Abstract
The discovery of new antimalarials with transmission blocking activity remains a key issue in efforts to control malaria and eventually eradicate the disease. Recently, high-throughput screening (HTS) assays have been successfully applied to Plasmodium falciparum asexual stages to screen millions of compounds, with the identification of thousands of new active molecules, some of which are already in clinical phases. The same approach has now been applied to identify compounds that are active against P. falciparum gametocytes, the parasite stage responsible for transmission. This study reports screening results for the Tres Cantos Antimalarial Set (TCAMS), of approximately 13,533 molecules, against P. falciparum stage V gametocytes. Secondary confirmation and cytotoxicity assays led to the identification of 98 selective molecules with dual activity against gametocytes and asexual stages. Hit compounds were chemically clustered and analyzed for appropriate physicochemical properties. The TCAMS chemical space around the prioritized hits was also studied. A selection of hit compounds was assessed ex vivo in the standard membrane feeding assay and demonstrated complete block in transmission. As a result of this effort, new chemical structures not connected to previously described antimalarials have been identified. This new set of compounds may serve as starting points for future drug discovery programs as well as tool compounds for identifying new modes of action involved in malaria transmission.
Introduction
The last decade has witnessed unprecedented progress in reducing the incidence of Plasmodium falciparum malaria, especially in Africa where the burden of disease is greatest. Continuous efforts to reduce malaria cases, such as insecticide treated nets, indoor residual spraying and artemisinin-based combination therapies (ACTs) have contributed to a significant decrease in malaria mortality, from an estimated 1,800,000 deaths in 2005 to 584,000 registered in 2014 [1]. Nevertheless, in order to achieve the ultimate goal of eradicating malaria, more effective tools will be required. These include efficacious vaccines and innovative antimalarial drugs with novel mechanisms of action displaying not only efficacy against resistant parasites but also transmission blocking potential.
The World Health Organization (WHO) currently recommends ACTs as the standard of care for uncomplicated malaria in endemic countries. However, since 2008 [2], artemisinin resistance has been described in western Cambodia, Thailand and Vietnam, threatening malaria control, treatment and elimination efforts worldwide [3]. In order to block transmission, ACTs must be combined with primaquine, the only drug recommended by the WHO at single low dose for this indication. However, primaquine safety is of particular concern given its hemolytic side effects in patients with glucose-6-phospate dehydrogenase (G6PD) deficiency. Consequently, there is an urgent need to develop alternative antimalarial drugs to treat acute malaria in individual patients and prevent the spread of infection in the population.
During the past decade, phenotypic screenings have focused on identifying compounds with activity against P. falciparum asexual blood stages, responsible for proliferation of the parasite in the human host and for the clinical symptoms of malaria. Millions of compounds have been tested and thousands of new molecules identified, some of which are currently undergoing development [4, 5]. As part of this effort, GlaxoSmithKline published the Tres Cantos Antimalarial Set (TCAMS), comprising 13,533 compounds resulting from the screening of nearly two million compounds from the GSK corporate collection [6]. The TCAMS set described high quality starting points demonstrating potent activity against malaria asexual stages and physicochemical traits suitable for the discovery of orally administrated drugs. Consequently, a number of new oral antimalarial agents are now under investigation [7].
The next challenge in malaria drug development is to identify molecules with activity against mature gametocytes, with consequent transmission blocking potential. Gametocytes are the parasite stage responsible for transmission of the disease through the Anopheline vectors [8]. In the case of P. falciparum malaria, gametocytes develop from committed schizonts over a period of 12 days [9]. Successive differentiation events lead to early gametocytes (stages I to III), susceptible to most of classical schizonticidal antimalarials. These finally develop into mature gametocytes (stages IV and V) which are insensitive to most commercial antimalarial drugs. Only the fully developed mature stage V gametocytes can infect Anopheles mosquitoes.
A drug discovery strategy to identify a new class of antimalarial agents with dual activity against both the asexual and gametocyte parasite stages is highly desirable. Consequently, a new assay to screen compounds for their potential to inhibit stage V gametocytes was developed, validated against standard antimalarials, and included in our test cascades [10]. This in vitro assay measures ATP intracellular levels as a surrogate of gametocyte viability. This robust and reliable assay was adapted to the high-throughput screening (HTS) format.
Here we describe the screening of the TCAMS compound set for activity against P. falciparum stage V gametocytes. To our knowledge, this is the largest screening campaign conducted using P. falciparum sexual stages. Hundreds of primary hits with activity against both blood stages and gametocytes were identified and analyzed, applying sequential and progressively more demanding chemical, biological and developability filters. Finally, selected compounds were evaluated for their impact on transmission reduction to the Anopheles stephensi mosquito vector using the standard membrane feeding assay (SMFA), and thereby validating the integrated approach presented in this work.
