Abstract
Corneal tissue displays the highest peripheral nerve density in the human body. Engineering of biomaterials to promote interactions between neurons and corneal tissue could provide tissue models for nerve/cornea development, platforms for drug screening, as well as innovative opportunities to regenerate cornea tissue. The focus of this study was to develop a co-culture system for differentiated human corneal stromal stem cells (dhCSSCs) and dorsal root ganglion neurons (DRG) to mimic the human cornea tissue interactions. Axon extension, connectivity, and neuron cell viability were studied. DRG neurons developed longer axons when co-cultured with dhCSSCs in comparison to neuron cultures alone. To assess the mechanism involved in the co-culture response, nerve growth factors secreted by dhCSSCs including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), and Neurotrophin-3 (NT-3) were characterized with greater focus on BDNF secretion. DhCSSCs also secreted collagen type I, an extracellular matrix molecule favorable for neuronal outgrowth. This co-culture system provides a slowly degrading silk matrix to study neuronal responses in concert with hCSSCs related to innervation of corneal tissue with utility towards human corneal nerve regeneration and associated diseases.
Keywords: cornea, neurons, co-culture, silk, collagen
Introduction
Neuronal innervation of cornea is essential for cornea functions including tear production, blinking, and protection [1]. The dense sensory nerve supply of cornea makes it the most sensitive organ in the body [2]. Corneal nerves are nerve bundles that enter the cornea at limbus [3] and then distribute in the stromal and epithelial layers [4, 5]. Corneal nerves are involved in a large range of diseases including corneal infection, leprosy and keratoconus [6] In addition corneal nerves injury is a side effect in operations including corneal transplantation and UV treatments [6, 7]. In response, current clinical solutions for corneal nerve dysfunction are limited to eye drops [8-10]. An in vitro corneal tissue model and improved understanding of cornea-nerve interactions would offer new options to study appropriate treatments.
To date, the evaluation of artificial cornea has mainly focused on the growth of stromal and epithelial cells, with limited studies including neurons that provide essential function of eye [1, 2]. Nerve regeneration should play a pivotal role in evaluating functional artificial cornea. Nerve regeneration has been investigated in collagen-based artificial cornea, where neuron dendrites were observed 4 years after the implantation of artificial cornea [11]. However the density of dendrites was much lower than healthy cornea and human donor transplantation. An in vitro corneal tissue model involving neurons would provide a closer step to a functionally active artificial cornea.
The current understanding of the organization and function of corneal nerves is based on histological and in vivo studies, which do not comprehend the ability to track the development and organization of neuron extensions [5]. There are only few in vitro 2D studies related to neuron-cornea interactions, showing that co-culture with corneal epithelial cells improved neuronal extensions [12]. However the field is still missing a model mimicking the structure of corneal tissue and related focus on integrating the unique features of corneal nerves.
Previous in vivo research has shown that corneal tissue interacts with nerve through soluble and insoluble factors. RNA for soluble neuron growth factors, including glial cell derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and neurotrophins-3(NT-3) were found to be expressed in corneal stromal and epithelial tissue[10]. Insoluble extracellular matrix (ECM) components including collagen types I, V, VI were also secreted by corneal resident cells, which ultimately modulated neuronal outgrowth [13, 14]. In vitro models of neuron interactions with corneal cells would provide a tool to study the role of ECM and soluble factors on neuronal extension and organization.
In the present study, an in vitro silk protein-based co-culture system was developed to assess dhCSSCs and DRG neuron interplay, with the goal of gaining a further understanding of the interactions between corneal stromal tissue and neuronal regeneration. The co-culture system was designed to closely mimic the anatomy of human corneal tissue, with thin silk films acting as the corneal stromal layer, and silk-collagen hydrogels containing DRG neurons resembling the surrounding corneal limbus tissue. Collagen is rich in cell adhesion sites that supports neurons attachment and outgrowth [13], while silk provides a more mechanically robust matrix [15]. Together these proteins provide a tunable system to stimulate neuron outgrowth. The hypothesis of this study was that DRG neurons co-cultured with dhCSSCs would have improved neuronal outgrowth compared to DRG neurons cultured alone. In this system the interactions between dhCSSCs and neurons will be investigated. This co-culture system is considered the starting point for a tissue platform for the in vitro modeling of corneal nerve regeneration, drug screening, as well as a disease model for functional testing of artificial cornea.
Materials and Methods
2.1 Preparation of Silk Solution
Silk solution was prepared from cocoons of Bombyx mori silkworm based on the procedures developed in our previous studies [16-18]. Silk cocoons were supplied by Tajima Shoji Co. (Yokohama, Japan) and boiled for 30 or 45 minutes in 0.02 M Na2CO3 solution (Sigma-Aldrich. St Louis, MO, USA). The boiled silk fibroin was rinsed with deionized water 3 times and dried overnight. The extracted silk was then dissolved in a 9.3M LiBr solution and dialyzed against distilled water for 2 days to obtain a silk fibroin aqueous solution (5~7% w/v).
2.2 Preparation of Silk Films
Patterned porous silk films were prepared using the procedure developed in our previous studies [19]. Silicone elastomer (PDMS) (Corning, Midland, MI, USA) was cast on optical dispersion angle grating (Edmund Optics, Inc., Great Barrington , NJ, USA). The PDMS was cured at 60? for 1h, and then peeled off from the grating, and rinsed in 70% EtOH followed by Milli-Q water. A 1% w/v silk solution with 0.035% w/v of polyethylene oxide (PEO) was prepared and cast on a PDMS mold and dried overnight. The film was annealed in a water filled desiccator at −20 mm Hg for 2.5 hours and then peeled off from the mold using our established methods [17]. The silk films were immersed in water for 2 days to extract the PEO and generate the pores.
