Abstract
Stroke is a leading cause of mortality and severe long-term disability worldwide. Development of effective treatment or new therapeutic strategies for ischemic stroke patients is therefore crucial. Ischemic stroke promotes neurogenesis by several growth factors including FGF-2, IGF-1, BDNF, VEGF and chemokines including SDF-1, MCP-1. Stroke-induced angiogenesis is similarly regulated by many factors most notably, eNOS and CSE, VEGF/VEGFR2, and Ang-1/Tie2. Important findings in the last decade have revealed that neurogenesis is not the stand-alone consideration in the fight for full functional recovery from stroke. Angiogenesis has been also shown to be critical in improving post-stroke neurological functional recovery. More than that, recent evidence has shown a highly possible interplay or dependence between stroke-induced neurogenesis and angiogenesis. Moving forward, elucidating the underlying mechanisms of this coupling between stroke-induced neurogenesis and angiogenesis will be of great importance, which will provide the basis for neurorestorative therapy.
Keywords: Stroke, neurogenesis, angiogenesis, ischemia, cell interaction
1. Introduction
Stroke is a leading cause of mortality and severe disability worldwide. Currently, the number of patients suffering from stroke is steadily increasing. The only proven therapy for acute ischemic stroke approved by the FDA is systemic thrombolysis with recombinant tissue plasminogen activator (rtPA). However, it must be administered within 4.5 hours after the onset of stroke for rtPA to be effective. As a result, the short therapeutic time window and the potential complication from intracranial hemorrhage benefit only a minority of stroke patients (Zhang and Chopp, 2009). Even with successful rtPA thrombolysis, most stroke survivors still suffer from permanent neurological functional deficits. Therefore, extending the therapeutic time window of rtPA or uncovering new therapeutic strategies for the treatment of ischemic stroke is highly sought after.
In the past decade, several independent research groups have reported that neurogenesis continues into adulthood (Wang and Jin, 2014). Neurogenesis is the process of producing new functional neurons from neural stem/progenitor cells (NSCs), including proliferation of endogenous NSCs, migration, and differentiation into mature functional neurons. It is well accepted now that neurogenesis occurs at two distinct regions in the intact brain throughout life: the subventricular zone (SVZ) of the lateral ventricles and subgranular zone (SGZ) in the dentate gyrus of the hippocampus (Wang and Jin, 2014). In pathological conditions such as ischemic stroke, enhanced neurogenesis has been reported in animal models of stroke and even in stroke patients (Jin et al., 2001; Jin et al., 2006; Thored et al., 2006), suggesting a potential avenue for the treatment of ischemic stroke. However, it is now apparent that neurogenesis is not the stand-alone consideration in the fight for full functional recovery from stroke. One should now also factor in the role of angiogenesis as it has been shown to be critical in improving post-stroke neurological functional recovery.
Angiogenesis, defined as new microvessel formation via branching off from pre-existing vessels (Carmeliet and Jain, 2011), is a multi-step biological process, including proliferation and sprouting of endothelial cells, formation of tube-like vascular structures, branching and anastomosis (Risau, 1997). Angiogenesis is found in the penumbra of the brain infarct region in animal models of stroke and even in the brains of stroke patients (Hayashi et al., 2003; Krupinski et al., 1994; Zhang et al., 2002). It has been reported that neurogenesis and angiogenesis occur in the brains of stroke patients and a positive correlation was seen between patient survival and density of microvessels (Krupinski et al., 1994). Several findings in addition to this prove that neurogenesis and angiogenesis are coupled processes after an insult such as ischemic stroke, and should be acknowledged and pursued as concurrent and non-mutually exclusive events to further develop neurorestorative therapy.
This mini-review aims to establish the underlying mechanisms of how ischemic stroke induces endogenous neurogenesis and angiogenesis, and then address the interplay between neurogenesis and angiogenesis after ischemic stroke.
2. Mechanisms underlying stroke-induced neurogenesis
In this section, we review cellular and molecular mechanisms underlying stroke-induced neurogenesis and how factors released by endothelial cells may participate in the process.
2.1 Proliferation
Endogenous NSC proliferation is the first step in stroke-induced neurogenesis. It was demonstrated that ischemic stroke is sufficient to increase the endogenous NSC proliferation to result in the expansion of the NSC pool (Tang et al., 2009). Ischemic stroke injury promotes the proliferation of NSCs and expands the NSC pool by regulating NSC cytokinetics such as shortening the length of the cell cycle to increase the percentage of proliferating cells (Zhang et al., 2006). As reported by Zhang et al., the proportion of actively dividing SVZ NSCs is about 15-21% in the adult rat brain. Stroke increases the proportion of proliferating SVZ cells to 24% just two days after stroke, and this proportion reaches a maximum level of 31% 7 days after stroke (Zhang et al., 2006). The length of the SVZ NSC cell cycle is 18-21 hours in the normal rat brain throughout its lifetime. However, stroke reduces the length of the cell cycle to 11 hours at 2 days after stroke onset.
