Abstract
This paper reports an innovative technique for reagents storage in microfluidic devices by means of a one-step UV-photoprintable ionogel-based microarray on non-modified polymeric substrates. Although the ionogel and the ink-jet printing technology are well published, this is the first study where both are used for long-term reagent storage in lab-on-a-chip devices. This technology for reagent storage is perfectly compatible with mass production fabrication processes since pre-treatment of the device substrate is not necessary and inkjet printing allows for an efficient reagent deposition process. The functionality of this microarray is demonstrated by testing the release of biotin-647 after being stored for 1 month at room temperature. Analysis of the fluorescence of the ionogel-based microarray that contains biotin-647 demonstrated that 90% of the biotin-647 present was released from the ionogel-based microarray after pumping PBS 0.1% Tween at 37 °C. Moreover, the activity of biotin-647 after being released from the ionogel-based microarray was investigated trough the binding capability of this biotin to a microcontact printed chip surface with avidin. These findings pave the way for a novel, one-step, cheap and mass production on-chip reagents storage method applicable to other reagents such as antibodies and proteins and enzymes.
I. INTRODUCTION
In recent years, there has been a growing interest and activity in the area of reagents storage on chip due to its potential to simplify significantly the automation of lab-on-a-chip (LoC) testing. Cost-efficiency, reduction of end-users intervention (i.e., reducing cross-contamination) thus improving results, reproducibility, and portability are only some of the advantages compared to off-chip manual handling of reagents. Nowadays, the study of methods and formulations for preserving and integrating reagents into microfluidic chips, so that they are stable over time, continues to have a high technical-scientific significance since not a single approach seems to solve the manipulation of reagents in microfluidics.1 In fact, various methods and materials have been described in the literature for integrating, storing and releasing reagents on chip in liquid, solid, and gelified state. For example, ThinXXS Microtechnology AG has developed the so-called "blister-on-chip technology," which allows the storage of liquid reagents directly into a microfluidic device. More examples of on-chip storage of liquids are the use of glass capillaries or ampoules2,3 and thermally actuated paraffin pumps.4 In the case of solid state reagents, the use of paraffin-encapsulated dry reagents,5 evaporative drying,6–8 and lyophilised reagents2,9 have also been reported. And, for gelified reagents, both stabilising and gelifying agents were added directly to the reagents of interest10,11 or gelification took place on chip by photopolymerisation.12,13 Of all the methods described above, it is often preferred to store reagents in dry state due to the advantages they offer over liquid reagents: longer-term stability due to fewer unwanted side reactions, the ability to store at room temperature without the need for refrigeration, lower risk of bacteria, easier handling, and to occupy less volume. However, in all cases, fabrication processes that allow for high-throughput, highly parallel and mass production of microfluidic chips containing reagents is a key issue, which still remains unsolved.1
Another important factor to consider in microfluidics is the immobilisation of molecules of interest on commonly used substrates. Polymeric substrates used in microfluidics usually require either chemical or physical surface treatments to increase both their hydrophilicity and biocompatibility to successfully immobilise biomolecules.14,15 Current alternative approaches to traditional surface modification of substrates are the use of UV irradiation16 and hydrogels,17 either used as separate techniques or in combination. Despite the fact that hydrogels are widely used as substrates for the development of microarrays, they have several drawbacks such as poor mechanical strength, fast dehydration behavior, and restricted permeability to certain biomolecules. Rendl et al.16 reported on oligonucleotides and thyroid peroxidase immobilisation by printing hydrogels on non-modified polycarbonate (PC), cyclic olefin copolymer (COC), cyclic olefin polymer (COP), and polypropylene (PP). Authors did not report any problem associated with hydrogels since their drops were of relatively high volume for ink-jet printing (i.e., drop of 400 pl with 150 μm diameter), but in fact smaller drops would suffer from rapid desiccation.