Results
Phenotypic screening against mature gametocytes
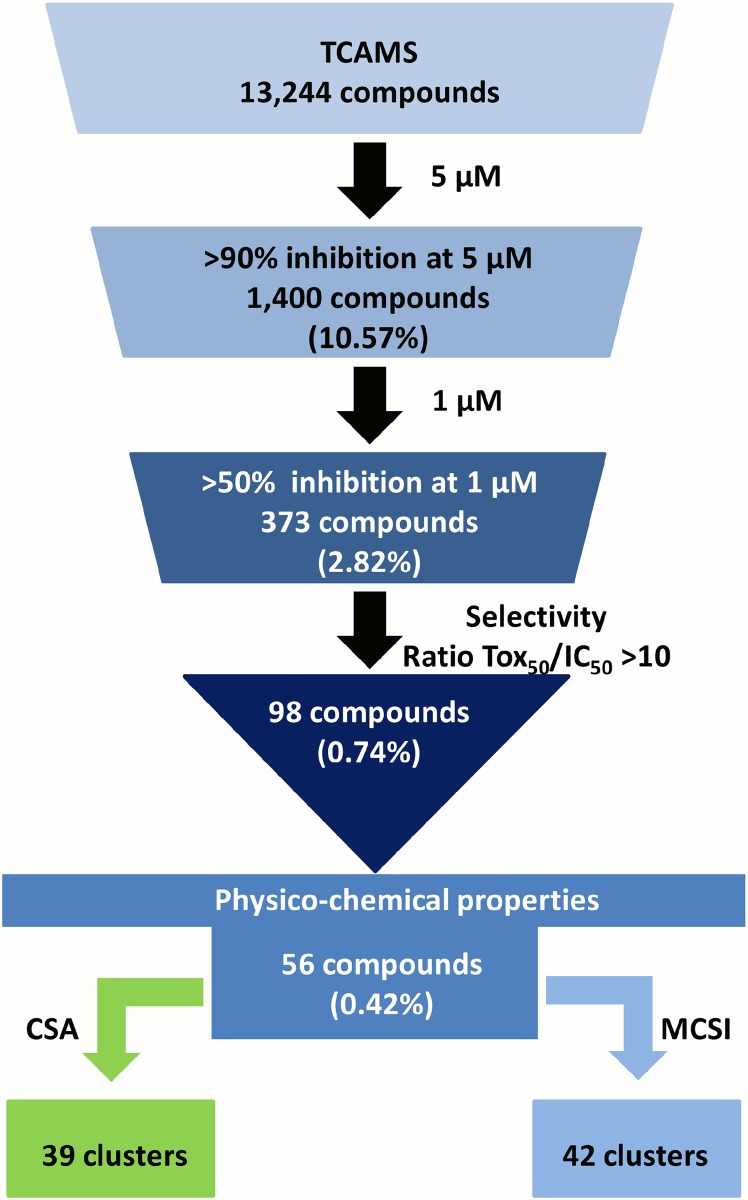
The research protocol for this study is described in Fig 1. Briefly, the TCAMS compound set was screened using a bioluminescence ATP assay [10] to identify molecules with dual activity against P. falciparum asexual forms and stage V gametocytes. Subsequently, the primary hits were analyzed by applying a cascade of sequential and increasingly more demanding biological and physicochemical filters.
Fig 1. Description of the critical path followed to generate and prioritize hits from TCAMS.
The initial screen of the entire TCAMS set identified 1400 compounds (10.57% hit rate) as positive hits for inhibition of mature gametocytes, based on a decrease in the level of ATP content by ≥ 90% at a single assay concentration of 5 μM. A summary of the HTS results is provided as S1 Table. The 1400 primary hits were tested in a second screen at a single concentration of 1 μM. A total of 373 compounds (2.82% hit rate) were identified that decreased ATP levels by ≥ 50% (S2 Table). An interference assay, using a panel of 167 compounds tested at 50 μM, showed no significant inhibition confirming that the assay had identified compounds with activity against mature gametocytes, rather than the BacTiter-Glo reaction itself (S3 Table).
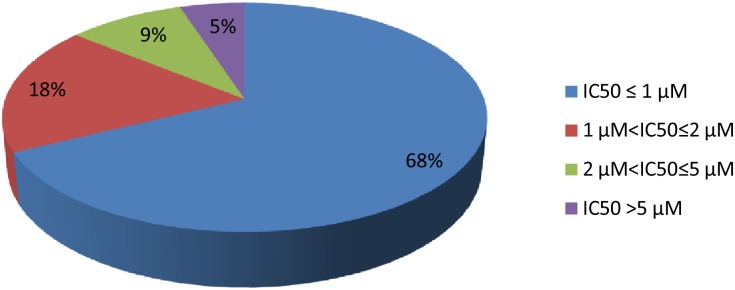
The 373 selected compounds were subsequently re-tested in a dose–response assay to determine IC50 values, with an average Z-prime factor of 0.55. There was excellent correlation between percent inhibition at 1 μM and the IC50 results. This suggests high reliability of the single point assays. As can be seen in Fig 2, nearly 70% of the hits displayed potencies ≤ 1 μM. However, there was no significant correlation between compound IC50 values obtained against gametocytes and the inhibition of growth of P. falciparum asexual forms as previously determined for TCAMS [6] (S4 Table).
Fig 2. Relative distribution of TCAMS primary hits based on P. falciparum IC50 in gametocytes.
In order to assess the antimalarial specificity of the 373 active compounds, their potential cytotoxicity was tested against HepG2 cells after 48 h of exposure using the same biomarker as for the gametocyte assays (depletion of intracellular ATP levels). Compounds were selected with potencies of < 2 μM from the dose–response curves in P. falciparum and a selectivity ratio > 10 in the cytotoxicity test (i.e. activity in the human hepatoma cell line divided by activity in gametocytes > 10). As a result, 98 compounds were progressed for subsequent analysis (S5 Table).
Chemical filtering, analog search and selection of drug-like molecules
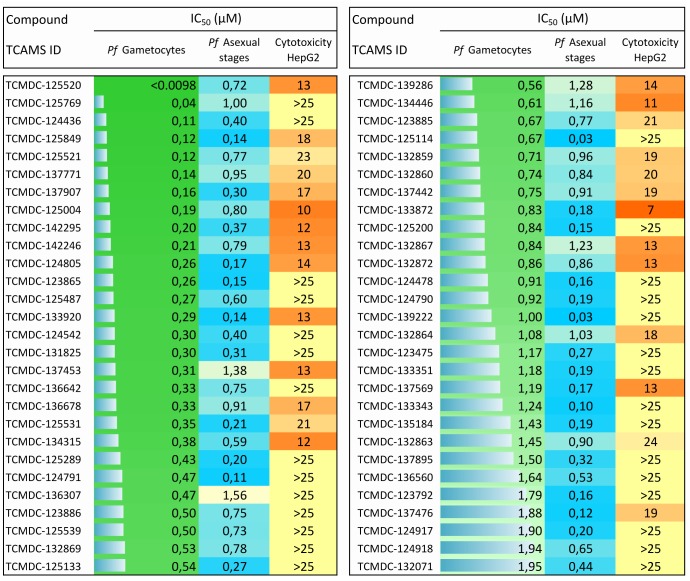
Hits were further narrowed down based on physicochemical parameters, attempting to select the most developable assets. Cutoffs, such as molecular weight ≤ 500, clogP ≤ 5, number of aromatic rings ≤ 4, number of rotatable bonds ≤ 10, number of hydrogen bond acceptors ≤ 10, and number of hydrogen bond donors ≤ 5, were used (Fig 1). A full description of the physicochemical properties of the 98 compounds is provided (S6 Table). This process led to prioritization of 56 drug-like compounds (S7 Table). Their biological activity and physicochemical properties are summarized in Figs 3 and 4.
