Abstract
Women constitute half of all smokers and many studies suggest that adult males and females differ in factors that maintain tobacco smoking, yet there is limited information about sex differences in nicotine reward during adolescence. Limited studies suggest that adolescent male rats self-administer more nicotine than adults, suggesting that drug administration during adolescence leads to different behavioral effects than during adulthood. In the present study, male rats developed a significant conditioned place preference (CPP) to lower doses of nicotine than females, regardless of age. In addition, adolescents were more sensitive than adults. In female rats, adolescents exhibited a CPP of greater magnitude than adult females. In males, the magnitude of the CPP did not differ as a function of age, but adolescents exhibited CPP to lower doses than adults. There also were differences in nicotinic acetylcholinergic receptor binding in nucleus accumbens and caudate putamen in response to nicotine across age and sex. These findings suggest that it is necessary to consider sex- and age-specific effects of drugs such as nicotine when developing strategies for improving smoking cessation treatments.
Keywords: Nicotine, conditioned place preference, adolescence, reward, nicotinic receptors
1. Introduction
Cigarette smoking is the leading cause of preventable death in the United States and approximately 20% of American adults are current cigarette smokers, with males (23%) having a slightly higher rate than females (18%) (CDC, 2010). The rate of cigarette smoking in high school students in the United States is only slightly less (17%) than that of American adults, with boys having a slightly higher rate than girls (20% vs. 15%, respectively) (NSDUH, 2010). These statistics are concerning because it is well known that approximately 80% people who start smoking before the age of 18 go on to become regular smokers as adults (CDC, 2003). In fact, children and adolescents may be especially susceptible to nicotine addiction as symptoms of dependence can emerge as early as the first time or first few times tobacco is used (DiFranza et al., 2000) and can even develop in adolescents not classified as daily smokers (i.e., weekly or monthly smokers) (Panday et al., 2007).
Although more males than females smoke cigarettes in both age groups, there is some evidence that female cigarette smokers may be more susceptible to the negative health consequences of tobacco use. For example, females metabolize nicotine faster (and, thus, must dose themselves accordingly), may be more likely to develop chronic obstructive pulmonary disorder (COPD), and have more difficulty with tobacco cessation (reviewed in Rahmanian et al., 2011) than male cigarette smokers. Further, nicotine craving may be more severe in adolescent females than in adolescent males (Panday et al., 2007). Therefore, it is important to understand sex and age differences in behaviors related to nicotine and tobacco, as well as nicotine reward.
Preclinical animal models are useful to examine whether biological sex differences and age differences have considerable effects on behaviors related to nicotine use and dependence (Carroll and Anker, 2010; Carroll et al., 2009; Lynch et al., 2002; O’Dell and Khroyan, 2009). During adolescence, subjects exhibit a unique pattern of behavioral and neurochemical responses to nicotine that are different in males and females. Examples of these age- and sex-specific behaviors and responses are described below.
Female adult rats acquire nicotine self-administration faster than males at the lowest training doses (Chaudhri et al., 2005; Donny et al., 2000; Lanza et al., 2004), and self-administer more intravenous infusions of nicotine per session than males (Rezvani et al., 2008). Although these sex differences seem to be diminished at the end of acquisition and during maintenance (Chaudhri et al., 2005; Donny et al., 2000; Lanza et al., 2004), females are more motivated to initially obtain nicotine as compared to males (Donny et al., 2000). If these findings extrapolate to humans, then perhaps women are more sensitive to lower doses of nicotine, and are more likely to continue tobacco use after fewer “tries” than are men. In adolescence, males administer more nicotine than during adulthood, after which rates decline to adult rates (Levin et al., 2007). In females, adolescents also administer more nicotine than adults, however, this difference is maintained into adulthood (Levin et al., 2003). Thus, starting nicotine during adolescents leads to different use patterns in adults compared to initiating administration in adulthood, and this is sex-dependent.
As sex differences in nicotine self-administration occur in adults, there also are sex differences in behavioral actions of nicotine in adolescents. Data from our laboratory have shown that adolescent females rapidly become sensitized to the locomotor-activating effects of nicotine, with significant effects seen on day 2 of treatment (Collins and Izenwasser, 2004). This finding is in contrast to adult female and male rats that exhibited significant sensitization beginning on day 5 of treatment. Further, adolescent male rats did not become sensitized to the locomotor-activating effects of nicotine within a 7-day treatment period, a finding that has been shown in our laboratory (Collins and Izenwasser, 2004; Collins et al., 2004a) and others (Schochet et al., 2004). Although the adolescent male and female rats were tested during the periadolescent period (postnatal day 28-40; Spear and Brake, 1983) in these studies, males and females have different rates of maturation (Ojeda et al., 1980; Ojeda et al., 1983) that could contribute to sex differences in nicotine self-administration and sensitization during adolescence. Similar to adults, adolescent female rats more easily acquire nicotine self-administration and express greater motivation to earn nicotine than adolescent male rats (Lynch, 2009).
