Abstract
Schizophrenia patients have abnormal neural responses to salient, infrequent events. We integrated event-related potentials (ERP) and fMRI to examine the contributions of the ventral (salience) and dorsal (sustained) attention networks to this dysfunctional neural activation. Twenty-one schizophrenia patients and 22 healthy controls were assessed in separate sessions with ERP and fMRI during a visual oddball task. Visual P100, N100, and P300 ERP waveforms and fMRI activation were assessed. A joint independent components analysis (jICA) on the ERP and fMRI data were conducted. Patients exhibited reduced P300, but not P100 or N100, amplitudes to targets and reduced fMRI neural activation in both dorsal and ventral attentional networks compared with controls. However, the jICA revealed that the P300 was linked specifically to activation in the ventral (salience) network, including anterior cingulate, anterior insula, and temporal parietal junction, with patients exhibiting significantly lower activation. The P100 and N100 were linked to activation in the dorsal (sustained) network, with no group differences in level of activation. This joint analysis approach revealed the nature of target detection deficits that were not discernable by either imaging methodology alone, highlighting the utility of a multimodal fMRI and ERP approach to understand attentional network deficits in schizophrenia.
Keywords: ERP, fMRI, Joint ICA, Oddball, Salience network
Highlights
-
•
Examined dorsal and ventral attention network activity to targets in schizophrenia
-
•
Joint ICA (jICA) analyses using ERP and fMRI during target detection was used.
-
•
Schizophrenia patients had reduced P300 ERP and fMRI activation to targets.
-
•
jICA revealed that P300 was linked to activity in the ventral (salience) network.
-
•
This linked component was significantly reduced in schizophrenia patients.
1. Introduction
Schizophrenia patients have deficits in many cognitive abilities, including the basic ability to separate important from distracting stimuli, such as distinguishing targets from nontargets. One widely-used task to examine dysfunctional target processing is the oddball task, in which infrequent target (“oddball”) stimuli are embedded in a stream of frequent nontarget (“standard”) stimuli. Schizophrenia patients have impairments on oddball tasks (Ford, 1999; Ford et al., 1994; Kiehl et al., 2005; Kim et al., 2009), as they do on similar tasks of target detection, including the continuous performance task (Cornblatt and Erlenmeyer-Kimling, 1985; MacDonald, 2008; Nuechterlein and Dawson, 1984), and visual search tasks (Silverstein et al., 2010). Efforts have been made to better understand the specific nature of the attentional impairment involved in this task (Ford et al., 2010). In the current study we examined the neural networks associated with dysfunctional oddball detection in schizophrenia using converging evidence from functional magnetic resonance imaging (fMRI) and event-related potentials (ERPs).
Two separate attentional processes are involved in target detection in oddball tasks: 1) maintaining attentional readiness, and 2) detecting an infrequent change in the environment. Dorsal and ventral attentional networks have been implicated in these separate processes (Corbetta and Shulman, 2002; Kim, 2014). The dorsal network includes activations in the inferior frontal junction (IFJ), medial intraparietal sulcus (IPS), superior parietal lobule (SPL), and middle temporal area (MT+). This network is thought to orient attention to the task in general, responding to both targets and nontargets (Kim, 2014). In contrast, the ventral network includes activations in the temporal parietal junction (TPJ), anterior insula (AI), anterior middle frontal gyrus (aMFG), bilateral anterior cingulate cortex (ACC), and supplementary motor area (SMA). This network is involved in detecting salient changes within the task (Corbetta and Shulman, 2002), and is specifically associated with detection of targets, but not nontargets. When presented with a task-relevant stimulus the ventral network is associated with attentional allocation to that stimulus to initiate an action (Palaniyappan and Liddle, 2012). Importantly, the Kim (2014) meta-analysis found the same ventral attention network activated across both auditory and visual oddball tasks.
Oddball studies yield a highly characteristic ERP, the P300, elicited by rare, cognitively-relevant (target) stimuli. The P300 is a positive deflection occurring approximately 300–600 ms after target presentation, and is largest over parietal areas. Source localization, though not as precise as fMRI, and intracranial studies have identified P300 neural generators located in several regions that partially overlap with the ventral attentional network, including the TPJ, posterior superior parietal regions, ACC, dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, and medial temporal regions (Bledowski et al., 2004; Jeon and Polich, 2003; Kiss et al., 1989; Machado et al., 2014; McCarthy and Wood, 1987; Mulert et al., 2004). Attention has also been shown to increase amplitudes of earlier, primarily sensory components, including the visual P100 and N100 (for review, see Herrmann and Knight, 2001) as well as components at Pz occurring earlier than the P300 (e.g., Clementz et al., 2008; Sponheim et al., 2006). These findings indicate that attention can enhance neural responses to targets, and thus salience detection, in early stages of processing.
There are well established fMRI (Gur et al., 2007; Kiehl et al., 2005) and P300 ERP (Bramon et al., 2004; Ford, 1999; Jeon and Polich, 2003) findings of deficits in schizophrenia on oddball tasks. Schizophrenia patients also exhibit deficits in early sensory ERP components (P100, N100) (Foxe et al., 2001; Martinez et al., 2012; Schechter et al., 2005), though they are less consistent than those seen for the P300. Thus, problems in target detection could possibly arise earlier in the processing stream, leading to down-stream deficits.
Despite the deficits seen within each imaging method, the relationship between abnormal ERP components and dysfunctional fMRI activation during oddball tasks in schizophrenia patients is not established (Kiehl et al., 2005). Examining this relationship between ERP and fMRI would allow us to discern which attentional network is specifically contributing to impairments in target detection. For example, fMRI alone implicates both dorsal and ventral systems in schizophrenia as abnormal in oddball tasks, but only one might be responsible for the problems in target detection.
Recent advances in computational neuroimaging have utilized a joint independent components analysis (jICA) approach to determine underlying neural structures and chronometry associated with a task by combining the temporal resolution of EEG with the spatial resolution of fMRI (Calhoun et al., 2006). One study examined EEG and fMRI in the same sample of patients using jICA, but it did not focus on P300 (Calhoun et al., 2010). No study to our knowledge has integrated visual oddball ERP and fMRI data in the same sample of patients and controls. Without such integration, we cannot know whether deficits in oddball tasks in schizophrenia are specific to the ventral or dorsal attentional networks, or whether they arise in early, primarily sensory brain regions.
The goal of the current study is to utilize fMRI and ERPs to examine whether schizophrenia is associated with a dysfunctional ventral and/or dorsal network during a visual oddball task. We hypothesized that: 1) patients will show reduced fMRI neural activation to targets relative to controls, 2) patients will show reduced ERP responses to targets relative to controls, and 3) that a joint ERP–fMRI analysis will reveal that dysfunctional P300 ERP in schizophrenia is associated with abnormalities in the ventral attention network.
2. Methods
2.1. Participants
EEG and fMRI data were collected from 21 schizophrenia patients and 22 healthy controls in separate sessions, with a median of 14 days between sessions. Patients were recruited from outpatient treatment clinics at the Greater Los Angeles VA (GLA) and the community. All patients were receiving second generation antipsychotic medication, mean (SD) chlorpromazine equivalent of 307 (153) mg/day (Andreasen et al., 2010). Patients met diagnostic criteria for schizophrenia based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First et al., 1996b). Psychiatric symptoms were evaluated using the 24-item University of California Los Angeles (UCLA) version of the Brief Psychiatric Rating Scale (BPRS; Ventura et al., 1995); we report total scores and means for the “positive symptom,” “negative symptom,” “agitation/mania,” and “depression/anxiety” factors (Kopelowicz et al., 2008). Healthy controls were recruited through internet postings, interviewed with the SCID-I and portions of the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II; First et al., 1996a), and excluded if they had any of the following Axis II disorders: avoidant, paranoid, schizoid, or schizotypal. Additional inclusion and exclusion criteria for both groups can be seen in the Inline Supplementary Methods.
