Abstract
Ecological carryover effects occur when an individual’s previous history and experiences explain their current performance. It is becoming clear that ecological carryover effects are a common phenomenon across taxa, and have the potential to play an important role in governing individual fitness and population dynamics. Carryover effects may reduce the success of conservation efforts aimed at slowing or reversing biodiversity loss. Failure to consider carryover effects might lead to erroneous conclusions about the effectiveness of conservation measures. We suggest that carryover effects are considered explicitly in threat assessment and conservation planning, in order to understand the long-term consequences of stressors, target efforts more effectively, and ensure that the success or failure of conservation efforts is tracked more accurately. We encourage proactive research focused on the proximate mechanisms underlying carryover effects, so that predictive measures of carryover effects in wild populations can be developed and refined. Finally, we suggest that in some cases, positive carryover effects could be exploited for conservation benefit. We conclude that the failure to consider carryover effects in conservation science and practice may put imperiled populations at further risk.
Keywords: Stress, Fisheries, Wildlife, Management, Latent effect, Delayed effect
Introduction
Consider a scenario where a regulatory body requires that a threat assessment be conducted to determine if a given selective fishing strategy is risk averse. Researchers evaluate the short-term survival of endangered anadromous fish that are captured and discarded in the marine realm, and determine that survival rates are 99 % after 48 h. Therefore, the selective fishing strategy is considered appropriate. However, when the fish are released in the ocean to continue their migration, the fish that were exposed to the selective fishery and released suffer high mortality rates during the transition from saltwater to freshwater. In this case, the effects of the interaction with the selective fishery were not apparent immediately, but generated a carryover effect that was manifested at a later time, in a different location, when the fish were exposed to an additional, natural stressor. The concept of carryover effects requires that those examining the consequences of various human activities and human-induced environmental changes ought to do so with a long-term view.
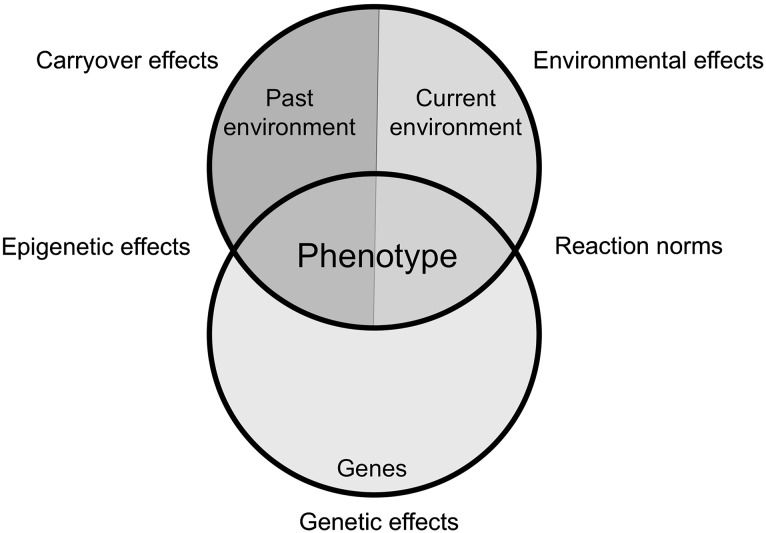
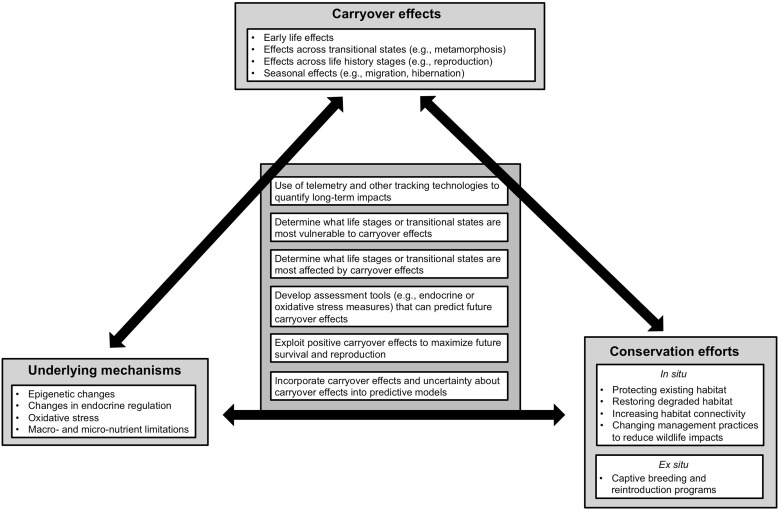
The use of the term ‘carryover effect’ within an ecological context first became widely applied from the perspective of migratory animals, when it became clear that the reproductive success of many migratory birds is influenced not just by the conditions on the breeding grounds, but by conditions during the overwintering period (Webster et al. 2002; Norris 2005; Norris and Marra 2007; Harrison et al. 2011). Based on these studies in migratory animals, ecological carryover effects were initially defined as events or processes occurring in one season, which consequently affect individual performance in a subsequent season (Norris 2005; Norris and Marra 2007; Harrison et al. 2011). However, the concept that environmental conditions at one life stage can have lasting impacts on phenotype during subsequent life stages has been studied within an ecological context since the late 1950s (e.g., Leslie 1959; Prout and McChesney 1985; Scott 1994). Delayed effects of environment on phenotype can occur not just across seasonal transitions (e.g., migration, hibernation, aestivation), but also across a range of biological transitions, such as those between life-history stages (e.g., maturation, reproduction) or distinct life-history states (e.g., metamorphosis, moltification) (see reviews by Beckerman et al. 2002; Benard and McCauley 2008). Therefore, we use an inclusive definition of carryover effects, as the delayed effects of the environment on phenotype (Box 1). Under this inclusive definition, the first hallmark of an ecological carryover effect is that previous experience predicts aspects of individual performance, which are relevant to fitness, such as somatic growth rate or reproductive output. The second hallmark of an ecological carryover effect is that the time points, between the experience that generates the carryover effect and the performance metric that is affected by the carryover effect, are separated by a biologically relevant transition within the lifetime of the individual (see review by O’Connor et al. 2014).
