Abstract
Previous work in our laboratories provides preclinical evidence that mixed-action delta/mu receptor glycopeptides have equivalent efficacy for treating pain with reduced side effect profiles compared to widely used mu agonist analgesics such as morphine. This study evaluated the rewarding and reinforcing effects of a lead candidate, mixed-action delta/mu agonist MMP-2200, using a conditioned place preference assay as well as a drug self-administration procedure in rats. In place conditioning studies, rats underwent a 2-week conditioning protocol and were then tested for chamber preference. Rats receiving MMP-2200, at previously determined analgesic doses, could not distinguish between the drug and saline-paired chamber, whereas rats receiving the opioid agonist morphine showed a strong preference for the morphine-paired chamber. In self-administration studies, rats were trained to respond for the high efficacy mu opioid receptor agonist fentanyl on an FR5 schedule of reinforcement. Following complete dose-response determinations for fentanyl, a range of doses of MMP-2200 as well as morphine were tested. Relative to the mu agonist morphine, MMP-2200 maintained a significantly lower number of drug infusions. To begin investigating potential molecular mechanisms for the reduced side effect profile of MMP-2200, we also examined βarrestin2 (βarr2) recruitment and chronic MMP-2200 induced cAMP tolerance and super-activation at the human delta and mu receptors in vitro. MMP-2200 efficaciously recruited βarr2 to both receptors, and induced cAMP tolerance and super-activation equivalent to or greater than morphine at both receptors. The in vivo findings suggest that MMP-2200 may be less reinforcing than morphine but may have some abuse potential. The reduced side effect profile cannot be explained by reduced βarr2 recruitment or reduced cAMP tolerance and superactivation at the monomeric receptors in vitro.
Keywords: drug self-administration, conditioned place preference, mixed-action opioids, delta opioid receptor (DOR), mu opioid receptor (MOR), glycopeptides, MMP-2200, fentanyl, morphine, rats, βarrestin2, cAMP, tolerance, super-activation
1. Introduction
Prescription opioid analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and steroids are frontline treatments for acute and chronic pain. However, inconsistent therapeutic effects and deleterious side effects limit their usefulness (Shalmi 2004). There is a clear need for more effective, safer and better-tolerated analgesic drugs to treat chronic pain. One promising strategy for development of more effective analgesics is to exploit interactions between delta and mu opioid receptors, using fixed-ratio mixtures of delta and mu agonists (Jiang et al. 1990; Adams et al. 1993; Su et al. 1998; Tallarida 2000; Stevenson et al. 2003, 2005) to create an optimum mixture that produces synergistic analgesic effects and additive or sub-additive side effects. A related drug development strategy is the design, synthesis and testing of single molecule mixed-action delta/mu agonists. Several laboratories have synthesized non-peptide small molecules as well as modified peptides based on the enkephalin scaffold that are metabolically stable and have more efficient blood-brain barrier penetration compared to the parent peptide. Glycosylation of these peptides has been shown to increase blood-brain barrier penetration in vitro and systemic bioavailability in vivo (Polt et al. 1994; Bilsky et al. 2000; Elmagbari et al. 2004). Although these glycosylated delta/mu agonists demonstrate broad-spectrum analgesic efficacy under inflammatory and neuropathic pain conditions (Lowery et al. 2011; Giuvelis et al., unpublished findings), their potential side effects have not been fully characterized.
One common side effect of mu opioid agonists is abuse liability and/or addiction (Fishman et al. 2004). Prescription mu opioids are not only abused, but also lead to considerable morbidity and death from overdose (DAWN 2009; CDC 2010). Given that mixed-action delta/mu agonists contain a measure of mu receptor-mediated efficacy, it is reasonable to assume that these drugs may also possess some degree of abuse liability. One way to test for potential abuse liability is by conditioned place preference (Bilsky et al., 1990). A more detailed analysis of the abuse-related reinforcing effects of drugs can be assessed using drug self-administration procedures (Mello and Negus 1996; Thomsen and Caine 2005; Panlilio and Goldberg 2007), and there is a high correlation between drugs that produce place preferences and are self-administered by rodents and non-human primates and drugs that are abused by humans (O’Connor et al. 2011).
Our laboratories have recently characterized the structure-activity profiles of a series of novel delta/mu glycopeptides (Elmagbari et al. 2004). Based on plasma stability and bioavailability, a lead analgesic candidate, MMP-2200, was selected for further therapeutic and side effect profiling in mice (Lowery et al. 2011). We found that MMP-2200 had potent and efficacious anti-nociceptive activity in mice, with reduced locomotor activation, tolerance, and dependence. However, the abuse liability of MMP-2200 has yet to be determined in rodents. Toward that end, the present studies characterized the rewarding and reinforcing effects of the mixed-action delta/mu agonist MMP-2200 in rats, using a conditioned place preference (CPP) assay and a drug self-administration procedure, respectively. We also characterized the molecular pharmacology of MMP-2200 activity in vitro in cells expressing the human delta or mu receptors by examining chronic MMP-2200 induced cAMP tolerance and super-activation, and βarrestin2 (βarr2) recruitment.
2. Materials and Methods
2.1 Subjects
Fifty adult male SASCO Sprague-Dawley rats (200–250g; Charles River Laboratories, Wilmington, Mass., USA) were used for the conditioned place preference study and were group housed (2–3 rats/cage) in individually ventilated Innovive rat cages (San Diego, CA, USA). Eight adult male Sprague Dawley rats (200–250g; Charles River Laboratories) were used for drug self-administration procedures and were individually housed in standard Plexiglas containers. All animals received food and water available ad libitum and were maintained in a temperature and humidity controlled colony on a 12-h light/dark cycle (lights on at 0700 and off at 1900). All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and procedures were approved by the University of New England Institutional Animal Care and Use Committee (IACUC). The health of the animals was assessed daily by laboratory technicians and animal care staff.