2.3 RGD Surface Modification
RGD functionalized on the silk films were made using methods from our previous works [19, 20]. The silk films were presoaked in MES buffer (Pierce, Woburn, MA, USA 100 mM borate 150 mM and NaCl, pH 6.5) for 30 min. A 1-ethyl-3-(dimethylaminopropyl)carbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) solution (0.5 mg/mL of EDC and 0.7 mg/mL of NHS in MES buffer, pH 6.5) was reacted with the –COOH groups from the aspartic and glutamic acid residues in the silk for 30 min at room temperature. These activated silk films were then washed with MES buffer 2 times and subsequently treated in 1 mg/mL Arg-Gly-Asp(RGD) solution (Bachem Inc.Torrance, California, USA).
2.3. Preparation of silk collagen Hydrogels
Collagen gels were prepared by adding 30μl of 10X DMEM (GIBCO, Life Technology, Grand Island, NY, USA) to 270 μl (4 mg/ml) acetic acid-type I collagen solution (rat-tail tendon, BD, Franklin Lake, NJ, USA), followed by neutralization with 1M NaOH (Sigma, USA). A 45 minutes boiled silk solution (2% w/v) was mixed with 10X DMEM (GIBCO) with ratio of 9 to 1, and then sonicated with a Digital Sonifier (Branson Ultrasonics, Danbury, CT, USA) at 20% amplitude for 20 seconds to generate silk hydrogels using our establish protocols [16]. The sonicated silk was mixed with neutralized collagen solution at ratios (v/v) of 1 to 2 and 2 to 1, we refer as 1/2 hydrogel and 2/1 hydrogel respectively.
2.4 Human Corneal Stromal Stem Cell Culture
Human corneal stromal stem cells (HCSSCs) isolated from collagenase digestion of limbal stromal tissue of human corneas unsuitable for transplant were obtained from the Center for Organ Recovery & Education (Pittsburgh, PA) [18]. HCSSCs were passaged 6 times before seeding. Cells were detached with 0.25% trypsin (GIBCO) solution and seeded on the surface of the sterilized patterned porous silk film with concentration of 15,000 cells /cm2. Cell seeding was accomplished by adding cell suspension dropwise on top of the film. The films were incubated for 30 minutes to allow cell attachment. Seeded silk films were cultured in proliferation medium containing DMEM/MCDB-201in the ratio of 3 to 2(v/v) with 2% fetal bovine serum, 10 ng/mL platelet-derived growth factor (PDGF-BB), 1 mg/ml lipid-rich bovine serum albumin (Albumax, Life Technologies, Grand Island, NY), 10 ng/mL epidermal growth factor (EGF), 5 mg/mL transferrin, 5 ng/mL selenous acid (ITS), 0.1 mM ascorbic acid-2-phosphate, 10−8 M dexamethasone, 100 IU/mL penicillin, 100 mg/mL streptomycin, 50 mg/mL gentamicin, and 100 ng/ml cholera toxin until con?uent (~2 days) [21] . After hCSSCs confluence, silk films were cut into circular shapes with a 12 mm diameter biopsy punch (McMaster-Carr, Robbinsville, NJ, USA) and anchored in 24 well tissue culture plate with 8 mm diameter Teflon rings (McMaster-Carr). HCSSCs were differentiated on the silk films into keratocytes (dhCSSCs) with differentiation medium using advanced DMEM (Life Technologies), containing 1.0 mM L-ascorbic acid-2-phosphate (Sigma-Aldrich, St Louis, MO, USA), 50 μg/ml gentamicin (Life Technologies), 2 mM L-alanyl-L-glutamine (Life Technologies), 100 μg/mL penicillin, 100 μg/ml streptomycin (Mediatech Inc., Manassas, VA, USA) 0.1 ng/ml transforming growth factor-beta3 (TGF-B3, Sigma-Aldrich), and 10 ng/ml basic fibroblast growth factor (FGF-2, Sigma-Aldrich) [21]. HCSSCs were also differentiated on 24 well tissue culture plates (TCP) for collagen staining and 6 well TCP for ELISA assays for neurotrophin secretion. with a concentration of 15,000cell/cm2 and cultured for 14 days and 22 days.
2.5 Chicken Dorsal Root Ganglion Cell Culture
DRG neurons were dissociated from Dorsal root ganglion from chicken embryos (University of Connecticut, Poultry Farm, CT, USA). The dissection and dissociation followed the protocol developed in our prior study [15]. Briefly, ganglia were trypsinized for 25 min and then centrifuged for 5 minutes at 1,500 rpm. The pellets were triturated for 3 rounds, 12 times for each round. The dissociated neurons were transferred to 100×200 mm2 tissue culture plates and incubated for 45 min, with neurons having less tendencies to attach to the bottom compared to other types of cells. The suspension was collected and centrifuged to isolate the neurons.
2.6 DRG Neuron Culture In Hydrogels
To achieve encapsulation, dissociated DRG neurons was added to sonicated silk solution (20 seconds, 20% amplitude). For silk collagen composition hydrogel, dissociated DRG neurons were added after mixing the sonicated silk solution and collagen solution. Cells were encapsulated in the hydrogel systems upon gelation. All samples were seeded with 100,000 cells/ml. DRG neurons were cultivated in DRG growth medium prepared from DMEM/F12 medium supplemented with FBS (10%) and nerve growth factor (NGF) (50 ng/mL, R&D Systems, Minneapolis, MN) for 7 days.