Box 1.
Individual phenotype is influenced by both genetic and environmental factors. Conservation science aims to maximize the survival and reproductive success of individuals and the persistence of threatened populations by addressing both genetic and environmental factors, and their interactions. For example, a conservation genetics approach might involve quantifying and conserving the range of genetic diversity within a population, since a more diverse genetic pool increases overall population fitness (Reed and Frankham 2003) and has a greater potential for adaptation in the face of environmental change (Lande and Shannon 1996). Understanding reaction norms, or the variation in phenotypic expression of a single genotype across a range of environmental conditions, might be useful for a conservation behaviorist interested in creating a habitat that maximizes the reproductive success of an individual in a captive breeding program (Buchholz 2007). However, in many cases, the survival and reproductive success of individuals is strongly influenced by previous environmental conditions. Environmental experiences can cause epigenetic effects, or changes to gene expression and regulation, which will permanently change individual phenotype (Feil and Fraga 2012). The past environment can also exert its influence through nongenetic mechanisms, such as micro and macronutrient limitations or oxidative stress, and these can be reversible or partially reversible effects, but equally important in dictating individual phenotype and fitness (Harrison et al. 2011; O’Connor et al. 2014). In the current manuscript, we discuss how considering carryover effects, or the delayed effects of the environment on phenotype, can be important for conservation. For example, the reproductive success of an individual may be dictated not by the environmental conditions during reproduction, but by previous conditions experienced during the nonreproductive season, which caused a micronutrient limitation to reproduction (Norris et al. 2004; Saino et al. 2004). In this example, a conservation physiology approach can provide physiological measures that reveal whether previous carryover effects occurred, and can be use to predict reproductive success
The study of ecological carryover effects has gained momentum in recent years, and an increasing number of studies demonstrate that individual fitness and population dynamics are strongly influenced by carryover effects. For example, many studies in migratory birds show that reproductive success on the breeding grounds is predicted by overwintering habitat (e.g., Norris et al. 2004; Saino et al. 2004). Effects on reproduction are not limited to seasonal effects; for example, in long-tailed manakins (Chiroxiphia linearis), male reproductive success is not determined by a male’s current social status, but instead, is higher in males with strong social ties in early life (McDonald 2007). Effects are also not limited to reproductive effects; for example, in Atlantic salmon (Salmo salar; Morgan and Metcalfe 2001) and brown trout (Salmo trutta; Johnsson and Bohlin 2006), starved fish reduce their growth rates, and when more food becomes available, the previously starved fish are able to compensate and ‘catch up’. However, this compensatory growth has a long-term cost, and fish that undergo food deprivation and subsequent compensatory growth ultimately show increased mortality over long timescales (Morgan and Metcalfe 2001; Johnsson and Bohlin 2006; Lee et al. 2013). The common theme in all of these examples is that environmental effects are often manifested at much longer timescales than previously suspected, and often after periods of apparent ‘recovery’ from the initial stressor.
Carryover effects represent a challenge to conservation outcomes precisely because they are manifested at long timescales, and after periods of apparent recovery. Conservation science is an interdisciplinary effort aimed at slowing or reversing biodiversity loss (Soulé 1985). Common conservation actions include both in situ efforts such as protecting existing habitat, restoring degraded habitat, increasing habitat connectivity (e.g., wildlife corridors), or changing management practices to reduce impact on wildlife (e.g., changing fishing gear to reduce bycatch, or installing bird-friendly glass in city skyscrapers to reduce migratory bird kills), and ex situ efforts such as captive breeding and reintroduction programs (Primack 2010). The success of conservation effort varies widely depending on the characteristics of the habitat and the species, population or ecosystem intended for protection, as well as socioeconomic factors (Brooks et al. 2006). Evidence-based evaluations are necessary to assess whether or not conservation efforts are successful (Kleiman et al. 2000; Sutherland et al. 2004; Ferraro and Pattanayak 2006), and the increasing evidence that carryover effects are important drivers of individual fitness (Harrison et al. 2011) and population-level processes (Frederiksen et al. 2008) suggests that conservation practitioners need to consider carryover effects in evaluations. However, limited finances and the necessity to prioritize efforts means that long-term evaluations are rare (James et al. 1999; Ferraro and Pattanayak 2006). In this article, we present the case that carryover effects represent a concern for conservation practitioners, and suggest that conservation actions will be improved by considering these effects. We then suggest approaches from conservation physiology (see Cooke et al. 2013) that can identify when carryover effects have occurred. Finally, we suggest that incorporating carryover effects into predictive models might provide a solution for including carryover effects in conservation planning even when long-term evaluations prove to be the cost effective or practical.
Ecological carryover effects complicate conservation
Ecological carryover effects represent an increasing challenge
As global environmental change increases and human populations expand, carryover effects are likely to increase. For example, climate change has been established as one of the great threats to biodiversity, with predicted changes exceeding the ability of many species to adapt or disperse (Thomas et al. 2004). However, rather than simply increasing mean temperature in a given region, climate change is likely to increase climate variability, and increase the frequency of extreme environmental events (Solomon et al. 2007). Research has identified that stochastic weather events can generate carryover effects that affect reproductive behavior and reproductive success (e.g., Dickey et al. 2008; Møller 2011), as well as drive population dynamics (e.g., Frederiksen et al. 2008) and life histories (e.g., Moreno and Møller 2011) of birds. While carryover effects of extreme weather events have been much less studied in other taxa, increasing stochastic weather events as a result of climate change are likely to play a critical role, generating carryover effects on reproduction and survival across all taxa. For example, amphibians are extremely sensitive to stochastic weather events, and increasing variability of weather patterns as a result of climate change is also expected to lead to declines in amphibian populations (Blaustein et al. 2010).