2.2 Conditioned Place Preference (CPP)
A non-biased place conditioning protocol was used for all assessments. The apparatus (Med Associates, St. Albans, VT) has two main conditioning compartments (28 × 21 × 21 cm) separated by a central area (12 × 21 × 21 cm). The compartments are differentiated by the color/wall pattern and flooring texture. One main compartment has black walls with white strips and rounded stainless steel rod (4.8 mm) flooring. The other main compartment has all white walls with stainless steel mesh flooring (1.25 × 1.25 cm grid). The center compartment is neutral gray in color with smooth PCV flooring.
The 11-day protocol (detailed in Table 1) consisted of a pre-conditioning phase, six days of drug conditioning and two test phases. On day one (pre-conditioning phase), drug-naïve rats were placed in the middle chamber with free access to both compartments for 15 min. The time spent (sec) in each compartment was recorded by computerized monitoring software (Med-PC). On days 2–4 and 8–10 (conditioning phase 1 and 2, respectively) rats were injected subcutaneously (s.c.) injection at a volume of 1 ml/kg bodyweight at 9:00am with MMP-2200 (3.2 or 10 mg/kg), morphine (1 mg/kg) or saline and then placed into the drug-paired chamber (black or white) for a 30 min conditioning session. At 1:00pm on conditioning days the same rats as above were all injected with saline (s.c.) and placed into the alternate chamber for a 30 min session (Bilsky and Giuvelis et al, unpublished findings). Treatments were counterbalanced as closely as possible between compartments, and doses and pre-treatment times were chosen based on previous analgesic data obtained in our laboratory. On days five and 11 (test phase 1 and 2, respectively) rats were tested as described above in the pre-conditioning phase. Following completion of test phase two, a preference/aversion score was calculated for each group as the difference between the times spent in the drug-paired compartment during the tests and pre-conditioning phases. The dependent variable for place conditioning was change from baseline, defined as time spent in drug-paired compartment during test phase (sec) minus time spent in drug-paired compartment during pre-conditioning phase. A two-way ANOVA with a Bonferroni post hoc test was then conducted to determine significance at the p ≤ 0.05 level.
Table 1.
cAMP Tolerance and Superactivation Values.
| Overnight Treatment | IC50 (nM) | IC50 Shift (fold) | cAMP Baseline (%) | |
|---|---|---|---|---|
| MOR | Vehicle | 11.9± 2.2 | 1.0 | 100 |
| Morphine | 44.2 ± 10.6* | 3.6 ± 0.29* | 151.8 ± 8.8* | |
| MMP2200 | 57.2 ± 14.8* | 5.0 ± 0.85* | 150.2 ± 11.6* | |
| DOR | Vehicle | 0.63 ± 0.16 | 1.0 | 100 |
| Morphine | 1.4 ± 0.62 | 2.3 ± 0.78 | 125.0 ± 5.5* | |
| MMP2200 | 4.2 ± 1.7# | 7.0 ± 2.3* | 119.4 ± 4.4* |
The numeric values from Fig. 4 are reported from each independent experiment (n=4) reported as mean ± SEM. This also includes the raw IC50 values. The fold shift and % baseline are calculated in relationship to each Vehicle value in each independent experiment.
= p<0.05 vs. Vehicle in each group by unpaired 2 tailed t-test.
= p=0.08 vs. Vehicle.
2.3 Drug Self-Administration
2.3.1 Apparatus
All studies were conducted in drug self-administration operant conditioning chambers (Med Associates, model MED-008-CT-B1) placed within sound-attenuating cubicles equipped with a house light and exhaust fan. Each chamber contained two response levers situated on the front wall of the chamber. A shallow steel cup situated between the two levers, and just above the floor, contained a reservoir for consumption of food pellets. A pellet dispenser delivered 45 mg food pellets (see food training). Stimulus lights were situated above each response lever and were programmed to signal the availability of drug or food. A drug infusion pump was mounted outside each individual chamber to deliver intravenous drug via Tygon tubing. A complete swivel system with tether (Camcaths, Cambridgeshire, GB) was mounted inside each chamber to allow for unconstrained movement of the animal.
2.3.2 Food training
Lever pressing was initially shaped during daily training sessions (30min day 1; 15 min days 2–8). Food training involved reinforcement of successive approximations of lever-press behavior with delivery of a food pellet. Once shaping was complete, 45 mg food pellets (Noyes brand) were available under a Fixed Ratio (FR) 1 schedule of reinforcement. Illumination of the stimulus light above the active lever served as a discriminative stimulus that the response-food delivery contingency was in place. Responding on the other inactive lever was counted but had no programmed consequences (animals were counterbalanced such that the left lever was active for half the rats and the right lever was active for the other half). A maximum of 50 food reinforcers was available during each daily training session. Once responding stabilized under the FR1 schedule (three consecutive days in which response rates varied by no more than 20% and at least 80% of responses emitted on the active lever), the FR requirement was raised to FR5 over the course of 3–6 sessions. After responding stabilized under the FR5 schedule, using the same criteria as above, rats underwent surgery for implantation of an i.v. catheter.
2.3.3 Surgery
Rats underwent surgery using aseptic techniques. In brief, animals were sedated with a 10-minute pretreatment of midazolam (5mg/ml i.p.) and then anesthetized with an isoflurane/oxygen vapor mixture and implanted with a chronic indwelling i.v. catheter into the right external jugular vein. The surgical procedure was based on methods described elsewhere (Thomsen and Caine, 2005). A single dose of the analgesic non-steroidal-anti-inflammatory drug ketoprofen (5 mg/kg s.c.) as well as the antibiotic amikacin (10 mg/kg s.c.) was administered immediately prior to surgery. Following surgery, animals were allowed to recover for seven days before training was resumed.