An alternative to overcome these limitations is the incorporation of ionic liquids (ILs) into polymer gels to form the so-called “ionogels.”18 The use of ionogel materials, polymer gels generated in the presence of an IL, could avoid several of the mentioned problems in reagent storage since ionogels are considered as a new class of hybrid materials that combine the physical properties of both the polymer gel and the physically entrapped IL within.19 Therefore, physical properties as their negligible vapour pressure, high thermal stability, and mouldable ionic conductivity could improve reagent storage in a pseudo-dry stage for microfluidic applications.20 Taking into account their ionic conductivity property, ionogels were previously ink-jet printed to generate semi-conductive, flexible materials with tunable physical properties. Nevertheless, in this case, the author uses the ink-jet printing process to generate lines instead of arrays.21
In this paper, we describe an innovative way of reagent storage in microfluidic chips. The innovation is not in the use of ink-jet printing nor in the ionogels, but on the application of both for the reagent storage application. The use of ink-jet printing combined with a one-step UV-photoprintable ionogel microarray fabrication method on non-modified thermoplastic substrates are two of the main advantages of this approach. The proposed technique is useful for disposable and point of care biochemical analysis LOC devices. We demonstrate good adhesion of ionogel matrix from 40 nl to 1 μl on to COP, COC, and PP and also the incorporation of ionogels as a reagent storage matrix has been demonstrated to be successful when keeping viability of biotin-647 for 1 month at room temperature.
II. EXPERIMENT
A. Microfluidic device fabrication
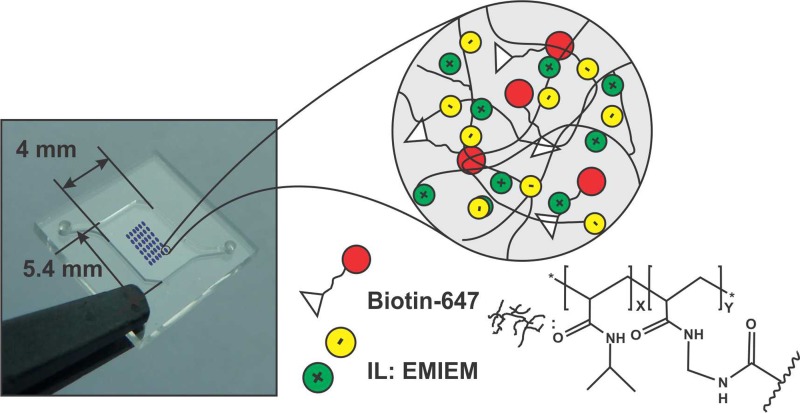
Injection moulding was performed with COP (Zeonoex/Zeonor, Germany), COC (Topas, Germany), and PP (ExxonMobil, Belgium) to obtain 10 × 10 × 1 mm3 microfluidic chips with an embedded microchamber of 10 μl volume. The main dimensions of the microchamber were: 5.4 mm width, 4 mm length, and 0.45 mm depth. The microchamber was sealed with a PCR compatible pressure sensitive adhesive film (PSA) (Adhesives Research, Ireland) at room temperature.
B. Ionogel microarray fabrication
The polymer composition used for the fabrication of the microarray was: N-isopropylacrylamide (NIPAM) as the thermo-sensitive monomer, 2,2-dimethoxy-2 phenylacetophenone as the photoinitiator, and N, N-methylenebisacrylamide as the crosslinker in a ratio of 88/6/6 (% w/w) (Sigma-Aldrich, Spain) in 1 ml of 1-ethyl-3-methylimidazolium ethyl sulfate ionic liquid (EMIEM) (Sigma-Aldrich, Spain). Avidin (Invitrogen, Spain) and biotin fluorescently conjugated to Atto-647N (Sigma-Aldrich, Spain) were used as the biomolecules models.
Biotin-647 was added to the polymer solution with a concentration of 0.22 μg ml−1. The mixture was dissolved in IPA in a 50%:50% ratio to achieve a suitable viscosity to be injected through the 21.5 × 21.5 μm2 ink-jet printer nozzles. An ink-jet printer Dimatix DMP-2800 (Fujifilm, United States) with 10 μl cartridges was employed to perform the printing of the biotin-647-ionogel mixture. A 6 × 6 array of 300 μm × 300 μm squares, with 500 μm pitch, was printed on COP, COC, and PP chambers (Figure 1). The total volume of each 300 μm × 300 μm printed spot is 4 nl. This is calculated by the programmed drop space in the ink-jet printer and the amount of drops that the machine jets to get a single spot. Considering these two parameters, an effective height of 57 μm can be estimated. Samples were exposed to UV light at 365 nm for 15 s rendering an array of ionogels containing biotin-647. It must be pointed out that due to the superficial tension of the ionogel, theoretically square fabricated spots turned into circular spots of 300 μm diameter (Figure 2).