Beyond climate change, more localized anthropogenic changes are also increasing, and are likely to generate carryover effects. Stressors in combination or succession can have different effects than any single stressor in isolation (e.g., Crain et al. 2008), and indeed, in many cases, carryover effects are only manifested when individuals face a subsequent challenge (e.g., O’Connor et al. 2010). Multiple environmental changes therefore increase the stressors that generate carryover effects, and the subsequent challenges that reveal them. Amphibians are perhaps the best-studied system for understanding the impacts of interactive stressors, and global amphibian declines have been linked to the interactions among climate change, disease outbreaks, increasing ultraviolet radiation, pesticide use, and habitat destruction, rather than to any single stressor or the simple sum of the parts (Blaustein and Kiesecker 2002). Multiple interacting stressors are also threatening migratory Pacific salmonids (Cooke et al. 2012; Miller et al. 2014). Both examples demonstrate the importance of considering carryover effects across the lifetime of wild individuals, particularly since individuals are increasingly likely to face subsequent stressors.
Some species may be more vulnerable to carryover effects
Conservation of migratory animals has historically been a particular concern for conservation scientists (e.g., Abell 2002; Martin et al. 2007; Bolger et al. 2008). Conservation efforts are complicated in species that cover vast distances, and are particularly difficult for species, such as highly migratory marine fishes (Miller 2007) and large ungulates (Bolger et al. 2008; Sawyer et al. 2009), which are exploited as they cross political as well as geographic boundaries. Conservation efforts typically focus on protecting critical habitats, such as breeding grounds, and there is always a concern that animals will migrate out of protected areas, and into areas where they might experience harvest, pollution, habitat alteration, or other adverse conditions (Abell 2002; Martin et al. 2007; Bolger et al. 2008). However, the prevalence of carryover effects in migratory species means that animals will carry stressors with them, even if protected areas or favorable habitat exist for some stages of the migration. There is clear evidence that stressors experienced during the winter and during migration influence reproductive success in songbirds and seabirds (e.g., Norris and Taylor 2006; Sørensen et al. 2009). Further, Calvert et al. (2009) reviewed a growing body of evidence that suggests that events occurring outside of the reproductive season are a critical driver of population dynamics for seasonal migrants across taxa, including birds, fish, and mammals. Thus, although birds have by far received the most research attention to date, carryover effects are likely an important consideration for all migratory species, and have the potential to interfere with or mask conservation efforts that are focused on a single region/jurisdiction or life-history stage. Similarly, long-lived animals experience a wide range of temporal conditions comparable to the wide range of geographic conditions experienced by highly migratory animals, and may also be vulnerable to carryover effects.
As an additional challenge, it has historically been logistically difficult to follow animals across broad spatial and temporal scales (Bowlin et al. 2010), and therefore it is difficult to accurately record the stressors experienced by these animals or understand the long-term consequences. Recent advances in telemetry provide tools for tracking animals across broad spatial and temporal realms (Webster et al. 2002; Rubenstein and Hobson 2004; Block 2005), but remain relatively expensive and impractical for broad-scale studies. The logistic challenges associated with tracking animals add to the concerns about carryover effects in conservation efforts for long-lived and highly migratory species.
Some conservation practices may generate carryover effects
Captive breeding programs are popular for species that face specific habitat threats, and for species that are commercially harvested, where captive-reared individuals supplement wild populations for harvesting. However, the captive environment or relocation stresses can generate carryover effects. In a dramatic example, the wild-born offspring of two hatchery fish had a relative reproductive success that was only 37 % of the reproductive success of wild steelhead trout (Araki et al. 2009). Although in this extreme example there are likely genetic differences between the hatchery and wild stocks that are contributing to these effects, there are also less dramatic examples where the experience of being raised in a captive environment changes the behavior and the physiology of animals, and makes them less fit for the wild environment in the long term. For example, captive rearing is known to result in reduced antipredator responses (e.g., McPhee 2004), as well as changes to other critical aspects of behavior such as social and mating behavior (Buchholz 2007). Captive rearing also affects the physiology of animals, and these effects may have long-term consequences. For example, captive-reared feather-tailed gliders (Acrobates pygmeus; small marsupials) have altered torpor relative to wild individuals, which makes them more susceptible to hypothermia (Geiser and Ferguson 2001).
Similarly, translocations are a common conservation tool (Fischer and Lindenmayer 2000), but translocations are also known to be very stressful for the translocated individuals (Dickens et al. 2010; Tarszisz et al. 2014), and have the potential to generate carryover effects that alter the long-term stress physiology of translocated individuals (Dickens et al. 2010), and therefore influence their long-term reproductive success and survival (Teixeira et al. 2007). Ultimately, such carryover effects of stress can reduce the success of translocation efforts (Armstrong and Seddon 2008). As a final example, ecotourism can raise awareness of conservation issues, and generate revenue and public/political will to support conservation efforts (Page et al. 2001). However, wildlife viewing can cause stress to populations that interact frequently with tourists (see review by Green and Higginbottom 2000), which may similarly change the stress physiology and generate carryover effects on the survival and reproductive output of viewed populations. Collectively, these examples demonstrate that some conservation practices can actively generate carryover effects, and without taking these carryover effects into consideration, the effectiveness of these conservation efforts will be compromised.
Incorporating ecological carryover effects into conservation action
Threat assessments need to consider carryover effects
Incorporating carryover effects into conservation requires that the various state changes that will be experienced by an organism are explicitly considered, as well as considering how other pervasive environmental challenges may lead to a carryover effect being manifested at a later time. This will require more comprehensive and longer-term evaluation of the effects of different stressors on organisms (i.e., conservation physiology; Cooke et al. 2013) on the part of conservation practitioners. With often-limited government resources, the burden of these increasingly comprehensive and longer-term evaluations will likely be placed on the proponent (e.g., the mining company, hydropower utility, fishing industry, etc.). However, this begs the question as to what can reasonably be expected of a proponent when evaluating their potential impact on wild organisms, and what happens in cases where retroactive action is necessary. More high-level threat assessments (e.g., IUCN Red List; Rodrigues et al. 2006) inherently consider a wide range of threats and stressors, but even here, there will be a few cases where there are appropriate data available to evaluate the extent to which carryover effects may be operating. In this case, it is quite apparent how carryover effects can complicate conservation. Incorporating carryover effects into conservation effectively requires both a framework for considering carryover effects in conservation efforts and also guidelines for defining reasonable limits to evaluations.