All participants had the capacity to give and provided written informed consent after all procedures were explained in accordance with procedures approved by the Institutional Review Boards at UCLA and GLA.
2.2. Procedures
2.2.1. fMRI task
Participants viewed images of two letters, X and K, that served as targets or nontargets. The target and nontarget letters were counterbalanced across subjects and recordings (i.e., EEG and fMRI). Stimuli were presented in a fast event-related design in three separate blocks using magnet-compatible goggles (Resonance Technology, Northridge, CA). Stimuli were displayed for 100 ms with an interstimulus interval that varied between 900 and 2900 ms. Participants were instructed to push a button on an MRI-compatible button box whenever they detected the target and had 3000 ms to make a response. Within each block a total of 150 stimuli were presented: 12% were targets (n = 18) and 88% were nontargets (n = 132). Null trials were interspersed throughout each block and consisted solely of a fixation point. Correct and incorrect trials were combined in the analyses.
2.2.2. EEG task
The same stimuli and parameters used in the fMRI paradigm were used for the EEG paradigm. Stimuli were presented on a 17-inch computer monitor 1 m from the participant. A total of 500 stimuli were presented: 12% were targets (n = 60) and 88% were nontargets (n = 440). Correct and incorrect trials were combined in the analyses.
2.2.3. fMRI analysis
Scanning was performed on a Siemens 3 T Trio (Erlangen, Germany) MRI scanner. Acquisition and preprocessing details can be found in the Inline Supplementary Methods. Statistical analyses were performed at the single subject level using a general linear model with fMRI Expert Analysis Tool (FEAT). Neural activity associated with targets vs. baseline was examined, within and between groups, using a whole brain approach, conducted using FSL's FLAME, stage 1 (Beckmann et al., 2003; Woolrich et al. 2004, Woolrich 2008). Activation maps were thresholded at p < 0.01, uncorrected for multiple comparisons, with an extent threshold of 36 contiguous voxels, corresponding to a false-positive discovery rate of <5% across the whole brain as estimated by Monte Carlo simulation (10,000 simulations) (Slotnick et al., 2003).
2.2.4. EEG analysis
EEG was acquired using a Neuroscan SynAmps2 amplifier (Compumedics USA, Charlotte, NC). Acquisition and preprocessing details can be found in the inline Supplementary Methods. ERP data were processed using BrainVision Analyzer 2.0 software (Brain Products, Germany). There were no significant between-group differences in the number of trials accepted for targets and nontargets (Table 1). The ERPs of interest were the early, sensory P100 and N100 components, and the later cognitive P300 response. Peaks were identified in the range of 60–100 ms for P100 and between 110 and 180 ms for N100; a 10 ms average around the peak was calculated. For the P100 and N100, activity was averaged separately over left (P5, P7, PO7) and right (P6, P8, PO8) electrodes. For the P300, peak positive amplitudes between 250–600 ms were analyzed at Pz. To examine group differences for the P100 and N100, separate repeated measure analyses of variance were used with stimulus type (target vs. nontarget) and hemisphere (left vs. right) as the within subject variable and group as the between subject variable; for the P300 hemisphere was not entered as a factor. An alpha level of p = 0.05 was used. We report partial eta-squared (ηp2) effect sizes.
Table 1.
Demographic information, symptom ratings, and oddball task behavioral and ERP results.
| Schizophrenia patients (n = 21) |
Healthy controls (n = 22) |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 46.2 | 10.9 | 41.5 | 7.7 |
| Education* | 12.6 | 1.1 | 14.4 | 1.8 |
| Parental education | 13.7 | 3.3 | 14.2 | 3.0 |
| Male:female | 19:2 | − | 19:3 | − |
| BPRS | ||||
| Total score | 40.1 | 7.6 | ||
| Factors (mean score per item) | ||||
| Positive symptoms | 1.8 | 0.6 | ||
| Negative symptoms | 1.6 | 0.8 | ||
| Depression/anxiety | 1.8 | 0.6 | ||
| Agitation/mania | 1.3 | 0.3 | ||
| Behavioral performance | ||||
| EEG accuracy (% out of 60) | 84.6 | 28.1 | 96.3 | 5.5 |
| fMRI accuracy (% out of 54) | 84.9 | 19.4 | 93.7 | 9.8 |
| EEG d-prime* | 3.97 | 1.40 | 4.79 | 0.62 |
| fMRI d-prime* | 3.71 |
0.97 |
4.44 |
0.78 |
| Number EEG trials accepted | ||||
| Targets | 53.8 | 8.5 | 51.5 | 12.4 |
| Nontargets | 390.7 |
63.5 |
375.3 |
93.4 |
| P300 amplitude (µV) | ||||
| Targets* | 2.36 | 1.34 | 4.40 | 2.37 |
| Nontargets | 1.27 | 1.20 | 1.71 | 0.95 |
p < 0.05 difference between groups.
To more completely visualize ERPs across the scalp, we computed statistical cluster plots (Molholm et al., 2002) across all electrodes composed of between-group comparisons separately for the target and nontarget stimuli. Two-tailed t-tests were computed for each time point and each electrode from −50 to +500 ms. To partially correct for potential Type 1 errors due to the number of comparisons, analyses were restricted to an alpha level of p < 0.01 for 10 consecutive time points (i.e., 20 ms) (Guthrie and Buchwald, 1991). The plots present significant t-values that exceeded this threshold.
2.2.5. Joint ERP/fMRI ICA analyses
To examine the attentional networks associated with the P300 we conducted a joint independent components analysis (jICA) using the Matlab-based Fusion ICA Toolbox (FIT; Calhoun et al., 2006). Joint ICA statistically combines data from multiple modalities collected from the same participants as a way to identify linked sources of activity. In this study we used jICA to examine intersubject covariation between spatial components in fMRI activation and temporal components in ERP waveforms. To provide spatial features of the oddball response, we utilized the fMRI contrast images of targets vs. implicit baseline. To provide the temporal data features, we used the ERP response to targets averaged over electrodes P5, P7, PO7, P6, P8, and PO8 to examine sensory components and at Pz to examine the cognitive component. Two separate jICAs were performed to examine the sensory and cognitive components.
Briefly, FIT first extracts features from each modality separately (i.e., EEG time-courses and fMRI spatial maps) across the combined groups (i.e., the algorithm is data-driven and initially blind to group membership), then performs between-group comparisons of these features (Calhoun et al., 2010). In this approach, each group's ERP and fMRI datasets are concatenated side-by-side; the sources associated with each are assumed to modulate the same way across groups and subjects within groups (i.e., equal linear covariation). Within groups, couplings between fMRI and EEG modalities were identified by components with shared loading parameters. Between groups comparisons were made based on differences of amplitude, latency and location of each data component. Each component was then tested for a significant difference between patients and controls using a two-sample t-test.
