Abstract
Background
The phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB, AKT)/mammalian target of rapamycin (mTOR) signaling pathway plays a critical role in angiogenesis and cell growth, proliferation, metabolism, migration, differentiation, and apoptosis. Genetic diversity in key factors of this pathway may influence protein function and signal transduction, contributing to disease initiation and progression. Studies suggest that MTOR rs1064261 and AKT rs1130233 polymorphisms are associated with risk and/or prognosis of multiple cancer types. However, this relationship with gastric cancer (GC) remains unclear. The aim of this study was to investigate the role of MTOR and AKT polymorphisms in the risk and prognosis of GC.
Methods
The Sequenom MassARRAY platform was used to genotype 1842 individuals for MTOR rs1064261 T→C and AKT rs1130233 G→A polymorphisms. ELISA was used to detect Helicobacter pylori antibodies in serum. Immunohistochemical analysis was used to detect total and phosphorylated MTOR and AKT proteins.
Results
The MTOR rs1064261 (TC+CC) genotype and the AKT rs1130233 (GA+AA) genotype were associated with increased risk of GC in men (P = 0.049, P = 0.030). In H. pylori-negative individuals, the AKT rs1130233 GA and (GA+AA) genotypes were related to increased risk of atrophic gastritis (AG; P = 0.012, P = 0.024). Notably, the AKT rs1130233 (GA+AA) genotype demonstrated significant interactions with H. pylori in disease progression from healthy controls (CON) to AG (P = 0.013) and from AG to GC (P = 0.049). Additionally, for individuals with the AKT rs1130233 variant, those in the H. pylori-positive group had higher levels of phosphorylated AKT (p-AKT) expression. The AKT rs1130233 genotype was found to be associated with clinicopathological parameters including lymph node metastasis and alcohol drinking (P<0.05).
Conclusion
MTOR rs1064261and AKT rs1130233 polymorphisms were associated with increased GC risk in males and increased AG risk in H. pylori-negative individuals. A significant interaction existed between the AKT rs1130233 genotype and H. pylori infection in CON→AG→GC disease progression. The AKT rs1130233 genotype influenced p-AKT protein expression in H. pylori-infected individuals.
Introduction
Gastric cancer (GC) is one of the most common cancers worldwide and a leading cause of cancer-related death [1]. The initiation and development of GC is a multistep process influenced by genetic and environmental factors. Studies have indicated that exogenous environmental factors including Helicobacter pylori (HP) infection, diet habits, smoking, alcohol drinking, and economic factors contribute to gastric carcinogenesis [2]. Under similar environmental conditions, individuals with different genetic background suffer different risks of cancer with diverse clinical outcomes. Until now, the interaction of genetic and environmental factors with gastric carcinogenesis has been largely unknown.
Polymorphisms are a class of genetic factors that participate in gastric carcinogenesis and determine inter-individual variations in GC risk. Genetic polymorphisms can weaken intrinsic protective mechanisms and increase the damage caused by environmental carcinogens. Carriers of susceptible genotypes are at a greater risk of developing cancer than those with resistant genotypes under similar conditions. Therefore, genetic factors play a crucial role in GC risk and clinical outcome.
The phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB, AKT)/mammalian target of rapamycin (MTOR) pathway regulates various cellular functions including growth, proliferation, migration, and apoptosis. Tapia et al. reported that the PI3K/AKT/MTOR pathway is activated in GC and that most proteins (phosphorylated and unphosphorylated forms) studied so far in this pathway are overexpressed in tumor tissues [3]. MTOR is a key member of the PI3K/AKT/MTOR pathway and a core metabolic signaling molecule [4, 5]. Single nucleotide polymorphisms (SNPs) may affect MTOR expression and transcriptional activity, thereby altering protein function. So far, a total of 14 polymorphisms in this gene have been studied, of which three SNPs (rs2295080, rs1883965, and rs2536) have been most widely reported. The rs2295080 polymorphism in the promoter region is reported to reduce the risk of renal cell carcinoma [6], GC [7], and prostate cancer [8] by downregulating endogenous protein expression. The rs1883965 polymorphism has been associated with an increased risk of GC [9] and esophageal squamous cancer [10]. Moreover, rs2536 polymorphism is related to a significantly increased risk of prostate cancer [8]. In addition, the MTOR rs11121704 polymorphism is associated with worse clinical parameters (death, metastasis, and chemotherapy resistance) [11]. There are 45 exons in the MTOR gene, and the rs1064261 polymorphism is located in exon 18. The T→C variation is located at the boundary of exon 18 and intron 19, but its relation with disease is still unclear.