Research is necessary to understand mechanisms
While long-term evaluations may be the best way to quantify carryover effects, these evaluations may not always be cost effective or practical. In those cases, we offer some potential solutions from proactive conservation physiology and conservation behavior research, recognizing that behavior and physiology are inherently connected whenever animals are studied in the wild (Cooke et al. 2014). One of the research priorities that will benefit conservation outcomes is to determine the mechanisms underlying carryover effects at the individual level. Macro and micronutrient limitations have been proposed as physiological mechanisms driving seasonal carryover effects by Harrison et al. (2011). O’Connor et al. (2014) also suggest that many of the mechanisms underlying life-history trade-offs are also likely driving carryover effects. In particular, both the endocrine systems (e.g., Ricklefs and Wikelski 2002) and oxidative stress (Monaghan et al. 2009; Beaulieu et al. 2013) have received a great deal of attention as potential mediators of life-history trade-offs, and are excellent candidates as mechanisms underlying carryover effects. With the predicted increase of extreme weather events, there is great opportunity to connect carryover effects to potential mechanisms using natural experiments. For example, Wingfield et al. (2011) reviewed the physiological mechanisms used by wild animals to cope with extreme environmental events. In particular, the authors identified attenuation of testosterone and other reproductive hormones, as well as insensitively to the glucocorticoid stress hormones are important adaptations that wild animals use to cope with unpredictable weather events. The regulation of these hormones therefore provides an excellent candidate mechanism to understand the carryover responses to stochastic environmental events. In many cases where carryover effects have been documented, the underlying mechanisms have not been investigated, and it is possible that many of the important mechanisms driving carryover effects remain undocumented. For example, epigenetic changes (i.e., functionally relevant changes to gene regulation as a result of the environment) have received a great deal of recent research attention in the medical sphere, and it is clear that gene regulation is sensitive to environmental changes, and changes in gene regulation have profound, long-term impacts on phenotype (Feil and Fraga 2012). Indeed, there has been recent interest in using epigenetic changes as biomarkers for contaminant exposure in ecotoxicology (Head et al. 2012), and these mechanisms may prove similarly useful in carryover effects research. From a behavioral perspective, state–space models of animal movement (Forester et al. 2007; Patterson et al. 2008) or mechanistic models of home range formation and behavior (Moorcroft et al. 2006; Van Moorter et al. 2009) explicitly incorporate an individual animal’s previous experience in order to understand individual decision-making. Such models are relevant to conservation issues such as reducing wildlife collisions on transportation corridors, and can be used to prevent or mitigate such losses.
Proactive research can identify specific mechanisms underlying carryover effects, which in turn can be used to develop accurate measures that indicate when carryover effects have occurred, in order to better predict the success of conservation programs that are associated with stress such as captive breeding (Snyder et al. 1996) and translocation programs (Teixeira et al. 2007), or to determine whether a specific individual is suitable for such programs (e.g., Mathews et al. 2005). Such tools are most valuable if they can identify when carryover effects have occurred before the consequences are manifested. As an example of such a tool, reflex impairment predicts whether fish that have been captured as bycatch in commercial fishing operations will survive if released (Davis 2010). In this case, the measure involves a quick assessment of fish reflexes such orientation, startle responses, fin erection, body flex upon restraint, gag response, and vestibular–ocular response, which together represent a comprehensive measure that is both inexpensive and accessible to conservation practitioners. Behavioral indicators such as habitat use or foraging behavior could also provide an effective early measure to indicate that a disturbance has occurred (see review by Berger-Tal et al. 2011). Other approaches such as the genomic tools that have been used to predict mortality and spawning success in sockeye salmon (Oncorhynchus nerka; Miller et al. 2011) are very promising as predictive tools, but remain relatively expensive and inaccessible, and highlight the need for continued research and collaborations between research institutions and conservation practitioners on either developing new tools, or finding ways to make existing tools more accessible.
Harnessing positive carryover effects
Most conservation efforts focus on identifying and mitigating various anthropogenic threats and stressors, including carryover effects. However, it is also theoretically possible to generate positive conservation outcomes by maximizing positive carryover effects. For example, behavioral strategies such as training captive-bred animals to fear predators can be used to increase the survival of captive-bred individuals once released (e.g., McLean et al. 1999). On a broader scale, stress during development can influence long-term reproductive output and survival in birds (Blas et al. 2007), and stressors that delay metamorphosis in amphibians can have population-level effects that are expressed only later in life, during the terrestrial stages (Chelgren et al. 2006). While this can be discouraging for conservation managers attempting to protect populations that have already experienced early-life stress, this research also implies that protecting critical life stages has the potential to generate positive carryover effects. For example, protecting key life stages from stressors could have permanent positive effects on physiological processes that would make individuals more resistant to subsequent stressors. Protecting key habitat by creating reserves is often a successful conservation strategy (e.g., Mosquera et al. 2000). One way to improve these reserves is to identify key life stages that are vulnerable to carryover effects, and then create reserves that target individuals during these key life stages. Such a practice has potential to generate positive carryover effects that increase reproductive output and enhance survival later in life for these individuals and populations, even as they move into areas that are affected by anthropogenic changes.