2.7 Co- culture of Differentiated hCSSCs with DRG Neurons
Co-culture was processed on the film-hydrogel system as well as TCP and transwell for cell morphology analyzes and metabolism activity assays. Co-cultivation on film-hydrogel system was started from culturing dhCSSCs on silk films for two weeks before initiating co-culture. The DRG neurons were incorporated into 300μl of 1/2 hydrogel with 100,000 cells/ml. The hydrogels were then added gently around the silk film (Figure 1). The samples were then incubated for 30 minutes to achieve gelation.
Figure 1.

Preparation of co-culture scaffold system. (A) Human corneal stromal stem cells (hCSSCs) were seeded on the patterned silk film with density of 15,000/cm2 and cultivated in proliferation medium to reach confluence. Differentiation of corneal stromal stem cell was then initiated to achieve differentiated hCSSCs (dhCSSCs). Silk film was then cut in to 12mm diameter before co-culture. Silk-collagen mix hydrogel containing DRG neuron was then applied around the silk film. The co-culture system was then cultivated in dhCSSCs differentiation medium. (B) The picture of co-culture system in 24 well tissue culture plastic after 7 days of co-cultivation. The silk-collagen hydrogel remain its integrity after co-culture, the silk film remain to be anchored by the hydrogel after 7 days of cultivation. Scale= 6mm
A tissue culture plastic (TCP) co-culture group in which dhCSSCs have direct contact with DRG neurons, and a transwell co-culture group where dhCSSCs and DRG neurons were separated but cultivated in the same system were also prepared. We refer to the groups as the TCP co-culture and transwell co-culture, respectively. The TCP co-culture group was prepared by seeding dhCSSCs at 15,000 cell/cm2 and DRG neurons at 30,000 cell/cm2 in 24 wells TCP (Corning, Corning, NY, USA). The transwell co-culture group was prepared by seeding dhCSSCs in the insert and DRG neurons in the well of 24 well polycarbonate membrane transwells (Corning, Corning, NY, USA) with the same concentrations as in the TCP co-culture group.
In order to analyze the uniqueness of dhCSSCs co-culture impact on neurons, human corneal epithelial cells (hCECs) were also co-cultured with DRG neurons with the same seeding method and cell density on silk film and hydrogel systems. All co-cultures were maintained for 7 days in hCSSC differentiation medium with 50 ng/ml of NGF.
2.8 Cell Metabolic Activity
Cell metabolic activity was characterized using Alamar Blue (Invitrogen, Grand Island, NY) assay. Before the assay, the inserts from the transwell co-culture group were removed in order to separate analyze the 2 types of cells. Alamar blue dye was added to the medium at a ratio of 1 to 10 in both the TCP and transwell co-culture groups and incubated for 2 hrs. Fluorescence was measured with excitation and emission wavelengths of 530 and 590 nm, respectively.
2.9 Immunohistochemical Staining
Anti β-tubulin-III staining was processed to observe neuronal extensions. Neurons cultivated in hydrogels were fixed on day 7, while the co-cultured samples were fixed on day 1,3,5 and 7 in 4% paraformaldehyde in PBS (Affymetrix Inc, Cleveland, Ohio, USA) for 45 min. Samples were then treated with 0.03% Triton (Sigma) X-100 in PBS for 30 minutes. Anti β-tubulin III rabbit antibody (Sigma) was diluted 1:500 in 10% FBS/ PBS solution, and applied on the samples. For quantifying collagen types I, V, VI secretion, dhCSSCs cultured on TCP were fixed on days 3, 7 and 14. Anti collagen type I, V and VI antibodies (Abcam, Cambridge, MA, USA) were diluted 1:200 in 10% FBS/PBS solution and applied on samples. All samples were maintained at 4? for 12 hours and then washed 3 times, with soaking in PBS for 10 minutes each time. Anti-rabbit IgG-FITC antibody produced in goat (Sigma) was diluted 1:200 in 2% FBS /PBS solution and added to samples and incubated at 37? for 1 hr. In order to observe dhCSSCs in co-culture conditions, F-actin filaments were stained with Alexa Fluor ® 568 Phalloidin (Life Technologies). Phalloidin was diluted 1:20 in 10% FBS/PBS solution and incubated at the same time as anti-rabbit IgG-FITC antibody. Scaffolds without cells were also stained as negative controls. Immunostained samples were imaged using a fluorescence microscope (Leica DMIL, Buffalo Grove, IL) with FITC filter (513-556nm) and Texas Red filter (604-644nm).
2.11 Neural Axon Length Measurements
Positive staining of β-tubulin III was determined at 10X magnification for four regions on each sample of DRG neurons cultivated in hydrogels and co-cultured with dhCSSC or hCECs from n=6 samples from 3 independent experiments. All the images were then converted into 8-bit tiff files using Image J (NIH). The neuron J routine was applied to measure the axon length. [22].
2.12 ECM Synthesis of dhCSSCs
Images were collected at 10X magnification for 4 regions from each sample with anti collagen type I, V, VI staining using n=4 from 3 independent experiments. The integral pixel density was measured by image J (NIH) for each image and normalized by dividing the cell number in the region.
