Abstract
Objective
Despite advances in stent technology for vascular interventions, in-stent restenosis (ISR) due to myointimal hyperplasia (MH) remains a major complication.
Approach and Results
We investigated the regulatory role of microRNAs in MH/ISR, utilizing a humanized animal model in which balloon-injured human internal mammary arteries (IMAs) with or without stenting were transplanted into RNU rats, followed by microRNA profiling. miR-21 was the only significantly up-regulated candidate. In addition, miR-21 expression was increased in human tissue samples from patients with ISR compared to coronary artery disease specimen. We systemically repressed miR-21 via intravenous FAM-tagged-LNA-anti-miR-21 (anti-21) in our humanized MH-model. As expected, suppression of vascular miR-21 correlated dose-dependently with reduced luminal obliteration. Further, anti-21 did not impede re-endothelialization. However, systemic anti-miR-21 had substantial off-target effects, lowering miR-21 expression in liver, heart, lung, and kidney with concomitant increased serum creatinine levels. We therefore assessed the feasibility of local miR-21 suppression using anti-21-coated stents. Compared to bare metal stents, anti-21-coated stents effectively reduced ISR, while no significant off-target effects could be observed.
Conclusion
This is the first study to demonstrate the efficacy of an anti-miR-coated stent for the reduction of ISR.
Keywords: Myointimal hyperplasia, restenosis, microRNA, local drug delivery, rat, humanized model
Introduction
A universal and common pathological process of cardiovascular diseases is myointimal hyperplasia (MH), characterized by augmented proliferation and migration of vascular smooth muscle cells (VSMC) and increased synthesis of extracellular matrix (ECM), which progressively obliterates the vessel lumen1. These cellular events are the result of a dedifferentiation process of VSMCs, which are capable of losing their contractile function and re-establishing a more undifferentiated synthetic geno- and phenotype2. This VSMC plasticity can be observed as a response to growth factors, cytokines, cell-cell contacts, lipids and ECM components and is defined by a distinct gene expression profile3, 4. VSMCs further exhibit hyperpolarized mitochondria and an imbalanced replication-apoptosis ratio, which makes them highly proliferative5. Prevention of this VSMC phenotype holds promise for alleviating myointimal hyperplasia and subsequent obliterative vascular diseases like in-stent restenosis (ISR).
MicroRNAs (miRNAs) are small, endogenous antisense RNAs that regulate gene expression typically via mRNA degradation or translational repression6. miRNA manipulation can lead to broad alterations in regulatory pathways at multiple levels, as a single miRNA is capable of binding multiple mRNAs. These effects can be cell type- or tissue-specific. Previous studies suggest that miRNAs are critically involved in MH and ISR7. Modulation of various miRNAs can inhibit myointimal growth, suggesting a therapeutic approach for vascular disease7,8.
Most in vivo studies examining MH have utilized rodent models, and systemic miRNA modulation carrying the risk for off-target effects9. In order to increase translational potential, we decided to utilize our previously described orthotopic transplant model5, 10, and now report a novel strategy for local anti-miR (−21) delivery engaging coatable drug eluting stents (DES).
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
miR-21 is up-regulated during myointima formation
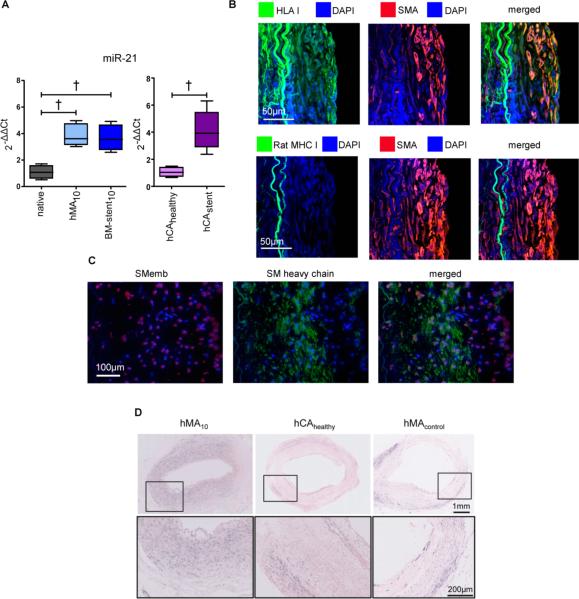
Balloon-injured human IMAs were implanted into the abdominal aortic position of athymic RNU rats (hMA model) to study MH as reported previously5, 10. In a second cohort, each IMA had a single bare metal stent (BM-stent) deployed prior to implantation. Ten days post-implantation, we retrieved the IMAs from both groups (denoted hMA10 and BM-stent10, respectively) and measured tissue expression of eight miRNAs previously shown to be associated with the proliferative response in vascular (patho-)physiology (miR-1, −21, −29b, −133a, −143, −145, −221, and −222)8, 11. Non-denuded native hMAs served as controls (“native”). Significant changes in miRNA expression were detected for all except one (miR-221) (Figure1A and Figure I in the online-only Data Supplement). Except for miR-21 (significantly up-regulated), all other remaining miRNAs were substantially repressed. miRNA expression levels were similar in the hMA10 and BM-stent10 groups for all miRs measured. Importantly, miR-21 expression was low in native hMA, and non-stenosed human coronary arteries (hCAhealthy), while previously-stented, diseased human coronary arteries (hCAstent) had similar miR-21 levels to the hMA10/BM-stent10 groups (Figure 1A), suggesting that our humanized in vivo model closely mimics human ISR. As miR-21 was substantially up-regulated, it appeared distinctly suitable for anti-miR-based therapeutic modulation.