Incorporating carryover effects in cases where mechanisms are unknown
Given that conservation biology is a field where action often needs to precede a complete body of scientific evidence (Sutherland et al. 2004), it may be most prudent to assume that carryover effects are the rule rather than the exception. In particular, in highly migratory animals and long-lived species, there may be a great deal of uncertainty about what stressors have been experienced by the populations in question. The proactive assumption that many individuals will have experienced stressors leading to carryover effects will provide better conservation results than ignoring carryover effects in conservation. For example, Dickens et al. (2010) suggest that stress during translocations is so widespread that it can be incorporated into conservation planning as a predictable side effect of the translocation process, and efforts can then be adjusted accordingly, and overall outcomes improved.
Ideally, carryover effects should be managed or mitigated during conservation efforts, and this may require some knowledge of underlying mechanisms. In cases where this is not possible, or where conservation action precedes complete scientific study, then carryover effects can still be incorporated into conservation efforts by including carryover effects in some planning and prediction models. For example, an excellent paper by Fefferman and Romero (2013) presents a mathematical model that estimates the population-level effects of cumulative physiological stressors. From this model, the authors conclude that populations under stress disproportionately rely on the oldest and most physically fit individuals for population persistence. Thus, protecting these key individuals may proactively mitigate some carryover effects in wild populations, without the need to fully elucidate and track all carryover effects that are occurring. Similarly, Ratikainen et al. (2008) presents a mathematical model for considering density-dependent effects sequentially, rather than instantaneously, which is far more realistic for predicting population dynamics in populations where carryover effects occur. Norris and Taylor (2006) also present models that incorporate carryover effects into estimates of population sizes. In this case, the authors are specifically interested in migratory populations, and carryover effects are estimated by looking at the slope of the relationship between winter habitat quality and individual reproductive success during the subsequent breeding season. These models provide far better estimates than models without carryover effects. Conceptually, these models are useful for any case where information about the conditions generating the carryover effects are known, and can be expanded to any carryover effect by looking at the slope of the relationship between the condition that generates the carryover effect and the performance metric of interest that is affected by these carryover effects.
In summary, in cases where mechanisms are unknown, or it is not feasible to complete full studies of carryover effects prior to implementing conservation efforts, it is likely far more realistic to assume that carryover effects are occurring rather than to assume the opposite. At the least, acknowledging that carryover effects might be associated with population declines (e.g., for threat assessments at various scales; Rodrigues et al. 2006) would be an important first step. Indeed, the inclusion of carryover effects into threat assessments is possible by means of the methods already available for incorporating uncertainty (Akçakaya et al. 2000). Moreover, assuming that carryover effects generate more variation in conservation success than that would otherwise occur will make predictions more conservative. By incorporating carryover effects into population models, these effects can be explicitly considered in conservation planning and can lead to better outcomes.
Conclusions
It is becoming clear that carryover effects are a common phenomenon across taxa, and have the potential to play an important role in governing individual fitness and population dynamics. Carryover effects may complicate conservation efforts if, for example, stressors experienced in degraded or captive environments carry over to influence reproductive success or survival in restored environments, and if management practices have carryover effects that are only manifested after the end of an evaluation period. These carryover effects can reduce conservation success, and ignoring carryover effects can lead to erroneous conclusions about the effectiveness of conservation efforts, or lead to decisions that are not sufficiently risk averse. We suggest a variety of physiological assessment and modeling methods that could be used to incorporate carryover effects into conservation planning when long-term monitoring is not practical, and present some key research questions for researchers and conservation practitioners to work together to address (Box 2). Incorporating carryover effects into conservation is not a simple and straightforward task. However, considering carryover effects explicitly in threat assessments and conservation planning, through either long-term evaluation or modeling, will target efforts more effectively, and ensure that the success of conservation efforts is tracked more accurately.
Box 2.
Carryover effects occur when the past environment, rather than the current environment, dictates individual performance in a given situation. Here, we have outlined some of the mechanisms connecting the cause (i.e., the past environmental condition) to the effect (i.e., the carryover effect manifested at a different time period) with examples of some of the life-history transitions that might separate the initial cause from the carryover effect. We have also outlined some common conservation efforts, and suggested research areas on carryover effects, which would benefit conservation outcomes
Acknowledgments
CMO is supported by the NSERC Postdoctoral Fellowship. SJC is supported by the Canada Research Chairs program, the NSERC Discovery Grant program, and Carleton University.
Biographies
Constance O’Connor
is a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellow at McMaster University (Hamilton, ON). Her research uses physiological and behavioral tools to understand evolutionary patterns in fish, and to understand how these might be influenced by current anthropogenic changes.
Steven Cooke
is an Associate Professor and Canada Research Chair of Environmental Science and Biology at Carleton University (Ottawa, ON), in the field of fish ecology and conservation physiology. Dr. Cooke is also an Adjunct Professor in the Biology Department at the University of Waterloo and an Affiliate Research Scientist at the Illinois Natural History Survey.
Contributor Information
Constance M. O’Connor, Email: coconn@mcmaster.ca
Steven J. Cooke, Email: steven.cooke@carleton.ca
References
- Abell R. Conservation biology for the biodiversity crisis: A freshwater follow-up. Conservation Biology. 2002;16:1435–1437. doi: 10.1046/j.1523-1739.2002.01532.x. [DOI] [PubMed] [Google Scholar]
- Akçakaya HR, Ferson S, Burgman MA, Keith DA, Mace GM, Todd CR. Making consistent IUCN classifications under uncertainty. Conservation Biology. 2000;14:1001–1013. doi: 10.1046/j.1523-1739.2000.99125.x. [DOI] [Google Scholar]
- Araki H, Cooper B, Blouin MS. Carry-over effect of captive breeding reduces reproductive fitness of wild-born descendants in the wild. Biology Letters. 2009;5:621–624. doi: 10.1098/rsbl.2009.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DP, Seddon PJ. Directions in reintroduction biology. Trends in Ecology & Evolution. 2008;23:20–25. doi: 10.1016/j.tree.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Beaulieu M, Thierry AM, González-Acuña D, Polito MJ. Integrating oxidative ecology into conservation physiology. Conservation Physiology. 2013;1:cot004. doi: 10.1093/conphys/cot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman A, Benton TG, Ranta E, Kaitala V, Lundberg P. Population dynamic consequences of delayed life-history effects. Trends in Ecology & Evolution. 2002;17:263–269. doi: 10.1016/S0169-5347(02)02469-2. [DOI] [Google Scholar]
- Benard MF, McCauley SJ. Integrating across life-history stages: Consequences of natal habitat effects on dispersal. The American Naturalist. 2008;171:553–567. doi: 10.1086/587072. [DOI] [PubMed] [Google Scholar]
- Berger-Tal, O., T. Polak, A. Oron, Y. Lubin, B.P. Kotler, and D. Saltz. 2011. Integrating animal behavior and conservation biology: A conceptual framework. Behavioral Ecology 22: 236–239. doi:10.1093/beheco/arq224.
- Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA. Stress response during development predicts fitness in a wild, long lived vertebrate. Proceedings of the National Academy of Sciences. 2007;104:8880–8884. doi: 10.1073/pnas.0700232104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein AR, Kiesecker JM. Complexity in conservation: Lessons from the global decline of amphibian populations. Ecology Letters. 2002;5:597–608. doi: 10.1046/j.1461-0248.2002.00352.x. [DOI] [Google Scholar]
- Blaustein AR, Walls SC, Bancroft BA, Lawler JJ, Searle CL, Gervasi SS. Direct and indirect effects of climate change on amphibian populations. Diversity. 2010;2:281–313. doi: 10.3390/d2020281. [DOI] [Google Scholar]
- Block BA. Physiological ecology in the 21st century: Advancements in biologging science. Integrative and Comparative Biology. 2005;45:305–320. doi: 10.1093/icb/45.2.305. [DOI] [PubMed] [Google Scholar]
- Bolger DT, Newmark WD, Morrison TA, Doak DF. The need for integrative approaches to understand and conserve migratory ungulates. Ecology Letters. 2008;11:63–77. doi: 10.1111/j.1461-0248.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Bowlin MS, Bisson IA, Shamoun-Baranes J, Reichard JD, Sapir N, Marra PP, Kunz TH, Wilcove DS, Hedenström A, Guglielmo CG, Åkesson SA, Ramenofsky M, Wikelski M. Grand challenges in migration biology. Integrative and Comparative Biology. 2010;50:261–279. doi: 10.1093/icb/icq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JS, Franzen MA, Holmes CM, Grote MN, Mulder MB. Testing hypotheses for the success of different conservation strategies. Conservation Biology. 2006;20:1528–1538. doi: 10.1111/j.1523-1739.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- Buchholz R. Behavioural biology: An effective and relevant conservation tool. Trends in Ecology & Evolution. 2007;22:401–407. doi: 10.1016/j.tree.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Calvert AM, Walde SJ, Taylor PD. Nonbreeding-season drivers of population dynamics in seasonal migrants: Conservation parallels across taxa. Avian Conservation and Ecology. 2009;4:5. [Google Scholar]
- Chelgren ND, Rosenberg DK, Heppell SS, Gitelman AI. Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecological Applications. 2006;16:250–261. doi: 10.1890/04-0329. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Hinch SG, Donaldson MR, Clark TD, Eliason EJ, Crossin GT, Raby GD, Jeffries KM, Lapointe M, Miller K, Patterson DA, Farrell AP. Conservation physiology in practice: How physiological knowledge has improved our ability to sustainably manage Pacific salmon during up-river migration. Philosophical Transactions of the Royal Society B. 2012;367:1757–1769. doi: 10.1098/rstb.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conservation Physiology. 2013;1:cot001. doi: 10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Blumstein DT, Buchholz R, Caro T, Fernández-Juricic E, Franklin CE, Metcalfe J, O’Connor CM, Cassidy St. Clair C, Sutherland WJ, Wikelski M. Physiology, behaviour and conservation. Physiological and Biochemical Zoology. 2014;87:1–14. doi: 10.1086/671165. [DOI] [PubMed] [Google Scholar]
- Crain CM, Kroeker K, Halpern BS. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters. 2008;11:1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- Davis MW. Fish stress and mortality can be predicted using reflex impairment. Fish and Fisheries. 2010;11:1–11. doi: 10.1111/j.1467-2979.2009.00331.x. [DOI] [Google Scholar]
- Dickens MJ, Delehanty DJ, Michael Romero LM. Stress: An inevitable component of animal translocation. Biological Conservation. 2010;143:1329–1341. doi: 10.1016/j.biocon.2010.02.032. [DOI] [Google Scholar]
- Dickey MH, Gauthier G, Cadieux MC. Climatic effects on the breeding phenology and reproductive success of an arctic-nesting goose species. Global Change Biology. 2008;14:1973–1985. doi: 10.1111/j.1365-2486.2008.01622.x. [DOI] [Google Scholar]
- Fefferman NH, Romero LM. Can physiological stress alter population persistence? A model with conservation implications. Conservation Physiology. 2013;1:cot012. doi: 10.1093/conphys/cot012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Fraga MF. Epigenetic and the environment: Emerging patterns and implications. Nature Reviews Genetics. 2012;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Ferraro PJ, Pattanayak SK. Money for nothing? A call for empirical evaluation of biodiversity conservation investments. PLoS Biology. 2006;4:e105. doi: 10.1371/journal.pbio.0040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Lindenmayer DB. An assessment of the published results of animal relocations. Biological Conservation. 2000;96:1–11. doi: 10.1016/S0006-3207(00)00048-3. [DOI] [Google Scholar]
- Forester JD, Ives AR, Turner MG, Anderson DP, Fortin D, Beyer HL, Smith DW, Boyce MS. State-space models link elk movement patterns to landscape characteristics in Yellowstone National Park. Ecological Monographs. 2007;77:285–299. doi: 10.1890/06-0534. [DOI] [Google Scholar]
- Frederiksen M, Daunt F, Harris MP, Wanless S. The demographic impact of extreme events: Stochastic weather drives survival and population dynamics in a long-lived seabird. Journal of Animal Ecology. 2008;77:1020–1029. doi: 10.1111/j.1365-2656.2008.01422.x. [DOI] [PubMed] [Google Scholar]
- Geiser F, Ferguson C. Intraspecific differences in behaviour and physiology: Effects of captive breeding on patterns of torpor in feathertail gliders. Journal of Comparative Physiology B. 2001;171:569–576. doi: 10.1007/s003600100207. [DOI] [PubMed] [Google Scholar]