Switching from asymmetric to symmetric NSC division may be another underlying mechanism, which contributes to the expansion of the NSC pool. Symmetric divisions generate two identical daughter cells that go into maintaining the NSC pool while asymmetric or self-renewing divisions generate one daughter cell and a differentiated cell such as a neuron or non-stem-cell progenitor (Chenn and McConnell, 1995; Gotz and Huttner, 2005; Smart, 1973). It was shown that in the adult rat, stroke briefly increases the number of dividing SVZ NSCs with vertical cleavage orientation, and decreases the number of SVZ NSCs with horizontal cleavage orientation (Zhang et al., 2004). This suggests that the NSCs switch from asymmetric to symmetric division to expand the NSC pool, whose numbers were shown to be significantly increased after stroke. Interestingly, 4 days after stroke, the frequency of neuronal phenotype in symmetrically divided cells increased to 47% from 33% in asymmetrically divided cells. Thus, it is possible to conclude that stroke increases the neuronal phenotype though no evidence was shown to what degree this phenotype is preferred versus other glial cell fates.
Several extracellular signals such as growth factors that regulate stroke-induced proliferation of NSCs have been identified and extensively examined.
FGF-2
The mRNA expression of fibroblast growth factor-2 (FGF-2) is upregulated significantly after ischemic stroke injury in the adult rat brain (Naylor et al., 2005) as well as patients who died from acute ischemic stroke (Navaratna et al., 2009). It was also shown that FGF-2 was localized to endothelial cells in these stroke patients (Issa et al., 2005). To illustrate the impact of the loss of endogenous FGF-2, Yoshimura et al. used mice genetically deficient in FGF-2 and showed that knocking out of FGF-2 resulted in reduction of ischemia-induced progenitor proliferation when compared with wild type mice (Yoshimura et al., 2001). However, after overexpression of FGF-2 by intraventricular (ICV) injection with a herpes simplex virus-1 amplicon vector carrying the FGF-2 gene, the number of proliferating NSCs in post-stroke mice increased significantly when compared to the wild type mice (Yoshimura et al., 2001). Furthermore, transplantation of FGF-2 gene-modified MSCs to ischemic rats resulted in enhanced neurogenesis and increased functional recovery (Ikeda et al., 2005). Based on these findings, endogenous production of FGF-2 by endothelial cells plays an important role in regulating post-stroke induced NSC proliferation.
IGF-1
Insulin-like growth factor-1 (IGF-1), primarily produced in the liver, plays a major role in brain development. It was reported that IGF-1 had a direct effect on proliferation of adult hippocampal NSCs in vitro, and the proliferative effect is relied upon the activation of the MAPK signaling pathway (Kalluri et al., 2007). Like FGF-2, the expression of IGF-1 protein and its receptor significantly increased after ischemic stroke insult (Yan et al., 2006). Inhibiting IGF-1 expression by ICV administration of the IGF-1 antibody led to the blockage of the stroke-induced proliferation of NSCs (Yan et al., 2006). In a more recent study (Zhu et al., 2009), Zhu and colleagues demonstrated neurotrophic and angiogenic properties of IGF-1 in vivo using a mouse model of permanent focal ischemia and overexpression of IGF-1 by stereotaxic injection with an adeno-associated viral (AAV) vector containing the IGF-1 gene. The results were two-fold. They reported significant increases in vascular density at 8 weeks post-stroke in the peri-infarct region, which corresponded with improved vascular perfusion locally, and in neurogenesis at 7 days post-stroke. The question to ask here is: Could it be possible that improved vascular density, and hence, perfusion, leads to increased neurogenesis?
BDNF
Among the many neurotrophic factors, the brain-derived neurotrophic factor (BDNF) has been most extensively studied for its role in adult neurogenesis. Intrahippocampal administration of BDNF in adult rats results in increased neurogenesis in the dentate gyrus and ICV infusion of BDNF promotes the production of new neurons in the adult olfactory bulb in the intact brain (Scharfman et al., 2005; Zigova et al., 1998). As demonstrated by Kokaia’s group, ischemic stroke insult induced the upregulation of BDNF and its receptor expression (Kokaia et al., 1998). Continuous intrastriatal delivery of BDNF via recombinant AAV gene transfer before ischemia in adult rats resulted in enhanced neurogenesis and better neurological functional recovery (Andsberg et al., 2002). A well-known study by Chen et al. found that endothelial nitric oxide synthase (eNOS) knock-out mice have decreased BDNF expression after stroke, suggesting the involvement of eNOS in the regulation of BDNF expression (Chen et al., 2005) mediated by endothelial cells and neurons (Li et al., 2014; Shin et al., 2004). The mechanism wherein eNOS regulates BDNF secretion is still largely unclear.
