Abstract
Background and objectives
While the literature supports the idea that multiple sclerosis (MS) and migraine are related, the exact mechanism(s) of this association is not well understood. Observations of increased contrast enhancing (CE) lesion activity in individual MS patients suffering from migraine prompted us to determine a relationship between migraine and MRI outcomes in a large cohort of MS patients.
Methods
We included 509 MS and 64 clinically isolated syndrome (CIS) patients and 251 age- and sex-matched healthy individuals (HIs) who obtained 3 T MRI and were assessed for history of migraine. Number and volume of T2, T1 and CE lesions and brain volume measures were determined. The MRI findings were analyzed adjusting for key covariates and correcting for multiple comparisons.
Results
More MS (22.2%) and CIS (17.2%) patients had migraine, compared to HIs (14.6%, p = 0.067). More MS patients with migraine presented with CE lesions compared to those without (35.4% vs. 23.7%, p = 0.013). MS migraine patients had significantly increased number (p = 0.019) and volume (p = 0.022) of CE lesions compared to those without. In the regression analysis, MS migraine patients had an increased number of CE lesions (B = 1.242, p = 0.001), specifically those with relapsing–remitting disease course (B = 1.377, p = 0.001). No significant association of other MRI measures and migraine was found in MS and CIS patients or in HIs.
Conclusions
Our findings suggest an increased inflammatory pathobiology in MS patients with migraine headaches requiring possibly more frequent MRIs and also more efficient anti-inflammatory treatment.
Keywords: Multiple sclerosis, Migraine MRI, Contrast-enhancing lesions, Controls
Highlights
-
•
We examined 509 MS and 64 CIS patients and 251 healthy individuals.
-
•
Subjects were assessed with 3 T MRI and for history of migraine.
-
•
More MS and CIS patients had migraine, compared to healthy controls.
-
•
More MS migraine patients presented with enhancing brain lesions compared to those without.
-
•
MS migraine patients had an increased number and volume of enhancing lesions.
1. Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system characterized by demyelination and axonal loss. Signs and symptoms can vary widely throughout the disease course. While sensitive, optic and motor symptoms predominate, other symptomatology can include urinary tract and bowel dysfunction, pain, fatigue, cognitive dysfunction and mood disorders (Samkoff and Goodman, 2011).
Several case–control studies have established a higher rate of headache in MS population compared to the healthy individuals (HIs) (Kister et al., 2010; Nicoletti et al., 2008; Vacca et al., 2007; Watkins and Espir, 1969; Zorzon et al., 2003). The potential link between MS and migraine has been hypothesized for over 40 years (Elliott, 2007; Watkins and Espir, 1969). It has been recently shown that MS patients had a three-fold higher frequency of migraine compared to the HIs (Kister et al., 2010). Another most recent meta-analysis found that migraine was more common in 1864 MS patients compared to the 261,563 control subjects, with an odds ratio of 2.6, however there was a heterogeneity between the examined studies (Pakpoor et al., 2012). On the contrary, another German study did not find a higher prevalence of headache or migraine in 491 MS patients compared to 447 controls (Putzki et al., 2009).
While the literature supports the idea that MS and migraine are related, the exact mechanism(s) of this association is not well understood (Kister et al., 2010; Nicoletti et al., 2008; Pakpoor et al., 2012; Vacca et al., 2007; Watkins and Espir, 1969; Zorzon et al., 2003). Is migraine simply a comorbidity of MS? Can it signal the onset of MS? If so, what is the underlying pathology? Is it increased frequency related to use of disease-modifying treatment (DMT)? These questions have important implications for diagnosis and treatment of MS and need to be addressed in the future studies. Several studies investigated frequency of migraine in relation to the clinical course of MS and determined that the migraine frequency is increased in patients with relapsing–remitting (RR) MS, whereas the tension-type headache is more frequent in patients with chronic progressive MS (D'Amico et al., 2004; Ergun et al., 2009; Moisset et al., 2013; Villani et al., 2008). A recent case report illustrated a young woman whose initial presentation was that of status migrainosus, after which she developed MS within 2 years (Alroughani et al., 2015). The term radiologically isolated syndrome (RIS) has been recently proposed to describe patients with atypical symptoms of MS who present with MRI features suggestive of underlying demyelinating pathology (Okuda et al., 2009). One of the most common complaints in RIS subjects which led to performance of the MRI examination was migraine type headache (Lebrun et al., 2008; Okuda et al., 2009). This suggests that migraine can be prominent symptom at early onset of the disease, in addition to have increased comorbidity frequency in RRMS patients.
It has been recently reported in a case study that a patient who had a history of migraine, experienced worsening of migraine-headache symptoms as the initial manifestation of MS, and showed concomitant asymptomatic contrast enhancing (CE) lesions on T1-weighted MRI after gadolinium (Gd) contrast injection (Lin et al., 2013). This case report raises question, as to the role of CE lesions in the presentation of migraine in MS patients. Our own clinical routine observations prompted us to investigate whether the number and volume of CE lesions are associated to increased frequency of migraine in MS patients. In addition, we aimed to determine if there is relationship between migraine and the presence of other lesion and global and tissue specific brain volume measures in patients with MS.
2. Methods
2.1. Subjects
This study used data from an ongoing prospective study of cardiovascular, genetic and environmental risk factors in MS that enrolled over 1000 patients with clinically isolated syndrome (CIS), MS, other neurological diseases and HIs (Kappus et al., 2015; O'Connor et al., 2012; Zivadinov et al., 2011). The inclusion criteria for this sub-study of migraine in MS were as follows: a) age 18–75 years old, b) having CIS or MS, or being HI and c) MRI examination performed at the time of the clinical visit (±30 days) with standardized 3 T MRI protocol. The exclusion criteria were as follows: a) the presence of a relapse or steroid treatment within 30 days preceding study entry for MS and CIS patients, b) presence of acute headache attack at the time of MRI, c) pre-existing medical conditions known to be associated with brain pathology and d) pregnancy.