Fig 3. Biological profile of the 56 prioritized compounds.
Bars and color intensity represent compound potency against P. falciparum gametocytes asexual stages, and cytotoxicity.
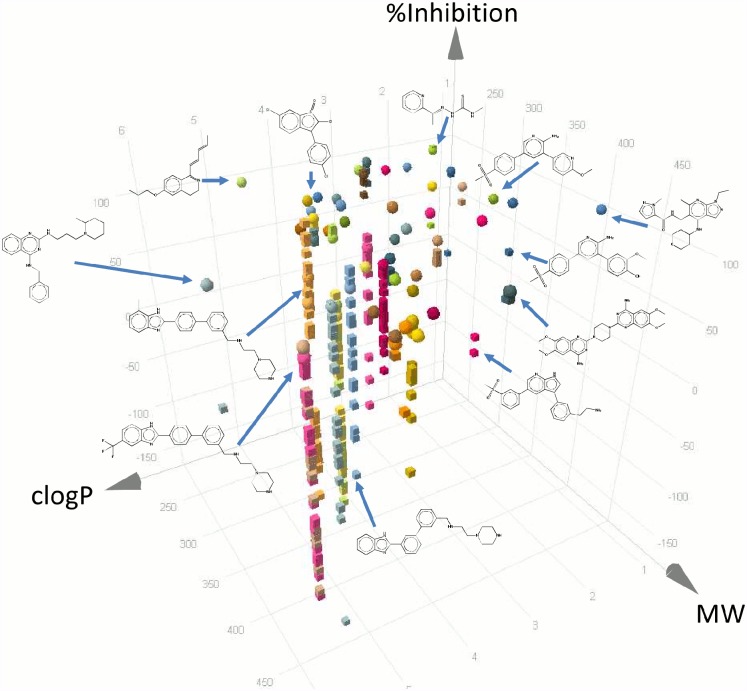
Fig 4. Three-dimensional plot displaying the structures, properties and parasitological activity of some of the 56 prioritized compounds and their analogs.
Selected compounds are represented as spheres, while the corresponding analogs (displayed at the same clogP and molecular weight as their proposed compound, but with their own inhibition value) are represented as cubes.
Sorting similar structures into clusters is a valuable tool in the drug discovery process. Two different approaches were applied to analyze the TCAMS chemical space around the 56 prioritized compounds.
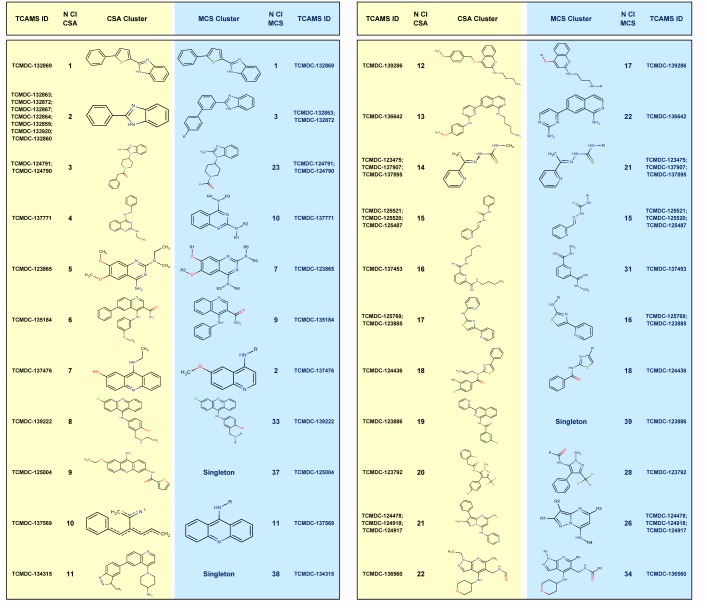
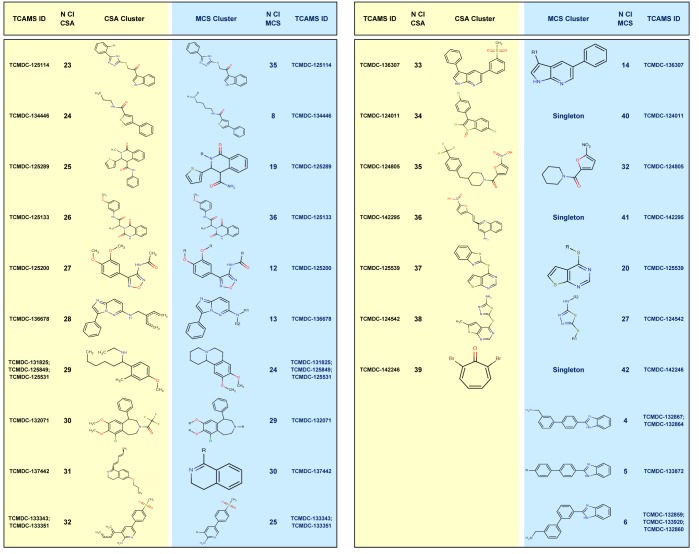
1. Similarity-based computational search of analogs (CSA) identified 25 clusters and 14 singletons (Fig 5a, Fig 5b and S8 Table), and retrieved 434 analogs associated to the different clusters (S9 Table).
Fig 5a. Chemical analysis of the 56 prioritized hits by CSA and MCSI methods.