VEGF
Vascular endothelial growth factor (VEGF) is an angiogenic protein that binds to its receptor on endothelial cells to induce angiogenesis. Evidence from recent studies suggests that VEGF also act directly on neuronal progenitor cells to produce neurogenic effect. VEGF receptors, such as Flk-1 and Flt-1 are expressed on neural progenitors in the adult SVZ and hippocampus (Maurer et al., 2003; Yang et al., 2003). A study by Jin et al. revealed that ICV infusion of VEGF into the adult rat brain promotes proliferation of NSCs in the SGZ and the SVZ area (Jin et al., 2002). Further, Sun and colleagues revealed that ICV administration of VEGF after stroke injury led to improved neurological performance and a significant reduction in infarct volume, suggesting that VEGF has an important role in post-stroke neurogenesis as well as angiogenesis (Sun et al., 2003).
2.2 Migration
In order for proliferating NSCs to contribute to functional recovery, it is necessary for these NSCs to migrate from their birthplace to the ischemic region. In the normal adult brain, the SVZ neuroblasts are destined to migrate to the olfactory bulb through the rostral migratory stream. In the stroke brain, many of these SVZ neuroblasts migrate from the neurogenic region of the SVZ through the brain parenchyma into the boundary of the infarct region (Arvidsson et al., 2002; Jin et al., 2003; Thored et al., 2006). This redirected migration is associated with cellular interactions between immature migrating neuroblasts, astrocytic processes and blood vessels (Yamashita et al., 2006). However, ischemic stroke also upregulates inhibitory molecules such as chondroitin sulfate proteoglycans (CSPGs), which block the migration of neuroblasts (Carmichael, 2005). On the other hand, stroke not only upregulates chemotactic factors for neuroblast migration, but also produce peri-infarct scar and barrier molecules to impede neuroblast migration. The net neuroblast migration is thus dependent upon the balance between inhibitory molecules and chemotactic factors.
The mechanism underlying this injury-induced redirected migration is unclear. Several receptor-ligand signaling pathways and molecular factors involved in the stroke-induced endogenous NSCs migration have been identified. These include stromal cell-derived factor-1 (SDF-1) and CXC chemokine receptor 4 (CXCR4), monocyte chemoattractant protein-1 (MCP-1), and matrix metalloproteinases (MMPs).
SDF-1 and CXCR4
Under physiological conditions, high levels of SDF-1 (CXCL12) secreted from ependymal cells perpetuates NSC quiescence (Sawada et al., 2014). After ischemic stroke, SDF-1 that is released from reactive astrocytes and vascular cells increases the state of activation of Type C and activated Type B NSCs (Kokovay et al., 2010). SDF-1 is a member of the alpha chemokine family, which has a crucial role in the mobilization and homing of hematopoietic stem cells to the bone marrow with its receptor CXCR4 (Hattori et al., 2003). A role for SDF-1 and its receptor CXCR4 in the directional migration of neuroblasts has been reported as well. Robin et al. showed that CXCR4 is expressed on NSCs and migrating neuroblasts after stroke. Inhibiting SDF-1α expression using an antibody against CXCR4 significantly reduced stroke-induced NSC migration (Robin et al., 2006). Ohab et al. also found that ICV administration of SDF-1 in ischemic mice promoted neuroblast migration after stroke and contributed to behavioral recovery (Ohab et al., 2006). Through a series of thorough investigations, Kokovay et al. further reported that neuroblasts homed to the vasculature, and were attracted by factors released by endothelial cells (Kokovay et al., 2010). Taken together, these studies demonstrated neuroblast migration toward the infarct boundary is dependent upon an SDF-1/CXCR4 mechanism through binding with the neurovasculature.