Subjects were assessed with structured environmental questionnaire followed by physical and neurological examination. Migraine data were collected from participants in an in-person interview by a trained interviewer, as well as by examination of the patients' medical records (Dolic et al., 2011). Migraine was defined according to the International Classification of Headache Disorders (ICHD-2) guidelines (Headache Classification Subcommittee of the International Headache Society, 2004). Subjects who had at least 5 headache attacks in the past that lasted 4–72 h, had at least two of the following characteristics (unilateral location, pulsating quality, moderate or severe pain intensity, aggravation by or causing avoidance of routine physical activity), presence of nausea/vomiting, and were not better accounted by another disorder were classified as migraine headache.
HIs were recruited from hospital personnel, spouses of MS patients or were respondents of local advertisements. By definition HIs were required to have a normal neurological examination and have a normal MRI health screen. Race/ethnicity was determined according to the US Census Bureau definitions.
The study protocol was approved by the local Institutional Review Board and all participants gave their written informed consent.
2.2. MRI acquisition and analysis
All subjects were examined on a 3T GE Signa Excite HD 12.0 Twin Speed 8-channel scanner (General Electric, Milwaukee, WI). MRI sequences included the axial dual fast spin-echo (FSE) T2/PD-weighted image (WI), 3D-spoiled-gradient recalled (SPGR) T1-WI, spin echo (SE) T1-WI pre- and post-contrast, and fluid attenuated inversion recovery (FLAIR) scans. Pulse sequence characteristics for 3 T MRI were as follows: all scans were acquired with a 256 × 256 matrix and a 25.6 cm field of view (FOV) for an in-plane resolution of 1 × 1 mm2 with a phase FOV of 75% and one average. Sequence specific parameters were as follows: for the T2/PD-WI: 3-mm-thick slices with no gap, TE1/TE2/TR = 12/95/3000 ms, echo train length = 14, flip angle = 90°; for the FLAIR scans, 3-mm thick slices with no gap, TE/TI/TR = 120/2100/8500 ms, flip angle = 90°; for 3D T1-WI, 1-mm thick slices with no gap, TE/TI/TR = 2.8/900/5.9 ms, flip angle = 10° and for SE T1-WI, 3-mm thick slices with no gap, TE/TR = 16/600 ms, flip angle = 90°. The SE T1-WI sequence was obtained after injection of a single dose intravenous bolus (0.1 mmol/kg gadolinium-DTPA) 5 min after administration of contrast agent only in MS and CIS patients (Kappus et al., 2015; Zivadinov et al., 2012).
The following MRI measures were collected: CE, T1 and T2 lesion number and lesion volumes (LVs), assessed by a semi-automated edge detection contouring/thresholding technique, as previously reported (Zivadinov et al., 2012). Measures of brain atrophy included normalized brain volume, normalized gray matter (GM) volume, normalized white matter (WM) volume, normalized cortical volume and normalized lateral ventricle volume (Kappus et al., 2015; Zivadinov et al., 2012), assessed by the SIENAX 2.6 cross-sectional software tool (Smith et al., 2002).
2.3. Statistical analysis
All data were analyzed using Statistical Package for Social Science (SPSS), version 20.0 (IBM, Armonk, NY). The data were analyzed both by disease group (MS, CIS and HIs) and by MS disease subtype (RR, SP and PP). Differences in demographic and clinical characteristics between subjects with and without migraine were analyzed using chi-square test, Mann–Whitney rank sum test and analysis of variance (ANOVA), as appropriate. In the analysis of covariance (ANCOVA), MRI outcomes were input as the dependent variables, and disease group or MS disease subtype, as the grouping measure with age, gender and DMT status selected as covariates. Given the colinearity between age and disease duration, the latter was not used as covariate.
Additionally, the data were also analyzed using a negative binomial regression given the non-normally distributed lesion data. The inputs into the model included the number of CELs as the dependent measure and migraine status, age, gender and DMT status as the independent variables. Regression analysis was applied to both the disease groups and MS subtypes. The Benjamini–Hochberg correction was used to control the false discovery rate and p-values < 0.05 were considered significant using two-tailed testing (Benjamini et al., 2001).
3. Results
3.1. Demographic and clinical characteristics
A total of 826 subjects who fulfilled inclusion and exclusion criteria were selected for this substudy of migraine and MRI. These included 320 patients with RRMS, 124 patients with secondary-progressive (SP) MS, 36 patients with primary-progressive (PP) MS, 64 patients with CIS and 251 HIs. As expected MS patients had higher age, longer disease duration and more advanced disability compared to CIS patients.
Tables 1 and 2 show the differences in the study groups between the subjects with or without migraine. In the MS group there was significantly more females with migraine (p = 0.01). The age of the MS patients with migraine was younger (p = 0.001) and disease duration was shorter (p = 0.024). In the MS disease subtype analyses, the SPMS patients with migraine were younger (p = 0.015), while the PPMS patients with migraine were younger (p = 0.032) and had lower disease duration (p = 0.039). The CIS patients with migraine were younger compared to those without migraine (p = 0.001).
Table 1.
Characteristics of subjects with and without migraine in patients with multiple sclerosis and clinically isolated syndrome, and in healthy individuals.