The AKT gene is critical for cell survival and encodes an important downstream effector of the PI3K/AKT/MTOR pathway that regulates key cellular functions including glucose metabolism and protein synthesis. AKT, alternatively known as AKT1, has five widely studied polymorphisms (rs3803300, rs1130214, rs2494732, rs2498804, and rs1130233); the first four have been linked to the risk or prognosis of nasopharyngeal carcinoma, oral squamous cell carcinoma, and non-small cell lung cancer. The rs1130233 polymorphism is located in exon 8 and the G→A variation is located at the boundary of exon 8 and intron 7. The AKT1 AA haplotype for both rs1130233 and rs2494732 is reported to confer an elevated risk of nasopharyngeal carcinoma [12]; the haplotype containing variant alleles of rs1130214 and rs3803300 polymorphisms significantly increases susceptibility to oral squamous cell carcinoma [13]. Carriers of the (GT+GG) genotype of AKT1 rs2498804 or the CT/TT genotype of AKT1 rs2494732 were found to have an increased risk of brain metastasis of non-small cell lung cancer [14]. AKT1 rs3803300, rs1130214, and rs2494732 have significant effects on survival in non-small cell lung cancer patients: patients with the rs3803300 G allele and rs1130214 G allele had shorter overall survival (OS) and disease-free survival (DFS) times [15].
Although variations in MTOR and AKT play important roles in gastric carcinogenesis, no study has investigated the relation of AKT polymorphism with GC risk and prognosis. In addition, the mechanistic link between MTOR rs1064261 and both cancer susceptibility and survival are unknown. To elucidate whether MTOR and AKT polymorphisms can serve as risk and/or prognostic markers for GC, we investigated MTOR rs1064261 and AKT rs1130233 polymorphisms in relation to GC risk and their interactions with H. pylori in a case–control study of 1842 subjects. In 205 individuals with sufficient data, clinicopathological parameters were analyzed to explore the association of these two polymorphisms with GC prognosis and thus identify new biomarkers for GC risk and prognosis.
Materials and Methods
Study design and study population
The design of this study was approved by the Human Ethics Committee of the First Affiliated Hospital of China Medical University (Shenyang, China). Each participant provided written informed consent during an epidemiological investigation. The study design included two parts (polymorphism and protein level analyses) and associations between polymorphisms and disease risk and prognosis were investigated. In the GC risk analysis, the GC patients were from the First Affiliated Hospital of China Medical University which obtained surgery operation resection or gastroscopic diagnosis/treatment between 2004 and 2013. The AG patients and controls were recruited from a health check program in Zhuanghe, Liaoning Province, between 2002 and 2013. All diagnoses were based on gastroscopic and histopathological examinations.
In addition, GC cases were classified into intestinal type and diffuse type based on Lauren’s classification [16, 17]; AG classification and staging were based on the new Sydney system [18, 19]. Control participants had normal stomach findings or gastritis only. The information about the smoking habit, alcohol consumption and family history were acquired by “face to face” questionnaire survey. The smokers and alcoholic drinks were defined as follow: if one smoked more than once per day and if this lasted more than 1 year, then this individual was defined as smokers, and this situation included current smokers and former smokers who had quit smoking for more than 1 year. Persons who did not satisfy this situation were defined as never smokers. If one consumed one bottle of beer or a fifth pound of liquor a day and if this situation lasted more than 1 year, then this person was considered drinkers. Individuals who did not fit this standard were defined as nondrinkers. Fasting venous blood was obtained from each participant and stored at −20°C as serum and clotted cells.
To further evaluate the relation of polymorphisms with clinicopathological parameters and survival of GC, we performed a prognostic analysis of GC patients for whom sufficient clinical data was available. Histology data were assessed according to Health Organization criteria and tumor–node–metastasis (TNM) staging of postoperative pathologic specimens was done according to 7th edition of the of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) (2010) criteria. Patients with distant metastasis before surgery, those who received radiotherapy or chemotherapy before surgery, and those with insufficient information for prognostic analysis were excluded. A sensitivity analysis was performed to test whether the excluded patients had effect to the survival analysis. Follow-up for all patients was completed in May 2014.
SNP genotyping
Genomic DNA was extracted from blood samples using the phenol–chloroform method [20] and diluted to working concentration of 50 ng/μl for MTOR rs1064261 T→C and AKT rs1130233 G→A genotyping. Samples were placed randomly in 384-well plates and analyzed in a blinded manner to disease status. Genotyping was performed using the Sequenom MassARRAY platform (Sequenom, San Diego, California, USA) according to the manufacturer’s instructions. For quality control, 5% of samples underwent repeated genotyping; the results were 100% consistent.
H. pylori serology examination
Serology analysis to detect H. pylori infection was performed using ELISA (H. pylori-IgG ELISA kit, BIOHIT, Helsinki, Finland), as described previously [21]. A reading of >34 enzyme immune units was regarded to indicate H. pylori positivity.