In previous research, it has been shown that a moderate dose of nicotine (i.e., 0.6 mg/kg) produces CPP in adolescent rats but not adult rats when male and female data were combined (Vastola et al., 2002). However, the dose used in this study was based on the weight of the salt rather than nicotine as a free base, so the amount of nicotine actually received by these animals was somewhat lower. Others found similar results, in which adolescents developed CPP to the highest tested doses (0.5-0.8 mg/kg nicotine base), but older adolescents and adults did not (Belluzzi et al., 2004; Brielmaier et al., 2008; Shram et al., 2006). Torres and colleagues (2008) described the same results as mentioned above, but also found that both adolescents and adults developed CPP to 0.2 mg/kg nicotine. These results were specific to males, as females were not tested. In females, adolescents developed maximal CPP to 0.6 mg/kg, whereas adults developed CPP to 1.2 mg/kg nicotine (Torres et al., 2009).
Sex differences in nicotine CPP have been examined in adult animals by several groups. It has been reported that adult male rats developed CPP in response to 0.1 and 0.2 mg/kg nicotine base (but not any higher doses), whereas adult females did not develop CPP to any of the doses tested (0.1-0.6 mg/kg base) (Yararbas et al., 2010). Results reported by Torres and colleagues (2009) were similar, in which male rats had maximal CPP to 0.2 mg/kg nicotine and females had maximal CPP to 1.2 mg/kg nicotine; males and females developed conditioned place aversion to 1.8 mg/kg. However, Wistar rats were used in the study by Torres, whereas Sprague-Dawley rats were used in the study by Yararbas. Comparisons between the two studies are somewhat limited because of potential strain differences. Further, sex differences in nicotine CPP are not species-dependent; as male and female differences also have been reported in mice. For example, both male and female mice developed significant CPP to 0.32 mg/kg nicotine, but CPP was greater in females than males at this dose (Isiegas et al., 2009). These results give credence to the idea that sex differences in nicotine reward are not specific to rats and that this likely is a general phenomenon. However, the current literature lacks reports in which effects of age and sex on nicotine reward have been addressed by directly comparing male and female adults and adolescents using nicotine conditioned place preference.
In light of this previous literature, it is clear that the rewarding effects of nicotine are different in male and female rats and that these differential responses likely will be age-specific. While males and females and adolescents and adults have been studied, full dose-response curves for nicotine CPP in all four groups have not yet been reported in a single study. In the present study, the rewarding effects of nicotine were measured using CPP to several doses of nicotine, such that full dose-response curves were attained in male and female adult and adolescent rats. In addition, the responsiveness of brain nicotinic acetylcholine receptors (nAChRs) to stimulation by nicotine was measured in each group to explore possible neurobiological correlates underlying any age and sex differences.
2. Methods
2.1. Subjects
Naïve periadolescent male (n = 110), periadolescent female (n = 52), adult male (n=72), and adult female (n= 68) Sprague-Dawley rats were used (Charles River, Wilmington, MA). All rats were housed in a light- (12 hr light/dark cycle with lights on at 7 a.m. and off at 7 p.m.), temperature- (21 ± 2 °C) and humidity-controlled vivarium (53 ± 13%). At the start of the experiment, male and female periadolescent rats (postnatal day (PND) 34) weighed an average of 126.1 ± 1.9 g and 117.3 ± 1.5 g respectively, and the adult male and female rats (PND 66) weighed an average of 325.7 ± 2.3 g and 221.7 ± 2.3 g respectively. All behavioral tests occurred during the light schedule between 8:30 a.m. and 5:00 p.m., with each group tested at the same hour every day and groups counterbalanced over the day. Food and water were available ad libitum, except during the 30-min conditioning and testing sessions. Male and female rats were studied in separate groups at different times, using identical methods. All experiments were carried out in accordance to the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1996 and were approved by the Institutional Animal Care and Use Committee.
2.2. Drugs
(–)-Nicotine hydrogen tartrate salt (Sigma-Chemical Co., Saint-Louis, MO) was dissolved in an isotonic saline solution (0.9% sodium chloride in water). Nicotine doses were expressed as the weight of the base. Nicotine was injected intraperitoneally (i.p.) in a volume of 1 ml/kg body weight.