| HIs (n = 251) |
MS (n = 509) |
CIS (n = 64) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| W/o migraine (n = 214) |
Migraine (n = 37) |
p-Value | W/o migraine (n = 396) | Migraine (n = 113) | p-Value | W/o migraine (n = 53) | Migraine (n = 11) | p-Value | |
| Female, n (%) | 136 (63.6) | 28 (75.7) | 0.135 | 266 (67.2) | 95 (84.1) | 0.01 | 38 (71.7) | 10 (90.1) | 0.181 |
| Age in years, mean (SD) | 43.0 (17.4) | 43.6 (13.9) | 0.852 | 46.9 (12.4) | 42.4 (13.0) | 0.001 | 39.9 (11.3) | 38.3 (9.9) | 0.001 |
| Age at onset in years, mean (SD) | NA | NA | NA | 32 (10.7) | 29.8 (10.5) | 0.066 | 36.0 (11.1) | 31.9 (10.5) | 0.266 |
| Disease duration in years, mean (SD) | NA | NA | NA | 15 (10.7) | 12.4 (9.6) | 0.024 | 1.3 (2.1) | 1.4 (2.2) | 0.115 |
| BMI, mean (SD) | 26.8 (5.5) | 26.9 (5.5) | 0.95 | 26.7 (5.5) | 26.5 (5.9) | 0.745 | 27.2 (6) | 26.5 (5.5) | 0.734 |
| Presence of DMT, n (%) | NA | NA | NA | 284 (71.7) | 83 (73.5) | 0.884 | 26 (49.1) | 4 (36.3) | 0.634 |
| Interferon-beta 1a | 140 (49.3) | 34 (41) | 20 (76.9) | 3 (75) | |||||
| Glatiramer acetate | 73 (25.7) | 27 (32.5) | 6 (23.1) | 1 (25) | |||||
| Natalizumab | 56 (19.7) | 18 (21.7) | 0 (0) | 0 (0) | |||||
| Mycophenolate mofetil | 6 (2.1) | 1 (1.2) | 0 (0) | 0 (0) | |||||
| Intravenous immunoglobulin | 5 (1.8) | 2 (2.4) | 0 (0) | 0 (0) | |||||
| Azathioprine | 3 (1.1) | 1 (1.2) | 0 (0) | 0 (0) | |||||
| Mitoxantrone | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | |||||
| EDSS, mean (IQR) | NA | NA | NA | 3.6 (2.2) | 3.2 (1.8) | 0.082 | 1.3 (.7) | 1.9 (1.7) | 0.092 |
| Race, n (%) | 0.168 | 0.337 | |||||||
| White | 183 (85.5) | 33 (89.2) | 373 (94.2) | 103 (91.2) | 48 (90.5) | 11 (100) | 0.771 | ||
| Hispanic/Latino | 2 (0.9) | 1 (2.7) | 4 (1.0) | 4 (3.5) | 2 (3.8) | 0 (0) | |||
| Black/African/American | 16 (7.5) | 2 (5.4) | 15 (3.8) | 5 (4.4) | 2 (3.8) | 0 (0) | |||
| Asian | 8 (3.7) | 0 (0) | 2 (0.5) | 0 (0) | 1 (1.9) | 0 (0) | |||
| American Indian/Alaska native | 0 (0) | 1 (2.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Other | 5 (2.3) | 0 (0) | 2 (0.5) | 1 (0.9) | 0 (0) | 0 (0) | |||
HIs—healthy individuals; MS—multiple sclerosis; CIS—clinically isolated syndrome; SD—standard deviation; EDSS—Expanded Disability Status Scale; DMT—disease modifying therapy; NA—not available; BMI—body mass index; IQR— interquartile range.
The comparison between the migraine and non-migraine subjects was performed using chi-square test, Mann–Whitney rank sum test and one-way analysis of variance. p-Values < 0.05 were considered significant (highlighted in bold).
Table 2.
Characteristics of subjects with and without migraine in patients with relapsing–remitting, secondary-progressive and primary-progressive multiple sclerosis.
| RRMS (n = 320) |
SPMS (n = 124) |
PPMS (n = 36) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| W/o migraine (n = 241) |
Migraine (n = 79) | p-Value | W/o migraine (n = 104) | Migraine (n = 20) | p-Value | W/o migraine (n=30) | Migraine (n = 6) | p-Value | |
| Female, n (%) | 159 (78.8) | 68 (86.1) | 0.001 | 77 (74) | 18 (90) | 0.122 | 17 (56.7) | 3 (50) | 0.764 |
| Age in years, mean (SD) | 44.6 (10.3) | 42.2 (11.4) | 0.081 | 54.7 (7.5) | 49.9 (10.6) | 0.015 | 56.0 (6.9) | 49.3 (4.7) | 0.032 |
| Age at onset in years, mean (SD) | 32.3 (9.2) | 30.2 (9.4) | 0.082 | 32.4 (11.1) | 29.2 (9.9) | 0.22 | 37.9 (10.8) | 42.4 (6) | 0.374 |
| Disease duration in years, mean (SD) | 12.4 (8.9) | 11.4 (8.7) | 0.401 | 22.4 (10.9) | 20.7 (9.8) | 0.527 | 18.1 (11.5) | 6.8 (4.5) | 0.039 |
| BMI, mean (SD) | 27.5 (5.6) | 27.0 (6) | 0.555 | 25.8 (5) | 24.7 (5.7) | 0.376 | 26.1 (4.2) | 26.0 (5.6) | 0.954 |
| Presence of DMT, n (%) | 204 (84.6) | 60 (75.9) | 0.579 | 72 (69.2) | 15 (75) | 0.831 | 13 (43.3) | 3 (50) | 0.85 |
| Interferon-beta 1a | 101 (49.5) | 24 (40) | 40 (55.6) | 6 (40) | 5 (38.5) | 1 (33.3) | 5 | ||
| Glatiramer acetate | 51 (25) | 21 (35) | 14 (19.4) | 5 (33.3) | 5 (38.5) | 2 (66.7) | |||
| Natalizumab | 45 (22.1) | 14 (23.3) | 12 (16.7) | 2 (13.3) | 0 (0) | 0 (0) | |||
| Mycophenolate mofetil | 3 (1.5) | 0 (0) | 3 (4.2) | 0 (0) | 1 (7.7) | 0 (0) | |||
| Intravenous immunoglobulin | 3 (1.5) | 1 (1.7) | 1 (1.4) | 1 (6.7) | 1 (7.7) | 0 (0) | |||
| Azathioprine | 0 (0) | 0 (0) | 2 (2.8) | 1 (6.7) | 1 (7.7) | 0 (0) | |||
| Mitoxantrone | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| EDSS, mean (IQR) | 2.6 (1.5) | 2.6 (1.4) | 0.884 | 5.6 (1.5) | 5.2 (1.9) | 0.25 | 5.7 (1.9) | 4.8 (1.2) | 0.298 |
| Race, n (%) | 0.385 | 0.851 | 0.071 | ||||||
| White | 225 (93.4) | 70 (88.6) | 100 (96.1) | 20 (100) | 29 (96.7) | 5 (83.3) | |||
| Hispanic/Latino | 3 (1.2) | 3 (3.8) | 1 (1.0) | 0 (0) | 0 (0) | 1 (16.7) | |||
| Black/African/American | 10 (4.1) | 5 (6.3) | 2 (1.9) | 0 (0) | 1 (3.3) | 0 (0) | |||
| Asian | 2 (0.8) | 0 | 0 | 0 (0) | 0 (0) | 0 (0) | |||
| American Indian/Alaska native | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 (0) | |||
| Other | 1 (0.4) | 1 (1.3) | 1 (1.0) | 0 (0) | 0 (0) | 0 (0) | |||
MS—multiple sclerosis; RRMS—relapsing–remitting; SPMS—secondary-progressive; PPMS—primary-progressive; SD—standard deviation; EDSS—Expanded Disability Status Scale; DMT—disease modifying therapy; NA—not available; BMI—body mass index; IQR—interquartile range.