3. Results
3.1. Demographic and clinical characteristics
Table 1 lists group demographics and BPRS ratings for patients. As most patient participants were recruited from VA clinics, the sample is predominantly male. Patients were clinically stable and exhibited mild clinical symptoms. Performance (accuracy and d-prime) during the EEG and fMRI tasks can be seen in Table 1.1
3.2. fMRI results
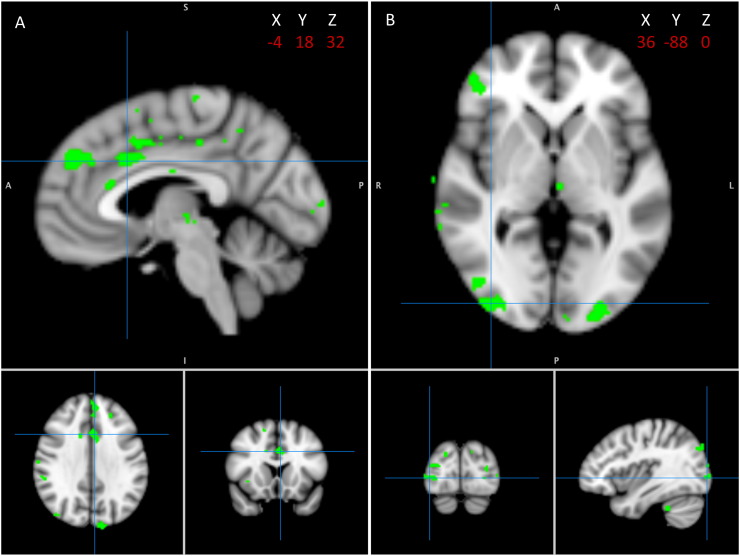
Comparing targets to implicit baseline, significant activation was seen in both groups in several regions (see Inline Supplementary Table S1 and Inline Supplementary Fig. S1). For group differences in levels of activation in the target vs. implicit baseline contrast, the control > patient contrast revealed significant differences in several brain regions corresponding to the ventral and dorsal networks. These included frontal regions (superior frontal gyrus, inferior frontal gyrus, frontal pole), parietal regions (supramarginal gyrus), ACC (Fig. 1A), as well as occipital regions (occipital pole, right LOC, precuneous) (Fig. 1B). The patient > control contrast revealed only one small significant cluster located in midbrain.
Table S1.
Regions of activation, maximum z-score, and MNI coordinates for within group effects and direct group contrasts for targets vs. baseline.
| Regions | Voxel # | Max z-score | Max X | Max Y | Max Z |
|---|---|---|---|---|---|
| Patients | |||||
| postcentral gyrus, left | 14388 | 5.56 | -42 | -24 | 46 |
| LOC, left | 2354 | 4.6 | -46 | -68 | -2 |
| paracingulate gyrus | 764 | 4.69 | 10 | 12 | 40 |
| justapositional lobule cortex | 740 | 4.66 | -10 | -8 | 44 |
| LOC, right | 541 | 3.76 | 30 | -66 | 26 |
| --- | 451 | 3.62 | 16 | -70 | -44 |
| LOC, right | 198 | 3.48 | 26 | -82 | 6 |
| --- | 155 | 3.19 | -38 | 0 | -28 |
| LOC, left | 132 | 3.45 | 30 | -60 | 40 |
| posterior cingulate gyrus | 72 | 2.94 | 6 | -22 | 30 |
| intracalcarine gryus | 70 | 3 | -14 | -74 | 10 |
| posterior cingulate gyrus | 62 | 3 | 10 | 22 | 44 |
| parahippocampul gyrus | 47 | 2.82 | 24 | 2 | -36 |
| --- | 36 | 2.96 | 32 | -14 | -6 |
| Controls | |||||
| postcentral gyrus, left | 15750 | 5.69 | -38 | -26 | 46 |
| LOC, right | 10045 | 5.5 | 48 | -74 | 0 |
| paracingulate, right | 3675 | 5.26 | 12 | 10 | 38 |
| --- | 347 | 3.26 | -16 | -56 | -44 |
| --- | 283 | 3.61 | -2 | -24 | -44 |
| frontal pole, right | 218 | 4.05 | -32 | 40 | 20 |
| --- | 56 | 3.71 | -32 | -52 | -30 |
| Patients > Controls | |||||
| --- | 49 | 2.93 | -12 | -32 | 16 |
| Controls > Patients | |||||
| occipital pole, left | 199 | 3.7 | -28 | -92 | 2 |
| superior frontal gyrus | 138 | 3.74 | -4 | 48 | 36 |
| occipital pole, left | 108 | 3.42 | -12 | -92 | 32 |
| anterior cingulate gyrus | 88 | 2.91 | -2 | 18 | 32 |
| frontal pole, right | 82 | 3.35 | 46 | 42 | 2 |
| anterior cingulate gyrus | 71 | 2.86 | -2 | 2 | 44 |
| middle frontal gyrus | 66 | 3.07 | -32 | -2 | 54 |
| LOC, right | 63 | 3.06 | 32 | -90 | 0 |
| inferior frontal gyrus, right | 58 | 3.24 | 54 | 26 | 20 |
| postcentral gyrus, right | 54 | 3.12 | 60 | -18 | 40 |
| LOC, right | 53 | 3.13 | 38 | -82 | 36 |
| precuneous | 51 | 2.74 | 2 | -52 | 48 |
| middle frontal gyrus, right | 45 | 3.02 | 28 | 8 | 52 |
| --- | 42 | 3.28 | 40 | -44 | -36 |
| precentral gyrus, right | 40 | 2.69 | 60 | 0 | 26 |
| supramarginal gyrus, right | 39 | 3.16 | 68 | -32 | 38 |
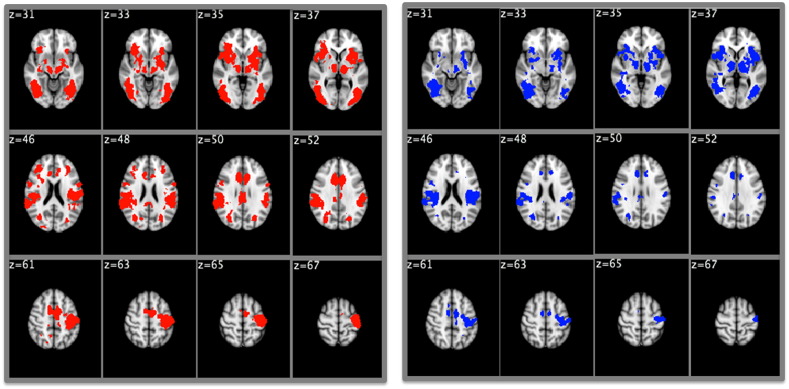
Fig. S1.
Whole brain analysis for the target vs. baseline contrast in healthy controls (red, left) and schizophrenia patients (blue, right). Reported activations were thresholded at p < .01, uncorrected for multiple comparisons, with an extent threshold of 36 contiguous voxels, corresponding to a false-positive discovery rate of < 5% across the whole brain.
Fig. 1.
Brain regions showing greater activity to target stimuli in healthy controls compared with schizophrenia patients. A) Crosshairs corresponding to anterior cingulate cortex on coordinates shown. B) Crosshairs corresponding to right lateral occipital cortex on coordinates shown. Reported activations were thresholded at p < .01, uncorrected for multiple comparisons, with an extent threshold of 36 contiguous voxels, corresponding to a false-positive discovery rate of <5% across the whole brain.