- Green RJ, Higginbottom K. The effects of non-consumptive wildlife tourism on free-ranging wildlife: A review. Pacific Conservation Biology. 2000;6:183. [Google Scholar]
- Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. Carry-over effects as drivers of fitness differences in animals. Journal of Animal Ecology. 2011;80:4–18. doi: 10.1111/j.1365-2656.2010.01740.x. [DOI] [PubMed] [Google Scholar]
- Head JA, Dolinoy DC, Basu N. Epigenetics for ecotoxicologists. Environmental Toxicology and Chemistry. 2012;31:221–227. doi: 10.1002/etc.1707. [DOI] [PubMed] [Google Scholar]
- James AN, Gaston KJ, Balmford A. Balancing the earth’s accounts. Nature. 1999;401:323–324. doi: 10.1038/43774. [DOI] [PubMed] [Google Scholar]
- Johnsson JI, Bohlin T. The cost of catching up: Increased winter mortality following structural growth compensation in the wild. Proceedings of the Royal Society of London B. 2006;273:1281–1286. doi: 10.1098/rspb.2005.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman DG, Reading RP, Miller BJ, Clark TW, Scott JM, Robinson J, Wallace RL, Cabin RJ, Felleman F. Improving the evaluation of conservation programs. Conservation Biology. 2000;14:356–365. doi: 10.1046/j.1523-1739.2000.98553.x. [DOI] [PubMed] [Google Scholar]
- Lande R, Shannon S. The role of genetic variation in adaptation ad population persistence in a changing environment. Evolution. 1996;50:434–437. doi: 10.2307/2410812. [DOI] [PubMed] [Google Scholar]
- Lee WS, Monaghan P, Metcalfe NB. Experimental demonstration of the growth rate-lifespan trade-off. Proceedings of the Royal Society of London B. 2013;280:20122370. doi: 10.1098/rspb.2012.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie PH. The properties of a certain lag type of population growth and the influence of an external random factor on a number of such populations. Physiological Zoology. 1959;32:151–159. [Google Scholar]
- Martin TG, Chadès I, Arcese P, Marra PP, Possingham HP, Norris DR. Optimal conservation of migratory species. PLoS One. 2007;2:e751. doi: 10.1371/journal.pone.0000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews F, Orros M, McLaren G, Gelling M, Foster R. Keeping fit on the ark: Assessing the suitability of captive-bred animals for release. Biological Conservation. 2005;121:569–577. doi: 10.1016/j.biocon.2004.06.007. [DOI] [Google Scholar]
- McDonald DB. Predicting fate from early connectivity in a social network. Proceedings of the National Academy of Science of the United States of America. 2007;104:10910–10914. doi: 10.1073/pnas.0701159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean IG, Hölzer C, Studholme BJ. Teaching predator-recognition to a naive bird: Implications for management. Biological Conservation. 1999;87:123–130. doi: 10.1016/S0006-3207(98)00024-X. [DOI] [Google Scholar]
- McPhee EM. Generations in captivity increases behavioral variance: Considerations for captive breeding and reintroduction programs. Biological Conservation. 2004;115:71–77. doi: 10.1016/S0006-3207(03)00095-8. [DOI] [Google Scholar]
- Miller KA. Climate variability and tropical tuna: Management challenges for highly migratory fish stocks. Marine Policy. 2007;31:56–70. doi: 10.1016/j.marpol.2006.05.006. [DOI] [Google Scholar]
- Miller KM, Li S, Kaukinen KH, Ginther N, Hamill E, Curtis JMR, Patterson DA, Sierocinski T, Donnison L, Pavlidis P, Hinch SG, Hruska KA, Cooke SJ, English KK, Farrell AP. Genomic signatures predict migration and spawning failure in wild Canadian salmon. Science. 2011;331:214–217. doi: 10.1126/science.1196901. [DOI] [PubMed] [Google Scholar]
- Miller KM, Teffer A, Tucker S, Li S, Schulze AD, Trudel M, Juanes F, Tabata A, Kaukinen KH, Ginther NG, et al. Infectious disease, shifting climates and opportunistic predators: Cumulative factors potentially impacting wild salmon declines. Evolutionary Applications. 2014;7:812–855. doi: 10.1111/eva.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP. Behavioral and life history responses to extreme climatic conditions: Studies on a migratory songbird. Current Zoology. 2011;57:351–362. [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. Ecology Letters. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Moorcroft PR, Lewis MA, Crabtree RL. Mechanistic home range models capture spatial patterns and dynamics of coyote territories in Yellowstone. Proceedings of the Royal Society of London B. 2006;273:1651–1659. doi: 10.1098/rspb.2005.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J, Møller AP. Extreme climatic events in relation to global change and their impact on life histories. Current Zoology. 2011;57:375–389. [Google Scholar]
- Morgan IJ, Metcalfe NB. Deferred costs of compensatory growth after autumnal food shortage in juvenile salmon. Proceedings of the Royal Society of London B. 2001;268:295–301. doi: 10.1098/rspb.2000.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera I, Côté IM, Jennings S, Reynolds JD. Conservation benefits of marine reserves for fish populations. Animal Conservation. 2000;3:321–332. doi: 10.1111/j.1469-1795.2000.tb00117.x. [DOI] [Google Scholar]
- Norris DR. Carry-over effects and habitat quality in migratory animals. Oikos. 2005;109:178–186. doi: 10.1111/j.0030-1299.2005.13671.x. [DOI] [Google Scholar]
- Norris DR, Marra PP. Seasonal interactions, habitat quality and population dynamics in migratory birds. Condor. 2007;109:535–547. doi: 10.1650/8350.1. [DOI] [Google Scholar]