FIG. 1.
Ink-jet printed ionogel microarray in the microfluidic device and representation of the composition of the ionogel spots.
FIG. 2.
Ink-jet printed microarray of ionogel on untreated COP. (a) After UV polymerisation (dry). (b) Just after being exposed to water solution but before the tested flow was applied. (c) While being washed at 400 μl min-1 with PBS 0.1% Tween (microchamber filled with PBS 0.1% Tween).
C. Microcontact printing of avidin
Direct immobilisation of avidin on COP over 5 μm square areas without any chemical modification was performed by microcontact printing (μCP). Briefly, avidin was adsorbed in PDMS stamps containing a pattern of 5 μm squares and transferred onto the polymer substrates. After stamping, the unexposed surface was blocked with 1% BSA (Sigma-Aldrich, Spain) to minimise unspecific binding and washed with deionised water. Initially, to examine homogenous transfer of avidin onto COP, avidin fluorescently labelled with Alexa Fluor 488 (avidin-488) (Invitrogen, Spain) was directly immobilised on COP, by μCP. Specific adsorption of avidin onto the patterned areas was examined by fluorescence microscopy (Aixo manager A1M, Zeiss, Germany).
III. RESULTS AND DISCUSSION
A. Characterisation of the ionogel microarray
Chemical characterisation and physical properties of the ionogel material were recently published by us.22 It is remarkable that the dehydrated ionogel and its homologous dehydrated hydrogel (same gel but without the IL) presented significantly different physical properties. For instance, the hydrogel is hard and brittle (often cracks when dry), while the ionogel remains soft with a homogeneous structure, since the EMIEM acts as a plasticiser.
Spots adhesion strength of the ionogel material to different substrates (COP, COC, and PP) was tested by flushing them with PBS 0.1% Tween (Sigma-Aldrich, Spain) at increasing flow rates from 10 up to 400 μl min−1. This characterisation of adhesion versus flow rate instead of shearing stress was considered as a parameter that the final user will be able to easily regulate during experiments, by just changing the pumping parameters. To do this characterisation based on flow rate, the array embedded in the microchamber was placed in a chip holder (microLIQUID, Spain), and connected to a syringe pump where different flow rates (10, 25, 50, 100, 200, 250, and 400 μl min−1) were tested. As can be seen in Figure 2, ionogel microarray spots neither change shape nor position after passing different flow rates of PBS 0.1%Tween through the chip from the inlet at the bottom left side of the chamber to the outlet at the top right.
Although the strong binding of the ionogel spots with the COP, COC, and PP surface is not fully understood yet, it is believed that this adhesion strength was due to the UV polymerisation of the ionogel directly to the, also in-situ UV activated, thermoplastic surface.23 When applying UV light to the array, that light activates the surface of the COP, COC, and PP, and generates radicals that can covalently react with the radicals formed in the polymer matrix of the ionogel by the 2,2-dimethoxy-2 phenylacetophenone photoinitiator. Moreover, it is proven that ionic liquids originate positive effects on catalysis processes by the formation of more reactive catalysts and stabilising reactive intermediates and transition states in the ionic liquids that could promote the covalent bond of the polymeric matrix of the ionogel to the activated thermoplastic surface.24
Taking into account the results obtained with this 6 × 6 arrays, we can assure that the spots withstand a minimum shear stress of 0.299 dyn/cm2 and a maximum of 0.598 dyn/cm2. These values have been calculated considering the largest and narrowest sections of the microchamber perpendicular to the flow direction. Then, an approximation to a rectangular chamber with these largest and narrowest sections has been done. Assumption of parallel plate flow has been possible because of the difference between width (3 and 6 mm) and height (450 μm) of these assumed largest and narrowest sections. Also, a velocity profile fully developed can be assured before the liquid reaches the first spot, because a distance shorter that 0.1 mm was calculated as necessary for the velocity profile to be fully developed. This means that spots with 300 μm × 300 μm area and 57 μm height can withstand at least a minimum shear stress of 0.299 dyn/cm2 and a maximum of 0.598 dyn/cm2.