MCP-1
Monocyte chemoattractant protein-1 (MCP-1), a chemokine of the CC family, was previously shown to interact with its receptor CCR2, which is widely expressed on NSCs to increase NSC migration in vitro (Wang and Jin, 2014). It was also reported to have a crucial role in guiding neuroblast migration after ischemic stroke. Yan and colleagues demonstrated that ischemic stroke in rats caused an increase of MCP-1 mRNA expression in activated astrocytes and microglia, which lasted for at least 3 days after reperfusion (Yan et al., 2007). This study also confirmed that migrating neuroblasts expressed its respective receptor, CCR2, in the adult rodent brain. Furthermore, after focal ischemia, knockout mice lacking either MCP-1 or CCR2 showed significantly decreased numbers of migrating neuroblasts from the ipsilateral SVZ to the injured striatum. So far, the mechanism for MCP-1/CCR2 dependent migration of neuroblasts has yet to be elucidated though activation of the PI3 kinase pathway seems to be a likely candidate (Katakowski et al., 2003; Turner et al., 1998).
MMPs
Matrix metalloproteinases (MMPs), a family of proteinases, are known to have a role in extracellular matrix (ECM) degradation and cell migration. Recently, the role of MMP in guiding SVZ neuroblasts migration has also been implicated. The expression of MMPs-3 and -9 in neuroblasts has been observed. Inhibition of function of these MMPs resulted in reduction in post-stroke neuroblast migration (Barkho et al., 2008). Using a coculture system consisting SVZ NSCs and brain endothelial cells from the adult mouse to mimic the microenvironment within the peri-infarct region, Wang et al. showed that the secretion of MMPs-2 and -9 on the vasculature via the activation of PI3K/Akt and ERK1/2 signaling pathways also contributes to the migration of neuroblasts (Wang et al., 2006). These findings indicate that neuroblasts may “digest” their way through the ECM as they migrate by secreting MMPs. More work is needed to determine how cell-cell interactions could utilize MMPs to aid in migration of neuroblasts to the ischemic lesion.
3. Mechanisms underlying stroke-induced angiogenesis
New vessels are generated in the brain through several ways, including angiogenesis, vasculogenesis, and collateral vessel growth. The overarching purpose for the generation of these new vessels is to increase collateral circulation as first line defense against ischemia. Mounting evidence indicates that angiogenesis play a crucial role in ischemic stroke brain repair and long-term functional recovery. In animal models of ischemic stroke, proliferation of endothelial cells at 12 to 24 hours after injury was seen (Hayashi et al., 2003; Marti et al., 2000). Capillary sprouting and new vessels were reported to continue to grow for at least 3 weeks in the ischemic boundary zones (Hayashi et al., 2003; Zhang et al., 2002). In ischemic stroke patients, Krupinski and group analyzed post-mortem brain tissues and found that angiogenesis also occur in the boundary of the ischemic site. They also noted that angiogenic activity occurred at 3 to 4 days after stroke (Krupinski et al., 1994). Thus, the activated angiogenesis is beneficial for the stroke-injured brain, as high levels of new vessel formation following stroke is correlated with better functional recovery and prolonged survival (Krupinski et al., 1994). The presence of angiogenic activity in the long run however, is still not examined. Since we know that angiogenesis is coupled to neurogenesis (Teng et al., 2008; Zhang et al., 2014), and that neurogenesis is dependent on new vessels for long-term survival (Font et al., 2010), it is possible to posit, albeit naïve, that angiogenic activity decreases in the long run, which makes it a possible causal factor for the high death rate of neuroblasts (at least 80%) in the ischemic boundary zone.
Several excellent reviews have been written involving angiogenesis under physiological conditions and after stroke; we urge the reader to refer to them accordingly (Beck and Plate, 2009; Carmeliet and Jain, 2011; Ergul et al., 2012; Font et al., 2010; Xiong et al., 2010). Here, we highlight a few well-studied factors that regulate post-stroke angiogenesis, including eNOS and CSE, VEGF and its receptor VEGFR2, and angiopoietin-1 (Ang-1) and its receptor Tie2.
eNOS and CSE
Upon proliferation and migration of endothelial cells, angiogenesis and vasodilation occurs, which are regulated by nitric oxide (NO) through eNOS generation and more recently, by hydrogen sulfide (H2S) through cystathionine-γ-lyase (CSE), an enzyme required for H2S synthesis (Coletta et al., 2012). Coletta et al. showed that the cooperative action of these two gases are required for angiogenesis and vasodilation to occur. By inhibiting eNOS or protein kinase G-I (PKG-I), the H2S-stimulated angiogenic response and vasorelexation were inhibited. Contrariwise, CSE silencing in bEnd3 immortalized mouse brain microvascular endothelial cells with shRNA abolished NO-stimulated accumulation of cGMP, angiogenesis, and acetylcholine-induced vasorelexation, suggesting the critical role of H2S in NO vascular activity. In aiming to improve angiogenesis after stroke, one might find it helpful to consider finding ways to reinstate the homeostatic balance of H2S and NO in the vasculature.