Figure 1. MiR-21 is involved in human myointimal hyperplasia.
(A) Human IMAs underwent either balloon denudation (hMA), or denudation and stenting (BM-stent) and were implanted into the abdominal aortic position of RNU rats to await myointima development. After 10 days, qRT-PCR measurement of miR-21 revealed significant overexpression in both hMA and BM-stent groups as compared to native IMA (n=10 (native), n=6 (hMA), n=6 (BM-stent), ANOVA with Bonferroni's post-hoc test). Likewise, miR-21 levels in diseased, stented human coronary artery (hCAstent n=5;) were similarly altered vs. normal vessels (hCAhealthy n=4; unpaired t-test). (B) Human origin of myointimal cells in the hMA model was confirmed by double-positive immunofluorescence staining for SMA and human leukocyte antigen class I (HLA I) and the lack of rat major histocompatibility complex class I (rat MHC I). (C) Smooth muscle cells (SMC) within the myointima of hMA28 showed expression of SMemb and/or SM heavy chain. Synthetic, SMemb positive cells were distributed more in neo-myointimal regions and SM-heavy chain positive, contractile SMCs were found near the elastic laminae. (D) Presence of miR-21 in hMA10 confirmed by in situ hybridization in comparison to un-denuded hMA (hMAcontrol) and uninjured healthy human coronary arteries (hCAhealthy; purple chromagen). † p<0.01.
SMA-positive myointimal cells in the hMA model co-localized with HLA I and did not express rat MHC I (Figure 1B), confirming human myointimal origin. SMemb (VSMC de-differentiation marker), and SM heavy chain (VSMC-specific marker) were found in the myointima of hMA28 vessels (immunofluorescence; Figure 1C), as was miR-21 in comparison to un-denuded control hMA and non-stenosed human coronary arteries when analyzed by in situ hybridization (ISH; Figure 1D). miR-21 was approximately 4-fold up-regulated in injured human arteries, and abundantly expressed in the VSMC-rich human myointima.
Systemic miR-21 repression
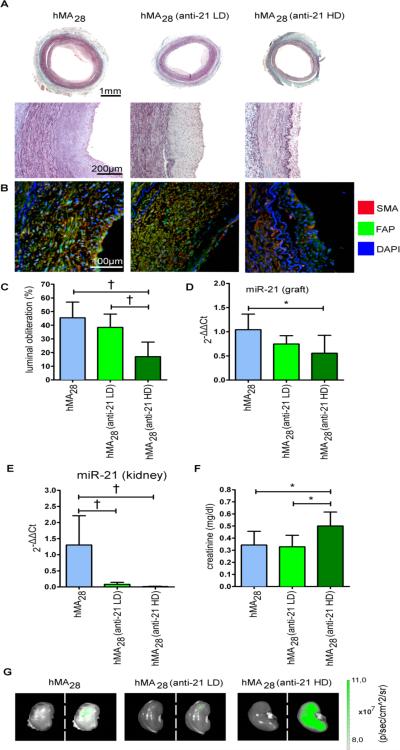
We sought to inhibit miR-21 in the hMA model through systemic administration. RNU rats received one intravenous dose of either 1mg/kg anti-21 in the low-dose (LD) or 5mg/kg anti-21 in the high-dose (HD) group, one day after hMA implantation. Vessels were retrieved after 28 days for histologic evaluation (Figure 2A). Untreated hMA28 arteries showed a circular, cell-rich myointima with increased extracellular matrix deposition. Myointimal area was reduced in both anti-21 treatment groups, an effect that was markedly more pronounced with HD treatment.