2.3. Nicotine Conditioned Place Preference Paradigm
Test chambers (40.64 width × 40.64 length × 30.5 cm height) were located in a dimly lit testing room adjacent to the colony room. A removable center barrier divided each chamber into two equal sized compartments, which were easily distinguishable by distinctive visual and tactile cues. On one side, the walls and the lid were white and the floor was smooth. On the other side, the walls and the lid were black and white striped, and the bottom was covered with a textured metal floor. Initially, a pretest was conducted on PND 34 in periadolescents and PND 66 in adults to determine the initial preference to both sides of the chamber. The amount of time spent on each side of the box was recorded during a 30-minute test session that occurred during the middle of the day. There were no significant differences in initial preference for one side over the other across groups. The following day marked the beginning of the conditioning phase. This phase was carried out over three consecutive days (from PND 35 to PND 37 in periadolescents and from PND 67 to PND 69 in adults), each of which consisted of morning and afternoon training sessions. In the morning, rats were injected with saline and then confined to one side by a dividing barrier for 30 minutes. In the afternoon, they received an injection of nicotine bin the opposite side for 30 minutes. Morning and afternoon sessions were separated by at least 4 hours. A range of nicotine doses was tested (0.01, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 mg/kg, i.p.) and different groups of rats were used for each dose (n= 8-16/group). This training schedule was chosen instead of training saline and nicotine on separate days because of the constraints involved in doing developmental studies, as has been described elsewhere (Badanich and Kirstein, 2004; Balda et al., 2006; Brenhouse and Andersen, 2008; Zakharova et al., 2009a; Zakharova et al., 2009c). On day 5, the testing phase occurred in the middle of the day, under the same conditions as the preconditioning phase, where the rats were able to move around freely between both sides of the box. The amount of time spent on each side of the box was recorded in a 30-minute test session.
2.4. Quantitative autoradiography
Rats that had received three daily injections of saline or 0.4 mg/kg nicotine (all groups received this dose, half the groups developed a significant place preference to it, and none developed an aversion) were sacrificed by decapitation after the test session on PND 38 in adolescents and PND 70 in adults for neurochemical analysis. Their brains were removed quickly and frozen by immersion in isopentane at −35 °C, then stored at −80 °C prior to slicing. Rostro-caudal sections were cut at − 20°C in a cryostat according to the rat brain atlas of Paxinos and Watson (1998). Slices (20 μm) from the prefrontal cortex and nucleus accumbens were thaw-mounted on gelatin/chromate-coated slides and stored at −80 °C prior to assay.
For the nicotinic acetylcholine receptor autoradiography assay, sections were thawed to room temperature and incubated for 40 min with 0.4 nM [125I]epibatidine in binding buffer (50 mM Tris–HCl, 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 2.5 mM CaCl2), as described previously (Tizabi and Perry, 2000). Sections were then washed twice in ice-cold buffer, dipped in ice-cold deionized water, and dried with a stream of cool dry air. Slides and standards (125I-labeled microscales, Amersham Corp., Arlington Heights, IL) were apposed to radiosensitive film for 24 hours at room temperature. Nonspecific binding was defined as binding in the presence of 300 μM (–)-nicotine hydrogen tartrate salt.
Films were developed in Kodak GBX developer and fixative, and autoradiograms were analyzed using a Macintosh-based image analysis system (NIH, Image 1.60 software).
2.5. Statistical analysis
Behavioral and neurochemical data were expressed as mean ± SEM. Conditioned place preference scores were expressed as the difference between the time in seconds spent in nicotine-paired side during the posttest minus the time spent in that side during the pretest. These CPP scores were analyzed using a three-way (dose × sex × age) analysis of variance (ANOVA) for the three training doses tested in all groups (i.e., 0.2, 0.4 and 0.6 mg/kg). All post hoc comparisons were carried out by the Fisher’s Protected Least Significant Difference (PLSD) analysis.
In addition to the ANOVAs to determine group differences, individual posttest minus pretest scores were analyzed using t tests to determine whether or not a significant preference or aversion occurred. By subtracting the pretest scores from the posttest scores, both preference and aversion for the nicotine-paired side could be determined. If there was no change in preference, the result was 0. To determine whether a significant preference (or aversion) had occurred in each group for each dose, the CPP scores were compared to 0 using t-tests. Positive values indicated that more time was spent in the nicotine-paired side after conditioning than during the pretest, whereas negative values indicated that a significant aversion for the nicotine-paired side occurred.
For each region of the autoradiography experiment, the relative activity was calculated per animal and a mean of relative activity ± SEM was calculated. All autoradiography data were analyzed by two-way ANOVA with group (age and sex considered together) and dose as the independent variables followed by Fisher’s PLSD. Differences were considered statistically significant with an alpha level of p≤0.05.