2.13 dhCSSCs Neurotrophin Secretion
In order to test dhCSSCs ability to secrete neurotrophins and analyze their respective effects on neurons, ELISA experiments were performed against the medium in dhCSSCs cultures. Briefly, dhCSSCs were cultivated in 6 well tissue culture plastic plate for 22 days; 1 ml medium aliquots were collected every 3 days from each well. Media samples were then concentrated with Amicon Ultra 10K Filter Devices (Millipore, Merck KGaA, Darmstadt, Germany). Elisa assays for BDNF, GDNF, NT-3, NGF were processed with human BDNF, GDNF, NT-3, NGF Duo set (R&D system, Minneapolis, MN, USA) on n= 3 from two independent experiments.
2.14 Statistical Analysis
All data analysis was performed with One way ANOVA with Dunnett post hoc test. Significance level was set at p<0.05. All experiments were run in at least triplicates for 2 independent experiments.
3. Results and Discussion
3.1 Design of co-culture scaffolds for DRGs and dhCSSCs
The design of the co-culture system allowed for the spatial confinement of the dhCSSCs and DRGs while maintaining both cell types (Figure 1A). Silk-collagen hydrogels seeded with the neurons was cast at the periphery of a silk film containing the cultured dhCSSCs (Figure 1A). A silk-collagen hydrogel maintained DRG neuron viability while providing a suitable elastic modulus [15] for neuronal extension (Figure 1B). Porous patterned silk films functionalized with RGD supported the aligned growth of the dhCSSCs as well as providing a transparent scaffold to mimic corneal tissue. The co-cultures were maintained for 1 week and during cultivation the silk films remained transparent and the hydrogels and scaffolds remained structurally stable (Figure 1B).
3.2 Optimization of Hydrogel Composition for DRG Neuronal Outgrowth
A silk-collagen hydrogel was chosen to support neuronal outgrowth. Collagen type I is a favorable substrate for many types of neurons [13, 23] while silk provided robust material to adjust the mechanical property of the hydrogel [15]. The optimal silk to collagen ratio for neuronal outgrowth was assessed. Silk alone, collagen alone, 1 to 2 silk-collagen hydrogel (1/2 hydrogel) l, and 2 to 1 silk-collagen hydrogel (2/1 hydrogel) were prepared. The concentration of silk and collagen in the 1/2 hydrogel was 6.67 mg/ml and 2.67 mg/ml, respectively, and in the 2/1 hydrogel these values were 13.33 mg/ml and 1.3 mg/ml, respectively.
Within the silk hydrogel system (Figure 2A), there was limited neuronal extension with an average axon length of ~30±14.8 μm after 7 days. In comparison, within the collagen hydrogels, an increase in axon extensions was observed (~210 ±78.6μm), although axon morphology was thinner on the collagen hydrogels when compared to the silk hydrogels. In the case of the composite hydrogels, the 1/2 hydrogel supported significantly longer axon extension compared to the other hydrogels with average axon length of 534±167.87μm after 7 days, while 2/1 hydrogels supported and average 312±144.972 μm axon extensions. This study corroborated our previous work where silk hydrogels provide 3 times longer neuronal extension than collagen hydrogels. Collagen concentration was reported previously to have an impact on neuronal extension with 1 mg/ml as the optimal concentration for axon extension [24]. However, the 1/2 hydrogel which contained 0.33 mg/ml of collagen provided longer axon length compare to the 2/1 hydrogel which contained 2.67 mg/ml of collagen. This result indicates that the silk fibroin provided some enhancement towards axon development. This may be due to primary (e.g., signaling) or secondary (mechanical, physical guidance) inputs. The results demonstrate that by varying the amount of silk relative to the collagen content in the hydrogels axon length could be tuned.
Figure 2.

DRG neuronal outgrowth in silk-collagen composite gels. (A) Fluorescence images of DRG neuronal outgrowth in (immunostained with anti-β-Tubulin III in green): (a) silk hydrogels, (b) silk/collagen=2/1 (2/1) hydrogel, (c) silk/collagen=1/2 (1/2) hydrogel, (d) collagen hydrogel. Images were taken on day 7 (Scale bar 100 μm) (B) Quantification of neuronal extension length. 1/2 hydrogel gave statistically longer neuronal outgrowth than other hydrogels. N=6 from 3 independent c cultures respectively. ***p<0.0001, one way ANOVA with Dunnett post hoc test.
3.3Effect of Co-culture on DRG Neuron Axon Extensions
The co-culture was maintained for 1 week and the results were compared with the DRG neuron cultured in 1/2 hydrogels alone. Staining for β-tubulin III showed that neurons co-cultured with dhCSSCs have denser and longer axons, as well as increased cell aggregation, compared to neuron culture alone (Figure 3A). Quantification of neuronal extension showed comparable axon length in both co-culture and monoculture systems for the first 3 days (Figure 3B). However, from days 5 to 7 the DRG neurons co-cultured with dhCSSCs reached an average length of 714 μm (±102μm), 35 times longer than DRGs culture alone. The fraction of neurons with extensions (Figure 3B,b) was significantly higher in the co-culture group compare to the monoculture throughout the entire cultivation time. Long and dense neuronal extensions appeared on day 7 in the co-culture system (Figure 3A).
Figure 3.
DRG neuronal outgrowth in co-culture with dhCSSCs and monocultures in 1/2 hydrogels. (A) Representative fluorescence images of DRG neurons monoculture and co-cultured with dhCSSCs. DRG neurons were immunostained with anti-β-Tubulin III in green on days 1, 3, 5 and 7 (Scale bars, 100μm). (B) (a) Quantification result indicated neuronal outgrowth during co-cultures with dhCSSCs are statistically longer than monocultures on day 7. N =3 from 3 independent cultures, respectively. **p<0.001***p<0.0001,one way ANOVA with Dunnett post hoc test. (b) Fraction of DRG neurons with extension in co-culture was statistical significantly more than DRG monocultures. N=3 from 3 independent cultures, respectively. ***p<0.0001 from one wayANOVA with Dunnett post hoc test.