The comparison between the migraine and non-migraine groups was performed using chi-square test, Mann–Whitney rank sum test and one-way analysis of variance. p-Values < 0.05 were considered significant (highlighted in bold).
3.2. Frequency of migraine
Hundred and thirteen (22.2%) MS patients, 11 (17.2%) of CIS patients and 37 (14.7%) of HIs had migraine (p = 0.067). In the MS disease subtype analyses, patients with RRMS had the highest rate of migraine (24.7%), followed by PPMS (16.7%) and SPMS (16.1%) (p = 0.108).
3.3. MRI differences in subjects with and without migraine
Table 3 and Fig. 1 show within disease group differences in subjects with and without migraine. More MS patients with migraine presented with CE lesions compared to those without (35.4% vs. 23.7%, p = 0.013). The mean number of CE lesions in MS patients with migraine was increased respect to those without (0.91 vs. 0.21, p = 0.019). The mean CE-LV was also increased in subjects with migraine respect to those without (125.3 vs. 31.2 mm3, p = 0.022). No other MRI lesion and brain volume MRI outcome differences were found in subjects with and without migraine within specific disease groups.
Table 3.
Within study group MRI comparisons between subjects with and without migraine.
| HIs (n = 251) |
MS (n = 509) |
CIS (n = 64) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| W/o migraine (n = 214) |
Migraine (n = 37) |
p-Value | W/o migraine (n = 396) | Migraine (n = 113) | p-Value | W/o migraine (n = 53) | Migraine (n = 11) | p-Value | |
| Presence of CE lesions | NA | NA | NA | 94 (23.7) | 40 (35.4) | 0.013 | 9 (17) | 2 (18.2) | 0.923 |
| Number of CE lesions | NA | NA | NA | 0.21 (0.74) | 0.91 (4.36) | 0.019 | 0.45 (1.25) | 0.10 (0.32) | 0.380 |
| CE-LV | NA | NA | NA | 31.2 (134.4) | 125.3 (572.6) | 0.022 | 65.8 (196.5) | 5.4 (17.1) | 0.326 |
| Number of T2 lesions | 2.7 (6.82) | 2.03 (4.5) | 0.505 | 29 (20) | 27.8 (19.8) | 0.401 | 22.2 (25.8) | 11 (6.9) | 0.170 |
| T2-LV | 277.1 (1200) | 113.5 (241.1) | 0.402 | 13,901.7 (16,530.2) | 13,546.2 (15,966.2) | 0.829 | 4406.7 (6111.7) | 1960.9 (1794.3) | 0.183 |
| Number of T1 lesions | NA | NA | NA | 11.21 (11.52) | 10.12 (12.33) | 0.412 | 4.14 (7.83) | 2.70 (4.88) | 0.469 |
| T1-LV | NA | NA | NA | 3021.6 (5528.6) | 3082.4 (7479.1) | 0.832 | 423.6 (731.8) | 369.3 (957.9) | 0.706 |
| NBV | 1533.8 (95.3) | 1551.9 (83) | 0.089 | 1474.5 (99.1) | 1484.9 (95.6) | 0.278 | 1543.4 (63.7) | 1580 (81.3) | 0.157 |
| NGMV | 780.2 (67.2) | 789.9 (52.7) | 0.191 | 734.4 (71.9) | 747.4 (68.2) | 0.651 | 784 (49) | 802.3 (59.9) | 0.444 |
| NWMV | 753.6 (44.1) | 762.1 (44.1) | 0.156 | 740.1 (66.8) | 737.5 (65) | 0.332 | 759.4 (44.1) | 777.7 (70.6) | 0.278 |
| NLVV | 34.3 (15.5) | 30.2 (9.2) | 0.107 | 49.6 (22.6) | 46 (22.1) | 0.954 | 35.2 (12.7) | 33.9 (19.2) | 0.999 |
| NCV | 636.1 (57.4) | 644.3 (46.3) | 0.231 | 594.3 (60.7) | 607.1 (56.9) | 0.819 | 633.4 (40.5) | 658.6 (47.7) | 0.115 |
HIs—healthy individuals; MS—multiple sclerosis; CIS—clinically isolated syndrome; CE—contrast enhancing; LV—lesion volume; NA—not available; NBV—normalized brain volume; NGMV—normalized gray matter volume; NWMV—normalized white matter volume; NLVV—normalized lateral ventricular volume; NCV—normalized neocortical volume.
All data are presented as mean and standard deviations. The presence of CE is reported as the number and percentage. Volumetric measures are presented in cubic millimeters (mm3) for LV and in cubic centimeters (cm3) for brain volumes.
The comparison between HIs, MS and CIS groups was performed using analysis of covariance with age, gender and disease-modifying treatment, as covariates. p-Values < 0.05 were considered significant (highlighted in bold) after correction for multiple comparisons.
