kim S, Helmke BP, Yu WW, Du KL, Lu MM, Strobeck M, Yu Q, Parmacek MS. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol Cell Biol. 2001;21:1336–1344. doi: 10.1128/MCB.2001.21.4.1336-1344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Dirksen WP, Babu GJ, Periasamy M. Differential vasoconstrictions induced by angiotensin II: the role of AT1 and AT2 receptors in isolated C57BL/6J mouse blood vessels. Am J Physiol Heart Circ Physiol. 2003;285:H2797–2803. doi: 10.1152/ajpheart.00466.2003. [DOI] [PubMed] [Google Scholar]
- 16.Swafford AN, Jr, Harrison-Bernard LM, Dick GM. Knockout mice reveal that the angiotensin II type 1B receptor links to smooth muscle contraction. Am J Hypertens. 2007;20:335–337. doi: 10.1016/j.amjhyper.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Response to lack of specificity of commercial antibodies leads to misidentification of angiotensin type-1 receptor protein. Hypertension. 2013;61:e32. doi: 10.1161/HYPERTENSIONAHA.112.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benicky J, Hafko R, Sanchez-Lemus E, Aguilera G, Saavedra JM. Six Commercially Available Angiotensin II AT(1) Receptor Antibodies are Non-specific. Cell Mol Neurobiol. 2012;32:1353–1365. doi: 10.1007/s10571-012-9862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhbandner S, Brummer S, Metzger D, Chambon P, Hofmann F, Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28:15–22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112:417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 21.Nickenig G, Jung O, Strehlow K, Zolk O, Linz W, Scholkens BA, Bohm M. Hypercholesterolemia is associated with enhanced angiotensin AT1-receptor expression. Am J Physiol. 1997;272:H2701–2707. doi: 10.1152/ajpheart.1997.272.6.H2701. [DOI] [PubMed] [Google Scholar]
- 22.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto S, Cassell MD, Sigmund CD. The brain Renin-Angiotensin system in transgenic mice carrying a highly regulated human Renin transgene. Circ Res. 2002;90:80–86. doi: 10.1161/hh0102.102272. [DOI] [PubMed] [Google Scholar]
- 24.Lazartigues E, Dunlay SM, Loihl AK, Sinnayah P, Lang JA, Espelund JJ, Sigmund CD, Davisson RL. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res. 2002;90:617–624. doi: 10.1161/01.res.0000012460.85923.f0. [DOI] [PubMed] [Google Scholar]
- 25.Cinato E, Mirotsou M, Sablitzky F. Cre-mediated transgene activation in the developing and adult mouse brain. Genesis. 2001;31:118–25. doi: 10.1002/gene.10014. [DOI] [PubMed] [Google Scholar]
- 26.Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM., Jr The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs. 2012;195:73–81. doi: 10.1159/000331413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tieu BC, Ju X, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Brasier AR, Tilton RG. Aortic adventitial fibroblasts participate in angiotensin-induced vascular wall inflammation and remodeling. J Vasc Res. 2010;48:261–272. doi: 10.1159/000320358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones JA, Beck C, Barbour JR, Zavadzkas JA, Mukherjee R, Spinale FG, Ikonomidis JS. Alterations in aortic cellular constituents during thoracic aortic aneurysm development: myofibroblast-mediated vascular remodeling. Am J Pathol. 2009;175:1746–1756. doi: 10.2353/ajpath.2009.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;12:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 31.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305:H1363–1372. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci USA. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng J, Wang Y, Liang A, Jia L, Du J. FSP-1 silencing in bone marrow cells suppresses neointima formation in vein graft. Circ Res. 2012;110:230–240. doi: 10.1161/CIRCRESAHA.111.246025. [DOI] [PubMed] [Google Scholar]
- 35.Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48:563–567. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, O’Brien JE, Jr, Mannion JD, Morrison RC, Chung W, Fard A, Zalewski A. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95:2684–2693. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen AT, Gomez D, Bell RD, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.