VEGF/VEGFR2 and Ang-1/Tie2
VEGF/VEGFR2 and the Ang-1/Tie2 have been shown to regulate angiogenesis in the ischemic boundary zone.
VEGF and the mRNA for its receptor VEGFR2 were shown to be upregulated after stroke, which exerted remarkable pro-angiogenic effects (Zhang et al., 2000). Zhang et al. found that VEGF administration 48 hours after stroke promoted the formation of newly formed vessels, increased vascular permeability, and hence, increased cerebral microvascular plasma perfusion in the penumbra of the ischemic rat cortex (Zhang et al., 2000). Congruent with their findings, they reported an improvement in neurological function in ischemic rats after late VEGF administration. Sun et al. also administered VEGF via ICV and found that von Willebrand factor-immunoreactive endothelial cell numbers increased, suggesting the enhancement of postischemic angiogenesis (Sun et al., 2003).
On the other hand, Lin et al. noted that after the induction of stroke, the temporal profiles of Ang-1/Tie2 were vastly different compared to other angiogenic genes like VEGF/VEGFR (Lin et al., 2000), in that Ang-1/Tie2 exert their actions during late stages of vascular development – vascular remodeling and maturation. Tie2 is found predominantly in endothelial cells, and is critical for vascular formation and maintenance. Beck and colleagues showed that after ischemia, the increase of Ang-1/Tie2 expression resulted in the maturation of newly formed vessels to stabilize functional brain vessels (Beck et al., 2000). In a follow up study, Lin et al. noted an upregulation on both the mRNA and protein levels of Tie2 receptors in the new vessels in the ischemic cortex just a few hours after stroke. They saw another peak at 3 days, which persisted for a week post-stroke (Lin et al., 2001). Further, Lin and group showed that Ang-1 mRNA was transiently expressed just after stroke, but reported a large increase 1 to 2 weeks post-stroke (Lin et al., 2000).
Interestingly, some groups have also reported that Ang-1/Tie2 and endogenous stimulation or exogenous administration of VEGF can act in combination to enhance angiogenesis and vascular integrity (Lin et al., 2001; Zacharek et al., 2007; Zhang and Chopp, 2002). Lin et al. found that VEGF shared a similar expression pattern with Ang-2, another protein of the angiopoietin family that can interact with Tie2; it was co-localized with Tie2 in the ischemic cortex where active vessel remodeling occurred (Lin et al., 2001). Zhu and colleagues further showed that Ang-2 and VEGF worked in concert to enhance angiogenesis after stroke, but this came at a cost: increased MMP-9 activity and inhibition of zonular occludens-1 (ZO-1) expression result in BBB disruption (Zhu et al., 2005). In a follow up study, Zhu and colleagues found that overexpressing VEGF and Ang-1 in adult male CD-1 mice increased vascular density, but maintained ZO-1 protein expression after stroke, suggesting differential effects of Ang-1 and Ang-2 with VEGF, in that Ang-1 maintains BBB integrity unlike Ang-2 (Zhu et al., 2006). In fact, enhanced angiogenesis were reported by combining VEGF and Ang-2 compared to VEGF alone (Zhu et al., 2005) or administering VEGF and Ang-1 at submaximal doses compared to maximal doses of either alone (Chae et al., 2000). Another study by Zacharek et al. investigated VEGF and Ang-1/Tie2 effects in bone marrow stromal cell (MSC) treatment of stroke (Zacharek et al., 2007). They found that MSC treatment increased VEGF and Ang-1/Tie2 expression in the ischemic boundary zone. In addition, knockdown of Tie2 expression in brain endothelial cells reduced MSC-induced capillary tube formation significantly, suggesting Tie2’s critical role in MSC-induced angiogenesis.
Although stroke-induced angiogenesis has been extensively studied, the array of proteins involved and the supposed interactions with one another significantly increases the complexity with regards to finding a suitable angiogenic-targeted treatment for stroke. Yet, we are hopeful moving forward that researchers will continue to uncover more subtleties in order to understand the mechanisms and effectively treat stroke.