"TCAMS ID" represents the reference of the hit compound at TCAMS. "CSA Cluster" refers to the chemical scaffold obtained using the similarity-based computational search of analogs approach. "N Cl CSA" is the number of cluster using CSA approach. "MCS Cluster" depicts the chemical scaffold obtained using the similarity-based medicinal chemistry search of analogs approach and "N Cl MCS" indicates the number of cluster using MCS approach.
Fig 5b. Chemical analysis of the 56 prioritized hits by CSA and MCSI methods.
"TCAMS ID" represents the reference of the hit compound at TCAMS. "CSA Cluster" refers to the chemical scaffold obtained using the similarity-based computational search of analogs approach. "N Cl CSA" is the number of cluster using CSA approach. "MCS Cluster" depicts the chemical scaffold obtained using the similarity-based medicinal chemistry search of analogs approach and "N Cl MCS" indicates the number of cluster using MCS approach.
2. Substructure-based medicinal chemistry scaffold indexing (MCSI) identified 36 clusters and 6 singletons (Fig 5a, Fig 5b and S8 Table), together with 1252 associated analogs within the TCAM set (S10 Table).
For each chemical cluster, the corresponding analogs presented different substitution patterns around the common chemical substructure, maximizing the probabilities of building structure–activity relationships for each chemical substructure.
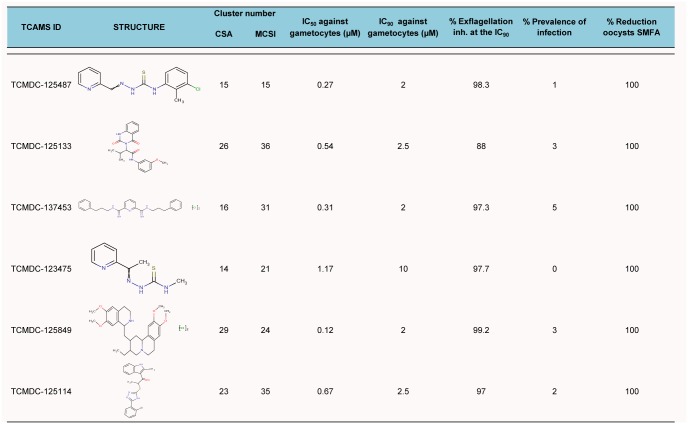
Confirmation of the transmission-blocking potential of selected hits in the SMFA
The in vitro gametocytocidal activity was validated ex vivo using the SMFA to test the efficiency of a selection of molecules in preventing the infection of mosquitoes. Six molecules displaying a wide range of anti-gametocyte activities varying from 0.12 to 1.17 μM, and representing great structural diversity within the drug-like space, were selected for evaluation in Anopheles stephensi mosquitoes. The transmission blocking potential of these molecules was measured as the percentage reduction in the prevalence of infection and mean oocyst intensity compared to DMSO-treated controls. As a previous step, the capability of these compounds to inhibit male gametocyte exflagellation was also tested. Following exposure to a concentration equivalent to the IC90 value obtained in the ATP assay, all six compounds blocked exflagellation, (≥ 88% inhibition, Fig 6).
Fig 6. Effect of selected TCAMS on P. falciparum oocyst intensity after treatment of mature gametocytes for 48 h.
In the figure, each red dot represents the total number of oocysts in a single mosquito midgut, and the green line depicts the mean oocyst intensity of infection. The table below the graph depicts the total number of full-fed mosquitoes dissected per treatment, the percentage infection prevalence, transmission blocking potential, mean oocyst intensity reduction, and the percentage inhibition of exflagellation at 48 h post-treatment. The percentage transmission blocking potential, mean oocyst intensity reduction and exflagellation inhibition were calculated by normalizing with values from the control group. Compounds showed a statistically significant decrease in oocyst distribution when compared to the control (p<0.0001). The data above are representative from one of the two independent experiments.
Figs 6 and 7 show the ability of selected compounds to prevent P. falciparum transmission to mosquitoes. The prevalence of infection after gametocyte treatment ranged from 5% to 0%, which corresponds to a block in transmission from 95% to 100% (Fig 6). An infection prevalence of 94% was observed in control mosquitoes. The inhibition of mosquito infection showed an excellent correlation with the IC90 values obtained in vitro (Fig 7). All the compounds produced a 100% reduction in mean oocyst numbers.
Fig 7. Summary of biological data for the 6 compounds evaluated in the SMFA.
Discussion
Several extensive small molecule screens against P. falciparum blood stages have been published, expanding the portfolio of potential candidates for treating acute malaria. Nevertheless, there is still a crucial need for antimalarial drugs that go beyond treating acute infections, and which have the potential to clear circulating gametocytes, thus contributing to eradicate the disease.
The main objective of this work was to identify promising antimalarials from the 13,533 molecules within the GSK TCAMS library which, in addition to demonstrated in vitro activity against asexual stages, could also display transmission blocking potential. Furthermore, to find drug-like molecules, screening based on developable properties was applied. In accordance to these objectives, the biological screens were performed following increasingly stringent filters. This approach enabled us to establish a primary list of 373 compounds with potencies in the nanomolar range against both asexual and sexual stages of P. falciparum.
To conduct such extensive gametocyte screens, some issues need to be resolved. For example, large numbers of mature gametocytes have to be generated in a reproducible manner and a robust assay with a fast and simple read-out suitable for HTS developed. Although several methods for assessing antimalarial activity in gametocytes have been published and used to screen compound collections [11–16], few have been validated by testing mosquito infectivity.
The ATP-bioluminescence assay used in this work fulfills the requirements of HTS [10]. Previously, the assay has been shown to yield a signal proportional to the number of viable cells [17, 18]. The possibility of selecting false-positive hits, i.e., molecules inhibiting the bioluminescence reaction rather than killing gametocytes, was examined. None of the tested compounds proved to be an inhibitor of the luciferase reaction. Another result supporting the robustness of our ATP gametocytocidal assay is the high re-test rate obtained (approximately 70%). Furthermore, in this study the ATP-bioluminescence assay was further validated ex vivo in the SMFA, currently considered the gold standard for assessing molecules with transmission blocking potential. A test selection of six positive hits identified using the ATP-bioluminescence assay had exceptional activity in preventing the infection of Anopheles stephensi mosquitoes. These findings strongly support the predictive value of this methodology.