Inline Supplementary Table S1.
Inline Supplementary Figure S1.
3.3. ERP and statistical cluster plot results
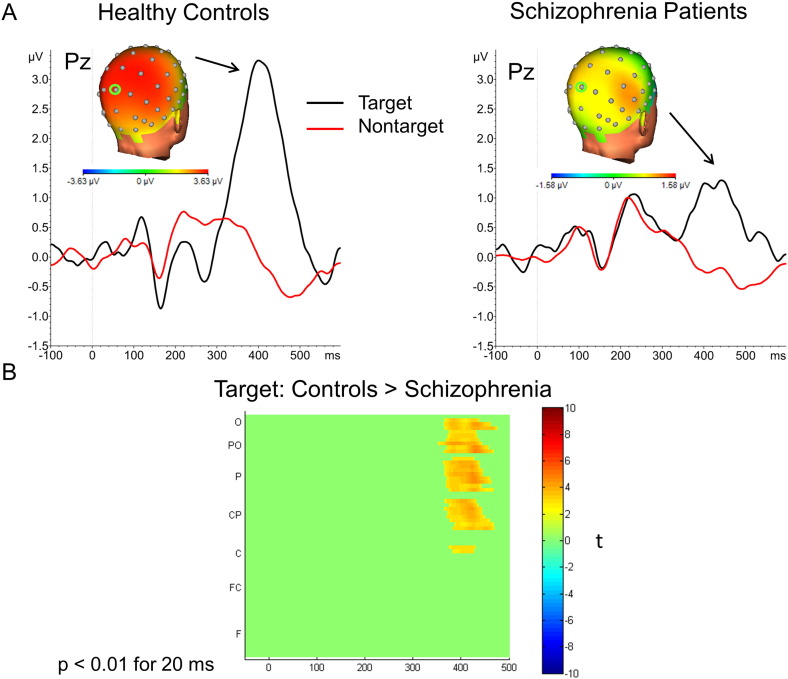
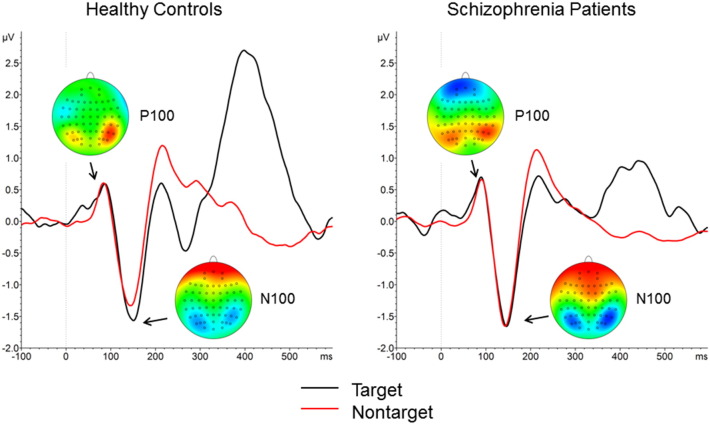
Fig. 2A shows grand average waveforms to targets and nontargets at electrode Pz and topographical maps for peak activity in response to targets for controls and patients. Grand average waveforms and topographical maps for the P100 and N100 can be found in Inline Supplementary Fig. S2. Mean peak P300 amplitudes for each group are shown in Table 1; mean peak P100 and N100 amplitudes can be found online in Inline Supplementary Table S2.
Fig. 2.
A) Event-related potentials (ERPs) at electrode Pz and corresponding topographical maps in healthy controls (left) and schizophrenia patients (right). ERPs waveforms in black = target, red = nontarget. B) Statistical cluster plot depicting running t-tests comparing ERP amplitudes to targets in the healthy control group vs. schizophrenia patient group. Significant effects exceeding an alpha level of 0.01, lasting for a minimum of 20 ms (10 data points at 500 Hz), are shown. The color bar depicts the direction of the difference, with green colors representing no significant difference. Time, plotted on the x-axis, between −50 to +500 ms is shown. The y-axis depicts regions of electrodes, moving from frontal (F) electrodes at the bottom of the axis to frontocentral (FC), central (C), central-parietal (CP), parietal (P), parietal-occipital (PO), and occipital (O) at the top of the axis.
Fig. S2.
Event-related potentials (ERPs) to for P100 and N100, pooled over electrodes P5, P7, PO7, P6, P8, and PO8, and corresponding topographical maps to target stimuli in healthy controls (left) and schizophrenia patients (right). ERP waveforms in black = target, red = nontarget.
Table S2.
P100 and N100 ERP amplitudes to target and nontarget stimuli.
| Schizophrenia Patients |
Healthy Controls |
|||
|---|---|---|---|---|
| Targets | Nontargets | Targets | Nontargets | |
| P100 | ||||
| Left | 0.88 (0.69) | 0.65 (0.77) | 0.93 (1.15) | 0.57 (0.46) |
| Right | 1.08 (1.11) | 0.86 (0.89) | 1.13 (1.27) | 0.96 (0.89) |
| N100 | ||||
| Left | -1.90 (1.57) | -1.86 (1.41) | -2.15 (2.62) | -1.75 (1.89) |
| Right | -2.22 (1.86) | -1.92 (1.50) | -2.23 (2.02) | -1.84 (1.32) |
Inline Supplementary Figure S2.
Inline Supplementary Table S2.
3.3.1. Sensory components
For the P100 there were main effects of hemisphere (F1, 41 = 7.21, p < 0.01, ηp2 = 0.15) and stimulus type (F1, 41 = 4.15, p < 0.05, ηp2 = 0.09). There was no significant main effect of group and no significant interactions. P100 was significantly greater in the right vs. left hemisphere and was significantly greater to targets vs. nontargets. For the N100 there was only a marginally significant main effect of stimulus type (F1, 41 = 3.80, p < 0.06, ηp2 = 0.09), with larger amplitudes to targets vs. nontargets. There was no significant main effect of group and no significant interactions.
3.3.2. Cognitive component
There were significant main effects of group (F1, 41 = 8.14, p < 0.01, η2 = 0.17) and stimulus type (F1, 41 = 88.71, p < 0.001, η2 = 0.68) and a significant group × stimulus type interaction (F1, 41 = 15.78, p < 0.001, η2 = 0.28). The interaction was due to controls having significantly greater P300 amplitudes than patients to target, but not nontarget, stimuli.
Statistical cluster plots comparing group amplitudes for target stimuli are shown in Fig. 2B. Controls had greater amplitudes to targets compared to patients in central-parietal to occipital electrodes at approximately 380–480 ms. Groups did not differ in their response to nontarget stimuli.
3.4. Joint analysis
3.4.1. Sensory components
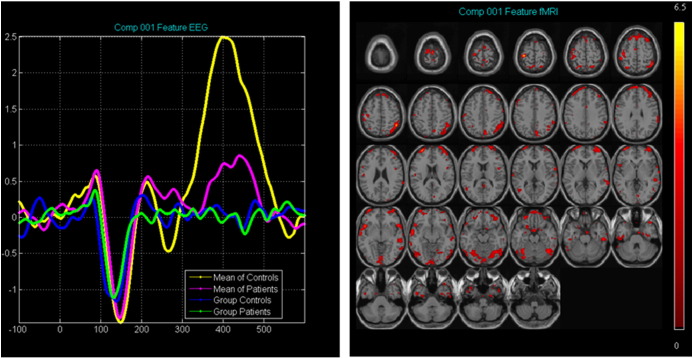
Results from the jICA of sensory ERPs and fMRI activity did not reveal any components that were significantly different between groups. However, one joint component, spanning the P100 and N100 responses, was identified and linked to activation primarily in the dorsal network, including the inferior frontal gyrus, inferior parietal lobule, middle temporal gyrus, and postcentral gyrus (see Inline Supplementary Fig. S3 and Inline Supplementary Table S3).