Figure 2. Systemic modulation of miR-21 inhibits myointima development, but causes off-target effects.
(A) To assess the effect of miR-21 modulation on myointimal hyperplasia, 1mg/kg (LD), 5mg/kg (HD) anti-miR-21 (anti-21) or vehicle were injected intravenously one day post-IMA-implantation. After 28 days, hMA-vessels were excised and stained with Masson's Trichrome. (B) Double immunofluorescence staining against SMA and FAP demonstrates similar myointimal composition in all groups. (C) Analysis of hMA vessels revealed marked luminal obliteration in the non-treated group, while anti-21-treated vessels showed less obliteration in a dose dependent manner (mean ± s.d., n=7 animals per group, ANOVA with Bonferroni's post-hoc test). (D) Sufficient inhibition of miR-21 in hMA vessel through anti-21 was confirmed by qRT-PCR. (mean ± s.d., n=7 (hMA), n=6 (anti-21 LD), n=6 (anti-21 HD), ANOVA with Bonferroni's post-hoc test). (E) Systemic miR-21 inhibition caused marked reduction of miR-21 expression in kidneys (mean ± s.d., n=6 (hMA), n=7 (anti-21 LD), n=7 (anti-21 HD), ANOVA with Bonferroni's post-hoc test). (F) This correlated with an increase in creatinine (mean ± s.d., n=7 animals per group, ANOVA with Bonferroni's post-hoc test). (G) Injected anti-21 was tracked via fluorescence imaging. High fluorescence signal was detected in HD-treated rat kidneys, indicating an accumulation of anti-21 (left: photo picture; right: overlay picture with fluorescence signal). * p<0.05, † p<0.01.
Confocal immunofluorescence staining revealed similar composition of the myointimas in all of the above groups (Figure 2B). Double immunofluorescence staining against SMA and Fibroblast activation protein (FAP) showed high myointimal SMC content with fibroblastic cells indicating an area of active tissue remodeling. Quantification of luminal obliteration confirmed significantly more luminal preservation in the anti-21 HD group compared to both untreated controls and the anti-21 LD group (Figure 2C). Effective knockdown of miR-21 in the hMA vessels with anti-21 HD treatment was confirmed by qRT-PCR (Figure 2D).
We next evaluated miR-21 off-target knockdown in other organs. As expected, we observed significant reduction of miR-21 expression in kidneys (Figure 2E), as well as liver, heart, and lungs (Figure IIA in the online-only Data Supplement). While other measured serum markers did not change significantly, creatinine elevation coincided with kidney miR-21 repression, indicating that systemic anti-21 HD administration negatively affects kidney function in our specific RNU rat model (Figure 2F). As the LNA-antisense was FAM-tagged, we were able to demonstrate (by fluorescence imaging) heavy renal accumulation of systemically-delivered anti-21 (Figure 2G). This potentially toxic side effect of anti-21 in kidneys should be taken into account when considering translational systemic miR-21 inhibition.