Furthermore, dhCSSCs and DRG neuron contact at the silk film-hydrogel interface was observed at day 7 (Supporting Figure 1A). Additionally, neurons were only seeded in the hydrogel, however, in some images (Support Figure 1B) neurons were observed to be located on silk film and formed connection with dhCSSCs nearby. This relocation might be due to dhCSSCs inducement of DRG neuron migration. The connections formed between dhCSSCs and DRG neurons illustrate that this system has potential of mimicking corneal stromal tissue and neuron interaction.
In order to study the ability of dhCSSCs to promote neuronal extension during co-culture, in comparison to other cell types, hCECs were also co-cultivated with DRG neurons using the same methods. In these cocultures, there was no significant improvement in axon length or fraction of neurons with extensions compared to the DRGs when cultured alone (Supporting Figure 2 A,B). Even though the 2D co-culture of neurons with corneal epithelial cells was reported to provide longer neuronal extensions [12] our results indicate that the neurons react differently in co-culture in scaffolds designed to mimic corneal anatomy. By co-culturing DRG neurons with dhCSSCs, neuronal extension was significantly increased, illustrating co-cultivation of DRGs with other types of cell may provide a method for improving neuronal extension other than modifying scaffolding materials.
To summarize, co-cultures of DRGs with dhCSSCs grown on patterned porous silk films supported the growth of both types of cell and increased axon extension. The combined gel-film scaffold design allowed directed neuronal outgrowth from a peripheral seeding location towards dhCSSC cells, with neuron-cornea cell contacts observed.
3.4 Synthesis of Collagen Types I, V,VI of dhCSSCs
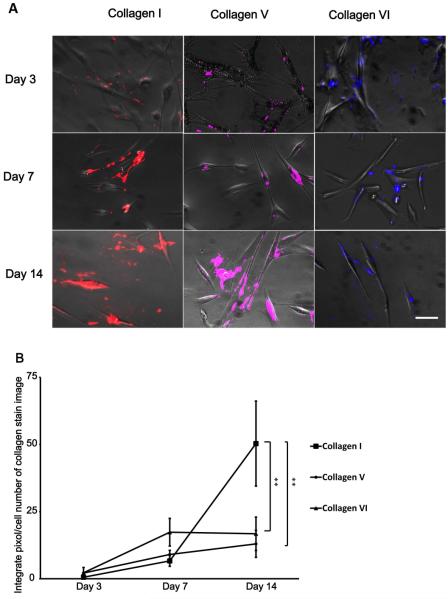
In order to further study the impact of ECM on neuronal outgrowth, hCSSCs without permeabilization were stained to study collagen I,V,VI secretion. These collagens have been detected from hCSSC cultures [20, 21] but the effect of collagen secretion on neuronal outgrowth has not been studied. The results indicated secretion of collagen types I,V,VI increased as time from days 3 to 14 (Figure 4A). Quantification of relative increase showed that collagen type I, V, and VI production increased 100, 6, and 8-times from days 3 to 14, respectively. All 3 types of collagens were mainly located on the dhCSSC cell bodies on day 3,and were secreted into intercellular spaces on day 7 and 14. Collagen type I secretion was ~5-times that of collagen types V and VI on day 14. Rat collagen type I was previously shown to promote neuronal outgrowth in culture [13], and may contribute to the increased neuronal outgrowth observed in the co-culture experiments. Conversely, collagen type V was found to inhibit axonal outgrowth by reducing cell adhesion [14, 24]. Collagen type VI affected Schwann cell differentiation, while its relationship with neuronal extension has not been reported [13]. Based on the known impact of collagen types I,V,VI to neurons [14, 25] the secretion of collagen type I from dhCSSCs is one of the reasons that neurons achieved longer extension during co-cultivation with dhCSSCs.
Figure 4.
Collagen types I, V, VI secretion from dhCSSCs. (A) Fluorescence images of Collagen types I, V, VI immunostained in red, purple and blue, respectively. Scale bars 50 μm. (B) Quantification of fluorescence signals of collagen types I,V,VI on days 3,7,14, normalized against samples with no cells, showed secretion of collagen I was statistically significant higher than collagen type V and VI. n=4 from 3 independent cultures, respectively.**p<0.001 from one way ANOVA with Dunnett post hoc test.