4. Coupling between neurogenesis and angiogenesis after ischemic stroke
Recent evidence has shed more light on the role of the brain vasculature in neurogenesis (Lacar et al., 2012; Shen et al., 2008; Tavazoie et al., 2008). Capillaries in the SVZ are shown to be permeable to diffusible molecules released from endothelial cells such as VEGF (Jin et al., 2002), FGF-2 (Biro et al., 1994), amongst many others. This is possible because pericytes and astrocytic end feet do not tightly envelope the capillaries in the region of the SVZ, thereby creating a special incomplete blood-brain barrier (BBB) (Tavazoie et al., 2008). Adult NSCs were also shown to express the laminin receptor α6β1 integrin (VLA6), which regulates NSC binding to endothelial cells, and its expression shown to decrease as the NSCs differentiate (Shen et al., 2008). Taken together, this suggests that regulation of neurogenesis is dependent upon the brain vasculature. To date, there is a fair amount of literature regarding vasculature-dependent adult neurogenesis, but we expect even more studies to be conducted since this is a relatively new concept introduced just a little over a decade ago by Louissant and colleagues’ pivotal work in adult canaries (Louissaint et al., 2002).
The processes of neurogenesis and angiogenesis after stroke are linked together and coordinated. For instance, neuroblasts migrate from the SVZ region to the infarct boundary where post-stroke angiogenesis occurs, and these neuroblasts migrate closely with cerebral vessels (Ohab et al., 2006; Thored et al., 2007). Many migrating neuroblasts are found to localize specifically to blood vessels in areas of active vascular sprouting and remodeling in the infarct boundary. Blocking normal and post-ischemic angiogenesis with 1 week of endostatin treatment showed significant decreases in the number of newborn endothelial cells and overall vascular density in the peri-infarct cortex compared to control (Ohab et al., 2006). This resulted in a 10-fold reduction in neuroblasts in the peri-infarct cortex 7 days after stroke compared to control, suggesting that angiogenesis and neurogenesis are causally linked within the post-stroke neurovascular niche (Ohab et al., 2006). A further study by Thored and colleagues revealed that even after 16 weeks from the stroke insult, there were occurrences of low-grade angiogenesis and increased vessel density in the adjacent injured striatum, whereby neuroblasts preferentially migrated toward (Thored et al., 2007), indicating that angiogenesis as well as the vasculature is important for migration during long-term neurogenesis. Using a co-culture system, Teng et al. endeavored to prove that angiogenesis and neurogenesis are coupled processes (Teng et al., 2008). To do this, they first co-cultured normal SVZ cells and rat brain endothelial cells (RBECs) taken from stroke rats to determine the effect of stroke-induced RBECs on SVZ cell proliferation and differentiation. Next, they determined the effect of soluble proteins released by stroke-induced NSCs on RBECs via incubation with the supernatant collected from a culture of normal and stroke-induced SVZ cells. The results demonstrated that stroke-induced RBECs increased NSC proliferation by 28%. Compared with normal RBECs, stroke-induced RBECs also expanded the neuronal population by 46% via reducing NSC differentiation into astrocytes through the upregulation and downregulation of Hes6 and Sox2, respectively. Furthermore, stroke-induced NSCs were reported to secrete high levels of VEGF, which enhanced capillary tube formation, but with the introduction of a VEGFR2 antagonist, angiogenesis was completely abolished. Taken together, these results suggest the interdependence of neurogenesis and angiogenesis after stroke, and that VEGF mediates this coupling, though the presence of other angiogenic factors that can be released by stroke-induced SVZ cells cannot be ruled out. Fairly recently, a study by Zhang et al. demonstrated using a whole-mount preparation of the lateral wall of the lateral ventricle that endothelial cell proliferation in the SVZ increased from 2% (in non-stroke mice) to 12% at 7 days and 14% at 14 days after stroke, and that newly-produced neuroblasts seen along the lateral wall of the lateral ventricle were intimately associated with the newly formed vessels (Zhang et al., 2014). Vascular volume also steadily increased from 2.6% of the total volume before the insult to 4.2, 4.9, and 5.7% at 14, 30, and 90 days after insult, respectively, of which the majority of newly formed vessels were capillaries. All in all, this study revealed long-term alterations in the NSC and vascular architecture of the adult SVZ.
The underlying mechanisms of how stroke-induced neurogenesis and angiogenesis are coupled together are yet to be elucidated. The endothelial cells activated by ischemic stroke in the ischemic boundary zone secrete several factors to regulate the biological activities of NSCs, especially the migration of neuroblasts. These endothelial cells produce SDF1α and MMPs. SDF1α is a C-X-C chemokine, which guide neuroblast migration towards the peri-infarct region by binding to its receptor CXCR4 found on neuroblasts (Robin et al., 2006). MMP, which digest the extracellular matrix and enable cells to penetrate, has been implicated in guiding neuroblast migration from the neurogenic region to the ischemic boundary (Lee et al., 2006). In addition to secreting chemokines to guide neuroblast migration, activated endothelial cells in the ischemic boundary secrete VEGF to promote neurogenesis. VEGF is not only an angiogenic growth factor, but also a strong neurogenic growth factor. VEGF can promote the proliferation of NSCs both in vitro and in the adult rat brain (Jin et al., 2002). NSCs in the SVZ can also promote angiogenesis by secreting several angiogenic factors such as VEGFR2, Ang-2, and FGF (Liu et al., 2007). Taken together, these data suggest that neurogenesis and angiogenesis are highly coordinated and coupled to enhance brain repair after ischemic stroke injury.