Local miR-21 repression using anti-21-eluting stents
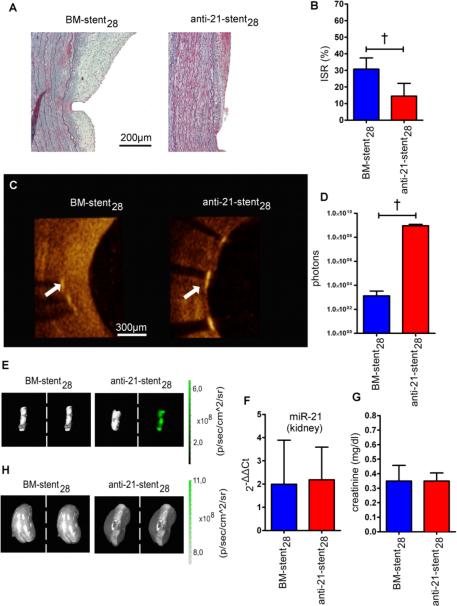
To minimize the above-noted off-target effects, we coated stents with anti-21 to permit local delivery to the injured hMA vessels. Successful stent coating with anti-21 was confirmed by FAM fluorescence and scanning electron microscopy (Figure III in the online-only Data Supplement). The total anti-21 amount coated onto each stent was similar to the systemic HD dosage (5mg/kg). Anti-21-stents were compared with otherwise identical BM-stents in the humanized model (Figure IIIB in the online-only Data Supplement), and ISR was assessed after 28 days. Trichrome staining revealed marked myointima formation within the BM-stent lumen, and much reduced myointima in anti-21-stented hMA (Figure 3A). Fixation and cutting protocols for stented vessels necessitated stent strut removal, leaving gaps adjacent to the internal elastic lamina. Despite this, ISR was clearly decreased in the anti-21-stent group as observed by histological quantification (Figure 3B). Optical coherence tomography (OCT) images obtained immediately after vessel retrieval and prior to fixation, also indicated markedly smaller myointimal growth within the anti-21-stent28 lumen (Movie S1, Figure 3C). Effective anti-21 uptake in the stented vessels was confirmed by fluorescence-imaging and -intensity analysis (Figures 3D-E). Screening for off-target regulation did not reveal any miR-21 expression changes in kidney (Figure 3F), liver, heart, or lung (Figure IIB in the online-only Data Supplement). Further, serum creatinine was unaffected by anti-21-stent placement (Figure 3G) and fluorescence confirmed no accumulation of anti-21 in the kidneys (Figure 3H).
Figure 3. Local inhibition of miR-21 prevents myointima development without exerting off-target effects.
(A) For local miR-21 inhibition, anti-21-coated stents were expanded into denuded IMAs, which were then implanted into RNU rats. Histological sections of stented vessel stained with Masson's Trichrome are shown 28 days after implantation. (B) Anti-21-stent coating resulted in significantly less luminal obliteration compared to the BM-stent group (mean± s.d., n=8 (BM-stent), n=6 (anti-21-stent), unpaired t-test). (C) OCT images revealed considerably less myointima formation in the anti-21-stent group (white arrow marks a stent strut, luminal side is to the right). (D) Fluorescent signal intensity measurements confirm anti-21 uptake in the anti-21-stent group (mean± s.d., n=5 per group, unpaired t-test). (E) Corresponding representative fluorescence images to signal intensity measurements shown in D. (F) miR-21 levels in kidneys were not affected by anti-21-coated stent delivery (mean± s.d., n=6 per group, unpaired t-test) and (G) serum creatinine showed no significant difference between the BM-stent and anti-21-stent groups (mean± s.d., n=8 (BM-stent), n=6 (anti-21-stent), unpaired t-test). (H) In fluorescence imaging, similar signals were detected in kidneys of both groups (left: photo picture; right: overlay picture with fluorescence signal). † p<0.01.
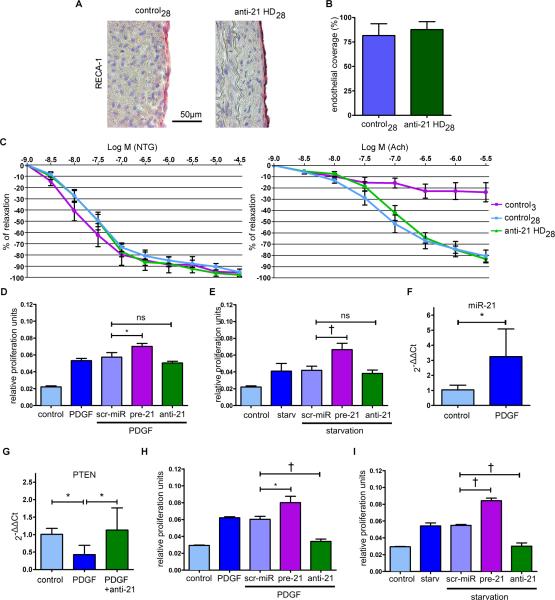
Anti-21 does not affect re-endothelialization
We investigated whether anti-21 treatment affects re-endothelialization of balloon-injured arteries. Lewis rats underwent balloon injury of their abdominal aortas and received the above-mentioned, highly effective anti-21 HD intravenously or remained untreated as control. Interestingly, after 28 days endothelial cell (EC)-specific RECA-1 staining revealed near complete re-endothelialization in both groups (Figures 4A-B). We also performed organ chamber experiments to assess the functional capacity of the regenerated endothelium to regulate vasodilatation (Figure 4C). Both control and anti-21-treated aortas showed similar endothelial-dependent and -independent relaxation characteristics. Together, these results indicate that re-growth of functionally intact endothelium in denuded arteries occurs despite anti-21 treatment. Of importance, in vitro proliferation of human coronary artery ECs (hCAECs) remained unaffected by anti-21 after applying different stimuli, such as serum starvation or platelet derived growth factor (PDGF) treatment (Figures 4D-E). Interestingly, only forced overexpression of miR-21 (pre-21 treatment) substantially increased MTT assay-detected EC proliferation (in comparison to scr-miR and anti-21; Figures 4D-E), indicating a less prominent regulatory effect of miR-21 inhibition in vascular ECs, an effect that was previously reported by others as well7, 12.