High hit rates were obtained on the HTS screening of TCAMS at 5 μM (10.57%) and at 1 μM (2.82%) compared with the typical range of values for random screenings (0.1–5%) [19]. The higher rates observed in this screen could be explained as the result of an 'enrichment’ of the antimalarial properties of the starting compound collection, as TCAMS is entirely composed of molecules active against asexual stages of Plasmodium.
In order to secure the early exclusion of potentially toxic molecules from the progression cascade, the 373 dual-acting compounds were tested in mammalian HepG2 cells, also using ATP depletion as readout methodology. The cytotoxicity assessment narrowed down the list of promising compounds to 98 molecules which displayed a selectivity ratio > 10. It is interesting that the percentage of compounds exhibiting inhibition > 50% at 10 μM in the HepG2 cell line was much lower in TCAMS overall (14.55%) than in the 373 dual-acting compounds (51.81%). This could be because mature gametocytes are non-dividing quiescent cells, while asexual stages are growing cells with an active metabolism. Compounds simultaneously targeting gametocytes and asexual stages could be affecting housekeeping processes also present in mammalian cells. This hypothesis suggests that it would be advisable to screen unbiased compound collections, not previously filtered by their activity in blood stages, for activity against gametocytes. Nevertheless, it is encouraging that 26.3% of the 373 dual-acting compounds displayed a selectivity ratio > 10, suggesting the existence of specific targets important for both asexual and sexual stages, but not present in mammalian cells.
There was no significant correlation between the potency of compounds in gametocytes compared with the activity in asexual forms [6]. A number of factors could explain this observation. Firstly, the inhibition data were obtained using quite different methodologies for each study (lactate dehydrogenase determination for asexual forms and ATP content for gametocytes). Another factor could be the low number of analogs composing the clusters studied, thus reducing statistical significance. There may also be differences in drug susceptibility between life-cycle stages or even different targets involved in asexual and sexual parasite stages.
Fifty-six molecules were prioritized in terms of drug-like and biological properties. The application of these filters does not guarantee success in identifying developable scaffolds, but aims to reduce the attrition risk in drug development. Two complementary chemoinformatic tools (CSA and MCSI) were applied to classify the selected molecules. While CSA was more efficient when the objective was hit expansion through the identification of active analogs, MCSI was the approach of choice for building structure–activity relationships around a common scaffold, which is very useful for a phenotype-based approach like TCAMS.
Hit structural analysis showed that few compounds included well-known single antimalarial scaffolds (quinacrine-like in TCMDC-137476 and TCMDC-137569) and double antimalarial sub-structures (amodiaquine-like + quinacrine-like in TCMDC-139222). Some other chemotypes described recently were also perceived (SAHH2-like in TCMDC-123865 [20]; MMV390048-like in TCMDC-133343 and TCMDC-133351 [21]; compound 13-like in TCMDC-125539 [22]; compound C2-1-like in TCMDC-123792 [23]; and SJ733-like in TCMDC-125289 [24]. However, most of the 56 selected molecules were new structures not connected to either a classical or recently described antimalarial scaffolds.
TCAMS is annotated with target information for each compound, if known. Annotations for the 56 compounds include two (TCMDC-123885 and TCMDC-125769) which have been reported as inhibitors of KCa2.2, a calcium-dependent K+ channel. Interestingly, recent bioinformatic analyses suggest the presence of homologues of calcium-activated K+ channels in the P. falciparum genome [25]. The only other compound with a target annotation is TCMDC-123886, reported as an adenosine A3 receptor ligand. The rationalization for this target is more problematic as there is little evidence for G-protein-coupled receptors (GPCRs) in P. falciparum. However, GCPRs are of ancient origin and present in almost all eukaryotic organisms so far examined [26]. Thus, further research is required to validate or exclude adenosine A3 as a target in P. falciparum. In addition, both TCMDC-123792 and TCMDC-125289 correspond to chemotypes that have been characterized as inhibitors of a P-type cation-ATPase (PfATP4) [23, 24]. The remaining 51 prioritized compounds lack target annotation in TCAMS. By publishing these novel structures and providing the associated data as open access, we hope to stimulate research into identifying validated targets for drugs acting against P. falciparum gametocytes.
The Malaria Box is a collection of 400 diverse compounds with confirmed activity against P. falciparum asexual forms [27]. This set has been assembled by Medicines for Malaria Venture (MMV) in an attempt to catalyze drug discovery and research in malaria and other neglected diseases. The collection is composed of molecules from St. Jude Children's Research Hospital, Novartis-GNF, commercial libraries, and GSK (140 molecules belonging to TCAMS). This set has been screened against mature gametocytes by other groups [12, 14–16], presenting a low degree of coincidence among them. Gathering the published hit lists, a total of 53 compounds from TCAMS were identified. When compared with our prioritized molecules, 15 structures were found to be in common. No more coincidences were found with those other screens, indicating a high degree of chemical novelty for the set of hits presented in this work.
Conclusion
Screening one of the largest antimalarial compound collections (TCAMS) identified molecules with activity against both P. falciparum asexual and gametocyte stages. The set of selected compounds with transmission-blocking potential includes well-known scaffolds as well as promising new chemical entities, showing a high degree of chemical novelty. Further parasitological, toxicological and pharmacokinetic studies will be required to identify antimalarial leads and pre-clinical candidates. Most importantly, the results suggest new starting points for malaria drug discovery and have been provided open access to enable further research into the prevention of malaria transmission.
Materials and Methods
Ethics statement
Human biological samples were ethically sourced, and their research use was in accordance with the terms of the informed consents. Written informed consent was obtained from the blood donors for the use of samples in this research.