As we have previously said, the binding of the ionogel spots to the COP, COC, and PP surface is related to the bonding forces that happen at the contact area between them. The intensity of these forces depends on the amount of monomers available during the photo-polymerization process and on the area where they appear. To check the effect of the quantity of reactives available during polimerisation, the same type of arrays but with 10-fold volume spots (40 nl) were printed (printing 10 layers one after each other) for different chips. Printing more layers leads to spreading of the spots and coalescence of them. Therefore, in order to test higher volume spots, up to 1 μl, single drops were pippetted directly with a standard micropipette onto other COP, COC, and PP chips. Then, the spots were UV-photopolymerised and exposed to a flow rate of 400 μl min-1 of PBS 0.1% Tween. These spots remained well adhered to COP, COC, and PP for the volumes investigated (40 nl and 1 μl). Ionogel arrays were let to soak in PBS 0.1%Tween up to four days, and, when checked at the same flow rate, adhesion strength remained intact.
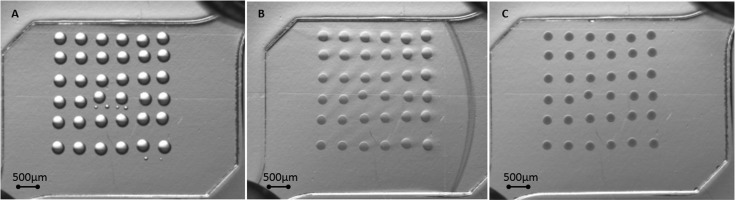
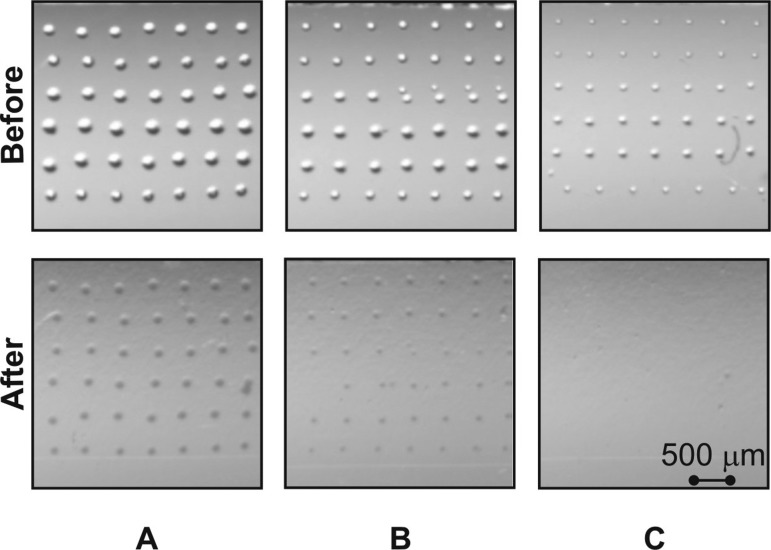
On the other hand, the minimum area necessary to obtain a successful ionogel microarray with proper adhesion to a COP, COC, and PP surface without surface functionalisation was investigated. Figure 3 shows different spots size (100, 150, and 200 μm diameter) before and after applying 25 μl min−1 flow of PBS 0.1% Tween. These spots had the same height, as only one layer was ink-jet printed, but the difference was only the contact area between the spot and the substrate. Reducing two times the contact area (for example, from 300 μm × 300 μm to 150 μm ×150 μm) keeping the same height of the spots means to reduce 4 times the bonding force, while the force of the liquid against the spot is reduced only two times. Results indicated that spots below 150 μm did not get enough surface contact area (ionogel-surface) to adhere properly to thermoplastic substrates and resist a flow rate of 25 μl min−1, which might be useful in microfluidics applications.
FIG. 3.
Photopolymerised ionogel microarrays with different spot sizes before and after being washed with PBS 0.1% Tween. (a) Spots of 200 μm, (b) spots of 150 μm, (c) spots of 100 μm diameter in the chamber of the microfluidic device.
It is interesting to mention that arrays with dimensions of spot sizes and volumes presented in this study were not achievable with the homologous hydrogel material (no IL). The hydrogel dried too fast to reach to the photopolymerisation step. When spots volume was big enough to allow the formation of the array and subsequent photopolymerisation, the generated array was not stable in the microfluidic device surface and all hydrogel spots were easily removed after the first washing step.