Figure 4. Anti-21 treatment does not impair re-endothelialization and abolishes stimulatory proliferative effects on SMCs in vitro.
(A) To assess the effect of anti-21 on vessel re-endothelialization, rat aortas underwent endothelial denudation via balloon injury. Animals in the treatment group received 5mg/kg anti-21 intravenously one day post-denudation. After 28 days, vessels were recovered and stained for endothelial cells. (B) There were no relevant differences in endothelial coverage between control28 and anti-2128 (mean± s.d., n=5 per group, unpaired t-test). (C)Endothelial function was assessed in organ chamber experiments (mean± SEM, n=6 (control28), n=6 (anti-21 HD28), n=3 (control3)). All three groups showed similar endothelial-independent relaxation characteristics, when stimulated with nitroglycerine. In contrast, stimulation with acetylcholine had no effect 3 days after vessel injury, while both control28 and anti-21 HD28 groups showed similar physiologic endothelial-dependent relaxation. (D) Proliferation assays with hCAECs after PDGF or 48h serum starvation (E) treatment revealed a negligible effect of miR-21 inhibition on EC proliferation (mean± s.d., quadruplicates of 3 independent experiments, unpaired t-test). (F) To determine the effect of PDGF on miR-21 expression in vessels, fresh pieces of IMA were stimulated with PDGF. 24h post-stimulation, miR-21 expression was significantly elevated (mean± s.d., n=6 (control), n=10 (PDGF), unpaired t-test) (G), while PTEN expression was suppressed. Concurrent transfection with anti-21 abolished this effect (mean± s.d., n=5 (control), n= 5 (PDGF), n=4 (PDGF+anti-21), ANOVA with LSD post-hoc test). (H) Inhibition of miR-21 with anti-21 caused diminished proliferation in PDGF-stimulated hCASMCs and (I) after 48h of serum starvation (mean± s.d., quadruplicates of 3 independent experiments, unpaired t-test). * p<0.05, † p<0.01.
Anti-21 inhibits proliferation of smooth muscle cells in vivo, ex vivo and in vitro
To confirm the observed anti-proliferative effect of miR-21 repression in VSMCs ex vivo, we stimulated fresh pieces of IMA with PDGF, an important driving factor for MH13 and miR-21 up-regulation in SMCs14 and fibroblasts15. PDGF not only increased miR-21 expression (Figure 4F), but also decreased PTEN (Figure 4G), a well-established target gene of miR-21 in vascular remodeling7. Simultaneous anti-21 treatment diminished PDGF-mediated PTEN suppression, confirming the intermediary and crucial role of miR-21 (Figure 4G). Inhibition of miR-21 abolished the pro-proliferative effect of PDGF (and serum starvation) on human coronary artery SMCs, whereas miR-21 overexpression substantially induced the pro-proliferative response (Figures 4H-I).
Conventional immunohistochemical analysis of explanted IMAs from our humanized in vivo model, in which rats were treated with systemic (high and low dose) anti-21, confirmed the inhibitory effect of the anti-21 treatment on SMC proliferation via de-repression of PTEN (Figure IV in the online-only Data Supplement). Transmural inflammation (as indicated by CD68-positive cells) was not impeded by anti-21 treatment in our model (Figure IV in the online-only Data Supplement).