The human blood was supplied by the Banc de Sang i Teixits (http://www.bancsang.net/) (Barcelona, Spain). The blood provision from Banc de Sang i Teixits was approved by the Clinical Research Ethics Committee of the Vall d’Hebron Hospital in Barcelona, Spain. The malaria project research was approved by the Ethics Research Committee of the Universidad Autónoma de Madrid, Spain.
Parasites and cultures
The 3D7A (MR4, MRA-151) and NF54 (kindly provided by Robert E. Sinden) P. falciparum strains were cultured using a method modified from that of Trager and Jensen [28], in a 5% CO2, 5% O2 atmosphere at 37°C [29].
Gametocytogenesis and purification of the mature gametocytes
Purified stage IV-V gametocytes were obtained as previously described [10]. Single point ATP bioluminescence assay and dose–response of positive hits was determined in accordance with the previously published protocol [10].
Compounds management
Compounds were dispensed using the Echo-CRS system. For the determination of single point activity, 250 or 50 nL from a 1 mM mother solution for 5 and 1 μM final concentration, respectively, were dispensed into the 384-well Greiner plate (ref. 781091), using the HTS GSK layout with 250 or 50 nL DMSO in column 6 (vehicle control) and 250 nL of 100 μM epoxomicin (background control, 0.5 μM final concentration) in column 18. For the dose–response analysis (determination of IC50), each row of the 384-well plate contained the serial 1/2 dilutions of two compounds (11 dilutions): the first one interrupted in column 6 and the second one in column 18 (vehicle and background controls, respectively). The plates were kept frozen until use.
Gametocytocidal assay
Once purified, gametocytes were counted using a Neubauer chamber, and the density was adjusted to 2.5 x 105 gametocytes/mL. Fifty μL of the gametocyte suspension (1.25 x 104 gametocytes) were added to each well of the 384-well plate containing the compounds, using a Multidrop combi (Thermo Electron Corporation). Plates were shaken for 5 sec to homogenize and then incubated at 37°C for 48 h in an atmosphere of 5% CO2, 5% O2, 90% N2. The ATP level of each well was determined using the BacTiter-Glo reagent (Promega) according to the recommendations of the manufacturer. The BacTiter-Glo assay generates a ‘glow-type’ luminescent signal produced by the luciferase reaction, which consists of mono-oxygenation of luciferin catalyzed by luciferase in the presence of Mg2+, ATP and molecular oxygen. The luminescence was measured using a plate reader (Victor V, Perkin Elmer). The background value of the assay was obtained as the average ATP level of 16 samples exposed to 1000-fold the IC50 of epoxomicin (0.5 μM). The background value was subtracted from every other value of the assay prior to further calculation. The average of 16 replicates of vehicle control (0.5% or 0.1% DMSO) represents 100% of signal. Related to this value, each test concentration was calculated as percentage of control. All the calculations were done using MS Excel software. The compounds which presented inhibition ≥ 90% at 5 μM were progressed to the assay at 1 μM. All the compounds active in the 1 μM single point assay (inhibition ≥ 50%) were tested to determine the exact IC50 value (concentration of compound which corresponds to 50% inhibition). These values were obtained from the dose–response curves plotted using Graph Pad software (version 6).
Interference assay
To discard possible false-positive results, we performed an interference assay in parallel. This evaluated the inhibition of the luciferase reaction by the compounds at a concentration of 50 μM, which is much higher than the maximum concentration tested in the screening assay (5 μM). Tests were done at a final concentration of ATP of 37.5 nM. BacTiter-Glo reagent (Promega) was added and luminescence was measured. The ATP solution was made by dissolving ATP powder (ATP standard from the ATPlite 1 step assay kit, reference 6016731, Perkin Elmer) in culture medium. The positive control used was the Luciferase Inhibitor I Calbiochem (Merck, 119113) at 100 μM.
Cytotoxicity
Recognition of drug-induced hepatotoxic potential early in the drug development cascade creates opportunities for ranking and prioritizing compounds with lower toxicity [30].
Cell line
HepG2, a human Caucasian hepatocellular carcinoma, was supplied by ECACC (ref. 85011430).
Routine culture
Cells were grown and maintained in EMEM (Sigma-Aldrich) supplemented with 2mM L-glutamine (Sigma-Aldrich) and 10% fetal calf serum (Perbio). Cultures were maintained at 37°C in a humidified incubator containing 5% CO2 and 95% air. Passages were routinely made upon reaching 80–90% confluence. For cytotoxicity experiments, cells were seeded onto 96-well clear bottom black plates coated with type I collagen (Biocoat, Becton Dickinson) at a cell density of 20,000 cells/well.
Measurement of cytotoxicity
To determine cytotoxic effects, represented by the Tox50 value (the concentration of drug that reduces cell viability by 50%), cells were exposed to serial dilutions of compounds for 48 h at 37°C. The assay medium was as previously described, but supplemented with 5% fetal calf serum instead of 10%. Following the 48 h drug exposure period, the intracellular ATP content was determined using the ATPlite 1 step kit (Perkin Elmer, 6016731). In the presence of ATP, luminescence is emitted as the reaction described earlier for the BacTiter-Glo kit. Percentages of inhibition were calculated relative to vehicle control wells.
Similarity-based computational search of analogs (CSA)
This chemical analytical method first comprised clustering of the 56 drug-like hits prioritized from the HTS into 39 clusters by using the Complete Linkage algorithm implemented in the Scaffold Hunter program [31]. These clusters were complemented with all the analogs in TCAMS showing a Tanimoto similarity ≥ 0.8 to any of the 56 prioritized compounds (molecules were represented by topological fingerprints in the RDKit cheminformatic toolkit [32]). These expanded clusters were characterized in terms of evidence of gametocyte activity in the neighborhood of the prioritized hits by calculating for each of them the hypergeometric probability p(m,n) of having m active compounds (> 50% inhibition at 5 μM) within the n members of the cluster. Larger clusters with an ‘unusually’ high proportion of actives are improbable and, therefore, will have a low value of p. These clusters show the largest opportunity for chemical expansion of the prioritized hits within TCAMS.