3. Results
3.1. Nicotine-induced Conditioned Place Preference
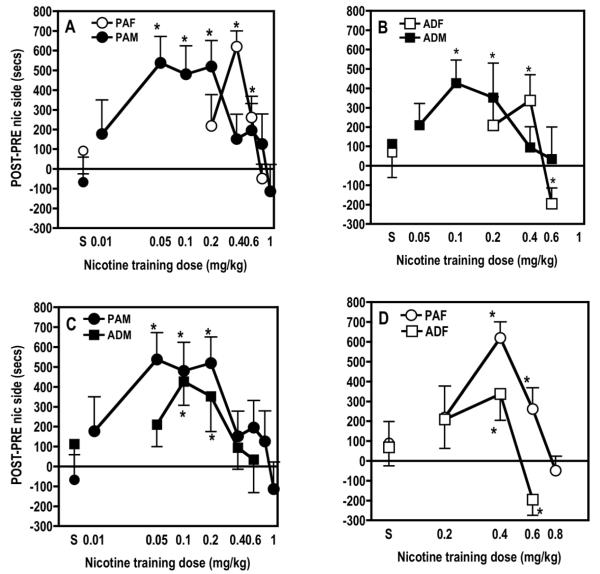
In periadolescent male rats (PAM), 3 days of conditioning induced a significant conditioned place preference (CPP) to low doses of nicotine. Specifically, the 0.05 [t(11)=3.989, p≤0.002], 0.1 [t(11)=3.352, p≤0.001], and 0.2 [t(13)=3.967, p≤0.002] mg/kg nicotine doses induced significant place preference in the PAM group whereas the higher doses of nicotine (0.4, 0.6, 0.8 and 1.0 mg/kg) and the lowest dose of nicotine (0.01 mg/kg) did not (Fig. 1A). In contrast, moderate doses of nicotine such as 0.4 [t(7)=8.286, p≤0.0001] and 0.6 mg/kg [t(8)=2.456, p≤0.04], induced a significant CPP in periadolescent female (PAF) rats (Fig. 1A). In addition, the dose-response curve for the PAM rats was shifted to the left compared to the PAF rats, but the maximal level of CPP achieved in both groups was equal. Thus, both periadolescent males and females have the ability to develop a CPP to nicotine under the same conditions, and nicotine has the same efficacy at producing CPP in both groups, but nicotine is more potent in adolescent males than in adolescent females. Interestingly, the dose-response curve for nicotine was steep and narrow in PAF, but in PAM it was broad and plateaued across several doses of nicotine that induced a preference (i.e., 0.05 to 0.2 mg/kg).
Fig. 1.
Effect of both gender and age on nicotine conditioned reward. The top graphs represent the dose-effect curve for nicotine conditioned place preference in (A) periadolescent male (PAM) versus female rats (PAF) and (B) in adult male (ADM) versus female (ADF) rats. The two bottom graphs compare the effect of age on this dose-effect curve (C, PAM versus ADM and D, PAF versus ADF). Data (means ± SEM) represent time spent in the nicotine-paired side after conditioning (POST) minus before conditioning (PRE) in seconds. Values above 0 reflect a conditioned place preference, while values below 0 indicate an aversion for the nicotine side. Seven nicotine doses (0.01, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8 or 1 mg/kg, IP) and saline (S) were tested in a between-session manner in the same conditions. N= 8-16 rats/group. *significant difference from 0 (p≤0.05).
Adult male rats (ADM) developed a significant place preference to 0.1 [t(11)=3.567, P≤0.01] and 0.2 [t(11)=2.606, p≤0.02] mg/kg nicotine whereas adult female rats (ADF) developed a significant CPP to only 0.4 mg/kg nicotine [t(11)=2.540, p≤0.03] (Fig. 1B). As in adolescents, adult males were more sensitive to lower doses of nicotine than were adult females as evidenced by the dose-response curve of ADM being shifted to the left as compared to ADF. However, ADF rats were more sensitive to the aversive effects of nicotine than male adult rats or adolescent rats, as shown by the development of a significant place aversion to 0.6 mg/kg [t(7)=2.422, p≤0.05)].
Within each age group, males and females express different responses to nicotine CPP, but there are comparisons that can be made between adults and adolescents within each sex as well. In male rats, both periadolescent and adult rats developed a significant CPP to 0.1 and 0.2 mg/kg nicotine; however, the ADM dose-response curve was narrower than that of the PAM rats, as evidenced by the significant CPP to 0.05 mg/kg nicotine in PAM but not ADM rats (Fig. 1C). In contrast to the male data, a difference in the efficacy of nicotine to produce CPP was observed between PAF and ADF rats (Fig. 1D). Nicotine produced higher levels of CPP in PAF than in ADF rats at 0.4 and 0.6 mg/kg nicotine. These results suggest that the same dose of nicotine is more efficacious at inducing reward in adolescent females as compared to adult females. In addition, it is important to highlight the fact that 0.6 mg/kg nicotine induced a significant preference in PAF rats, but a significant aversion in ADF rats. Clearly, adolescent females are more sensitive to rewarding effects of nicotine and adult females are more sensitive to aversive effects of the drug. Thus, age modulates the expression of nicotine CPP in males and females differently.