Discussion
The treatment of patients with ISR remains challenging, despite recent advances in device technology and pharmacological treatment. BM stents are still widely used during percutaneous coronary interventions (PCI), despite their considerably high risk of ISR, especially when utilized for complex pathologic lesions or difficult anatomic scenarios16. The use of anti-proliferative drug-eluting stents (DES) substantially decreased myointimal proliferation and thus ISR. Several generations of DES have entered the clinic and markedly reduced the burden, particularly of early ISR. However, late ISR after DES placement remains a significant problem associated with delayed re-endothelialization due to cell-unspecific effects and prolonged thrombogenicity17. Thus, the discovery of highly effective treatments that allow local delivery and mainly target SMC-dominant MH, while not interrupting the re-endothelization process, remain very appealing. The major aim of this study was to technically assess the feasibility of using miR modulation locally delivered from a DES to prevent a certain vascular response to injury, in our case ISR.
Vascular remodeling is a term widely used for adaptive structural changes of the vasculature to different types of injury. Myointima formation may develop after PCI and stenting of diseased arteries and leads to ISR. Numerous miRNAs have previously been suggested to play a role in proliferation-associated vascular diseases. Out of 8 miRNA candidates assessed in this study, all except for one miRNA (miR-221) were substantially altered in injured (hMA groups) as well as stented hMAs (BM-stent groups) compared to controls. All miRNAs but miR-21 (increased expression) were significantly reduced in response to injury.
The other miRNAs that were down-regulated included the muscle cell-enriched miR-1 and miR-133, which are crucial mediators in muscular development and in response to vascular injury18. miR-29b, which has been identified as an important mediator of the fibrotic response in the vasculature by directly targeting several collagen isoforms as well as other important components of the extracellular matrix such as elastin and fibrillin119, 20. The miR-143/-145 cluster is particularly important in its ability to perform phenotypic switching in VSMCs in response to vascular injury21, 22. MiR-221 and miR-222 have been shown to be prominently involved in vascular proliferation by targeting p27 (Kip1) and p57 (Kip2) as well as the stem cell factor c-Kit in VSMCs23.
Of importance for treatment considerations, miR-21 was the only miRNA in our study that was significantly up-regulated in response to injury. miRNAs with augmented expression in response to injury and disease are amenable for antisense oligonucleotide (anti-miR) inhibition and may therefore present promising therapeutic targets on the molecular level. Therapeutic overexpression of suppressed miRs is currently less attractive, and the delivery of miRNA-mimics or pre-miRNAs for miRNA induction remains problematic. While LNA-based miRNA-mimics are becoming available in the near future, traditional delivery has involved lenti- or adenoviral approaches, presenting risks common to gene therapy24. Some newer studies from the cancer field have explored packaging synthetic miRNA duplexes within lipid nanoparticles, and have been quite successful with this approach in murine models25.
A major difference between the classical pharmacologic approach, and the novel field of miRNA-based therapeutics is that the latter may regulate entire gene or protein networks8. Recent animal and human efficacy data are promising, suggesting that anti-miRs have the potential to become a new class of drugs26,27. Anti-miRs have several advantages for drug development, including their small molecular size and the frequent conservation of their target miRNAs across species6.
miR-21 is considered to be among the most readily inducible vascular miRNAs, associated with increased cell proliferation and decreased apoptosis in the vessel wall28,12. In a previous study, antisense-mediated inhibition of miR-21 using pluronic gel reduced myointima in balloon-injured rat carotid arteries7. The authors showed that PTEN and Bcl-2 up-regulation were involved in the anti-miR-21-mediated cellular effects7. Similarly, in vein graft failure studies, miR-21 was significantly up-regulated following engraftment in mouse, pig, and human models. Genetic deletion of miR-21 in mice and grafted veins limited the proliferative response and reduced myointima formation29.
Our anti-21-eluting stent was highly effective in reducing VSMC proliferation and alleviating myointimal hyperplasia and ISR. Importantly, we did not observe miR-21 suppression in other organs, nor did we observe off-target side effects with this approach. Anti-21, in contrast to the currently employed DES medications such as rapamycin30, did not delay vessel re-endothelization, a side effect believed to be the major cause of stent thrombosis and late ISR16. In our study, systemic anti-21 allowed re-coverage of balloon-injured rat aortas with functionally intact endothelium. In addition, anti-21 treatment did not inhibit EC proliferation in vitro. This anti-21 effect is most likely due to the very low concentration of miR-21 in the endothelium compared to its expression levels in VSMC, being particularly enriched in the myointima and vessel media in response to injury and stent deployment12.