Substructure-based medicinal chemistry scaffold indexing (MCSI)
Substructure-based medicinal chemistry scaffold indexing was carried out to facilitate structure–activity relationship studies for prioritized hits from the HTS campaign. This method used as starting points the 39 clusters obtained with the CSA method as well as the corresponding analogs identified for these clusters. For each cluster and its corresponding analogs, an initial proposed scaffold was optimized to yield the minimum common chemical structure shared by the highest number of compounds included in TCAMS. The optimization step was done by adding or subtracting small structural fragments from the previous proposed scaffolds until the optimized scaffold was present in the structure of the highest number of compounds included in TCAMS. In a further step, analysis of the results obtained for these compounds in the initial screen (whole set of compounds tested at a single concentration, 5 μM) (see S1 Table) led to the identification of ‘active’ TCAMS analogs, defined as those compounds not only displaying the defined chemical scaffold but also showing > 50% inhibition in the primary screening campaign, and therefore expected to present an IC50 in the low single-digit micromolar range.
Exflagellation assay
The exflagellation assay was performed as described by Delves et al. [33], with some modifications. Briefly, 200 μL samples of mature P. falciparum NF54 gametocytes culture in the presence of the corresponding test compound were incubated at 37°C for 48 h in an atmosphere of 5% CO2, 5% O2, 90% N2 in 1.5 mL microfuge tubes. After the incubation period, cells were settled down and medium was carefully removed. The cell pellets were resuspended in 15 μl of complete ookinete medium (RPMI medium with 25 mM HEPES, 50 mg/L hypoxanthine, 2 g/L sodium bicarbonate, 100 μM xanthurenic acid, 20% human serum) pre-warmed at 37°C. Once parasites were resuspended in ookinete medium, they were introduced in a chamber of a FastRead disposable hemocytometer slide and placed on a horizontal surface at room temperature (21°C), to allow cells to homogeneously settle, forming a tight monolayer. After 14 min in the chamber, exflagellation was observed at 10X magnification, and the number of exflagellation centers per field was recorded.
Standard membrane feeding assay (SMFA)
Gametocyte cultures of the P. falciparum NF54 strain, kindly provided by Robert Sinden (Imperial College London), were induced at 0.5% asexual parasitemia and 4% hematocrit (RBCs from the Spanish Red Cross) at 40 mL final volume in T75 flasks. Gametocyte production media comprised RPMI 1640 (Sigma, #R5886), 30 mM bicarbonate (Sigma-Aldrich, #S5761) and 5 mM hypoxanthine (Gibco, #11067–030). Media were made complete with 5% human serum A+ (Interstate Blood Bank, USA) and 5% AlbuMAX II (Gibco, #11021–037). Cultures were maintained in a gassed incubator at 37°C with 5% CO2, 5% O2, 90% N2 for 14–17 days post-induction, with daily medium replacement without the addition of fresh RBCs. Percentages of mature gametocytes were assessed by microscopic counts (Leica, DM2000) of Giemsa-stained thin blood smears, and functional microgametocytes were assessed for exflagellation activity at room temperature. To test exflagellation, mature gametocyte cultures were centrifuged at 1800 rpm for one minute. The infected RBC pellet was gently mixed with an equal volume of pre-warmed human serum and introduced onto a slide with a coverslip, followed by incubation at room temperature for 10–15 min. The average exflagellation centers per field at 10X magnification was determined by the exflagellation assay described above [33]. Gametocyte counts and exflagellation assays were performed both before and after gametocyte drug treatment (at the time of SMFA).
Two days before gametocyte treatment with compound, spent media was removed and fresh Gametocyte Treatment Medium consisting of RPMI1640 with L-glutamine and 25 mM HEPES (Gibco, #13018–031), 10 mM glucose (Sigma-Aldrich, #G8270), 20 mM bicarbonate (Sigma-Aldrich, #S5761) and 5 mM hypoxanthine (Gibco, #11067) was added. Media were made complete with 10% of human A+ serum (Interstate Blood Bank). Five ml of mature gametocyte culture at day 15 post-induction was transferred into each well of a pre-warmed 6-well plate. The selected TCAMS compounds were prepared fresh as a stock solution of 10 mM in 100% of DMSO and added to the mature gametocytes at the concentrations described in Fig 7. Gametocytes were exposed to the compound for a total duration of 48 h. At 24 h post-drug exposure, a fixed volume (3 mL) of spent medium was removed and fresh medium was re-added, accompanied by compound replenishment to obtain the required final concentration. Untreated gametocytes with the same final concentration of DMSO (0.1%) as in treated gametocytes were processed in parallel.
On the day of the feed, cultures were centrifuged at 2500 g for 3 min at 37°C, diluted 1:1 with 100% packed cell volume of fresh RBCs, and finally formulated as artificial mosquito blood meals at 50% hematocrit with pre-warmed human serum. All steps were performed at 37°C. Prepared blood meals were fed in duplicate to 4–6 days old female An. stephensi mosquitoes for 30–40 min via Parafilm membrane attached to glass feeders (Fisher Scientific, #12831283) connected to a 37°C circulating water bath. Fed mosquitoes were maintained in an incubator at 27°C and 75% relative humidity with 12 h light/dark cycles. At 7–8 days post-feeding, mosquitoes with fully developed ovaries were dissected for midguts (Leica, M80) and incubated in 0.2% mercurochrome solution in distilled water for 10–15 min. The total number of oocysts in individual midguts was counted using a light microscope (Leica, DM2000) under a 10X objective (100X magnification). Both infection prevalence (percentage of mosquitoes with one or more oocysts) and mean oocyst intensity of infection were defined in each treatment. The percentage block in transmission and the percentage reduction in mean oocyst intensity were calculated after normalizing to the Control DMSO-treated sample.