3.5 Neurotrophin Secretion from dhCSSCs
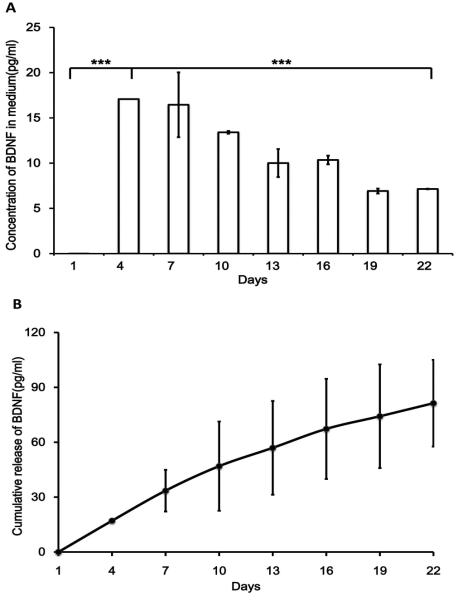
Beside insoluble ECM molecules, neurotrophins secreted from dhCSSCs were quantified using ELISA. In order to improve accuracy, the medium collected from dhCSSCs culture was concentrated with a 10K protein filter. Among the 4 types of neurotrophins tested, only BDNF was found to be secreted from dhCSSCs(Figure 5). The signals of GDNF, NT-3 and NGF from medium samples were not significantly higher than blank control, and lower than the detection range. RT-PCR study for corneal stromal tissue had previously detected mRNA expression of GDNF,NT-3,NGF,BDNF [10]. However, there was no protein assay for neurotrophin secretion of dhCSSCs tested during in vitro culture. From ELISA result (Figure 5 A), BDNF secreted from dhCSSCs during the first week was ~0. 3 and ~2.5 times higher than the second and third week. The concentration of neurotrophin for DRG viability and neuronal outgrowth improvement during in vitro cultivation is usually greater than 1ng/mL [26-28]. In this study the accumulated secretion of BDNF over 22 days is only 80 pg/ml (Figure 5B). Thus, the BDNF secreted from dhCSSCs during co-culture may have limited effect on the promotion of neuronal extension.
Figure 5.
Elisa assay for BDNF secretion from dhCSSCs cultured on tissue culture plastic. (A) The concentration of BDNF in medium of dhCSSCs culture from day 1 to day 22. Day 4 and 7 have the highest concentration, decreased on day 10,13,16 and remain on low level on day 19 and 22. (B) The cumulative release of BDNF from day 1 to day 22. The cumulative release of BDNF reached 90pg/ml after 22 days of culture. N=3 from 2 independent culture, respectively. ***p<0.0001 from one way ANOVA with Dunnett post hoc test.
3.6 Cell Metabolism in TCP and Transwell Co-cultures of dhCSSCs and DRG Neurons
In this study, dhCSSCs was found to promote neuronal outgrowth through neurotrophin secretion and ECM production. However the separate contributions of soluble and insoluble factors remains unclear. In order to study this, cell metabolic activity was measured for co-cultures on TCP where cells are exposed to both soluble and insoluble factors and for transwell co-culture groups where only soluble factors were available. Alarmar blue results indicated that both types of co-cultures provided higher neuronal metabolism activity compared to the neuron monocultures (Figure 6 A,B). In the transwell co-culture group (Figure 6A), however, the cell activity of neurons in co-culture was not statistically different to the monoculture, suggesting thatsoluble factor alone is not sufficient to promote neuronal viability. The activity of neurons in the TCP co-culture group was 2 times higher than TCP monoculture and 2 times higher than the transwell co-culture group,after correction for the dhCSSC contribution (Figure 6B). This demonstrates that direct contact is necessary for dhCSSCs to promote DRG neuronal extension with collagen secretion of dhCSSCs playing an important role in promoting DRG neuronal extension.
Figure 6.
Cell metabolism activity of DRG neurons co-cultured with dhCSSCs and monocultures in 1/2 hydrogel. Fluorescence intensity from Alamar blue assay was normalized against day 0. (A) Transwell tissue culture plastic co-cultures showed higher activity compared to DRG neuron monocultures on TCP. N=6 from 3 independent cultures respectively. (B) TCP co-cultures revealed cell activity in DRG neurons co-cultured with dhCSSCs and dhCSSCs monocultures were statistically significantly higher than DRG neuron monocultures, n=6 from 3 independent cultures respectively. ***p<0.0001 from one way ANOVA with Dunnett post hoc test.
This is the first in vitro study to show the promotion of corneal stromal cells effect on neuronal extension. Beside corneal stromal cells, co-cultivation of neuronal cells with other cell types has been previously reported to improve neuronal extension. Mesenchymal stem cells (MSCs) increased axon elongation by secreting BDNF and β-NGF, which contribute to neuron survival and regeneration [29]. Neural stem and precursor cells were also found to improve neuronal regeneration and survival through the secretion of fibronectin, laminin and neurotrophins [30]. Furthermore, astrocytes and microglia were found to protect metabolically impaired neurons[31, 32]. The 2D co-culture of neurons with Schwann cells and corneal epithelial cell also provides longer neuronal extension [7, 12].
Although media supplementation with nerve growth factors, such as NGF, GDNF, BDNF, NT-3, can lead to longer neuronal extension [32, 33], data shown from this study indicate that ECM components also play an important role in promoting neuron development. Similarly, co-cultivation of neurons with other types of cells might provide an improved understanding of mechanisms of nerve development and regeneration in different tissues.
Conclusions
A co-culture system was established to enable both dhCSSCs and DRG neurons to grow, organize, and interact in culture. Using a two-component gel/film system based on silk and collagen, the structure of the cornea could be mimicked in vitro allowing to study the interactions between dhCSSCs and DRG neurons. The impact of dhCSSCs on neuronal outgrowth was observed and co-cultures significantly increased neuronal extensions likely due to collagen type I expression by the dhCSSCs. The slow degrading silk system provides a robust in vitro model tissue system for longer-term studies of physiological and pathological mechanisms at the base of cornea neuron development and interactions.