2.3.4 Drug self-administration training
After 7 days recovery from surgery, the high efficacy mu opioid agonist fentanyl was available as the reinforcer during three-hour sessions five days/week. Sessions began with a noncontingent “priming” infusion of the available drug dose in a 56μl volume. Responding on the initial food-trained lever was reinforced under a FR1/time out 20 sec schedule with an i.v. infusion of fentanyl (0.0032 mg/kg/infusion). Fentanyl was available until baseline criteria were met (three consecutive sessions with a minimum of 15 reinforcers earned, no more than 20% variation in number of reinforcers earned between three sessions, at least 80% responses on the active lever). Once responding stabilized under the FR1 schedule, the response requirement was raised to FR5 over the course of 4–7 sessions. Following stable fentanyl self-administration under the FR5 schedule, saline substitution was initiated for 1–3 sessions until responding decreased to at least 50% of fentanyl-maintained baseline rates. Baseline fentanyl responding was then re-established followed by determination of a full dose-effect function (see below) using a within-subjects design.
2.3.5 Drug testing
All test sessions began with a non-contingent “priming” infusion of the available drug dose. Test sessions were conducted no more than twice each week and were separated by at least 48 hr. On Mondays, Wednesdays and Fridays, the solution available for self-administration was either saline or the training dose of fentanyl (0.0032 mg/kg/infusion), and substitution doses were tested on Tuesdays and Thursdays. Following determination of the fentanyl dose-effect function (0.00032 – 0.01 mg/kg/infusion), a range of doses of the delta/mu opioid agonist MMP-2200 (0.032–1 mg/kg/infusion) was tested. Given that MMP-2200 has a much slower onset than fentanyl, and similar onset and duration of action as morphine (Elmagbari et al., 2004; Lowery et al., 2011), a range of doses of morphine (0.01–0.32mg/kg/infusion) was also tested. The primary dependent variable for drug self-administration was number of drug infusions. Statistical analysis was accomplished with one-factor ANOVA. A significant ANOVA was followed by the Duncan post hoc test. Significance was set a priori at p ≤ 0.05.
2.4 cAMP Tolerance and Superactivation
2.4.1 Cell line creation and cell culture
Chinese hamster ovary (CHO) cells stably expressing N-terminal hemagglutinin tagged human mu (GeneCopoeia, Rockville, MD) or delta (Missouri S&T cDNA Resource Center, Rolla, MO) receptor cDNA constructs were transfected by electroporation, selection with 500 μg/mL of geneticin, and high expressing populations selected by fluorescence assisted cell sorting flow cytometry (full cell line characterizations performed [data not shown]; receptor expression: mu = 434.5 fmol/mg, delta = 445.2 fmol/mg). Cells were maintained in DMEM/F-12 50/50 mix w/L-glutamine and 15 mM HEPES containing 10% FBS, 1× penicillin/streptomycin, and 500μg/mL geneticin in a humidified incubator (5% CO2, 37°C).
2.4.2 Drug treatment and cAMP assay
For the experiments, cells were plated into 96-well plates (5,000–8,000 cells/well) and grown overnight in the same medium and conditions as described above. The cells were then treated with either drug or vehicle for 20 hrs. For the mu, the drug concentrations were 25 fold of the EC50 value (5 μM for MMP-2200, 1 μM for morphine), and for the delta, 5 fold of the EC50 value for MMP-2200 (70 nM), and a non-normalized concentration of 10 μM for morphine. These normalized concentrations were established by empirical testing, and based off of earlier cAMP tolerance work (data not shown, Ananthan et al. 2012).
After chronic treatment, the cells were washed two times with serum free medium and then incubated with serum free medium containing 500 μM IBMX (Tocris). After a 20 min incubation at 37°C, serum free medium containing 500 μM IBMX, 100 μM forskolin (Enzo Life Sciences), and an agonist concentration curve (DAMGO for mu, SNC80 for delta) were added and then incubated for 15 min at 37° C. The reaction was terminated by removing the medium and adding 60 μL of ice-cold assay buffer (50 mM Tris-HCl pH 7.4, 100 mM NaCl, 5 mM EDTA). Plates were sealed with boiling mats and then boiled at 95°C for 10 min. Plates were then centrifuged at 3,200rpm, 4° C, for 15min to remove debris. 50μL of lysate was transferred to a 96-well plate. Lysate was incubated with ~1pmol [3H]-cAMP (Adenosine 3′, 5′-Cyclic Phosphate, Ammonium Salt, [2,8-3H], PerkinElmer), and 7 μg of protein kinase A (Sigma Aldrich) with 0.05% BSA. The reaction was incubated at room temperature for 1 hr. The reactions were harvested onto GF/B filter plates (PerkinElmer) via rapid filtration by a 96-well plate Cell Harvester (Brandel) and washed 3 times with ice-cold water. Filter plates were dried, 40μL of scintillation cocktail (Microscint-20) was added to each well, and then counted in a TopCount microplate scintillation counter (PerkinElmer).