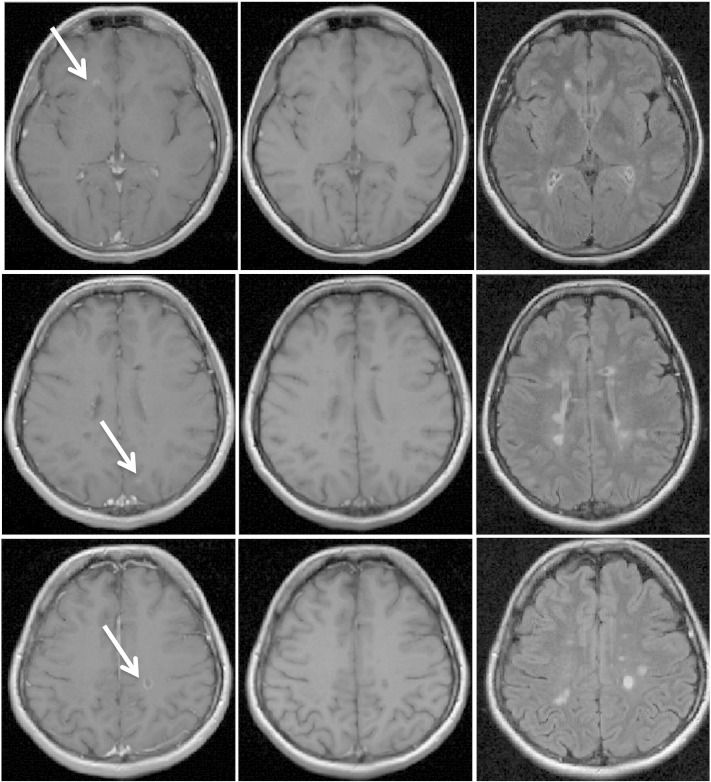
Fig. 1.
Representative MRI images of a 32 years old female relapsing–remitting multiple sclerosis patient with disease duration of 11 and 9 years history of migraine. On the left are displayed T1-weighted spin echo post-contrast images (after 5 min delay), in the middle are shown T1-weighted spin echo pre-contrast images and on the right are displayed representative fluid attenuation inversion recovery images. There are 3 visible contrast enhancing lesions (white arrows) in different brain lobes and hemispheres.
Table 4 shows within MS disease subtype study MRI outcome differences in subjects with and without migraine. More RRMS patients with migraine presented with CE lesions compared to those without (41.8% vs. 28.2%, p = 0.035). RRMS patients with migraine had increased mean number of CE lesions (1.19 vs. 0.27, p = 0.023) and CE-LV (164.4 vs. 41.1 mm3, p = 0.02) compared to those without. No other MRI lesion and brain volume outcome differences were found in subjects with and without migraine within MS disease subtypes.
Table 4.
Within disease course MRI comparisons between subjects with and without migraine.
| RRMS (n = 320) |
SPMS (n = 124) |
PPMS (n = 36) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| W/o migraine (n = 241) |
Migraine (n = 79) | p-Value | W/o migraine (n = 104) | Migraine (n = 20) | p-Value | W/o migraine (n=30) | Migraine (n = 6) | p-Value | |
| Presence of CE lesions | 68 (28.2) | 33 (41.8) | 0.035 | 26 (25) | 7 (35) | 0.354 | 0 (0) | 0 (0) | NA |
| Number of CE lesions | 0.3 (0.84) | 1.19 (4.9) | 0.023 | 0.07 (.3) | 0 (0) | 0.224 | 0 (0) | 0 (0) | 0.806 |
| CE-LV | 41.1 (144.4) | 164.4 (651.9) | 0.02 | 6.1 (28.6) | 0 (0) | 0.324 | 1.6 (8.5) | 0 (0) | 0.806 |
| Number of T2 lesions | 27.1 (19.3) | 28.2 (17.9) | 0.951 | 33.8 (19.7) | 29.4 (24.1) | 0.241 | 31.8 (24.4) | 15.3 (11.7) | 0.081 |
| T2-LV | 11,421.3 (14,030.7) | 12,163.3 (14,300) | 0.732 | 19,357.2 (17,541.4) | 19,850.3 (20,378.2) | 0.701 | 17,256.3 (19,859.5) | 10,457.4 (12,508.5) | 0.413 |
| Number of T1 lesions | 10.1 (10.8) | 9.7 (11.9) | 0.638 | 15.1 (12.3) | 12.4 (14.1) | 0.352 | 11.6 (12.7) | 11.3 (10.4) | 0.98 |
| T1-LV | 2424.2 (4769.6) | 3020.2 (8106.5) | 0.417 | 5050.6 (7378.7) | 4338.9 (6419.1) | 0.580 | 2236.3 (2554.0) | 2015.9 (2725.2) | 0.850 |
| NBV | 1492.8 (88.5) | 1488.8 (96.5) | 0.187 | 1414.7 (77.4) | 1428.5 (70.7) | 0.832 | 1430.9 (93.9) | 1485.9 (60.8) | 0.688 |
| NGMV | 742.5 (66.3) | 748.9 (64.7) | 0.747 | 697.5 (58.9) | 710 (54.5) | 0.59 | 715.7 (48.4) | 732.3 (57.5) | 0.521 |
| NWMV | 750.3 (60.9) | 740 (67.2) | 0.144 | 717.2 (71.6) | 718.6 (61.2) | 0.825 | 715.3 (71.3) | 753.6 (76.2) | 0.423 |
| NLVV | 45.8 (20.1) | 45.6 (22.8) | 0.402 | 60.3 (22.7) | 54.2 (23.6) | 0.353 | 57 (26.8) | 43 (8.8) | 0.494 |
| NCV | 600.1 (55.5) | 609.8 (52.7) | 0.822 | 564.6 (50.9) | 570 (41.8) | 0.903 | 580.4 (41.9) | 593.4 (55.3) | 0.619 |
MS—multiple sclerosis; RRMS—relapsing–remitting; SPMS—secondary-progressive; PPMS—primary-progressive; SD—standard deviation; CE—contrast enhancing; LV—lesion volume; NA—not available; NBV—normalized brain volume; NGMV—normalized gray matter volume; NWMV—normalized white matter volume; NLVV—normalized lateral ventricular volume; NCV—normalized neocortical volume.