Fig. S3.
Results from the fMRI/ERP jICA analysis for the sensory components. A) Average ERP waveforms for controls (yellow) and patients (pink) overlaid onto the temporal aspect of the identified joint component for controls (blue) and patients (green). B) 2D rendering of the spatial aspect of the joint component. Activation can be seen in inferior frontal gyrus, inferior parietal lobule, middle temporal gyrus, and postcentral gyrus, areas included in the dorsal network.
Table S3.
Regions of joint P100-N100 ERP/fMRI activation to targets, corresponding Brodman Area, volume, and maximum z-score MNI coordinates.
| Regions | Brodman Area | L/R Volume (cc) | Max value (x, y, z) |
|---|---|---|---|
| Precentral Gyrus | 4 | 0.2/0.0 | 6.3 (-32, -22, 66)/-999.0 (0, 0, 0) |
| * | * | 0.1/0.0 | 3.6 (-28, -72, -12)/-999.0 (0, 0, 0) |
| Inferior Frontal Gyrus | 47 | 0.2/0.1 | 4.9 (-50, 15, -6)/4.1 (53, 17, -6) |
| Superior Frontal Gyrus | 6, 10 | 0.3/0.0 | 4.9 (-34, 59, 15)/-999.0 (0, 0, 0) |
| Inferior Parietal Lobule | 40 | 0.0/0.3 | -999.0 (0, 0, 0)/4.7 (44, -50, 54) |
| Middle Temporal Gyrus | 21 | 0.2/0.0 | 4.4 (-63, -4, -7)/-999.0 (0, 0, 0) |
| Declive | * | 0.3/0.0 | 4.3 (-30, -65, -12)/-999.0 (0, 0, 0) |
| Middle Frontal Gyrus | * | 0.1/0.1 | 4.2 (-28, 65, 13)/4.2 (38, 62, 1) |
| Postcentral Gyrus | 3 | 0.1/0.0 | 4.0 (-42, -19, 54)/-999.0 (0, 0, 0) |
| Precuneus | 7 | 0.0/0.1 | -999.0 (0, 0, 0)/4.0 (4, -72, 42) |
| Inferior Temporal Gyrus | * | 0.0/0.1 | -999.0 (0, 0, 0)/4.0 (55, -24, -21) |
| Fusiform Gyrus | 37 | 0.0/0.1 | -999.0 (0, 0, 0)/4.0 (42, -63, -12) |
| Sub-Gyral | * | 0.0/0.1 | -999.0 (0, 0, 0)/3.9 (38, -63, -10) |
| Lingual Gyrus | 18 | 0.0/0.1 | -999.0 (0, 0, 0)/3.7 (4, -86, -9) |
| Superior Temporal Gyrus | 22 | 0.1/0.0 | 3.7 (-59, 6, -5)/-999.0 (0, 0, 0) |
| Precentral Gyrus | 4 | 0.2/0.0 | 6.3 (-32, -22, 66)/-999.0 (0, 0, 0) |
Inline Supplementary Figure S3.
Inline Supplementary Table S3.
3.4.2. Cognitive component
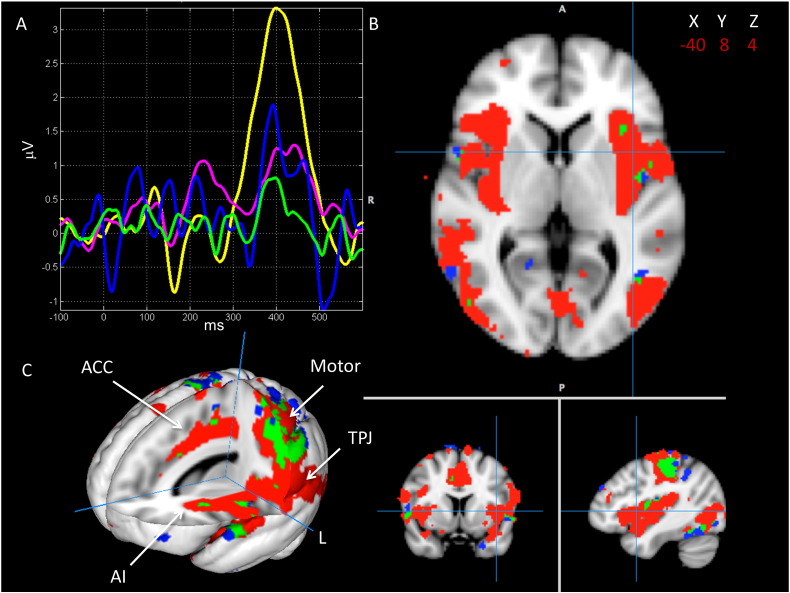
Results from the jICA of ERPs at Pz and fMRI activity to target stimuli are presented in Fig. 3. Only one joint component showed a significant between-group difference (p < 0.05) on the group loading parameters, suggesting a difference in the degree/magnitude of the linked fMRI-EEG brain features. Fig. 3A shows the ERP portion of the linked component, which maps onto the P300 response and is greater in controls. Fig. 3B and C shows spatial activation associated with this linked component for controls and patients, as well as overlapping activity between groups. Activation in regions corresponding to the ventral attention network, including the frontal operculum, AI, ACC, and TPJ, was observed in controls (in red). Patients (in blue) showed limited activity in these regions; minimal overlap (in green) with controls was observed. Areas of overlapping activation were seen mainly in motor cortex, i.e., pre- and postcentral gyri. Talairach coordinates of activations associated with this component for both groups are shown in Table 2. None of the other seven identified joint components differed significantly between groups and none included the ventral network.
Fig. 3.
Results from the fMRI/ERP jICA analysis at electrode Pz, showing the joint component with a significantly different loading parameter (p < 0.05) for controls vs. patients. A) Average ERP waveforms for controls (yellow) and patients (pink) overlaid onto the temporal aspect of the identified joint component for controls (blue) and patients (green). B) 2D rendering of the spatial aspect of the joint component for controls (red), patients (blue), and areas of group overlap (green). Crosshairs centered on left insula on MNI coordinates as shown. C) 3D rendering of the spatial component. Arrows point to activation in anterior cingulate cortex (ACC), anterior insula (AI), and temporo-parietal junction (TPJ) and motor cortex.
Table 2.