2.4.3 cAMP data analysis
The resulting values were quantitated for actual cAMP content by the use of a cAMP standard curve. The data was normalized to the maximum cAMP inhibition in the chronic vehicle treated cells, and nonlinear curves fit using Prism (GraphPad) (3 parameter non-linear curve, Hill Slope = 1.0, Y = Bottom + (Top-Bottom)/(1+10ˆ(X-LogIC50))). The resulting IC50 values of cAMP inhibition were calculated for each independent experiment. The IC50 tolerance fold shift was calculated for each drug versus the vehicle treated controls. Due to shifting absolute baselines between experiments, the fold shift was calculated within each experiment, thus the standard error of the vehicle group was lost in these calculations (i.e. each value for the vehicle group would be 1.0). The cAMP baseline was also determined from each experiment by the top of the fitted curve, and the baseline normalized to chronic vehicle treatment within each experiment. Unpaired two-tailed t-tests were used to compare each set of values (mean ± SEM) to the vehicle control. Significance was set at p < 0.05.
2.5 Qualitative βarr2 recruitment
The model systems used for this assay were U2OS cells expressing the human mu or delta receptor, and a βarr2-EGFP fusion protein (a kind gift from Dr. Larry Barak, Duke University). The cells were maintained in MEM media with 10% FBS, 1× penicillin/streptomycin, 200 μg/mL of geneticin, and 100 μg/mL of zeocin in a humidified incubator (37°C, 5% CO2). Approximately 40,000 cells from each line were seeded into collagen I coated glass bottom microscopy culture dishes (MatTek, Ashland, MA) and allowed to recover overnight. On the day of the experiment, the cells were serum starved for 1 hour in serum free MEM, and imaged using a Leica SP5 confocal microscope. The cells were imaged live, and the same cells were imaged before (0 min.) and after addition of 10 μM of positive control drug (SNC80 for delta, DAMGO for mu receptor) or MMP-2200. The cells were imaged periodically over a 10 minute time course, and βarr2 recruitment was visualized as bright green punctae.
2.6 Drugs
MMP-2200 (acetate salt) was synthesized by Dr. Robin Polt’s laboratory using previously published methods (Elmagbari et al., 2004). Morphine sulfate was generously provided by Mallinckrodt (St. Louis, MO). Fentanyl citrate was purchased from Spectrum Chemical Company (Gardena, CA). All drugs were dissolved in sterile saline (0.9% NaCl).
3. Results
3.1 Conditioned place preference (CPP) of morphine and MMP-2200
All rats during the pre-conditioning phase spent approximately equal time in both the white and black compartments and less time in the central, gray compartment. Test sessions showed rats conditioned with saline exhibited little to no preference for one compartment over the other having a mean change from baseline of −29.0 sec and +0.42 sec in test sessions one and two, respectively. Rats conditioned with morphine showed a significant place preference for the compartment they received the drug in. Morphine treated rats had a mean change from baseline of +83.4 sec (test session one) and +96.62 sec (test session two) which was significantly different from saline treated animals after the analysis by a two-way ANOVA, F = 6.187, p = 0.0013 (see Fig 1). Rats receiving MMP-2200 at either 3.2 or 10 mg/kg exhibited no preference for either compartment during test sessions.
Fig. 1.
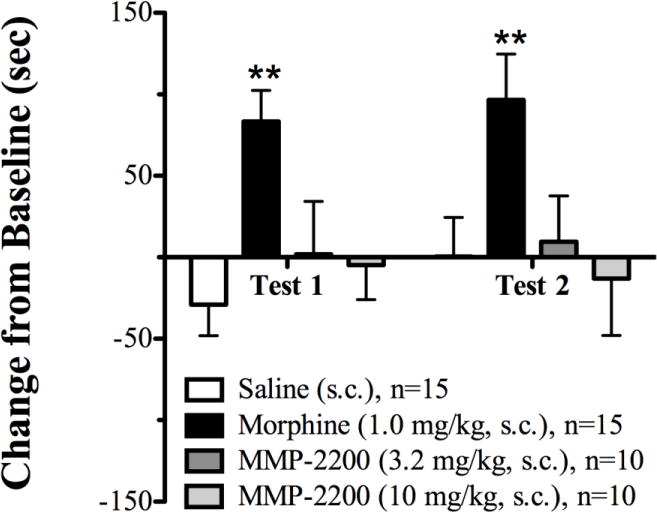
Place conditioning scores (change from baseline in sec) for the mu opioid agonist morphine and the mixed-action delta/mu glycopeptide MMP-2200. ** indicates significantly greater than saline (p ≤ 0.01).
3.2 Self-administration of fentanyl, morphine and MMP-2200
Rats were given the opportunity to self-administer a range of doses of fentanyl (0.00032–0.01 mg/kg), morphine (0.01–0.32 mg/kg), and the delta/mu agonist MMP-2200 (0.032–1 mg/kg). All three drugs produced characteristic inverted U-shaped dose-effect functions (see Fig. 2). Statistical analysis revealed that fentanyl (0.001 and 0.0032 mg/kg/infusion), and morphine (0.1 and 0.32 mg/kg/infusion) maintained significantly greater responding than saline (F = 9.122, df = 4, p < 0.0001; F = 18.504, df = 4, p < 0.0001, respectively). Although the peak of the MMP-2200 dose effect curve was lower than that of fentanyl and morphine, the 0.32 mg/kg dose of MMP-2200 maintained significantly greater responding than saline (F = 5.033, df = 4, p < 0.01). Peak doses of all three compounds were also compared (see Fig. 3), and MMP-2200 maintained significantly lower rates of responding than morphine but not fentanyl (F = 26.91, df = 3, p < 0.0001).
Fig. 2.
