Abstract
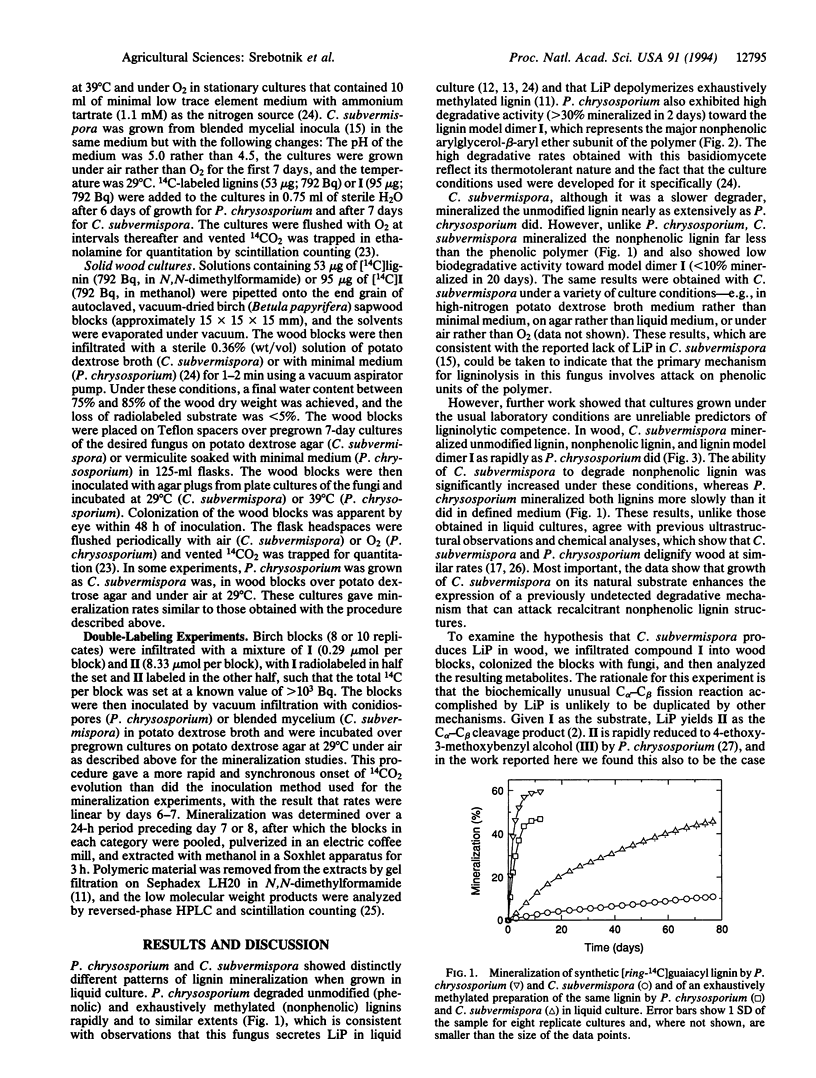
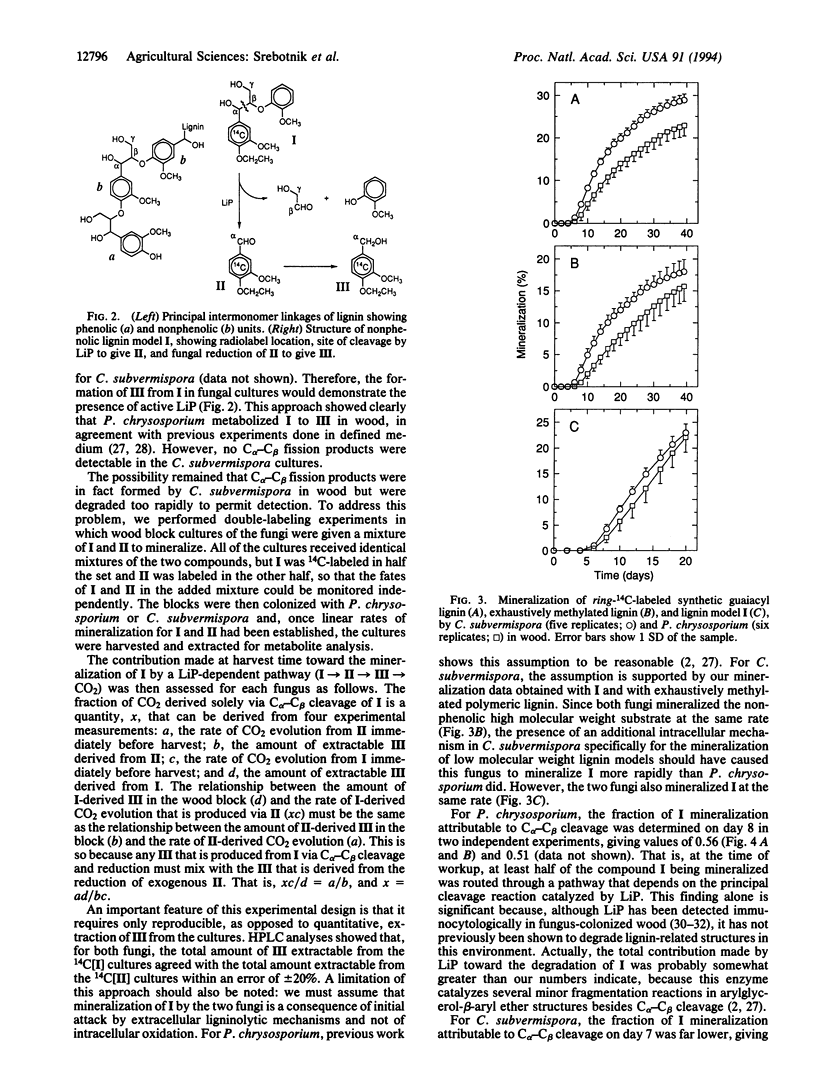
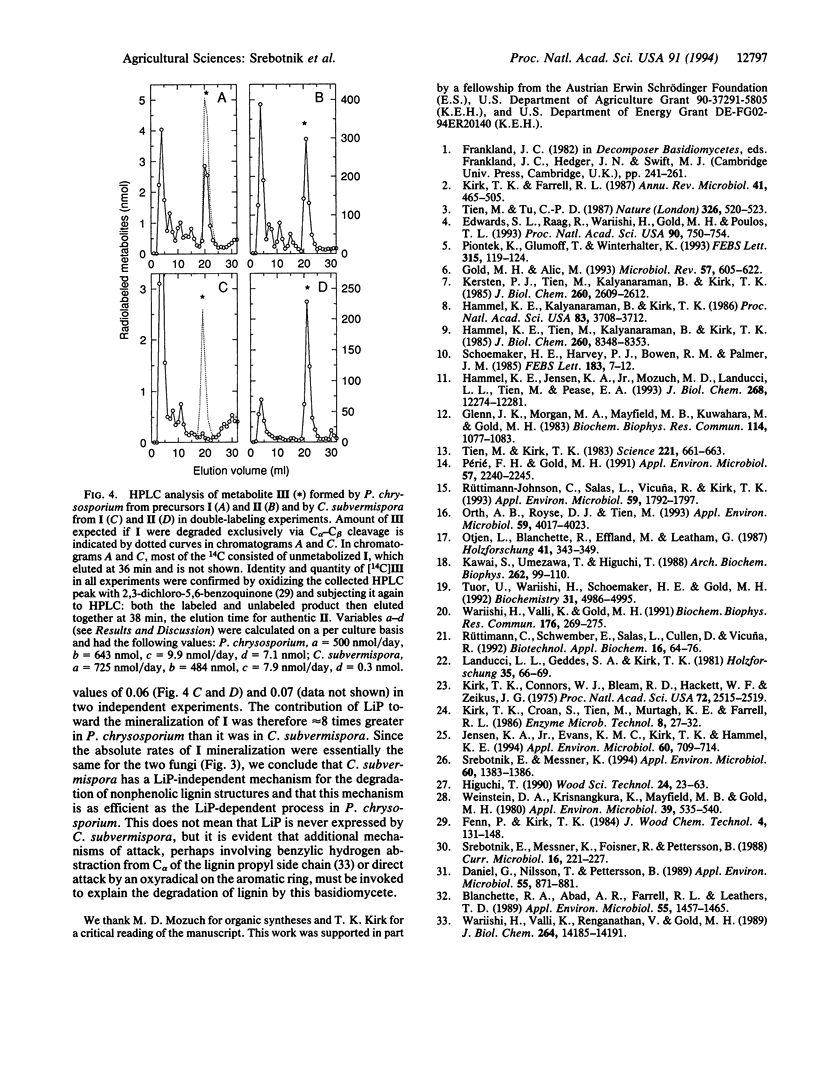
Lignin peroxidases (LiPs) are likely catalysts of ligninolysis in many white-rot fungi, because they have the unusual ability to depolymerize the major, recalcitrant, non-phenolic structures of lignin. Some white-rot fungi have been reported to lack LiP when grown on defined medium, but it is not clear whether they exhibit full ligninolytic competence under these conditions. To address this problem, we compared the abilities of a known LiP producer, Phanerochaete chrysosporium, with those of a reported nonproducer, Ceriporiopsis subvermispora, to degrade a synthetic lignin with normal phenolic content, a lignin with all phenolic units blocked, and a dimer, 1-(4-ethoxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol, that represents the major nonphenolic structure in lignin. P. chrysosporium mineralized all three models rapidly in defined medium, but C. subvermispora showed appreciable activity only toward the more labile phenolic compound under these conditions. However, in wood, its natural environment, C. subvermispora mineralized all of the models as rapidly as P. chrysosporium did. Defined media therefore fail to elicit a key component of the ligninolytic system in C. subvermispora. A double-labeling experiment with the dimeric model showed that a LiP-dependent pathway was responsible for at least half of dimer mineralization in wood by P. chrysosporium but was responsible for no more than 6-7% of mineralization by C. subvermispora in wood. Therefore, C. subvermispora has mechanisms for degradation of nonphenolic lignin that are as efficient as those in P. chrysosporium but that do not depend on LiP.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchette R. A., Abad A. R., Farrell R. L., Leathers T. D. Detection of lignin peroxidase and xylanase by immunocytochemical labeling in wood decayed by basidiomycetes. Appl Environ Microbiol. 