5. Conclusion
Ischemic stroke induces coordinated endogenous neurogenesis and angiogenesis, which contributes to brain repair. The potential mechanisms that trigger augmented endogenous neurogenesis and angiogenesis after stroke are becoming increasingly known. Ischemic stroke promotes the proliferation of NSCs by a variety of growth factors including FGF-2, IGF-1, BDNF, and VEGF. After NSC proliferation, neuroblasts migrate from the SVZ neurogenic niche to the injured brain region through the production of SDF-1, MCP-1, and MMPs. Stroke-induced angiogenesis is regulated by many factors most notably eNOS and CSE, VEGF/VEGFR2, and Ang-1/Tie2. Furthermore, stroke-induced angiogenesis has been shown to couple with and enhance post-stroke neurogenesis. Moving forward, elucidating the underlying mechanisms of the coupling between stroke-induced neurogenesis and angiogenesis will be of great importance, which will provide the basis for neurorestorative therapy.
Fig. 1. Key pathways potentially involved in the coupling of neurogenesis and angiogenesis after ischemic stroke.
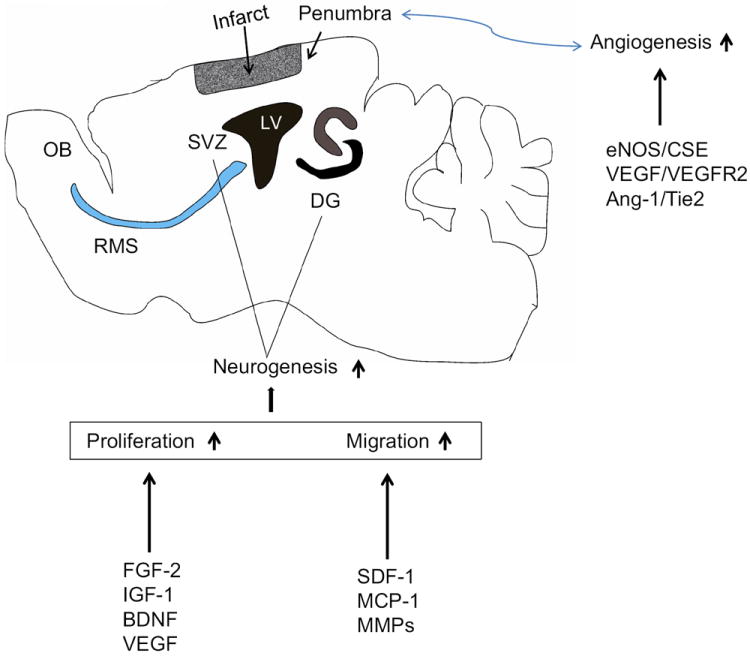
highlights.
Stroke is a leading cause of mortality and severe long-term disability worldwide.
Stroke-induced neurogenesis and angiogenesis are regulated by many factors.
Stroke-induced neurogenesis and angiogenesis are highly dependent on each other.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (81371396) to QZ, National Natural Science Foundation of China (81400954) to LR, and by the National Institute of Health (NIH) grants AG21980 and NS057186 to KJ.