In addition to simple t-tests to determine whether a place preference or aversion was induced at individual doses of nicotine, we wanted to examine the individual effects of and interactions between the variables of sex, age, and nicotine dose. Therefore, a three-way ANOVA was used to analyze the data from the three training doses that were tested in all groups (i.e., 0.2, 0.4 and 0.6 mg/kg; it seemed unnecessary to purposefully test multiple ineffective low doses of nicotine in female rats). This analysis revealed a significant sex × dose × age interaction [F(2, 119) = 3.121, p≤0.05). Two-way ANOVAs were used to analyze data within each sex (with dose and age as independent variables) and within each age (with dose and sex as independent variables). In males, there was an overall significant effect of dose [F(2, 72)=3.873, p≤0.05]. Fisher’s PLSD post hoc tests showed that the 0.2 mg/kg dose induced a significantly greater preference than did the 0.4 mg/kg or 0.6 mg/kg dose (p≤0.05). There was not a significant age difference or age × dose interaction in male rats.
In female rats, there was a significant effect of dose [F(2, 47)=7.558, p≤0.001] and post hoc tests showed that there was a greater preference for the 0.4 mg/kg dose than for either the 0.2 or 0.6 mg/kg doses. In addition there was a significant effect of age [F(1, 47)=6.915, p≤0.01], with adolescents exhibiting a greater preference for nicotine than adults.
In adolescent rats, there were no main effects of sex or dose, but there was a sex × dose interaction [F(2, 61)=4.859, p≤0.01]. Post hoc tests showed that PAF rats had a greater preference for the 0.4 mg/kg dose of nicotine than did PAM rats. No differences in preference were observed for the other two doses. In contrast to the adolescent rats, in adults, neither the main effects of sex or dose nor the interaction between these two variables was significant.
3.2. Nicotinic Acetylcholine Receptor Binding
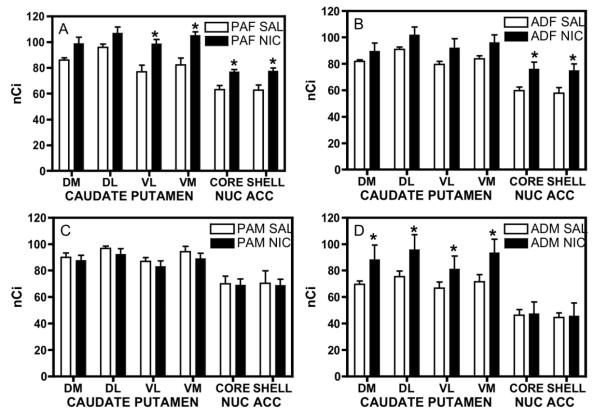
Brains from PAM, PAF, ADM, and ADF rats that were injected with either saline or 0.4 mg/kg nicotine for three days were assayed for binding of [125I]epibatidine in the caudate putamen and nucleus accumbens. Across the caudate putamen and nucleus accumbens there were significant effects of group and of nicotine dose but no group × dose interactions (Fig. 2).
Fig. 2.
nACh receptor binding in male and female periadolescent and adult rats injected with 0.4 mg/kg nicotine or vehicle for three days. (A) Periadolescent female rats (PAF) pretreated with nicotine had significantly higher nACh receptor densities in the ventral quadrants of the caudate putamen and in the nucleus accumbens core and shell than rats treated with vehicle. (B) In adult female rats (ADF), there were significant increases in nACh receptor densities in only the core and shell of the nucleus accumbens. (C) There were no significant changes in nACh receptor density in either the caudate putamen or the nucleus accumbens in periadolescent male rats (PAM) after nicotine treatment. (D) In adult male rats (ADM) pretreated with nicotine for three days compared to adult rats pretreated with vehicle nACh receptor densities were increased in the caudate putamen, but not in the nucleus accumbens. nACh receptor densities were measured in four quadrants of the rostral caudate putamen (DM: dorsomedial, DL: dorsolateral, VL: ventrolateral, VM: ventromedial) and the nucleus accumbens core and shell. *indicates a significant difference from rats pretreated with vehicle, P≤ 0.05 compared to vehicle
There were significant effects of group in the dorsolateral [F(3,49)=3.26, p≤0.05] and ventrolateral [F(3,49)=2.94, p≤0.05] regions of the caudate putamen, and in the core [F(3,49)=12.27, p≤0.0001] and shell [F(3,49)=10.97, p≤0.0001] of the nucleus accumbens. Post hoc tests showed that the ADM rats had significantly less nicotine binding density in the caudate putamen than the ADF and PAF rats, and less binding density in the nucleus accumbens than all other groups.
Significant effects of nicotine were found in the dorsomedial [F(1,49)=6.239, p≤0.05], dorsolateral [F(1,49)=6.09, p≤0.01], ventrolateral [F(1,49)=8.02, p≤0.01], and ventromedial [F(1,49)=10.81, p≤0.01] quadrants of the caudate putamen and in the nucleus accumbens core [F(1,49)=5.52, p≤0.05] and shell [F(1,49)=4.29, p≤0.05). Overall, nicotine increased binding density in at least one brain area in all groups except for the periadolescent male rats (Fig. 2C), where nicotine had no effect. Post-hoc testing showed that in the adolescent (Fig. 2A) and adult (Fig. 2B) female rats, nicotine increased nAChR density in the nucleus accumbens core and shell; whereas in males, nicotine did not alter binding in either area of the nucleus accumbens. It is interesting to note that this dose of nicotine (0.4 mg/kg) produced a significant CPP in the female rats of both ages, but not in the male rats, suggesting that place preference might be mediated by the nucleus accumbens. In adult males (Fig. 2D), there were significant increases in nACh receptor density in all four quadrants of the caudate putamen which suggests that for this group, the caudate putamen might mediate other effects of nicotine that do not include nicotine reward.