B. Viability of the stored biomolecules
As a proof of concept, the ability of the generated ionogel-based microarray as a scaffold for the storage of biomolecules at room temperature in a COP microfluidic device was investigated. Biotin-647, which is a small and relatively stable molecule, was embedded in the ionogel matrix, as previously described in Section II, and its affinity for avidin was checked using fluorescence microscopy (Leica DMLM (Germany), Hamamatsu C9100 (Japan), and Leica Y5 (Germany). Streptavidin or avidin-biotin interaction constitutes a good platform for engineering studies. A large number of studies reported in the literature demonstrate the continued interest in this model in biological and chemical systems.25
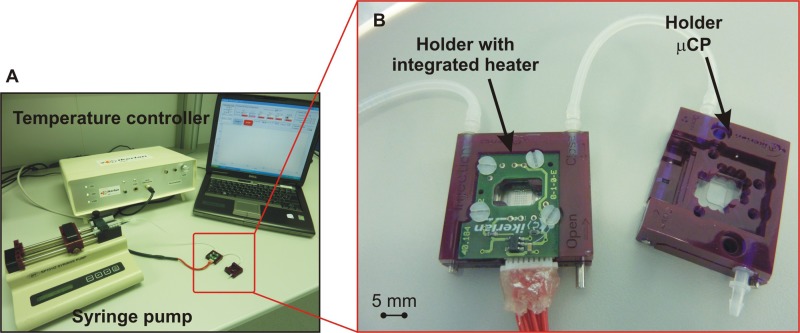
Two COP chips were placed in identical holders and connected in series as shown in Figure 4. A COP chip was printed following the layout described in Section II with a final concentration of 0.11 μg ml−1 biotin-647 embedded in the ionogel matrix. After UV polymerisation, the chamber containing the array was sealed with PSA and stored at room temperature for a month before being tested.
FIG. 4.
(a) Set-up used during the viability study. Two holders connected in series by commercially available silicone tubes. (b) Left: Ink-jet printed ionogel microarray containing biotin-647 on a COP chip and a holder with an integrated heater; right: a COP chip with a mCPed microarray of avidin.
The ionogel microarray was synthesised using N-isopropylacrylamide, a thermosensitive material that shrinks above its lower critical solution temperature (ca. 32 °C).20 10 μl of PBS were injected in the COP chamber and heated at 37 °C for 3 min in order to promote shrinkage of the ionogel microarray and so improve the releasing of the biotin-647 stored in the array. The actuation of the ionogels is possible—thanks to the specific physicochemical characteristics of the poly(N-isopropylacrylamide) (NIPAAm) present in the ionogel. This polymer shrinks and expands due to dramatic structural changes that occur at the so-called lower critical solution temperature (LCST), which is around 32 °C in water. In an aqueous environment, individual NIPAAm chains show a hydrophilic behavior with an expanded coil structure below the LCST, but as the temperature increases above 32 °C, hydrophobic isopropyl groups are exposed, undergoing a reversible conformational change to form compact globule shapes, reducing its volume and expelling water out of its structure. Therefore, small molecules that are not covalently attached to the polymer matrix and that are soluble in water are also driven out of the ionogel.22 It can be concluded that other type of molecules that do not physically or chemically interact with the ionogel matrix should undergo the same kind of behavior.
Subsequently, the solution was transferred to the second COP chamber by means of a syringe pump at a flow rate of 25 μl min−1, where a pattern of 5 × 5 μm2 squares of avidin were previously immobilised by microcontact printing technique.
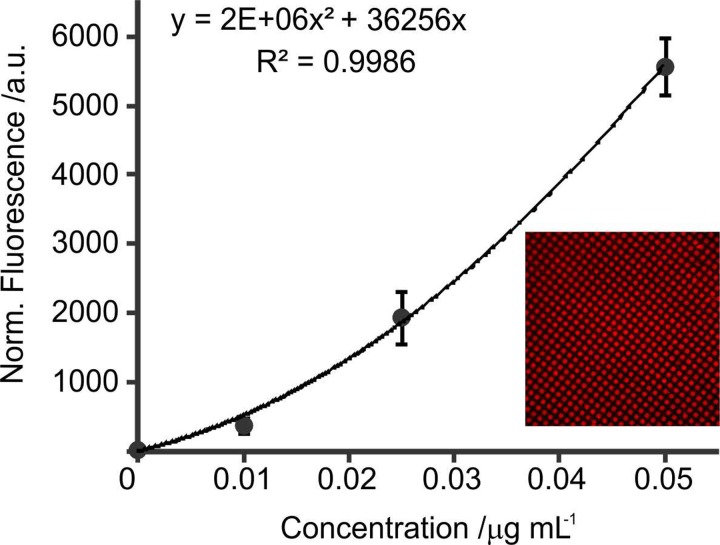
PBS 0.1% Tween at a flow rate of 25 μl min−1 allowed enough residence time for releasing biotin-647 biomolecules from ionogels spots and for binding to immobilised avidin present in the second chip. The concentration of biotin-647 released from the ionogel microarray, capable of binding to the μCP-ed patterned surface, was calculated using the calibration curve of Figure 5. Considering that fluorescence intensity is function of biotin-647 concentration bound to the avidin, the intensities at different concentrations of biotin-647 over different patterned areas of avidin were measured.