- Norris DR, Taylor CM. Predicting the consequences of carry-over effects for migratory populations. Biology Letters. 2006;2:148–151. doi: 10.1098/rsbl.2005.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliffe LM. Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proceedings of the Royal Society of London B. 2004;271:59–64. doi: 10.1098/rspb.2003.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Gilmour KM, Arlinghaus R, Hasler CT, Philipp DP, Cooke SJ. Seasonal carryover effects following the administration of cortisol to a wild teleost fish. Physiological and Biochemical Zoology. 2010;83:950–957. doi: 10.1086/656286. [DOI] [PubMed] [Google Scholar]
- O’Connor CM, Norris DR, Crossin GT, Cooke SJ. Biological carryover effects: Linking common concepts and mechanisms in ecology and evolution. Ecosphere. 2014;5:art28. doi: 10.1890/ES13-00388.1. [DOI] [Google Scholar]
- Page SJ, Dowling RK, Page SJ. Ecotourism. Upper Saddle River, NJ: Pearson Education Limited; 2001. [Google Scholar]
- Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J. State–space models of individual animal movement. Trends in Ecology & Evolution. 2008;23:87–94. doi: 10.1016/j.tree.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Primack RB. Essentials of Conservation Biology. 5. Sunderland, MA: Sinauer Associates; 2010. [Google Scholar]
- Prout T, McChesney F. Competition among immatures affects their adult fertility: Population dynamics. American Naturalist. 1985;126:521–558. doi: 10.1086/284436. [DOI] [Google Scholar]
- Ratikainen II, Gill JA, Gunnarsson TG, Sutherland WJ, Kokko H. When density dependence is not instantaneous: Theoretical developments and management implications. Ecology Letters. 2008;11:184–198. doi: 10.1111/j.1461-0248.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- Reed DH, Frankham R. Correlations between fitness and genetic diversity. Conservation Biology. 2003;17:230–237. doi: 10.1046/j.1523-1739.2003.01236.x. [DOI] [Google Scholar]
- Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends in Ecology & Evolution. 2002;17:462–468. doi: 10.1016/S0169-5347(02)02578-8. [DOI] [Google Scholar]
- Rodrigues AS, Pilgrim JD, Lamoreux JF, Hoffmann M, Brooks TM. The value of the IUCN Red List for conservation. Trends in Ecology & Evolution. 2006;21:71–76. doi: 10.1016/j.tree.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Rubenstein DR, Hobson KA. From birds to butterflies: Animal movement patterns and stable isotopes. Trends in Ecology & Evolution. 2004;19:256–263. doi: 10.1016/j.tree.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Saino N, Szép T, Ambrosini R, Romano M, Møller AP. Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proceedings of the Royal Society of London B. 2004;271:681–686. doi: 10.1098/rspb.2003.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer H, Kauffman MJ, Nielson RM, Horne JS. Identifying and prioritizing ungulate migration routes for landscape-level conservation. Ecological Applications. 2009;19:2016–2025. doi: 10.1890/08-2034.1. [DOI] [PubMed] [Google Scholar]
- Scott DE. The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology. 1994;75:1383–1396. doi: 10.2307/1937462. [DOI] [Google Scholar]
- Snyder NF, Derrickson SR, Beissinger SR, Wiley JW, Smith TB, Toone WD, Miller B. Limitations of captive breeding in endangered species recovery. Conservation Biology. 1996;10:338–348. doi: 10.1046/j.1523-1739.1996.10020338.x. [DOI] [Google Scholar]
- Solomon S, Qin D, Manning M, Alley RB, Berntsen T, Bindoff NL, Chen Z, Chidthaisong A, Gregory JM, Hegerl GC, et al. Technical Summary. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, et al., editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Sørensen MC, Hipfner JM, Kyser TK, Norris DR. Carry-over effects in a Pacific seabird: Stable isotope evidence that pre-breeding diet quality influences reproductive success. Journal of Animal Ecology. 2009;78:460–467. doi: 10.1111/j.1365-2656.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- Soulé ME. What is conservation biology. BioScience. 1985;35:727–734. doi: 10.2307/1310054. [DOI] [Google Scholar]
- Sutherland WJ, Pullin AS, Dolman PM, Knight TM. The need for evidence-based conservation. Trends in Ecology & Evolution. 2004;19:305–308. doi: 10.1016/j.tree.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Tarszisz E, Dickman CR, Munn AJ. Physiology in conservation translocations. Conservation Physiology. 2014;2:cou054. doi: 10.1093/conphys/cou054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CP, De Azevedo CS, Mendl M, Cipreste CF, Young RJ. Revisiting translocation and reintroduction programmes: The importance of considering stress. Animal Behaviour. 2007;73:1–13. doi: 10.1016/j.anbehav.2006.06.002. [DOI] [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, Ferreira de Siqueira M, Grainger A, Hannah L, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Van Moorter B, Visscher D, Behamou S, Börger L, Boyce MS, Gaillard J-M. Memory keeps you at home: A mechanistic model for home range emergence. Oikos. 2009;118:641–652. doi: 10.1111/j.1600-0706.2008.17003.x. [DOI] [Google Scholar]
- Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT. Links between worlds: Unraveling migratory connectivity. Trends in Ecology & Evolution. 2002;17:76–83. doi: 10.1016/S0169-5347(01)02380-1. [DOI] [Google Scholar]
- Wingfield JC, Kelley JP, Angelier F. What are extreme environmental conditions and how do organisms cope with them? Current Zoology. 2011;57:363–374. [Google Scholar]