All data are presented as mean and standard deviations. The presence of CE is reported as the number and percentage. Volumetric calculations are presented in cubic millimeters (mm3) for LV and in cubic centimeters (cm3) for brain volumes.
The comparison between RRMS, SPMS and PPMS groups was performed using analysis of covariance with age, gender and disease-modifying treatment, as covariates. p-Values < 0.05 were considered significant (highlighted in bold) after correction for multiple comparisons.
3.4. Regression analysis
Given the non-normally distributed lesion data, we used negative binomial regression to validate the ANCOVA results. MS patients with migraine had increased number of CE lesions (B = 1.242, p = 0.001), but not CE-LV (B = 1.320, p = 0.133) compared to those without migraine. RRMS patients with migraine had increased number of CE lesions (B = 1.377, p = 0.001), but not CE-LV (B = 1.490, p = 0.098) compared to those without migraine.
4. Discussion
This is the largest case–control study to date that investigated the association between migraine and MRI outcomes in MS patients. We found that there is an increased frequency of CE lesions in MS patients with migraine, specifically within the RRMS disease subtype. Given that our clinical/MRI assessments were performed on MS patients with a stable clinical status and in absence of acute headache attack prior to MRI examination, the current findings suggest that having migraine comorbidity may increase level of blood–brain-barrier (BBB) disruption in RRMS patients.
Presence of CE lesions is an indicator of inflammation and breakdown of the BBB, and MRI hallmark for diagnosis and monitoring of MS. Migraine is a disorder characterized by a strong vascular component in which vasoconstriction is followed by vasodilation, mediated by underlying inflammatory cytokines and/or neuropeptides (Silberstein, 2004). On the other hand, cardiovascular risk factors, including smoking, hypertension, hyperlipidemia, overweight/obesity, diabetes and heart disease are associated with MS (Kappus et al., 2015; Karmon et al., 2012). The pathophysiology of migraine is complex and variety of mediating mechanisms have been proposed including changes in levels of magnesium, calcium and glutamate, as well 5-HT, which stimulates the release of substance P and calcitonin gene related peptide (Silberstein, 2004). While our study did not assess any one of these factors, our findings do suggest that alterations of the BBB may compromise the microenvironmental vascular regulation. Indeed, previous studies suggested that the pathogenesis of migraine includes an inflammatory component (Longoni and Ferrarese, 2006). The inflammation of the meninges is an accepted component of the migraine process with release of vasogenic substances such as calcitonin gene-related peptide, substance P, neurokinin A, vasoactive intestinal peptide, and nitric oxide (Buzzi et al., 1991). In the last decade, it has been established that highly inflammatory cortical demyelination is also present and common in early MS, topographically associated with prominent meningeal inflammation and may even precede the appearance of classic WM plaques in some MS patients (Lucchinetti et al., 2011). Therefore, future work should explore the association between meningeal inflammation and BBB in MS patients with and without migraine.
In line with previous studies, we found that migraine is present at higher rates in patients with RRMS, compared to those with chronic progressive MS disease subtype (D'Amico et al., 2004; Ergun et al., 2009; Moisset et al., 2013; Villani et al., 2008). These findings are of interest in the context of our results that showed that only RRMS patients had increased number and volume of CE lesions. This may suggest that increased inflammatory component of the disease, usually present in earlier disease stages, may contribute to the development of migraine. However, the present study was not designed to collect information on the temporal relationship between onset of migraine and MS onset. The fact that no association of CE lesions and migraine was found in CIS patients and that frequency of migraine was higher in RRMS vs. CIS in the present study, indicates that migraine may be, at least in part, a comorbidity of MS disease process which is related to more severe BBB damage. A meta-analysis study demonstrated that the number of CE lesions over the first 6 months of follow-up increased the relative risk of relapse occurrence in the subsequent year (Kappos et al., 1999). It has been shown that patients in the relapsing phase have significantly more migraine attacks than those in the remitting phase (Ergun et al., 2009). Indeed, there were no differences in CE lesions in patients with SP and PPMS with and without migraine in the present study. However, it has to be underlined that PPMS patient did not present any CE lesions. While the present study was cross-sectional in design, it will be interesting to monitor the occurrence of CE lesions and migraine attacks in future longitudinal prospective studies using serial MRI.
Several previous MRI studies aimed to establish whether a specific locations of lesions in MS patients is associated with the presence of migraine. One study showed that MS patients with migraine had more lesions in red nucleus, substantia nigra and periaqueductal GM compared to MS patients without migraine (Tortorella et al., 2006). Another study confirmed that MS patients who have midbrain plaques, in close proximity to the periaqueductal GM have a four-fold increase in migraines compared to MS patients without plaques (Gee et al., 2005). These studies aimed to explain the onset of migraine symptoms by interruption of circuits involved in modulating pain pathways. As CE lesions rarely form in those brain areas, the present study poses an alternative hypothesis as to the presence of migraine in MS, by demonstrating that the underlying widespread inflammatory process may be also an initial trigger in some of the MS patients. It is also possible that these findings are not mutually exclusive, and that both the lesion location and the inflammatory process contribute to pain and migraine onset. In line with a previous study (Kamson et al., 2012), we did not find that MS patients or HIs with and without migraine differed significantly in T2 or T1 lesion burden, although the RRMS patients with migraine in the present study showed somewhat greater T2- and T1-LVs despite shorter disease duration compared to those without migraine.