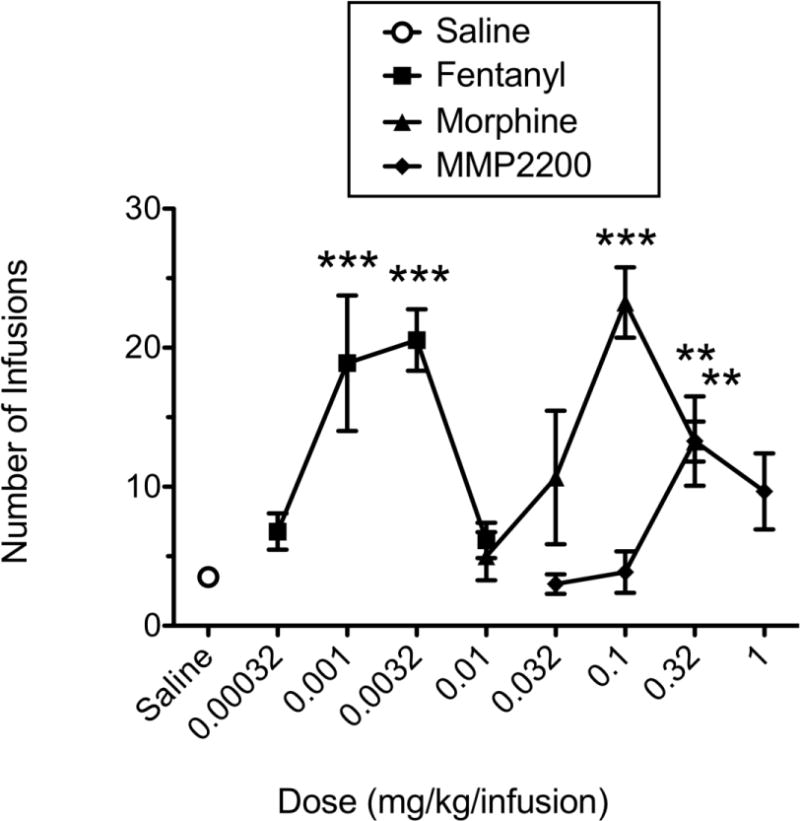
Dose-effect curves for IV self-administration of the mu opioid agonists fentanyl and morphine, and the mixed-action delta/mu glycopeptide MMP-2200 under a FR5-TO-20 sec schedule of reinforcement. ** indicates significantly greater than saline (p ≤ 0.01); *** indicates significantly greater than saline (p ≤ 0.001).
Fig. 3.
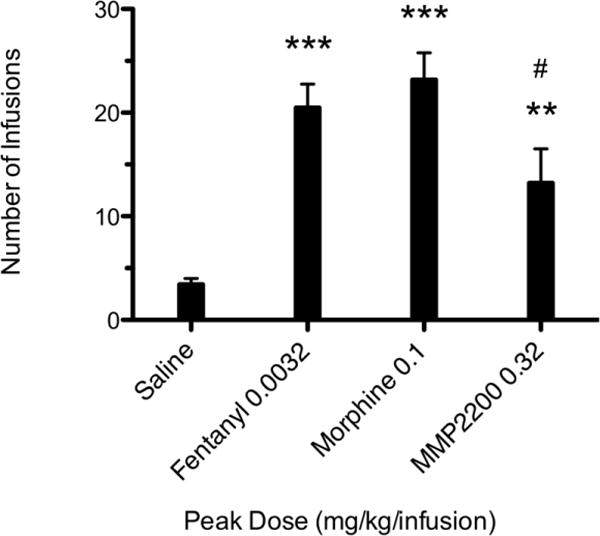
Peak doses for fentanyl, morphine and MMP2200. ** indicates significantly greater than saline (p ≤ 0.01); *** indicates significantly greater than saline (p ≤ 0.001). # indicates significantly less than morphine (p ≤ 0.05).
3.3 cAMP Tolerance and superactivation
(Avidor-Reiss et al. 1995; Varga et al. 2003). We tested the ability of chronic MMP-2200 to induce cAMP tolerance and super-activation at mu and delta receptors. After 20 hr treatment with vehicle, morphine control, or MMP-2200, we found that MMP-2200 induced cAMP tolerance and super-activation at both mu and delta receptor to a similar or greater extent than morphine (Fig. 3, Table 2). The IC50 fold shift (tolerance) and baseline increase (super-activation, dependence) was significantly increased with MMP-2200 treatment (p < 0.05) at both receptors (Fig. 3 B, C, E, F; Table 2), and statistically the same as morphine in all except for IC50 fold shift at delta (morphine was not different from vehicle there). The numeric values are reported in Table 2, and importantly, the raw IC50 values mirror the effects seen in the fold shift analysis, except for MMP-2200 at delta receptor (p = 0.08). MMP-2200 induced a 5.0 fold tolerance and 150.2% super-activation at mu, and a 7.0 fold tolerance and 119.4% super-activation at delta.
3.4 βarrestin2 recruitment
Using human mu and delta expressing U2OS cells with a βarr2-EGFP fusion protein, we demonstrate robust recruitment of βarr2 by the control ligands DAMGO and SNC80 (Fig. 4). MMP-2200 (10 μM) also induced robust βarr2 recruitment to the mu and delta receptors with indistinguishable qualitative efficacy and time course (full time course data not shown) from the control ligands (Fig. 4).
Fig. 4.