FIG. 5.
Calibration curve of biotin-647 attached to microcontact printed avidin. Fluorescence intensity versus concentration of biotin-647. The inset shows a detail of 5 × 5 μm2 square pattern of avidin with biotin-647 selectively bound.
From the calibration curve, it was determined that the concentration of biotin-647 bound to avidin was 0.02 μg ml−1. The total amount of biotin available in the array was calculated to be 15.84 × 10−5 μg (36 spots multiply by 40 nl, volume of a single spot, and by 0.11 μg ml−1, concentration of Biotin in the spot). Dividing this amount of biotin in 10 μl of solution that reaches the μ-CPed patterned surface ensures a final concentration of 0.016 μg ml−1. Our results show a small deviation with the calculated one for the calibration curve. The deviation could be explained by experimental errors related to the preparation of biotin solutions on different days by different operators, that not all the biotin is able to bind to the avidin, and to the accuracy of the volume of the drops from the inkjet printer. This experiment proves that biotin-647 is still functional and capable of binding to avidin even after being stored in the ionogel array for more than one month at room temperature.
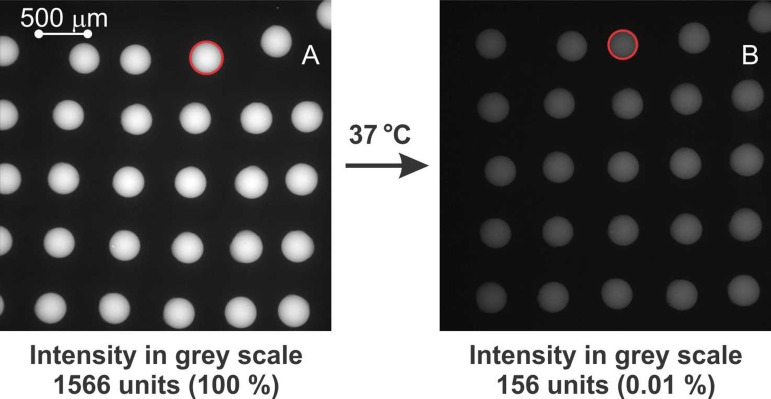
The analysis of the fluorescence intensity of the biotin-647 stored in the ionogel spots before and after pumping PBS 0.1% at 37 °C shows a decrease of 90% on fluorescence intensity when the biotin-647 was released from the ionogel-based microarray sports due to elution in the PBS solution and supported by the thermoactuation of the ionogel (see Figure 6). There is a linear relation of the fluorescence intensity of the ionogels with the concentration of Biotin-947 up to 80 μg ml−1, and there is not appreciable decrease on fluorescence intensity when placing the ionogel arrays in PBS 0.1% solution at r.t and stop flow. Therefore, the 90% decrease of fluorescence intensity is believed to be due to the release of the biotin-647 from the ionogel when flowing the PBS 0.1% at 37 °C. Other possible mechanisms that could influence fluorescence intensity are: (1) the solvent interchange within the ionogel, which in this case did not cause any perturbation, and (2) the influence of the temperature. It is well known, the good thermal and chemical stability of biotin-647 as described by the provider; therefore, we can dismiss temperature effects during our mild experimental conditions.
FIG. 6.
Fluorescence images of biotin-647 inside an ionogel-based microarray. (a) Before pumping 200 μl PBS 0.1% Tween. (b) After pumping 200 μl PBS 0.1% Tween at 37 °C. The red circles represent the dimensions of a spot before and after thermal actuation (i.e., 7% difference in diameter).