Past studies have suggested that migraine is associated with GM pathology (Rocca et al., 2006), although these findings were based on a small number of subjects and there were other studies showing conflicting results (Matharu et al., 2003). It has been demonstrated that migraine subjects do not present with cortical lesions (Absinta et al., 2012). Brain volumetry findings from the current study suggest that there is no significant difference in the GM and WM in HIs, MS and CIS groups between migraine and non-migraine subjects. Furthermore we did not find a significant difference in GM volumes by MS disease subtype. In terms of overall impact on MS severity, previous studies have found no significant correlation between level of disability and the presence of headache (D'Amico et al., 2004), which was confirmed in the current study.
It has been shown that treatment of MS with interferon-beta is associated with higher rate of migraine, but this increase is mainly due to the exacerbation of preexisting migraine and is less commonly associated with the new onset (Khromov et al., 2005; Villani et al., 2008; Villani et al., 2012). This is further supported by the fact that patients receiving interferon-beta may experience exacerbation of their migraine symptoms. As migraine may be iatrogenically triggered by use of DMT, we used treatment status, as a covariate in all our analyses. We further explored the differences between interferon-beta and other DMTs in MS patients with and without migraine (data not shown) and found no associations.
In addition, neuropathic pain is frequent in patients with MS and can be concomitantly present with migraine (Moisset et al., 2013). In a recent study, 32% of the MS patients who presented both with migraine and neuropathic pain, had more severe pain and lower health-related quality of life than MS patients with either migraine or neuropathic pain alone (Moisset et al., 2013). The pain intensity in MS patients with migraine was higher (6.0 ± 0.1) than that of neuropathic pain (4.9 ± 0.1). Moreover, in agreement with the present study, the migraine MS patients were younger and had more likely RRMS. This indicates that neuropathic pain and migraine pain may be mediated by different mechanisms and that optimal treatment for management of the migraine pain needs more attention at individual patient level.
There are several limitations to this study which warrant consideration. Firstly, the timeline between the presentation of migraine and imaging was not well established, therefore it was not possible to assess the correlation between MRI outcomes and frequency of migraine attacks or migraine onset. This study utilized data from an ongoing prospective study of cardiovascular, environmental and genetic risk factors in clinically stable MS patients who performed their MRI within 30 days of entering the study. However, the proximity of the MRI to the manifestation of migraine symptoms was consistent on an individual basis, as we excluded MS patients with acute headache attack at the time of MRI. Secondly, because subtype of migraine (migraine with aura, etc) is difficult to distinguish, and number of migraine attacks is difficult to capture retrospectively outside of specialty headache clinic, we focused only on collecting information about diagnosis of migraine. In addition, we did not apply most recent ICHD-3, which was not available at the time when this study was designed (Headache Classification Subcommittee of the International Headache Society, 2013). Thirdly, the association of increased CE lesion activity in MS patients with migraine does not prove causation, especially because we were not able to apply a contrast agent to the HIs in this study, which prevented us to understand whether migraine subjects without MS may show signs of BBB disruption. Nevertheless, findings from current study should encourage further research using longitudinal design, to explore the occurrence of CE lesion activity in active and chronic migraine MS, CIS and RIS subjects. Fourthly, the present study did not investigate the onset of migraine symptoms by location of CE lesions and future studies should investigate this topic. Finally, we did not investigate the onset of migraine symptoms by location of CE lesions and future studies should investigate this topic.
In conclusion, MS patients with migraine had a greater CE lesion activity and this was specifically manifested in RRMS patients. Our findings suggest an increased inflammatory pathobiology in MS patients with migraine headaches requiring possibly more frequent MRIs and also more efficient anti-inflammatory treatment.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Absinta M. Patients with migraine do not have MRI-visible cortical lesions. J. Neurol. 2012;259(12):2695–2698. doi: 10.1007/s00415-012-6571-x. 22714135 [DOI] [PubMed] [Google Scholar]
- Alroughani R. Status migrainosus as an initial presentation of multiple sclerosis. Springerplus. 2015;4:28. doi: 10.1186/s40064-015-0818-9. 25646151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. 11682119 [DOI] [PubMed] [Google Scholar]
- Buzzi M.G. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991;30(11):1193–1200. doi: 10.1016/0028-3908(91)90165-8. 1663596 [DOI] [PubMed] [Google Scholar]
- D'Amico D. Prevalence of primary headaches in people with multiple sclerosis. Cephalalgia. 2004;24(11):980–984. doi: 10.1111/j.1468-2982.2004.00790.x. 15482362 [DOI] [PubMed] [Google Scholar]
- Dolic K. Risk factors for chronic cerebrospinal venous insufficiency (CCSVI) in a large cohort of volunteers. PLOS One. 2011;6(11):e28062. doi: 10.1371/journal.pone.0028062. 22140507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D.G. Migraine in multiple sclerosis. Int. Rev. Neurobiol. 2007;79:281–302. doi: 10.1016/S0074-7742(07)79012-8. 17531846 [DOI] [PubMed] [Google Scholar]
- Ergün U. Headaches in the different phases of relapsing–remitting multiple sclerosis: a tendency for stabbing headaches during relapses. Neurologist. 2009;15(4):212–216. doi: 10.1097/NRL.0b013e3181906fc9. 19590381 [DOI] [PubMed] [Google Scholar]