Supplementary Material
Supporting Figures
Supporting Figure 1: DRG neurons and dhCSSCs form connections after 7 days of co-cultivation. Fluorescence images of DRG neurons and dhCSSCs (immune-stained with anti-β-Tubulin III in green for viewing DRG neurons and F-actin in red for viewing dhCSSCs) A: 10x image of DRG neuron formed connection with dhCSSCs at the interface of silk collagen hydrogel and silk film (interface shown with white line). DRG neuronal extension contact with the dhCSSCs is shown with arrows. B: 20X image of DRG neuron was observed outside of silk collagen hydrogel and formed direct contact with dhCSSCs. Scale=200μm
Supporting Figure 2: DRG neuronal outgrowth in co-culture with hCECs and monocultures in S/C=1/2 composite gels. (A) Representative fluorescence images of DRG neurons alone and co-cultured with hCECs. DRG neurons were immunostained with anti-β-Tubulin III in green on days 1,3,5,7. (Scale bars 100 μm). (B) (a) Quantification of neuronal extensions of DRG neurons co-cultured with hCECs was not statistically different than monocultures on days 1,3,5,7. N =3 from 3 independent cultures from one way ANOVA with Dunnett post hoc test. (b) Fraction of DRG neurons with extensions did not show statistical differences in co-cultures with hCECs compared to monocultures. N=3 from 3 independent cultures, respectively, one way ANOVA with Dunnett post hoc test.
Supporting Figure 3: 10 X images of controls for immunohistochemistry staining. A: TCP control stained with anti collagen type I in green. B: TCP control stained with anti collagen type V in green. C: TCP control stained with anti collagen type VI in green. D: silk film control stained for F-actin in red. E: silk hydrogel control stained with anti-β-Tubulin III in green. F: 1/2 hydrogel control stained with anti-β-Tubulin III in green. G: 2/1 hydrogel control stained with anti-β-Tubulin III in green. H: Collagen hydrogel control stained with anti-β-Tubulin III in green. Images were collected from N=3 for 2 independent experiments. Scale bars 200 μm.
Acknowledgments
The authors thank Dr. James L Funderburgh for providing human corneal stromal stem cell source and Dr. Min Tang-Schomer, Dr. Ying Chen and Erica Palma for insightful discussions and help with editing. The authors also thank the NIH (R01 EY020856) (R01 EY016415).
References
- [1].Heigle T, Pflugfelder S. Aqueous tear production in patients with neurotrophic keratitis. Cornea. 1996;15:135–8. doi: 10.1097/00003226-199603000-00005. [DOI] [PubMed] [Google Scholar]
- [2].Nishida T, Chikama T, Sawa M, Miyata K, Matsui T, Shigeta K. Differential contributions of impaired corneal sensitivity and reduced tear secretion to corneal epithelial disorders. Japanese Journal of Ophthalmology. 2012;56:20–5. doi: 10.1007/s10384-011-0105-4. [DOI] [PubMed] [Google Scholar]
- [3].Shaheen B, Bakir M, Jain S. Corneal nerves in health and disease. Survey of Ophthalmology. 2014;59:263–85. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marfurt C, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Experimental Eye Research. 2010;90:478–92. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- [5].Muller L, Marfurt C, Kruse F, Tervo T. Corneal nerves: structure, contents and function. Experimental Eye Research. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- [6].Hicks C, Fitton J, Chirila T, Crawford G, Constable I. Keratoprostheses: advancing toward a true artificial cornea. Survey of Ophthalmology. 1997;42:175–89. doi: 10.1016/s0039-6257(97)00024-6. [DOI] [PubMed] [Google Scholar]
- [7].Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. The Journal of Neuroscience. 2003;23:4967–74. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yoon K, You I, Im S, Jeong T, Park Y, Choi J. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114:1637–42. doi: 10.1016/j.ophtha.2006.12.014. [DOI] [PubMed] [Google Scholar]
- [9].Foster T, Puskas B, Mandelbaum B, Gerhardt M, Rodeo S. Platelet-rich plasma: from basic science to clinical applications. American Journal of Sports Medicine. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- [10].You L, Kruse F, Volcker H. Neurotrophic factors in the human cornea. Intestigative Ophthamology and Visual Science. 2000;41:692–702. [PubMed] [Google Scholar]
- [11].Fagerholm P, Lagali N, Ong J, Merrett K, Jackson W, Polarek J, et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35:2420–7. doi: 10.1016/j.biomaterials.2013.11.079. [DOI] [PubMed] [Google Scholar]
- [12].Kowtharapu B, Stahnke T, Wree A, Guthoff R, Stachs O. Corneal epithelial and neuronal interactions: role in wound healing. Experimental Eye Research. 2014;125:53–61. doi: 10.1016/j.exer.2014.05.006. [DOI] [PubMed] [Google Scholar]
- [13].Hubert T, Grimal S, Carroll P, Fichard-Carroll A. Collagens in the developing and diseased nervous system. Cellular and Molecular Life Sciences. 2009;66:1223–38. doi: 10.1007/s00018-008-8561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chernousov MA, Stahl RC, Carey DJ. Schwann cell type V collagen inhibits axonal outgrowth and promotes Schwann cell migration via distinct adhesive activities of the collagen and noncollagen domains. Journal of Neuroscience. 2001;21:6125–35. doi: 10.1523/JNEUROSCI.21-16-06125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hopkins A, De Laporte L, Tortelli F, Spedden E, Staii C, Atherton T, et al. Silk hydrogels as soft substrates for neural tissue engineering. Advanced Functional Materials. 2013;23:5140–9. [Google Scholar]