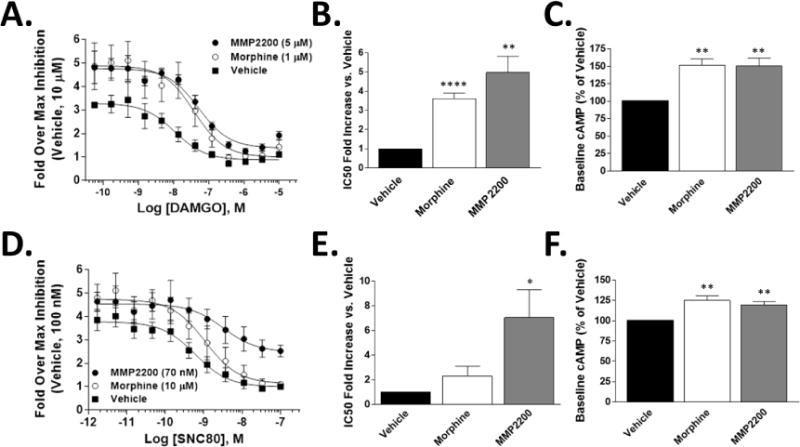
CHO cells expressing human mu (A-C) or delta receptor (D-F) were treated for 20 hrs with vehicle, morphine (1 μM for mu, 10 μM for delta), or MMP-2200 (5 μM for mu, 70 nM for delta). The cells were then stimulated with forskolin (100 μM) and an agonist concentration curve (DAMGO for mu, SNC80 for delta), and cAMP levels quantified. Sample size n=4 independent experiments. Values calculated from each individual experiment, reported as mean ± SEM. *, **, **** = p<0.05, 0.01, 0.0001 via unpaired 2 tailed t-test vs. Vehicle group. A) Concentration curves for mu receptor experiments. The mean from each individual experiment (n=4) used as a single value for the summary curve. B) The fold IC50 shift vs. the Vehicle value is reported for mu receptor (A), a marker of tolerance. C) The percent increase in cAMP baseline over the Vehicle for mu receptor (A) is reported, which is equally elevated (~150%) in both morphine and MMP-2200. D) Concentration curves for delta receptor experiments. The mean from each individual experiment (n=4) used as a single value for the summary curve. E) The fold IC50 shift vs. the Vehicle value is reported for delta receptor (D), a marker of tolerance. F) The percent increase in cAMP baseline over the Vehicle for delta receptor (D) is reported, which is equally elevated (~120%) in both morphine and MMP-2200.
4. Discussion
The present paper provides an initial characterization of the rewarding and reinforcing effects of the novel mixed-action delta/mu glycopeptide MMP-2200 in rats. The main findings were that MMP-2200 did not produce a conditioned place preference under the conditions examined, and maintained significantly lower levels of drug self-administration, relative to the mu agonist morphine, but not fentanyl. These in vivo data suggest that although MMP-2200 may have weaker reinforcing efficacy than the standard mu opioid receptor analgesic morphine, MMP-2200 can produce reinforcing effects and therefore may have some abuse potential. However, the lower peak rates of MMP-2200 self-administration may be a function of other factors than drug-induced reinforcing effects, including it’s long duration of action (Lowery et al., 2011; Giuvelis et al., unpublished findings), and the parameters of the current self-administration protocol which used an FR schedule of reinforcement with short post-reinforcer time-out periods. We also found that MMP-2200 is fully efficacious at recruiting βarr2 to the human mu and delta receptors, and induced equivalent or greater cAMP tolerance and super-activation than morphine at both human mu and delta receptors. These in vitro findings are in potential conflict with our earlier studies demonstrating decreased tolerance and dependence in mice (Lowery et al. 2011). This discrepancy is discussed in further detail below.
The differential effects of MMP-2200 in place conditioning vs. self-administration in the present report, may be due to a number of variables, including route of administration (SC vs. IV), drug pre-exposure resulting in learned behaviors (pre-exposure to both food and drugs in self-administration only), and passive vs. active drug administration (reviewed in Bardo and Bevins, 2000; Tzschentke, 1998). Regarding the latter variable, dopamine efflux has been shown to be greater in animals that actively self-administer drug compared to animals that passively receive drug (Hemby et al., 1997; Di Ciano et al., 1998) and this greater dopamine release may facilitate drug taking in self-administration procedures in rodents. The behavioral pharmacology of MMP-2200 has also been assessed in rhesus monkeys (Do Carmo et al. 2008). These authors demonstrated that MMP-2200 did not maintain drug self-administration at any dose tested. The reason that MMP-2200 maintains limited self-administration in rats but not in monkeys is unknown, but may be due to differences in experimental procedures, CNS bioavailability, species, and/or opioid receptor distributions in the central nervous system (Mennicken et al. 2003; Sharif and Hughes 1989; Mansour et al. 1988).
It is known that tolerance and dependence are two of several diagnostic criteria for opioid use disorder in humans (DSM-5), and tolerance and dependence are often included in preclinical assessments of abuse liability (Carter and Griffiths, 2009). In our earlier studies, we demonstrated that MMP-2200 induced less behavioral tolerance and dependence (as well as lower levels of locomotor stimulation) in mice, and these findings provided evidence to suggest that MMP2200 may have lower abuse liability than standard mu opioid analgesics. To further investigate the mechanisms of reduced tolerance and dependence, the current study examined potential molecular mechanisms for the side effect decreases. Specifically, this study assessed the effects of MMP2200 in a cellular assay that has been used to examine tolerance and dependence to opioid-induced inhibition of cAMP second-messenger signaling. Opioids which produce tolerance and dependence such as morphine induce an IC50 tolerance shift in vitro after chronic treatment, as well as super-activation of baseline cAMP levels, at both mu and delta receptors (Avidor-Reiss et al. 1995; Varga et al. 2003). Drugs that do not induce these in vitro markers of tolerance and dependence are correlated with reduced tolerance and dependence in vivo (Ananthan et al. 2012). Surprisingly, MMP-2200 induced a tolerance shift and cAMP super-activation greater than or equal to morphine at both mu and delta receptors (Fig. 5 and Table 2). This suggests that the mechanism of reduced MMP-2200 tolerance and dependence in vivo (Lowery et al. 2011) may not be at the level of the individual mu and delta receptors. Possibilities for this mechanism include mu/delta interaction in the same cells (including heterodimerization) which is not captured by our separate cell models, neuronal network effects such as mu and delta containing neurons modulating each others activity at a level above the individual receptor, or additional cellular regulators found in neurons but not CHO cells that could modulate the induction of tolerance and dependence at the receptor level.