Off-target effects in organs in which miRNA-modulators assimilate to a much higher extent than the targeted vasculature are a much-debated compromise of antisense oligonucleotide therapeutics. Anti-miR-21 treatment in our ISR studies increased creatinine levels in RNU rats, which might be due to the immune-incompetent state of the rats, and thus model-specific. Of note, previous studies evaluating the development of kidney fibrosis in mice showed protective effects of miR-21 inhibition31, 32. Of importance, these studies were mainly conducted in diseased mice to prevent or reverse kidney fibrosis, and thus were not performed in un-diseased and otherwise healthy rodents. miR-21 plays an important role in controlling cell apoptosis in various cells types (such as tubular epithelial cells; TEC). Inhibition of miR-21 in TEC increases cell death33-35, which could be an explanation of our observation in the RNU rats.
We believe this proof-of-concept and technical assessment study for the efficacy and safety of anti-21-eluting stents demonstrates the feasibility of local delivery for miRNA-based therapeutics being applicable for vascular diseases. The method could potentially be optimized by modulating the release kinetics and dosing of the anti-miR, or by potentially combining multiple anti-miRs on a single stent. Future trials should address these issues, as well as its long-term efficacy in preventing the occurrence of late ISR.
Supplementary Material
Significance.
microRNA-based therapeutics have the potential of becoming a whole new class of drugs. However, the systemic form of their administration carries a substantial risk for off-target effects on organ systems (e.g., liver, kidney), in which these modulators assimilate to a much higher extent than the targeted tissue, such as the vasculature in our case. The utilization of local forms of delivery, such as drug eluting stents could significantly elevate the therapeutic potential of microRNA modulation in cardiovascular pathologies.
Acknowledgements
We thank Christiane Pahrmann, Claudia Bergow and Hartwig Wieboldt for their technical assistance. We also thank Martin W. Bergmann for his support with the OCT videos. Special thanks to Ethicon for providing the suture material. The confocal images were taken at the umif, University Medical Center Hamburg-Eppendorf (Bernd Zobiak).
Sources of funding
D.W. received a graduate student stipend “Individualized Cardiovascular Medicine” from the Cardiovascular Research Center (CVRC) at the University Medical Center Hamburg-Eppendorf and a student stipend from the German Center for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Luebeck. T.D. received the Else Kröner Excellence Stipend from the Else-Kröner-Fresenius-Stiftung (2012_EKES.04). P.S.T. is supported by funding from the National Institutes of Health (1HL-105299, 1HL122939 ). S.S. received research grants from the Fondation Leducq (CDA 2013-2015; S.S.) and the Deutsche Forschungsgemeinschaft (DFG) (SCHR992/3-1, SCHR992/4-1). L.M. is a Ragnar Söderberg fellow in Medicine (M-14/55), and received funding from the Karolinska Institute Cardiovascular Program Career Development Grant and the Swedish Heart-Lung-Foundation (20120615, 20130664, 20140186).
Abbreviations
- BMS
bare metal stent
- CAD
coronary artery disease
- DES
drug eluting stent
- ECM
extracellular matrix
- FAM
fluorescein
- FAP
fibroblast activation protein alpha
- hCA
human coronary artery
- HD
high dose
- HLA I
human leukocyte antigen I
- hMA
humanized mammary artery model
- IMA
internal mammary artery
- ISH
in situ hybridization
- ISR
in-stent-restenosis
- LD
low dose
- LNA
locked nucleic acid
- MH
myointimal hyperplasia
- MHC I
major histocompatibility complex I
- miR
microRNA
- mRNA
messenger RNA
- OCT
optical coherence tomography
- PDGF
platelet derived growth factor
- qRT-PCR
quantitative real time polymerase chain reaction
- RNU
Rowett nude (rats)
- SMA
smooth muscle cell α-actin
- VSMC
vascular smooth muscle cell
Footnotes
Disclosures
None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiological reviews. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa M, Sakomura Y, Ueda M, Kimura K, Manabe I, Ishiwata S, et al. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. 1997;96:82–90. doi: 10.1161/01.cir.96.1.82. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circulation research. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 5.Deuse T, Hua X, Wang D, Maegdefessel L, Heeren J, Scheja L, et al. Dichloroacetate prevents restenosis in preclinical animal models of vessel injury. Nature. 2014;509:641–644. doi: 10.1038/nature13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. Micrornas: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. Microrna expression signature and antisense-mediated depletion reveal an essential role of microrna in vascular neointimal lesion formation. Circulation research. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 8.Small EM, Frost RJ, Olson EN. Micrornas add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maegdefessel L, Spin JM, Adam M, Raaz U, Toh R, Nakagami F, et al. Micromanaging abdominal aortic aneurysms. International journal of molecular sciences. 2013;14:14374–14394. doi: 10.3390/ijms140714374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua X, Deuse T, Michelakis ED, Haromy A, Tsao PS, Maegdefessel L, et al. Human internal mammary artery (ima) transplantation and stenting: A human model to study the development of in-stent restenosis. Journal of visualized experiments : JoVE. 2012:e3663. doi: 10.3791/3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: The good and bad of micrornas. American journal of physiology. Heart and circulatory physiology. 2013;304:H1050–1059. doi: 10.1152/ajpheart.00267.2012. [DOI] [PubMed] [Google Scholar]