Footnotes
Conflict of interest
The authors have no conflict of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andsberg G, et al. Neuropathological and behavioral consequences of adeno-associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiology of disease. 2002;9:187–204. doi: 10.1006/nbdi.2001.0456. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, et al. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–49. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, et al. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. The American journal of pathology. 2000;157:1473–83. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–96. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- Biro S, et al. Expression and subcellular distribution of basic fibroblast growth factor are regulated during migration of endothelial cells. Circ Res. 1994;74:485–94. doi: 10.1161/01.res.74.3.485. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx : the journal of the American Society for Experimental. NeuroTherapeutics. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae JK, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–8. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–75. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–41. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Coletta C, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–6. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke. 2012;43:2270–4. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6:238–44. doi: 10.2174/157340310791658802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leukemia & lymphoma. 2003;44:575–82. doi: 10.1080/1042819021000037985. [DOI] [PubMed] [Google Scholar]
- Hayashi T, et al. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–80. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Ikeda N, et al. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 2005;36:2725–30. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- Issa R, et al. Expression of basic fibroblast growth factor mRNA and protein in the human brain following ischaemic stroke. Angiogenesis. 2005;8:53–62. doi: 10.1007/s10456-005-5613-8. [DOI] [PubMed] [Google Scholar]
- Jin K, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Molecular and cellular neurosciences. 2003;24:171–89. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Jin K, et al. Evidence for stroke-induced neurogenesis in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri HS, Vemuganti R, Dempsey RJ. Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. The European journal of neuroscience. 2007;25:1041–8. doi: 10.1111/j.1460-9568.2007.05336.x. [DOI] [PubMed] [Google Scholar]
- Katakowski M, et al. Phosphoinositide 3-kinase promotes adult subventricular neuroblast migration after stroke. J Neurosci Res. 2003;74:494–501. doi: 10.1002/jnr.10775. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, et al. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Experimental neurology. 1998;154:289–301. doi: 10.1006/exnr.1998.6888. [DOI] [PubMed] [Google Scholar]
- Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–73. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke; a journal of cerebral circulation. 1994;25:1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Lacar B, et al. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. J Neurosci. 2012;32:16435–48. doi: 10.1523/JNEUROSCI.1457-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3491–5. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ST, et al. Endothelial nitric oxide synthase protects neurons against ischemic injury through regulation of brain-derived neurotrophic factor expression. CNS Neurosci Ther. 2014;20:154–64. doi: 10.1111/cns.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, et al. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2000;20:387–95. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- Lin TN, et al. Induction of Tie-1 and Tie-2 receptor protein expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2001;21:690–701. doi: 10.1097/00004647-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Liu XS, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:564–74. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, et al. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–60. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Marti HJ, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–76. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MH, et al. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neuroscience letters. 2003;344:165–8. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- Navaratna D, et al. Mechanisms and targets for angiogenic therapy after stroke. Cell Adh Migr. 2009;3:216–23. doi: 10.4161/cam.3.2.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M, et al. Preconditioning-induced ischemic tolerance stimulates growth factor expression and neurogenesis in adult rat hippocampus. Neurochemistry international. 2005;47:565–72. doi: 10.1016/j.neuint.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, et al. A neurovascular niche for neurogenesis after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Robin AM, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:125–34. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Sawada M, Matsumoto M, Sawamoto K. Vascular regulation of adult neurogenesis under physiological and pathological conditions. Front Neurosci. 2014;8:53. doi: 10.3389/fnins.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Experimental neurology. 2005;192:348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, et al. Immunohistochemical localization of endothelial and inducible nitric oxide synthase within neurons of cattle with rabies. J Vet Med Sci. 2004;66:539–41. doi: 10.1292/jvms.66.539. [DOI] [PubMed] [Google Scholar]
- Smart IH. Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J Anat. 1973;116:67–91. [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. The Journal of clinical investigation. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, et al. Effect of neural precursor proliferation level on neurogenesis in rat brain during aging and after focal ischemia. Neurobiol Aging. 2009;30:299–308. doi: 10.1016/j.neurobiolaging.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–71. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Thored P, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Turner SJ, et al. The CC chemokine monocyte chemotactic peptide-1 activates both the class I p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2alpha. J Biol Chem. 1998;273:25987–95. doi: 10.1074/jbc.273.40.25987. [DOI] [PubMed] [Google Scholar]
- Wang B, Jin K. Current perspectives on the link between neuroinflammation and neurogenesis. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9523-6. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, et al. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. The European journal of neuroscience. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Yan YP, et al. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1213–24. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yang SZ, et al. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003;274:851–6. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5874–9. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–91. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, et al. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004;24:5810–5. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, et al. Reduction of the cell cycle length by decreasing G1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:857–63. doi: 10.1038/sj.jcbfm.9600237. [DOI] [PubMed] [Google Scholar]
- Zhang RL, et al. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS One. 2014;9:e113972. doi: 10.1371/journal.pone.0113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–6. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. The Journal of clinical investigation. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:379–92. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. The Lancet Neurology. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, et al. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1528–37. doi: 10.1038/jcbfm.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36:1533–7. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]
- Zhu Y, et al. Effects of angiopoietin-1 on vascular endothelial growth factor-induced angiogenesis in the mouse brain. Acta Neurochir Suppl. 2006;96:438–43. doi: 10.1007/3-211-30714-1_90. [DOI] [PubMed] [Google Scholar]
- Zigova T, et al. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Molecular and cellular neurosciences. 1998;11:234–45. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]