4. Discussion
The purpose of these studies was to examine effects of sex and age on conditioned place preference (CPP) to multiple doses of nicotine and on the density of nicotinic acetylcholine receptors (nAChR) in the caudate putamen and nucleus accumbens of adolescent and adult male and female rats. The present data show that adolescent and adult males (PAM and ADM, respectively) develop place preferences to lower doses of nicotine than age-matched females. Further, there were age-specific responses within each sex. The present study allowed a comparison of full dose response curves for nicotine CPP across both sex and age with the ability to directly compare the results in adolescents and adults of both sexes in the same experiment. These data, combined with our previous studies show the importance of testing multiple drug doses for CPP and further, that using a single dose of drug for CPP studies can lead to incorrect conclusions about differences across groups (Zakharova et al., 2009a; Zakharova et al., 2009c).
4.1 Age differences
In male rats, adolescents developed CPP to a broader range of nicotine doses than did adults, although the maximal efficacy of nicotine reward was the same at both ages. In females, nicotine was more efficacious at inducing CPP in adolescents than in adults. In addition, in adult females, a high dose of nicotine (0.6 mg/kg) led to a significant place aversion.
An interesting outcome in our study was that male and female periadolescent rats failed to develop an aversion to nicotine, even after conditioning with a relatively high dose of nicotine (e.g., 1 mg/kg, base, i.p.). These data confirm a previous study in Wistar rats showing that even a dose of 1.8 mg/kg (base, s.c.) failed to induce a significant aversion in both periadolescent males and females (Torres et al., 2009; Torres et al., 2008). Thus, both female and male adolescent rats seem to be not very sensitive to the aversive effects of nicotine. It is interesting that the increased efficacy in adolescent vs adult female rats is retained across strains of rat. Previously, it was shown that female adolescent rats were more sensitive than adults to nicotine reward in Wistar rats (Torres et al., 2009) and the same finding was found in Sprague-Dawley rats in the current study. Overall, however, it does appear that the female Sprague-Dawley rats are more sensitive to nicotine reward than were the Wistars. In contrast, this did not appear to be the case in males.
4.2 Sex differences
In these studies, both during adolescence and adulthood, male rats were more sensitive than females to nicotine reward. In adults, male rats exhibited a significant CPP to doses as low as 0.1 mg/kg nicotine, whereas, a significant CPP was observed only at 0.4 mg/kg in female adults. Similarly, in adolescents, a low dose of 0.05 mg/kg nicotine produced a significant CPP in adolescent males, whereas in females, only 0.4 mg/kg and higher produced a significant CPP. It is interesting to note that in both age groups, the curves for the male rats were considerably broader than for females, with multiple doses producing a significant CPP. In females, the curves were steep, with the descending limb returning rapidly to zero, or in the case of the adult females, to a significant aversion. Whether or not anxiety plays a role in mediating this aversive effect is not known. However, nicotine has a bimodal effect on anxiety, inducing behavioral and neurochemical anxiolytic-like effects or anxiogenic-like effects, depending upon experimental conditions (Balfour, 1991; O’Dell and Khroyan, 2009; Slawecki et al., 2003). It is possible that these effects on anxiety may be involved in the development of aversion to nicotine in the adult females.
These findings are in contrast to our previous studies showing that both adolescent and adult female rats are more sensitive than males to cocaine reward (Zakharova et al., 2009c). This difference between nicotine and cocaine is somewhat unexpected whether one considers that nicotine and cocaine mediate reward predominantly via activation of the mesolimbic dopaminergic system, as demonstrated by previous animal studies (e.g. Corrigall, 1991; Corrigall et al., 1992). There are however, findings suggesting that the situation may be more complex for nicotine in that the rewarding properties of intra-VTA nicotine may be mediated though a non-dopaminergic substrate while the aversive properties of nicotine may be dependent on mesolimbic DA transmission (Laviolette et al., 2002; Laviolette and van der Kooy, 2004). Since this has not been studied in females, it is unknown whether or not there may be sex differences involved in the activation of these two different pathways induced by nicotine that could account for the differential data.