- 12.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, et al. Microrna-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Science translational medicine. 2012;4:122ra122. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirois MG, Simons M, Edelman ER. Antisense oligonucleotide inhibition of pdgfr-beta receptor subunit expression directs suppression of intimal thickening. Circulation. 1997;95:669–676. doi: 10.1161/01.cir.95.3.669. [DOI] [PubMed] [Google Scholar]
- 14.Alshanwani A, Riches K, Wood I, Turner N, Porter K. 168 exploring the role of microrna-21 on human saphenous vein smooth muscle cell function. Heart. 2014;100(Suppl 3):A96. [Google Scholar]
- 15.Polajeva J, Swartling FJ, Jiang Y, Singh U, Pietras K, Uhrbom L, et al. Mirna-21 is developmentally regulated in mouse brain and is co-expressed with sox2 in glioma. BMC cancer. 2012;12:378. doi: 10.1186/1471-2407-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. Journal of the American College of Cardiology. 2014 doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 17.Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: An observational study of drug-eluting versus bare-metal stents. Journal of the American College of Cardiology. 2006;48:2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Townley-Tilson WH, Callis TE, Wang D. Micrornas 1, 133, and 206: Critical factors of skeletal and cardiac muscle development, function, and disease. The international journal of biochemistry & cell biology. 2010;42:1252–1255. doi: 10.1016/j.biocel.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, et al. Microrna-29 in aortic dilation: Implications for aneurysm formation. Circulation research. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 20.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, et al. Inhibition of microrna-29b reduces murine abdominal aortic aneurysm development. The Journal of clinical investigation. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the mir143/145 gene cluster. The Journal of clinical investigation. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. Mir-145 and mir-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microrna-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. The Journal of biological chemistry. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra PK, Tyagi N, Kumar M, Tyagi SC. Micrornas as a therapeutic target for cardiovascular diseases. Journal of cellular and molecular medicine. 2009;13:778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, et al. Systemic delivery of tumor suppressor microrna mimics using a neutral lipid emulsion inhibits lung tumors in mice. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rooij E, Olson EN. Microrna therapeutics for cardiovascular disease: Opportunities and obstacles. Nature reviews. Drug discovery. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of hcv infection by targeting microrna. The New England journal of medicine. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, et al. Microrna-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 29.McDonald RA, White KM, Wu J, Cooley BC, Robertson KE, Halliday CA, et al. Mirna-21 is dysregulated in response to vein grafting in multiple models and genetic ablation in mice attenuates neointima formation. European heart journal. 2013;34:1636–1643. doi: 10.1093/eurheartj/eht105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen BX, Ma FY, Luo W, Ruan JH, Xie WL, Zhao XZ, et al. Neointimal coverage of bare-metal and sirolimus-eluting stents evaluated with optical coherence tomography. Heart. 2008;94:566–570. doi: 10.1136/hrt.2007.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, et al. Anti-microrna-21 oligonucleotides prevent alport nephropathy progression by stimulating metabolic pathways. The Journal of clinical investigation. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R, et al. Mir-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–674. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang A, Liu Y, Shen Y, Xu Y, Li X. Mir-21 modulates cell apoptosis by targeting multiple genes in renal cell carcinoma. Urology. 2011;78:474, e413–479. doi: 10.1016/j.urology.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov D, Liu H, et al. Delayed ischemic preconditioning contributes to renal protection by upregulation of mir-21. Kidney international. 2012;82:1167–1175. doi: 10.1038/ki.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microrna signature of renal ischemia reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.