Red circles in Figure 6 show the thermoactuation of the ionogel microarray (not considering the z-axis possible actuation of the ionogel due to their 3D structure). Spots diameter before washing and thermal actuation was 305,7 ± 7 μm (n = 5) (A) and after washing with PBS 0.1% at 37 °C spots size was reduced to 285,9 ± 5 μm (n = 5) (B), which represents a reduction of 7% in diameter (measured at the x-y-axis). As it was previously reported by us,21 ionogel macrostructures were found to shrink 31% when not attached to any surface. In this study, the shrinking effect was less pronounced probably due to the constriction generated in the ionogel spots when attached to the microfluidic surface. As explained above, the mechanism of shrinking of the ionogel promotes the release of water from the ionogel matrix. Therefore, since the biotin-647 is water-soluble (at our experimental concentrations), it will leave the ionogel matrix as well. Moreover, it is believed that the new, more hydrophobic, environment generated within the ionogel matrix at 37 °C also promotes the release of the biotin-647.
IV. CONCLUSIONS
An innovative way of reagent storage and on demand release for microfluidic devices has been demonstrated. The use of ink-jet printing to generate an array of thermoactuable ionogel materials containing the biomolecules of interest, combined with the low cost of non-modified thermoplastic substrates, is one of the main advantages of this novel method. Additionally, this approach introduces a simple and effective technique for high-throughput reagent storage reservoirs production.
Washing and thermoactuation of the ionogel-based microarrays ensured the release of the stored biotin-647. The viability of using ionogel materials as reagent storage matrices has been demonstrated to be successful when keeping the activity of biotin-647 at room temperature for one month. Future work will demonstrate the viability of other biomolecules such as antibodies, enzymes, and proteins that are more useful in diagnostic/analytical application.
ACKNOWLEDGMENTS
This work was supported by Gobierno Vasco, Dpto. Industria, Innovación, Comercio y Turismo under ETORTEK 2013 with Grant No. IE13-360. F.B.L. acknowledges the Ramón y Cajal Programme (Ministerio de Economía y Competitividad) and the European Union's Seventh Framework Programme for research, technological development, and demonstration under Grant Agreement No. 604241.
References
- 1. Hitzbleck M. and Delamarche E., Chem. Soc. Rev. 42, 8494–8516 (2013). 10.1039/c3cs60118h [DOI] [PubMed] [Google Scholar]
- 2. Lutz S., Weber P., Focke M., Faltin B., Hoffmann J., Muller C., Mark D., Roth G., Munday P., Armes N., Piepenburg O., Zengerle R., and von Stetten F., Lab Chip 10, 887–893 (2010). 10.1039/b921140c [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann J., Mark D., Lutz S., Zengerle R., and von Stetten F., Lab Chip 10, 1480–1484 (2010). 10.1039/b926139g [DOI] [PubMed] [Google Scholar]
- 4. Bodén R., Lehto M., Margell J., Hjort K., and Schweitz J. A., J. Micromech. Microeng. 18, 075036 (2008). 10.1088/0960-1317/18/7/075036 [DOI] [Google Scholar]
- 5. Kim J., Byun D., Mauk M. G., and Bau H. H., Lab Chip 9, 606–612 (2009). 10.1039/B807915C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris L. F., Rainey P., Castro-López V., O'Donnell J. S., and Killard A. J., Analyst 138, 4769–4776 (2013). 10.1039/c3an00401e [DOI] [PubMed] [Google Scholar]
- 7. Garcia E., Kirkham J. R., Hatch A. V., Hawkins K. R., and Yager P., Lab Chip 4, 78–82 (2004). 10.1039/b308914b [DOI] [PubMed] [Google Scholar]