Regions of joint P300 ERP/fMRI activation to targets, corresponding Brodman area, volume, and maximum z-score MNI coordinates.
| Regions | Brodman area | Volume (cc) | Max value (x, y, z) |
|---|---|---|---|
| Patients | |||
| Postcentral gyrus | 3, 40 | 0.5/0.1 | 5.6 (−30, −28, 66)/3.7 (55, −17, 14) |
| Superior temporal gyrus | 22 | 0.1/0.0 | 4.7 (−53, 11, −6)/−999.0 (0, 0, 0) |
| Sub-gyral | * | 0.0/0.2 | −999.0 (0, 0, 0)/4.6 (42, −65, −10) |
| Fusiform gyrus | 37 | 0.2/0.4 | 4.4 (−38, −53, −18)/3.9 (38, −53, −14) |
| Precentral gyrus | 4 | 0.3/0.0 | 4.4 (−57, −17, 41)/−999.0 (0, 0, 0) |
| Medial frontal gyrus | 6 | 0.1/0.0 | 4.2 (−4, −3, 55)/−999.0 (0, 0, 0) |
| Inferior frontal gyrus | * | 0.1/0.0 | 4.1 (−53, 15, −6)/−999.0 (0, 0, 0) |
| Culmen | * | 0.1/0.0 | 4.0 (−34, −51, −16)/−999.0 (0, 0, 0) |
| Superior frontal gyrus | 6 | 0.0/0.1 | −999.0 (0, 0, 0)/3.8 (6, 1, 66) |
| Middle occipital gyrus | * | 0.0/0.1 | −999.0 (0, 0, 0)/3.6 (42, −68, −8) |
| Controls | |||
| Rectal gyrus | 11 | 0.2/0.0 | 4.9 (−2, 18, −19)/−999.0 (0, 0, 0) |
| Postcentral gyrus | 1, 2, 3, 4, 40 | 3.0/1.3 | 4.9 (−57, −19, 41)/3.8 (57, −28, 18) |
| Precentral gyrus | 4, 6, 44 | 2.1/0.1 | 4.4 (−57, −18, 38)/2.8 (63, −14, 38) |
| Inferior parietal lobule | 40 | 0.5/1.2 | 3.1 (−59, −34, 22)/4.0 (67, −28, 24) |
| Cingulate gyrus | 24, 32 | 2.0/1.2 | 3.9 (−4, 8, 36)/3.5 (4, 4, 40) |
| Medial frontal gyrus | 6, 32 | 0.5/0.4 | 3.9 (0, −1, 50)/3.5 (4, 1, 53) |
| Inferior frontal gyrus | 44, 45, 47 | 0.1/1.2 | 2.3 (−36, 23, 3)/3.8 (34, 25, 1) |
| Paracentral lobule | 31 | 0.3/0.0 | 3.7 (−4, −11, 43)/−999.0 (0, 0, 0) |
| Precuneus | 7 | 0.1/0.8 | 2.7 (−6, −70, 39)/3.6 (12, −70, 46) |
| Posterior cingulate | 23, 30 | 0.1/0.1 | 2.8 (−4, −65, 12)/3.4 (2, −61, 14) |
| Insula | 13, 40 | 0.8/1.0 | 3.1 (−32, 23, 3)/3.4 (34, 21, 3) |
| Cuneus | * | 0.1/0.0 | 3.3 (−2, −66, 9)/−999.0 (0, 0, 0) |
| Middle temporal gyrus | 37, 39 | 0.3/0.0 | 3.0 (−48, −66, 7)/−999.0 (0, 0, 0) |
| Extra-nuclear | 47 | 0.0/0.1 | −999.0 (0, 0, 0)/3.0 (36, 21, −1) |
| Superior temporal gyrus | 22, 38, 41, 42 | 0.6/1.1 | 3.0 (−53, −59, 16)/3.0 (67, −32, 18) |
| Superior frontal gyrus | 6 | 0.1/0.0 | 3.0 (−10, 11, 64)/−999.0 (0, 0, 0) |
In post-hoc analyses we explored the relationship between the joint component, BPRS ratings, performance (d′), and antipsychotic chlorpromazine equivalents. There was a significant negative correlation between the joint component and ratings of unusual thought content, (Spearman's rho = −0.45, p < 0.05), indicating higher levels of unusual thought content were associated with lower joint activation levels. No other significant correlations were found with BPRS items or factors. In patients, the joint component was not significantly correlated with d′ for EEG or fMRI (Spearman's rho = 0.26 and 0.38, respectively, p's > 0.11). In controls, the joint component was significantly correlated with d′ for EEG (Spearman's rho = 0.47, p < 0.04), but not for fMRI (Spearman's rho = 0.27, p < 0.26). However, given that performance was at or near ceiling in a majority of the controls the significant correlation should be interpreted with caution. There was no significant relationship between chlorpromazine equivalent and the joint component (Spearman's rho = −0.36, p < 0.19).
4. Discussion
The current study used a multimodal imaging approach to examine the association between the ventral and dorsal attentional networks and target detection in schizophrenia using an oddball task, yielding several findings. First, fMRI revealed that both groups activated regions associated within the dorsal and ventral attention networks, with group differences evident in both, including frontal, parietal, and occipital regions. Second, ERP indicated that patients exhibited reduced P300 responses to targets in comparison to controls, but the groups did not differ in ERPs to nontargets. Furthermore, while attention enhanced the P100 and N100, there were no between-group differences in these early sensory components. Finally, joint activation in the P300 ERP and fMRI using an ICA approach demonstrated that target detection was associated with activation specific to the ventral attention network, and this joint ERP–fMRI component was significantly smaller in schizophrenia patients vs. healthy controls. Taken together, schizophrenia patients exhibited neural deficits in target detection that are reflected in reduced P300 and associated with dysfunction in the ventral attentional network.
The joint spatiotemporal analysis revealed notable findings that were not discernible when analyzing the ERP and fMRI results separately. Based on results from fMRI alone it was difficult to determine which attentional process (i.e., orienting to salient stimuli in the environment versus sustaining general attention to the task) was dysfunctional during target detection because both ventral and dorsal networks were activated. By combining the spatial advantages of fMRI with the temporal advantages of ERPs, we determined that the ventral network specifically was associated with the P300 response. The jICA results revealed that this component showed activation in the frontal operculum, AI, ACC, and TPJ, which correspond to regions in the ventral attention network (Kim, 2014), in which patients exhibited a significant decrease in magnitude and amplitude. In contrast, deficits were not seen in patients in early sensory ERP components nor in the linked ERP/fMRI component. These findings suggest that 1) dysfunction specific to the ventral attention network serves as a neural substrate for impaired target detection in schizophrenia; and 2) early sensory processing of the targets was associated with processing in the dorsal network and, importantly, was not dysfunctional in the patients with schizophrenia. Given that our method focused on visual target detection in an oddball task, it remains to be seen whether a similar pattern of results is present when using other visual task paradigms or other sensory modalities. However, based on the results in the Kim (2014) meta-analysis that the ventral network is activated using both auditory and visual oddball paradigms, it is plausible that such a general deficit could occur.
Target detection during an oddball task has traditionally been thought to assess several different but related constructs, including allocation of attentional resources, updating of working memory, and salience (Donchin and Coles, 1988; Mathalon et al., 2010; Polich, 1989; Sutton et al., 1967). The main regions of patient–control differences we found in the joint analysis during oddball detection, including ACC, AI, and TPJ, have been associated with target detection based on salience. The ACC has been associated with action selection and execution, as well as adaptive actions in response to salient signals (Harsay et al., 2012). The AI has been shown to be involved with evaluating motivational and emotional salience and serves as an intermediary between processing of external information and internal cognitive-motivational states (Seeley et al., 2007). The ACC and AI have shown to be functionally connected during various tasks that involve orienting to and processing salient emotional, social, and cognitive stimuli (Harsay et al., 2012). Finally, the TPJ has also been associated with salience detection, as well as pain and stimulus-driven attention (Downar et al., 2002; Kucyi et al., 2012). These three key regions have been associated with the so-called “salience network.” The ventral attentional network and the salience network are closely related, and may, in fact, comprise a single network (Kucyi et al., 2012).