Fig. 5.
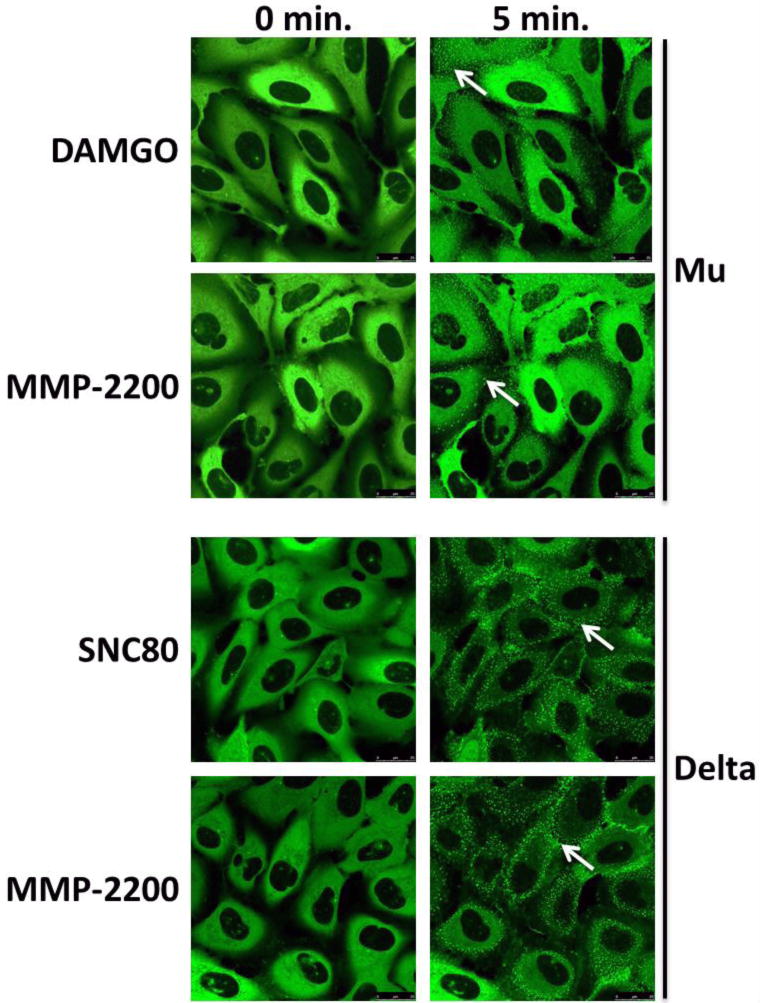
U2OS cells expressing the human mu or delta receptor and a βarr2-EGFP fusion protein were serum starved and then live imaged by confocal microscopy. Cells were imaged before (0 min.) and after (5 min.) the addition of 10 μM control agonist (DAMGO for mu, SNC80 for delta) or MMP-2200. βarr2 recruitment was visualized as bright green punctae (white arrows). Equivalent qualitative recruitment was seen between MMP-2200 and control agonist at both the mu and delta. 3 independent experiments performed, representative images shown.
βarr2 recruitment has also been associated with mu-mediated tolerance and dependence in mice, and functionally selective mu agonists that do not effectively recruit βarr2 have been shown to have an improved therapeutic index (Raehal et al. 2005; DeWire et al. 2013). We thus tested the ability of MMP-2200 to recruit βarr2, and found that MMP-2200 recruited βarr2 to the mu and delta receptors with full efficacy (Fig. 4). These results suggest that MMP-2200 is not biased against βarr2, and the results in vivo are likely not mediated via this mechanism. However, as this is a qualitative assay, we cannot rule out the possibility that there could be a potency bias.
Future molecular studies will involve studying cAMP tolerance and super-activation in mu/delta heteromer cells, as well as markers of tolerance and super-activation in primary neurons. We will also further explore the molecular mechanisms of MMP-2200 activity, as our results suggest a more complex and thus potentially interesting mechanism behind the observed decrease in tolerance, dependence, and other opioid receptor mediated side effects.
Highlights.
MMP-2200 did not produce conditioned place preference.
MMP-2200 maintained lower levels of drug self-administration relative to morphine.
MMP-2200 induced cAMP tolerance/super-activation at delta and mu receptors.