Earlier reports showed that adolescent males developed place preference to doses between the range of 0.2 to 0.8 mg/kg nicotine (Belluzzi et al., 2004; Brielmaier et al., 2008; Shram et al., 2006; Torres et al., 2008). These doses are higher than the ones to which a significant CPP was observed in the present study. A major difference between the earlier studies and the present experiment is the housing conditions. In the previous studies, animals were housed in groups of four, as opposed to the pair-housed animals in our study. We have shown previously that housing conditions (adding toys or increasing number of rats per cage) can alter cocaine conditioned place preference in adolescent male rats, with decreased reward seen as the number of rats is increased (Zakharova et al., 2009b). Similarly, it has been shown that housing conditions alter MDMA reward such that only single housed animals exhibit a significant CPP (Meyer et al., 2002). Thus, it is likely that housing conditions played a role in leading to these different results in nicotine CPP across studies.
In the present study, the female adult rats appeared to be more sensitive to the aversive effects of nicotine than the other groups, as shown by the development of a significant aversion in females and an absence of aversive effects at the same dose in males. Differences in the rate of metabolism rate in adult female and male rats could be in part explained why females are more sensitive to aversive behavioral effects than males (Kyerematen et al., 1988). Although it has been shown that in humans, females metabolize components of cigarette smoke faster than males, female rats display a slower nicotine metabolism than male rats, thus a longer half-life for nicotine, and a larger volume of distribution of nicotine in the brain. In consequence, females display an increased susceptibility to CNS toxicity of nicotine relative to males.
4.3 nAChRs
Three injections of nicotine (0.4 mg/kg) increased nAChR density in the nucleus accumbens of both adolescent and adult females. In contrast to the females, there were no changes in the nucleus accumbens in response to this dose of nicotine in either adolescent or adult males. It is interesting to note that both groups of females but neither group of males exhibited a significant CPP to this dose of nicotine. Thus, it suggests the possibility that changes in nAChRs in the nucleus accumbens subsequent to the conditioning phase of the CPP may be associated with the development of a significant CPP to nicotine.
In adolescent females, there were significant increases in nAChRs in the caudate putamen in addition to the nucleus accumbens subsequent to the three nicotine injections (each 0.4 mg/kg) during the conditioning phase, and this was not the case in the adult females. Although both groups exhibited a significant CPP to 0.4 mg/kg nicotine, the effect was greater in the adolescents. Thus, it may be that both the caudate putamen and the nucleus accumbens play roles in mediating nicotine CPP.
In adult male rats, there were increases seen across the caudate putamen. These increases are consistent with previous studies showing that continuously infused nicotine produced an upregulation of nicotinic receptor binding in adult male rats (Nguyen et al., 2003; Trauth et al., 1999) and mice (Marks et al., 1985; Pauly et al., 1991; Pauly et al., 1996). Further, this is consistent with increases seen in brains from smokers compared to non-smokers (Court et al., 1998; Perry et al., 1999). Since studies in human adolescent males have not been reported, it is not known whether the lack of effect holds true in humans.
It is interesting that in adolescent male rats, there were no changes in receptor density subsequent to administration of nicotine. In a previous study where this same dose of nicotine was administered for 7 days, there were no changes in locomotor activation by nicotine in adolescent male rats, while adolescent female rats, adult male rats and adult female rats all became sensitized to the effects of nicotine (Collins and Izenwasser, 2004). This behavioral sensitization was accompanied by a significant increase in receptor densities across the caudate putamen in the adult male rats, but not in the adolescents (Collins et al., 2004b). The current study confirms and extends these findings by showing that the effect of nicotine on nAChRs in adult, but not in adolescent males, occurs with only 3 days of nicotine administration. Other studies have shown that nicotinic acetylcholine receptor binding is upregulated in the cerebral cortex, midbrain, and hippocampus in adolescent rats after continuous infusion or twice-daily injections of 0.6, 2, or 6 mg/kg nicotine for one week (Abreu-Villaca et al., 2003) or after continuous infusion of 6 mg/kg nicotine for 17 days when rats were past the periadolescent period (Trauth et al., 1999). Thus, it is possible that changes in nicotinic receptor binding after nicotine treatment in periadolescent rats occur, if at all, in different brain regions than in rats in later stages of adolescence or adult rats. It is interesting to note that in adolescent male rats that began nicotine self-administration during adolescence there were higher levels of a4b2 receptor binding in the striatum than in rats that began self-administering nicotine during adulthood (Levin et al., 2007). There was, however, no correlation between receptor level and self-administration in the adolescents, as opposed to the adults. These studies, together with the present data suggest that there may be differential regulation of nicotine reward and reinforcement in adult and adolescent male rats.
This study highlights the importance of examining full-dose response curves for all groups under study in the place preference paradigm. Had we tested only a single dose of nicotine, we could have concluded that one group or another did not exhibit nicotine reward, and this conclusion would have differed depending upon which dose of nicotine had been tested. While parts of these results are confirmatory, the findings extend prior studies in Wistar rats to Sprague-Dawley rats by showing that adolescents are more sensitive to nicotine reward. In addition, the present results show that nicotine reward is accompanied by differential changes in brain nACH receptors in male and female adolescent and adult rats with a direct comparison. If these findings extend to humans, they would suggest that males are more sensitive to lower doses of nicotine, over a broader range than are females, and that this is especially true for adolescents. Perhaps this is the reason that more men than woman smoke cigarettes and why smoking often begins in adolescence – there is a broader range of nicotine doses that is rewarding in males than in there is in females, and in adolescents as compared to adults. It will be important to keep these sex differences in mind when developing prevention and cessation strategies in teenagers and adults.