Interestingly, it has been proposed that dysfunction in the salience network may be a key part of the pathophysiology of schizophrenia, resulting in the incorrect assigning of salience to irrelevant internal or external signals that then lead to the formation of a primary delusion (Palaniyappan and Liddle, 2012). For example, reduced volumes in the ACC and AI in schizophrenia have been associated with worse delusions and hallucinations (Palaniyappan et al., 2011), and a meta-analysis of fMRI and positron emission tomography studies also implicated insular dysfunction in patients with active auditory hallucinations (Jardri et al., 2011). Post-hoc analyses indicated that the magnitude of the joint component was significantly negatively correlated with unusual thought content (i.e., delusions). This finding, though not able to withstand corrections for multiple tests, is consistent with the hypothesis that salience network dysfunction is associated with positive symptoms in schizophrenia.
The only other oddball study using jICA on fMRI and ERP in schizophrenia (Calhoun et al., 2010) used auditory stimuli. That study found a deficit in schizophrenia in the joint component between the auditory N2 ERP and bilateral fronto-temporal fMRI activation (including superior frontal gyrus, superior temporal gyrus, middle temporal and frontal gyri, inferior frontal gyrus, and precentral gyrus). The second largest group difference was associated with the P300 joint component; however, the difference did not reach significance and they did not report the associated spatial network. Differences in patient and control samples and methods (i.e., auditory vs. visual stimuli) between that paper and the current paper may explain the different findings. It remains to be seen whether abnormalities in the ventral attention network in schizophrenia appear across visual and auditory modalities, as well as across various attentional tasks.
Some limitations of the study should be mentioned. First, all patients were taking antipsychotic medication at the time of testing which may have affected the results. However, it has been reported that P300 (Jeon and Polich, 2003) and oddball fMRI activation (Kiehl et al., 2001) may improve on antipsychotic medication. In the current study, chlorpromazine equivalents were not significantly associated with performance, the P300 or the joint component, thus making any potential effect of medication on the findings unlikely. Second, EEG and fMRI recordings occurred in separate sessions, though attempts were made to keep the time between recording sessions brief. Changes in symptoms could have occurred and affected the results; however, the sample was clinically stable. Third, jICA was restricted to a limited subset of electrodes, failing to take into account the full amount of temporo-spatial information contained in the high-density ERP data, which is an acknowledged limitation of the FIT program. However, electrodes were chosen that best represent the components of interest. Finally, our choice of using an implicit baseline rather than non-targets as the main contrast to targets is a potential limitation. While a target vs. nontarget contrast would be the ideal method to separate out neural activity generated specifically by targets as opposed to objects in general (i.e., both targets and nontargets), this contrast was not easily interpretable. There were wide-spread differences in activation observed in both groups (though no significant between group effects), most likely due to deactivation associated with the frequent, repetitive nontargets presented. Despite these limitations the results of the current study add valuable information to understanding spatio-temporal neural dynamics of target detection in schizophrenia.
In summary, the current study replicates and extends prior research on visual target detection in schizophrenia. The findings with the joint use of ERP and fMRI suggest that deficits in an oddball task in schizophrenia are associated with dysfunction specifically in the ventral attention network. This joint approach revealed the nature of deficits that were not discernable by either imaging methodology alone, highlighting the utility of a multimodal approach to understand attentional network deficits in schizophrenia by combining fMRI with ERP.
Funding
This work was supported by a Veterans Affairs (VA) Career Development Award (Wynn 0001) to JKW; VA Merit Review Grant (I01CX000497) and National Institutes of Mental Health (MH58262) to JMF; and National Institutes of Mental Health grants MH43292 and MH065707 to MFG. For generous support, we also thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund. The funding sources had no role in: the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Acknowledgements
The authors thank Poorang Nori and Crystal Gibson for assistance in data collection. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
The response window to targets (3000 ms) overlapped with the onset of non-targets. This could potentially result in a response to a non-target being registered as a response to the target. When limiting RTs to ≤1000 ms (i.e., the RT that could potentially overlap with the 1 s ITI) there was no appreciable change in accuracy rates, with the modal and median difference between the limited vs. full data set being zero. Furthermore, when examining RTs to targets for each of the three ITIs (1, 2 or 3 s) separately, only two participants had a maximum RT slower than 1000 ms for the 1 s ITI, and no participants had a slower RT than 2000 ms for either the 2 or 3 s ITI. These analyses reassure us that, while a couple of responses might have been slower or potentially in response to the distractor after a target, the vast majority of participants were responding faster than 1000 ms and responding almost exclusively to the target.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.07.004.
Appendix A. Supplementary data
Inline Supplementary Methods
Inline Supplementary Results
References
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. 19897178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. 14568475 [DOI] [PubMed] [Google Scholar]
- Bledowski C., Prvulovic D., Hoechstetter K., Scherg M., Wibral M., Goebel R., Linden D.E. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J. Neurosci. 2004;24(42):9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. 15496671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramon E., Rabe-Hesketh S., Sham P., Murray R.M., Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res. 2004;70(2–3):315–329. doi: 10.1016/j.schres.2004.01.004. 15329307 [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Kiehl K.A. Neuronal chronometry of target detection: fusion of hemodynamic and event-related potential data. Neuroimage. 2006;30(2):544–553. doi: 10.1016/j.neuroimage.2005.08.060. 16246587 [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Wu L., Kiehl K., Eichele T., Pearlson G. Aberrant processing of deviant stimuli in schizophrenia revealed by fusion of FMRI and EEG data. Acta Neuropsych. 2010;22(3):127–138. doi: 10.1111/j.1601-5215.2010.00467.x. 21331320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz B.A., Wang J., Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J. Neurosci. 2008;28(50):13411–13418. doi: 10.1523/JNEUROSCI.4095-08.2008. 19074014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. 11994752 [DOI] [PubMed] [Google Scholar]
- Cornblatt B.A., Erlenmeyer-Kimling L. Global attentional deviance as a marker of risk for schizophrenia: specificity and predictive validity. J. Abnorm. Psychol. 1985;94(4):470–486. doi: 10.1037//0021-843x.94.4.470. 3865953 [DOI] [PubMed] [Google Scholar]
- Donchin E., Coles M.G.H. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988;11(03):357–427. [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J. Neurophysiol. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. 11784775 [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbons M., Spitzer R.L., Williams J.B.W. Biometrics Research Department; New York: 1996. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. [Google Scholar]