Acknowledgments
This research was supported by a University of New England faculty grant and by NIAMS, NIH AR054975 to G.W.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JU, Tallarida RJ, Geller EB, Adler MW. Isobolographic superadditivity between delta and mu opioid agonists in the rat depends on the ratio of compounds, the mu agonist and the analgesic assay used. J Pharmacol Exp Ther. 1993;266:1261–7. [PubMed] [Google Scholar]
- Ananthan S, Saini SK, Dersch CM, Xu H, McGlinchey N, Giuvelis D, Bilsky EJ, Rothman RB. 14-Alkoxy- and 14-acyloxypyridomorphinans: μ agonist/δ antagonist opioid analgesics with diminished tolerance and dependence side effects. J Med Chem. 2012;55:8350–63. doi: 10.1021/jm300686p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Bayewitch M, Levy R, Matus-Leibovitch N, Nevo I, Vogel Z. Adenylylcyclase supersensitization in mu-opioid receptor-transfected Chinese hamster ovary cells following chronic opioid treatment. J Biol Chem. 1995;270:29732–8. doi: 10.1074/jbc.270.50.29732. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Marglin SH, Reid LD. Using antagonists to assess neurochemical coding of a drug’s ability to establish a conditioned place preference. Pharm, Biochem, Behav. 1990;37:425–431. doi: 10.1016/0091-3057(90)90007-5. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem. 2000;43:2586–90. doi: 10.1021/jm000077y. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;(Suppl):S14–25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Unintentional drug poisoning in the United States National Center for Injury Prevention and control. Centers for Disease Control and Prevention; 2010. [Google Scholar]
- Codd EE, Carson JR, Colburn RW, Stone DJ, Van Besien CR, Zhang SP, Wade PR, et al. JNJ-20788560, a selective delta opioid receptor agonist, is a potent and efficacious antihyperalgesic agent that does not produce respiratory depression, pharmacologic tolerance, or physical dependence. J Pharmacol Exp Ther. 2009;329:241–51. doi: 10.1124/jpet.108.146969. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–17. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur J Neurosci. 1998;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders (DSM-5) Fifth. American Psychiatric Association Publishing; Washington, DC: pp. 540–542. [Google Scholar]
- Do Carmo GP, Polt R, Bilsky EJ, Rice JC, Negus SS. Behavioral pharmacology of the mu/delta opioid glycopeptide MMP2200 in rhesus monkeys. J Pharmacol Exp Ther. 2008;326:939–48. doi: 10.1124/jpet.108.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Abuse Warning Network (DAWN) (HHS Publication No. (SMA) 11-4659 DAWN Series D-35).National estimates of drug-related emergency department visits. 2009 [Google Scholar]
- Elmagbari NO, Egleton RD, Palian MM, Lowery JJ, Schmid WR, Davis P, Navratilova E, et al. Antinociceptive structure-activity studies with enkephalin-based opioid glycopeptides. J Pharmacol Exp Ther. 2004;311:290–97. doi: 10.1124/jpet.104.069393. [DOI] [PubMed] [Google Scholar]
- Fishman SM, Condon J, Holtsman M. Common opioid-related side effects. In: Warfield CA, Bajwa ZH, editors. Principles and practice of pain medicine. 3. McGraw-Hill; 2004. pp. 612–615. [Google Scholar]
- Jiang Q, Mosbery HI, Porreca F. Modulation of the potency and efficacy of mu-mediated antinociception by delta agonists in the mouse. J Pharmacol Exp Ther. 1990;254:683–89. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracelluar dopamine concetnrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharm. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. Pharmacokinetics and pharmacodynamics of seven opioids in p-glycoprotein-competent mice: Assessment of unbound brain EC50,u and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther. 2007;323:346–55. doi: 10.1124/jpet.107.119560. [DOI] [PubMed] [Google Scholar]
- Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ. In vivo characterization of MMP-2200, a mixed delta/mu opioid agonist, in mice. J Pharmacol Exp Ther. 2011;336:767–78. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–14. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Menkens K, Bilsky EJ, Wild KD, Portoghese PS, Reid LD, Porreca F. Cocaine place preference is blocked by the δ-opioid receptor antagonist, naltrindole. Eur J Pharmacol. 1992;219:345–46. doi: 10.1016/0014-2999(92)90319-y. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–60. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev. 2011;35:912–38. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and investigative tool. Addiction. 2007;102:1863–70. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polt R, Porreca F, Szabo LZ, Bilsky EJ, Davis P, Abbruscato TJ, Davis TP, et al. Glycopeptide enkephalin analogues produce analgesia in mice: Evidence for penetration of the blood-brain barrier. Proc Natl Acad Sci. 1994;91:7114–18. doi: 10.1073/pnas.91.15.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- Seppala MD, Berge KH. The addicted physician: A rational response to an irrational disease. Minn Med. 2010;93(2):46–9. [PubMed] [Google Scholar]
- Shalmi CL. Opioids for non-malignant pain – issues and controversy. In: Warfield CA, Bajwa ZH, editors. Principles and practice of pain medicine. McGraw-Hill; 2004. pp. 601–611. [Google Scholar]
- Sharif NA, Hughes J. Discrete mapping of brain mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides. 1989;10:499–522. doi: 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- Spina L, Longoni R, Mulas A, Chang KJ, Di Chiara G. Dopamine-dependent behavioral stimulation by non-peptide delta opioids BW373U86 and SNC 80: 1. Locomotion, rearing and stereotypies in intact rats. Behav Pharmacol. 1998;9:1–8. [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS. Opioid interactions in rhesus monkeys: Effects of delta + mu and delta + kappa agonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther. 2003;307:1054–64. doi: 10.1124/jpet.103.056515. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Rice KC, Negus SS. Interactions between delta and mu opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: Studies with SNC80 and heroin. J Pharmacol Exp Ther. 2005;314:221–31. doi: 10.1124/jpet.104.082685. [DOI] [PubMed] [Google Scholar]
- Su YF, McNutt RW, Chang KJ. delta-Opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J Pharmacol Exp Ther. 1998;287:815–23. [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Chapman & Hall/CRC Press; Boca Raton, Florida: 2000. [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rodents. Current Protocols in Neuroscience. 2005 doi: 10.1002/0471142301.ns0920s32. Unit 9.20. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik MK, Stropova D, Sugiyama M, Grife V, Hruby VJ, Rice KC, Roeske WR, Yamamura HI. Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic delta-opioid agonist treatment. J Pharmacol Exp Ther. 2003;306:109–15. doi: 10.1124/jpet.103.049643. [DOI] [PubMed] [Google Scholar]