- Gee J.R. The association of brainstem lesions with migraine-like headache: an imaging study of multiple sclerosis. Headache. 2005;45(6):670–677. doi: 10.1111/j.1526-4610.2005.05136.x. 15953299 [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. 23771276 [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl. 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. 14979299 [DOI] [PubMed] [Google Scholar]
- Kamson D.O. Volumetric comparisons of supratentorial white matter hyperintensities on FLAIR MRI in patients with migraine and multiple sclerosis. J. Clin. Neurosci. 2012;19(5):696–701. doi: 10.1016/j.jocn.2011.07.044. 22440862 [DOI] [PubMed] [Google Scholar]
- Kappos L. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet. 1999;353(9157):964–969. doi: 10.1016/s0140-6736(98)03053-0. 10459905 [DOI] [PubMed] [Google Scholar]
- Kappus N. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2015 doi: 10.1136/jnnp-2014-310051. 25722366 [DOI] [PubMed] [Google Scholar]
- Karmon Y. Arterial, venous and other vascular risk factors in multiple sclerosis. Neurol. Res. 2012;34(8):754–760. doi: 10.1179/1743132812Y.0000000077. 22971465 [DOI] [PubMed] [Google Scholar]
- Khromov A. Migraines linked to interferon-beta treatment of multiple sclerosis. Am. J. Phys. Med. Rehabil. 2005;84(8):644–647. doi: 10.1097/01.phm.0000171012.86932.10. 16034235 [DOI] [PubMed] [Google Scholar]
- Kister I. Migraine is comorbid with multiple sclerosis and associated with a more symptomatic MS course. J. Headache Pain. 2010;11(5):417–425. doi: 10.1007/s10194-010-0237-9. 20625916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun C. Unexpected multiple sclerosis: follow-up of 30 patients with magnetic resonance imaging and clinical conversion profile. J. Neurol. Neurosurg. Psychiatry. 2008;79(2):195–198. doi: 10.1136/jnnp.2006.108274. 18202208 [DOI] [PubMed] [Google Scholar]
- Lin G.Y. Multiple sclerosis presenting initially with a worsening of migraine symptoms. J. Headache Pain. 2013;14:70. doi: 10.1186/1129-2377-14-70. 23937696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni M., Ferrarese C. Inflammation and excitotoxicity: role in migraine pathogenesis. Neurol. Sci. 2006;27(Suppl. 2):S107–S110. doi: 10.1007/s10072-006-0582-2. 16688611 [DOI] [PubMed] [Google Scholar]
- Lucchinetti C.F. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 2011;365(23):2188–2197. doi: 10.1056/NEJMoa1100648. 22150037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu M.S. No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur. J. Neurol. 2003;10(1):53–57. doi: 10.1046/j.1468-1331.2003.00510.x. 12534993 [DOI] [PubMed] [Google Scholar]
- Moisset X. Migraine headaches and pain with neuropathic characteristics: comorbid conditions in patients with multiple sclerosis. Pain. 2013;154(12):2691–2699. doi: 10.1016/j.pain.2013.07.050. 23911697 [DOI] [PubMed] [Google Scholar]
- Nicoletti A. Headache and multiple sclerosis: a population-based case–control study in Catania, Sicily. Cephalalgia. 2008;28(11):1163–1169. doi: 10.1111/j.1468-2982.2008.01662.x. 18727645 [DOI] [PubMed] [Google Scholar]
- O'Connor K. Patterns of dietary and herbal supplement use by multiple sclerosis patients. J. Neurol. 2012;259(4):637–644. doi: 10.1007/s00415-011-6226-3. 21898138 [DOI] [PubMed] [Google Scholar]
- Okuda D.T. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72(9):800–805. doi: 10.1212/01.wnl.0000335764.14513.1a. 19073949 [DOI] [PubMed] [Google Scholar]
- Pakpoor J. Meta-analysis of the relationship between multiple sclerosis and migraine. PLOS One. 2012;7(9):e45295. doi: 10.1371/journal.pone.0045295. 23024814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzki N. Prevalence of migraine, tension-type headache and trigeminal neuralgia in multiple sclerosis. Eur. J. Neurol. 2009;16(2):262–267. doi: 10.1111/j.1468-1331.2008.02406.x. 19138330 [DOI] [PubMed] [Google Scholar]
- Rocca M.A. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37(7):1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. 16728687 [DOI] [PubMed] [Google Scholar]
- Samkoff L.M., Goodman A.D. Symptomatic management in multiple sclerosis. Neurol. Clin. 2011;29(2):449–463. doi: 10.1016/j.ncl.2011.01.008. 21439453 [DOI] [PubMed] [Google Scholar]
- Silberstein S.D. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl. 2):2–7. doi: 10.1111/j.1468-2982.2004.00892.x. 15595988 [DOI] [PubMed] [Google Scholar]
- Smith S.M. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. 12482100 [DOI] [PubMed] [Google Scholar]
- Tortorella P. Assessment of MRI abnormalities of the brainstem from patients with migraine and multiple sclerosis. J. Neurol. Sci. 2006;244(1–2):137–141. doi: 10.1016/j.jns.2006.01.015. 16530789 [DOI] [PubMed] [Google Scholar]
- Vacca G. Multiple sclerosis and headache co-morbidity. A case–control study. Neurol. Sci. 2007;28(3):133–135. doi: 10.1007/s10072-007-0805-1. 17603764 [DOI] [PubMed] [Google Scholar]
- Villani V. Primary headache and multiple sclerosis: preliminary results of a prospective study. Neurol. Sci. 2008;29(Suppl. 1):S146–S148. doi: 10.1007/s10072-008-0908-3. 18545918 [DOI] [PubMed] [Google Scholar]
- Villani V. The impact of interferon beta and natalizumab on comorbid migraine in multiple sclerosis. Headache. 2012;52(7):1130–1135. doi: 10.1111/j.1526-4610.2012.02146.x. 22486199 [DOI] [PubMed] [Google Scholar]
- Watkins S.M., Espir M. Migraine and multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1969;32(1):35–37. doi: 10.1136/jnnp.32.1.35. 5774132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurol. 2011;77(2):138–144. doi: 10.1212/WNL.0b013e318212a901. 21490322 [DOI] [PubMed] [Google Scholar]
- Zivadinov R. Abnormal subcortical deep-gray matter susceptibility-weighted imaging filtered phase measurements in patients with multiple sclerosis: a case–control study. Neuroimage. 2012;59(1):331–339. doi: 10.1016/j.neuroimage.2011.07.045. 21820063 [DOI] [PubMed] [Google Scholar]
- Zorzon M. Risk factors of multiple sclerosis: a case–control study. Neurol. Sci. 2003;24(4):242–247. doi: 10.1007/s10072-003-0147-6. 14658040 [DOI] [PubMed] [Google Scholar]