1989 Jun;55(6):1457–1465. doi: 10.1128/aem.55.6.1457-1465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G., Nilsson T., Pettersson B. Intra- and Extracellular Localization of Lignin Peroxidase during the Degradation of Solid Wood and Wood Fragments by Phanerochaete chrysosporium by Using Transmission Electron Microscopy and Immuno-Gold Labeling. Appl Environ Microbiol. 1989 Apr;55(4):871–881. doi: 10.1128/aem.55.4.871-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. L., Raag R., Wariishi H., Gold M. H., Poulos T. L. Crystal structure of lignin peroxidase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):750–754. doi: 10.1073/pnas.90.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Alic M. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol Rev. 1993 Sep;57(3):605–622. doi: 10.1128/mr.57.3.605-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel K. E., Jensen K. A., Jr, Mozuch M. D., Landucci L. L., Tien M., Pease E. A. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993 Jun 15;268(17):12274–12281. [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Substrate free radicals are intermediates in ligninase catalysis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3708–3712. doi: 10.1073/pnas.83.11.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel K. E., Tien M., Kalyanaraman B., Kirk T. K. Mechanism of oxidative C alpha-C beta cleavage of a lignin model dimer by Phanerochaete chrysosporium ligninase. Stoichiometry and involvement of free radicals. J Biol Chem. 1985 Jul 15;260(14):8348–8353. [PubMed] [Google Scholar]
- Jensen K. A., Evans K. M., Kirk T. K., Hammel K. E. Biosynthetic Pathway for Veratryl Alcohol in the Ligninolytic Fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1994 Feb;60(2):709–714. doi: 10.1128/aem.60.2.709-714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Umezawa T., Higuchi T. Degradation mechanisms of phenolic beta-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch Biochem Biophys. 1988 Apr;262(1):99–110. doi: 10.1016/0003-9861(88)90172-5. [DOI] [PubMed] [Google Scholar]