- 8. Stevens D. Y., Petri C. R., Osborn J. L., Spicar-Mihalic P., McKenziea K. G., and Yager R., Lab Chip 8, 2038–2045 (2008). 10.1039/b811158h [DOI] [PubMed] [Google Scholar]
- 9. Rohrman B. A. and Richards-Kortum R. R., Lab Chip 12, 3082–3088 (2012). 10.1039/c2lc40423k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laouenan F., Monsalve L. G., Goiriena A., Agirregabiria M., and Ruano-Lopez J. M., Procedia Eng. 47, 1484 (2012). 10.1016/j.proeng.2012.09.434 [DOI] [Google Scholar]
- 11. Sun Y., Høgberg J., Christine T., Florian L., Monsalve L. G., Rodriguez S., Cao C., Wolff A., Ruano-Lopez J. M., and Bang D. D., Lab Chip 13, 1509–1514 (2013). 10.1039/c2lc41386h [DOI] [PubMed] [Google Scholar]
- 12. Manage D. P., Lauzon J., Atrazev A., Chavali R., Samuel R. A., Chan B., Morrissey Y. C., Gordy W., Edwards A. L., Larison K., Yanow S. K., Acker J. P., Zahariadis G., and Pilarski L. M., Lab Chip 13, 2576–2584 (2013). 10.1039/c3lc41419a [DOI] [PubMed] [Google Scholar]
- 13. Ikami M., Kawakami A., Kakuta M., Okamoto Y., Kaji N., Tokeshi M., and Baba Y., Lab Chip 10, 3335–3340 (2010). 10.1039/c0lc00241k [DOI] [PubMed] [Google Scholar]
- 14. Zhao Z., Peytavi R., Diaz-Quijada G. A., Picard F. J., Huletsky A., Leblanc E., Frenette J., Boivin G., Veres T., Dumoulin M. M., and Bergeron M. G., J. Clin. Microbiol. 46, 3752–3758 (2008). 10.1128/JCM.00377-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Mahony C. C., Gubala V., Gandhiraman R. P., Daniels S., Yuk J. S., MacCraith B. D., and Williams D. E., J. Biomed. Mater. Res. A 100A(1), 230–235 (2012). 10.1002/jbm.a.33268 [DOI] [PubMed] [Google Scholar]
- 16. Sun Y., Perch-Nielsen I., Dufva M., Savourin D., Bang D. D., Hogberg J., and Wolff A., Anal. Bioanal. Chem. 402, 741–748 (2012). 10.1007/s00216-011-5459-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rendl M., Bönisch A., Mader A., Schuh K., Prucker O., Brandstetter T., and Rühe J., Langmuir 27, 6116–6123 (2011). 10.1021/la1050833 [DOI] [PubMed] [Google Scholar]
- 18. Curto V. F., Fay C., Coyle S., Byrne R., O'Toole C., Barry C., Hughes S., Moyna N., Diamond D., and Benito-López F., Sens. Actuators, B 171–172, 1327–1334 (2012). 10.1016/j.snb.2012.06.048 [DOI] [Google Scholar]
- 19. Susan M. A., Kaneko T., Noda A., and Watanabe M., J. Am. Chem. Soc. 127, 4976–4983 (2005). 10.1021/ja045155b [DOI] [PubMed] [Google Scholar]
- 20. Curto V. F., Scheuermann S., Owens R., Ranganathan V., MacFarlane D. R., Benito-Lopez F., and Diamond D., Phys. Chem. Chem. Phys. 16(5), 1841–1849 (2014). 10.1039/C3CP52845F [DOI] [PubMed] [Google Scholar]
- 21. Delaney J. T., Liberski A. R., Perelaer J., and Schubert U. S., Macromol. Rapid Commun. 31(22), 1970–1976 (2010). 10.1002/marc.201000336 [DOI] [PubMed] [Google Scholar]
- 22. Benito-Lopez F., Antoñana-Díez M., Fabio Curto V., Diamond D., and Castro-López V., Lab Chip 14(18), 3530–3538 (2014). 10.1039/C4LC00568F [DOI] [PubMed] [Google Scholar]
- 23. Gandhiraman R. P., Volcke C., Gubala V., Boyle C., Basabe-Desmonts L., Dotzler C., Toney M. F., Iacono M., Nooney R. I., Daniels S., James B., and Williams D. E., J. Mater. Chem. 20(20), 4116–4127 (2010). 10.1039/b925737c [DOI] [Google Scholar]
- 24. Lee J. W., Shin J. Y., Chun Y. S., Jang H. B., Song C. E., and Lee S. G., Acc. Chem. Res. 43(7), 985–994 (2010). 10.1021/ar9002202 [DOI] [PubMed] [Google Scholar]
- 25. Dundas C. M., Demonte D., and Park S., Appl. Microbiol. Biotechnol. 97, 9343–9353 (2013). 10.1007/s00253-013-5232-z [DOI] [PubMed] [Google Scholar]