- [16].Wang X, Kluge J, Leisk G, Kaplan D. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–64. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hu X, Shmelev K, Sun L, Gil E, Park S, Cebe P, et al. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules. 2011;12:1686–96. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Du Y, Funderburgh M, Mann M, SundarRaj N, Funderburgh J. Multipotent stem cells in human corneal stroma. Stem cells (Dayton, Ohio) 2005;23:1266–75. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gil E, Park S, Marchant J, Omenetto F, Kaplan D. Response of human corneal fibroblasts on silk film surface patterns. Macromolecular Bioscience. 2010;10:664–73. doi: 10.1002/mabi.200900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gil E, Mandal B, Park S, Marchant J, Omenetto F, Kaplan D. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials. 2010;31:8953–63. doi: 10.1016/j.biomaterials.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wu J, Du Y, Mann M, Yang E, Funderburgh J, Wagner W. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Engineering Part A. 2013;19:2063–75. doi: 10.1089/ten.tea.2012.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meijering E, Jacob M, Sarria J, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry. 2004;58:167–76. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- [23].Leclere P, Norman E, Groutsi F, Coffin R, Mayer U, Pizzey J, et al. Impaired axonal regeneration by isolectin B4-binding dorsal root ganglion neurons in vitro. The Journal of Neuroscience. 2007;27:1190–9. doi: 10.1523/JNEUROSCI.5089-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Willits R, Skornia S. Effect of collagen gel stiffness on neurite extension. Journal of Biomaterials Science Polymer Edition. 2004;15:1521–31. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- [25].Sajanti J, Bjorkstrand A, Finnila S, Heikkinen E, Peltonen J, Majamaa K. Increase of collagen synthesis and deposition in the arachnoid and the dura following subarachnoid hemorrhage in the rat. Biochima et Biophysica Acta. 1999;1454:209–16. doi: 10.1016/s0925-4439(99)00016-2. [DOI] [PubMed] [Google Scholar]
- [26].Vitale P, Braghetta P, Volpin D, Bonaldo P, Bressan G. Mechanisms of transcriptional activation of the col6a1 gene during Schwann cell differentiation. Mechanisms of Development. 2001;102:145–56. doi: 10.1016/s0925-4773(01)00303-3. [DOI] [PubMed] [Google Scholar]
- [27].Tucker K, Meyer M, Barde Y. Neurotrophins are required for nerve growth during development. Nature Neuroscience. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- [28].Moore K, MacSween M, Shoichet M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Engineering. 2006;12:267–78. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- [29].Bae J, Han H, Youn D, Carter J, Modo M, Schuchman E, et al. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem cells (Dayton, Ohio) 2007;25:1307–16. doi: 10.1634/stemcells.2006-0561. [DOI] [PubMed] [Google Scholar]
- [30].Kaselis A, Treinys R, Vosyliute R, Satkauskas S. DRG axon elongation and growth cone collapse rate induced by Sema3A are differently dependent on NGF concentration. Cell and Molecular Neurobiology. 2014;34:289–96. doi: 10.1007/s10571-013-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fan C, Wang H, Chen D, Cheng X, Xiong K, Luo X, et al. Effect of type-2 astrocytes on the viability of dorsal root ganglion neurons and length of neuronal processes. Neural Regeneration Research. 2014;9:119–28. doi: 10.4103/1673-5374.125339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Daud M, Pawar K, Claeyssens F, Ryan A, Haycock J. An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials. 2012;33:5901–13. doi: 10.1016/j.biomaterials.2012.05.008. [DOI] [PubMed] [Google Scholar]
- [33].Lindsay R. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. Jounal of Neuroscience. 1988;8:2394–405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures
Supporting Figure 1: DRG neurons and dhCSSCs form connections after 7 days of co-cultivation. Fluorescence images of DRG neurons and dhCSSCs (immune-stained with anti-β-Tubulin III in green for viewing DRG neurons and F-actin in red for viewing dhCSSCs) A: 10x image of DRG neuron formed connection with dhCSSCs at the interface of silk collagen hydrogel and silk film (interface shown with white line). DRG neuronal extension contact with the dhCSSCs is shown with arrows. B: 20X image of DRG neuron was observed outside of silk collagen hydrogel and formed direct contact with dhCSSCs. Scale=200μm
Supporting Figure 2: DRG neuronal outgrowth in co-culture with hCECs and monocultures in S/C=1/2 composite gels. (A) Representative fluorescence images of DRG neurons alone and co-cultured with hCECs. DRG neurons were immunostained with anti-β-Tubulin III in green on days 1,3,5,7. (Scale bars 100 μm). (B) (a) Quantification of neuronal extensions of DRG neurons co-cultured with hCECs was not statistically different than monocultures on days 1,3,5,7. N =3 from 3 independent cultures from one way ANOVA with Dunnett post hoc test. (b) Fraction of DRG neurons with extensions did not show statistical differences in co-cultures with hCECs compared to monocultures. N=3 from 3 independent cultures, respectively, one way ANOVA with Dunnett post hoc test.
Supporting Figure 3: 10 X images of controls for immunohistochemistry staining. A: TCP control stained with anti collagen type I in green. B: TCP control stained with anti collagen type V in green. C: TCP control stained with anti collagen type VI in green. D: silk film control stained for F-actin in red. E: silk hydrogel control stained with anti-β-Tubulin III in green. F: 1/2 hydrogel control stained with anti-β-Tubulin III in green. G: 2/1 hydrogel control stained with anti-β-Tubulin III in green. H: Collagen hydrogel control stained with anti-β-Tubulin III in green. Images were collected from N=3 for 2 independent experiments. Scale bars 200 μm.