- Kersten P. J., Tien M., Kalyanaraman B., Kirk T. K. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. J Biol Chem. 1985 Mar 10;260(5):2609–2612. [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Orth A. B., Royse D. J., Tien M. Ubiquity of lignin-degrading peroxidases among various wood-degrading fungi. Appl Environ Microbiol. 1993 Dec;59(12):4017–4023. doi: 10.1128/aem.59.12.4017-4023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek K., Glumoff T., Winterhalter K. Low pH crystal structure of glycosylated lignin peroxidase from Phanerochaete chrysosporium at 2.5 A resolution. FEBS Lett. 1993 Jan 4;315(2):119–124. doi: 10.1016/0014-5793(93)81146-q. [DOI] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüttimann-Johnson C., Salas L., Vicuña R., Kirk T. K. Extracellular Enzyme Production and Synthetic Lignin Mineralization by Ceriporiopsis subvermispora. Appl Environ Microbiol. 1993 Jun;59(6):1792–1797. doi: 10.1128/aem.59.6.1792-1797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebotnik E., Messner K. A simple method that uses differential staining and light microscopy to assess the selectivity of wood delignification by white rot fungi. Appl Environ Microbiol. 1994 Apr;60(4):1383–1386. doi: 10.1128/aem.60.4.1383-1386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Tu C. P. Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature. 1987 Apr 2;326(6112):520–523. doi: 10.1038/326520a0. [DOI] [PubMed] [Google Scholar]
- Tuor U., Wariishi H., Schoemaker H. E., Gold M. H. Oxidation of phenolic arylglycerol beta-aryl ether lignin model compounds by manganese peroxidase from Phanerochaete chrysosporium: oxidative cleavage of an alpha-carbonyl model compound. Biochemistry. 1992 Jun 2;31(21):4986–4995. doi: 10.1021/bi00136a011. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Renganathan V., Gold M. H. Thiol-mediated oxidation of nonphenolic lignin model compounds by manganese peroxidase of Phanerochaete chrysosporium. J Biol Chem. 1989 Aug 25;264(24):14185–14191. [PubMed] [Google Scholar]
- Weinstein D. A., Krisnangkura K., Mayfield M. B., Gold M. H. Metabolism of Radiolabeled beta-Guaiacyl Ether-Linked Lignin Dimeric Compounds by Phanerochaete chrysosporium. Appl Environ Microbiol. 1980 Mar;39(3):535–540. doi: 10.1128/aem.39.3.535-540.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