The Mann-Whitney U-test was used to compare the statistical significance between the different treatments and the control.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSM)
(XLSM)
Acknowledgments
We are grateful to Dr. Javier Gamo for critically reading the manuscript and making valuable suggestions. Copy editing was provided by Naomi Richardson of Magenta Communications Ltd and was funded by GlaxoSmithKline.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funder, GlaxoSmithKline, provided support in the form of salaries for authors [MJA, SL, JL, GC, JMC, JR, CG and EH], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.World Health Organization. World Malaria Report 2014: WHO Press; 2014 [cited 2015 March]. Available: http://www.who.int/malaria/publications/world_malaria_report_2014/en/.
- 2. Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359(24):2619–20. 10.1056/NEJMc0805011 . [DOI] [PubMed] [Google Scholar]
- 3. Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371(5):411–23. 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, et al. Chemical genetics of Plasmodium falciparum . Nature. 2010;465(7296):311–5. 10.1038/nature09099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(26):9059–64. 10.1073/pnas.0802982105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, et al. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465(7296):305–10. 10.1038/nature09107 . [DOI] [PubMed] [Google Scholar]
- 7. Calderon F, Barros D, Bueno JM, Coteron JM, Fernandez E, Gamo FJ, et al. An invitation to open innovation in malaria drug discovery: 47 quality starting points from the TCAMS. ACS Med Chem Lett. 2011;2(10):741–6. 10.1021/ml200135p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wells TN, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8(11):879–91. 10.1038/nrd2972 . [DOI] [PubMed] [Google Scholar]
- 9. Smith TG, Lourenco P, Carter R, Walliker D, Ranford-Cartwright LC. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum . Parasitology. 2000;121 (Pt 2):127–33. . [DOI] [PubMed] [Google Scholar]
- 10. Lelievre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, et al. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence "transmission blocking" assay. PLoS One. 2012;7(4):e35019 10.1371/journal.pone.0035019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lucantoni L, Duffy S, Adjalley SH, Fidock DA, Avery VM. Identification of MMV malaria box inhibitors of Plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob Agents Chemother. 2013;57(12):6050–62. 10.1128/AAC.00870-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duffy S, Avery VM. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J. 2013;12:408 10.1186/1475-2875-12-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Alessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, et al. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J Antimicrob Chemother. 2013;68(9):2048–58. 10.1093/jac/dkt165 . [DOI] [PubMed] [Google Scholar]
- 14. Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the malaria box. Antimicrob Agents Chemother. 2014;58(2):811–9. 10.1128/AAC.01500-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS One. 2014;9(8):e105817 10.1371/journal.pone.0105817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, et al. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother. 2014;58(12):7292–302. 10.1128/AAC.03666-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cree IA, Andreotti PE. Measurement of cytotoxicity by ATP-based luminescence assay in primary cell cultures and cell lines. Toxicology in Vitro. 1997;11(5):553–6. 10.1016/S0887-2333(97)00060-X [DOI] [PubMed] [Google Scholar]
- 18. Kangas L, Gronroos M, Nieminen AL. Bioluminescence of cellular ATP: a new method for evaluating cytotoxic agents in vitro. Med Biol. 1984;62(6):338–43. Epub 1984/01/01. . [PubMed] [Google Scholar]
- 19.Drug Discovery Through High-Throughput Screening Technique. General Chemicals; [cited 2015 March]. General Chemicals. Available: http://www.worldofchemicals.com/15/chemistry-articles/rapid-lead-compounds-discovery-through-high-throughput-screening.html.
- 20. Crowther GJ, Napuli AJ, Gilligan JH, Gagaring K, Borboa R, Francek C, et al. Identification of inhibitors for putative malaria drug targets among novel antimalarial compounds. Mol Biochem Parasitol. 2011;175(1):21–9. 10.1016/j.molbiopara.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flannery EL, Chatterjee AK, Winzeler EA. Antimalarial drug discovery—approaches and progress towards new medicines. Nat Rev Microbiol. 2013;11(12):849–62. 10.1038/nrmicro3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Avery VM, Bashyam S, Burrows JN, Duffy S, Papadatos G, Puthukkuti S, et al. Screening and hit evaluation of a chemical library against blood-stage Plasmodium falciparum . Malar J. 2014;13:190 10.1186/1475-2875-13-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaidya AB, Morrisey JM, Zhang Z, Das S, Daly TM, Otto TD, et al. Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum. Nature communications. 2014;5:5521 10.1038/ncomms6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jimenez-Diaz MB, Ebert D, Salinas Y, Pradhan A, Lehane AM, Myrand-Lapierre ME, et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(50):E5455–62. 10.1073/pnas.1414221111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prole DL, Marrion NV. Identification of putative potassium channel homologues in pathogenic protozoa. PLoS One. 2012;7(2):e32264 10.1371/journal.pone.0032264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fredriksson R, Schioth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol. 2005;67(5):1414–25. . [DOI] [PubMed] [Google Scholar]
- 27.Open access Malaria Box. Medicines for Malaria Venture; 2015 [cited 2015 June]. Available: http://www.mmv.org/malariabox.
- 28. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–5. . [DOI] [PubMed] [Google Scholar]
- 29. Van Huyssen W, Rieckmann KH. Disposable environmental chamber for assessing the drug susceptibility of malaria parasites. Trop Med Parasitol. 1993;44(4):329–30. . [PubMed] [Google Scholar]
- 30. Miret S, De Groene EM, Klaffke W. Comparison of in vitro assays of cellular toxicity in the human hepatic cell line HepG2. J Biomol Screen. 2006;11(2):184–93. 10.1177/1087057105283787 . [DOI] [PubMed] [Google Scholar]
- 31. Wetzel S, Klein K, Renner S, Rauh D, Oprea TI, Mutzel P, et al. Interactive exploration of chemical space with Scaffold Hunter. Nat Chem Biol. 2009;5(8):581–3. 10.1038/nchembio.187 . [DOI] [PubMed] [Google Scholar]
- 32.RDKit: Cheminformatics and Machine Learning Software. Sourceforge; 2014 [cited 2014 July]. Available: http://www.rdkit.org.
- 33. Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, et al. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother. 2013;57(7):3268–74. 10.1128/AAC.00325-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSM)
(XLSM)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.