Highlights.
Male rats find lower doses of nicotine rewarding than female rats
In males, adolescent rats respond to lower doses of nicotine than adults
Female adolescent rats exhibit a greater maximal reward than female adults
CPP is accompanied by changes in nicotinic receptor binding in the nucleus accumbens and caudate putamen
Fig. 3.
Representative autoradiograms for data expressed in Fig. 2.
Acknowledgments
The animals used in this study were maintained and the studies were conducted in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1985. This work was supported by the National Institute on Drug Abuse and the NIH Office of Research on Women’s Health (grant DA 024584-0002).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–49. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Kirstein CL. Nicotine administration significantly alters accumbal dopamine in the adult but not in the adolescent rat. Annals of the New York Academy of Sciences. 2004;1021:410–7. doi: 10.1196/annals.1308.054. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–9. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The influence of stress on psychopharmacological responses to nicotine. Br J Addict. 1991;86:489–93. doi: 10.1111/j.1360-0443.1991.tb01795.x. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–95. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–5. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav. 2008;89:94–100. doi: 10.1016/j.pbb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–8. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- CDC Tobacco use among middle and high school students--United States, 2002. MMWR Morb Mortal Weekly Report. 2003;52:1096–8. [PubMed] [Google Scholar]
- CDC Vital Signs: Current Cigarette Smoking among Adults Aged ≥ 18 Years - United States, 2009. Morbidity and Mortality Weekly Report. 2010;59:1135–40. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–66. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–62. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Developmental Brain Research. 2004a;153:175–87. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. European Journal of Pharmacology. 2004b;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Understanding brain mechanisms in nicotine reinforcement. British Journal of Addiction. 1991;86:507–10. doi: 10.1111/j.1360-0443.1991.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–9. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tob Control. 2000;9:313–9. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob Res. 2009;11:851–8. doi: 10.1093/ntr/ntp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyerematen GA, Owens GF, Chattopadhyay B, deBethizy JD, Vesell ES. Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos. 1988;16:823–8. [PubMed] [Google Scholar]
- Lanza ST, Donny EC, Collins LM, Balster RL. Analyzing the acquisition of drug self-administration using growth curve models. Drug Alcohol Depend. 2004;75:11–21. doi: 10.1016/j.drugalcdep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. Journal of Neuroscience. 2002;22:8653–60. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–65. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–9. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Meyer A, Mayerhofer A, Kovar KA, Schmidt WJ. Rewarding effects of the optical isomers of 3,4-methylenedioxy-methylamphetamine (‘Ecstasy’) and 3,4-methylenedioxy-ethylamphetamine (‘Eve’) measured by conditioned place preference in rats. Neurosci Lett. 2002;330:280–4. doi: 10.1016/s0304-3940(02)00821-2. [DOI] [PubMed] [Google Scholar]
- NSDUH . Results from the 2009 National Survey on Drug Use and Health: National Findings. DHHS; Rockville, MD: 2010. [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–8. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Advis JP, Andrews WW. Neuroendocrine control of the onset of puberty in the rat. Fed Proc. 1980;39:2365–71. [PubMed] [Google Scholar]
- Ojeda SR, Aguado LI, Smith S. Neuroendocrine mechanisms controlling the onset of female puberty: the rat as a model. Neuroendocrinology. 1983;37:306–13. doi: 10.1159/000123565. [DOI] [PubMed] [Google Scholar]
- Panday S, Reddy SP, Ruiter RA, Bergstrom E, de Vries H. Nicotine dependence and withdrawal symptoms among occasional smokers. J Adolesc Health. 2007;40:144–50. doi: 10.1016/j.jadohealth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Rahmanian SD, Diaz PT, Wewers ME. Tobacco Use and Cessation Among Women: Research and Treatment-Related Issues. J Womens Health (Larchmt) 2011 doi: 10.1089/jwh.2010.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154:885–97. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology. 2004;175:265–73. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacology Biochemistry & Behavior. 2003;75:355–61. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Perry DC. Prenatal nicotine exposure is associated with an increase in [125 I]epibatidine binding in discrete cortical regions in rats. Pharmacology Biochemistry & Behavior. 2000;67:319–23. doi: 10.1016/s0091-3057(00)00379-8. [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206:303–12. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–63. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Research. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology & Behavior. 2002;77 doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58:374–82. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent rats. Behavioural Brain Research. 2009a;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Miller JS, Unterwald EM, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009b;163:890–7. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacology Biochemistry & Behavior. 2009c;92:131–4. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]