- First M.B., Gibbons M., Spitzer R.L., Williams J.B.W. Biometrics Research Department; New York: 1996. Users Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders-Research Version (SCID-1, Version 2.0, February 1996 Final Version) [Google Scholar]
- Ford J.M. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. 10554581 [PubMed] [Google Scholar]
- Ford J.M., Roach B.J., Miller R.M., Duncan C.C., Hoffman R.E., Mathalon D.H. When it's time for a change: failures to track context in schizophrenia. Int. J. Psychophysiol. 2010;78(1):3–13. doi: 10.1016/j.ijpsycho.2010.05.005. 20580752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.M., White P., Lim K.O., Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol. Psychiatry. 1994;35(2):96–103. doi: 10.1016/0006-3223(94)91198-3. 8167215 [DOI] [PubMed] [Google Scholar]
- Foxe J.J., Doniger G.M., Javitt D.C. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12(17):3815–3820. doi: 10.1097/00001756-200112040-00043. 11726801 [DOI] [PubMed] [Google Scholar]
- Gur R.E., Turetsky B.I., Loughead J., Snyder W., Kohler C., Elliott M., Pratiwadi R., Ragland J.D., Bilker W.B., Siegel S.J., Kanes S.J., Arnold S.E., Gur R.C. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am. J. Psychiatry. 2007;164(3):442–449. doi: 10.1176/ajp.2007.164.3.442. 17329469 [DOI] [PubMed] [Google Scholar]
- Guthrie D., Buchwald J.S. Significance testing of difference potentials. Psychophysiology. 1991;28(2):240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. 1946890 [DOI] [PubMed] [Google Scholar]
- Harsay H.A., Spaan M., Wijnen J.G., Ridderinkhof K.R. Error awareness and salience processing in the oddball task: shared neural mechanisms. Front. Hum. Neurosci. 2012;6:246. doi: 10.3389/fnhum.2012.00246. 22969714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C.S., Knight R.T. Mechanisms of human attention: event-related potentials and oscillations. Neurosci. Biobehav. Rev. 2001;25(6):465–476. doi: 10.1016/s0149-7634(01)00027-6. 11595268 [DOI] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. 20952459 [DOI] [PubMed] [Google Scholar]
- Jeon Y.W., Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. 14696723 [DOI] [PubMed] [Google Scholar]
- Kiehl K.A., Laurens K.R., Duty T.L., Forster B.B., Liddle P.F. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38(1):133–142. 11321614 [PubMed] [Google Scholar]
- Kiehl K.A., Stevens M.C., Celone K., Kurtz M., Krystal J.H. Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biol. Psychiatry. 2005;57(9):1029–1040. doi: 10.1016/j.biopsych.2005.01.035. 15860344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.I., Mathalon D.H., Ford J.M., Mannell M., Turner J.A., Brown G.G., Belger A., Gollub R., Lauriello J., Wible C., O'Leary D., Lim K., Toga A., Potkin S.G., Birn F., Calhoun V.D. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr. Bull. 2009;35(1):67–81. doi: 10.1093/schbul/sbn133. 19074498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta-analysis. Hum. Brain Mapp. 2014;35(5):2265–2284. doi: 10.1002/hbm.22326. 23900833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss I., Dashieff R.M., Lordeon P. A parieto-occipital generator for P300: evidence from human intracranial recordings. Int. J. Neurosci. 1989;49(1–2):133–139. doi: 10.3109/00207458909087048. 2514154 [DOI] [PubMed] [Google Scholar]
- Kopelowicz A., Ventura J., Liberman R.P., Mintz J. Consistency of brief psychiatric rating scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41(2):77–84. doi: 10.1159/000111551. 18033976 [DOI] [PubMed] [Google Scholar]
- Kucyi A., Hodaie M., Davis K.D. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J. Neurophysiol. 2012;108(12):3382–3392. doi: 10.1152/jn.00674.2012. 23019004 [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., III Building a clinically relevant cognitive task: case study of the AX paradigm. Schizophr. Bull. 2008;34(4):619–628. doi: 10.1093/schbul/sbn038. 18487225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado S., Arias-Carrión O., Sampaio I., Bittencourt J., Velasques B., Teixeira S., Nardi A.E., Piedade R., Ribeiro P. Source imaging of P300 visual evoked potentials and cognitive functions in healthy subjects. Clin. E.E.G. Neurosci. 2014:1–7. doi: 10.1177/1550059413514389. 24615930 [DOI] [PubMed] [Google Scholar]
- Martínez A., Hillyard S.A., Bickel S., Dias E.C., Butler P.D., Javitt D.C. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb. Cortex. 2012;22(6):1282–1293. doi: 10.1093/cercor/bhr195. 21840846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon D.H., Hoffman R.E., Watson T.D., Miller R.M., Roach B.J., Ford J.M. Neurophysiological distinction between schizophrenia and schizoaffective disorder. Front. Hum. Neurosci. 2010;3:70. doi: 10.3389/neuro.09.070.2009. 20140266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G., Wood C.C. Intracranial recordings of endogenous ERPs in humans. Electroencephalogr. Clin. Neurophysiol. 1987;39:331–337. 3477444 [PubMed] [Google Scholar]
- Molholm S., Ritter W., Murray M.M., Javitt D.C., Schroeder C.E., Foxe J.J. Multisensory auditory–visual interactions during early sensory processing in humans: A high-density electrical mapping study. Cogn. Brain Res. 2002;14(1):115–128. doi: 10.1016/s0926-6410(02)00066-6. 12063135 [DOI] [PubMed] [Google Scholar]
- Mulert C., Jäger L., Schmitt R., Bussfeld P., Pogarell O., Möller H.J., Juckel G., Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22(1):83–94. doi: 10.1016/j.neuroimage.2003.10.051. 15109999 [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Dawson M.E. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10(2):160–203. doi: 10.1093/schbul/10.2.160. 6729409 [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Liddle P.F. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012;37(1):17–27. doi: 10.1503/jpn.100176. 21693094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L., Mallikarjun P., Joseph V., White T.P., Liddle P.F. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol. Med. 2011;41(8):1701–1708. doi: 10.1017/S0033291710002205. 21144116 [DOI] [PubMed] [Google Scholar]
- Polich J. P300 from a passive auditory paradigm. Electroencephalogr. Clin. Neurophysiol. 1989;74(4):312–320. doi: 10.1016/0168-5597(89)90061-0. 2471632 [DOI] [PubMed] [Google Scholar]
- Schechter I., Butler P.D., Zemon V.M., Revheim N., Saperstein A.M., Jalbrzikowski M., Pasternak R., Silipo G., Javitt D.C. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin. Neurophysiol. 2005;116(9):2204–2215. doi: 10.1016/j.clinph.2005.06.013. 16055375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. 17329432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S.M., Berten S., Essex B., All S.D., Kasi R., Little D.M. Perceptual organization and visual search processes during target detection task performance in schizophrenia, as revealed by fMRI. Neuropsychologia. 2010;48(10):2886–2893. doi: 10.1016/j.neuropsychologia.2010.05.030. 20678981 [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Moo L.R., Segal J.B., Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn. Brain Res. 2003;17(1):75–82. doi: 10.1016/s0926-6410(03)00082-x. 12763194 [DOI] [PubMed] [Google Scholar]
- Sponheim S.R., McGuire K.A., Stanwyck J.J. Neural anomalies during sustained attention in first-degree biological relatives of schizophrenia patients. Biol. Psychiatry. 2006;60(3):242–252. doi: 10.1016/j.biopsych.2005.11.017. 16460700 [DOI] [PubMed] [Google Scholar]
- Sutton S., Tueting P., Zubin J., John E.R. Information delivery and the sensory evoked potential. Science. 1967;155(3768):1436–1439. doi: 10.1126/science.155.3768.1436. 6018511 [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. 18407525 [DOI] [PubMed] [Google Scholar]
- Ventura J., Nuechterlein K.I., Subotnik K., Gilbert E. 1995. Symptom Dimensions in Recent-onset Schizophrenia: The 24-Item Expanded BPRS. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. 15050594 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inline Supplementary Methods
Inline Supplementary Results