Detection of MTOR and AKT proteins in tissue
Immunohistochemical analysis was used to determine the expression of total and phosphorylated MTOR and AKT (p-MTOR and p-AKT) proteins in 65 formalin-fixed, paraffin-embedded GC tissue samples. Tissue samples were cut into 4-μm-thick sections and mounted onto poly-l-lysine-coated glass slides. After citric acid antigen retrieval, primary antibody was incubated with tissue sections at 4°C overnight (dilution concentration: MTOR and p-MTOR 1:100, AKT 1:300, and p-AKT 1:50, all were purchased from Cell Signaling Technology). After three 5-min washes in phosphate buffer saline, tissue sections were incubated with biotinylated secondary antibody (Maixin, Fujian, China) and streptavidin–biotin peroxidase for 10 min each at 37°C. For negative controls, primary antibody was replaced with PBS buffer.
Statistical analysis
Statistical analysis was performed using SPSS (version 18.0) statistical software (SPSS, Chicago, IL, USA). Hardy–Weinberg equilibrium (HWE) was first evaluated in healthy controls. Adjusted odds ratios and 95% confidence intervals (CIs) for the relation between both polymorphisms and disease risk were calculated by multivariable logistic regression, with adjustment for gender, age, and H. pylori infection status. In a stratified analysis, when stratified by age, the sex and H.pylori infection status were adjusted; when stratified by sex, the age and H.pylori infection status were adjusted; and when stratified by H.pylori infection status, the sex and age were adjusted. The likelihood ratio test was performed to assess the interaction effects of genotype and H. pylori on disease risk by comparing the model involving only the main effects of gender, age, H. pylori status, and genotype with the full model (also containing the interaction term of genotype with H. pylori status). Pearson’s χ2 test was used to evaluate the relation of different genotypes with the clinicopathological parameters of GC. Fisher’s exact method was used when the expected frequency was less than five. The Kaplan–Meier method was used to visualize OS by genotype group. The log-rank test was used to investigate differences in survival distributions. Univariable and multivariable Cox proportional hazards models were performed to calculate the crude or adjusted hazards ratios and 95% CIs for each genotype to estimate the effect on survival with or without adjustment for confounding factors. Logistic regression analysis was used to explore the effect of polymorphisms on protein expression adjusted by gender, age, and H. pylori status. Two-tailed P values of <0.05 were considered statistically significant.
Results
Baseline patient characteristics
The demographic and clinical characteristics of the 1842 individuals involved in the risk analysis comprising 483 GC patients, 686 AG patients and 673 healthy controls are shown in Table 1. Both SNPs were present in HWE; the genotype distribution is listed in Table 2. The result of the sensitivity analysis was shown in S1 Table, which verified the excluded patients had no effect to the survival analysis. The demographic and clinical characteristics of the 205 GC patients involved in the survival analysis including age, sex, Borrmann classification, Lauren’s classification, TNM stage, growth pattern, invasion depth, lymph node metastasis, smoking, drinking, family history, and H. pylori infection are shown in Table 3.
Table 1. The basic messages of the objects.
| Variability | Gastric mucosa status | ||||
|---|---|---|---|---|---|
| CON | AG | GC | |||
| Intestinal-type GC | Diffuse-type GC | ||||
| For SNP study | |||||
| N | 673 | 683 | 461 | 138 | 200 |
| Age | P<0.001 | ||||
| Mean±SD | 52.93±9.90 | 55.03±9.20 | 58.95±11.24 | 60.54±10.56 | 58.21±12.50 |
| Median | 53 | 56 | 58 | 59 | 58 |
| Range | 17–85 | 16–82 | 26–87 | 31–84 | 26–87 |
| Gender | P<0.001 | ||||
| Male | 342(50.8) | 386(56.3) | 316(68.0) | 107(76.4) | 125(61.9) |
| Female | 331(49.2) | 300(43.7) | 149(32.0) | 33(23.6) | 77(38.1) |
| H.pylori | P<0.001 | ||||
| Positive | 146(21.8) | 403(59.6) | 249(52.1) | 78(56.1) | 105(50.2) |
| Negative | 523(78.2) | 273(40.4) | 229(47.9) | 61(43.9) | 104(49.8) |
| For protein expression study | |||||
| N | 65 | ||||
| Age | |||||
| Mean±SD | 55.9±11.4 | ||||
| Median | 56 | ||||
| Range | 31–82 | ||||
| Gender | |||||
| Male | 42(64.6) | ||||
| Female | 23(35.4) | ||||
| H.pylori | |||||
| Positive | 25(38.5) | ||||
| Negative | 40(61.5) | ||||
Abbreviations: CON, control; AG, atrophic gastritis; GC, gastric cancer; SD, standard deviation; N, number of the objects.
Table 2. Association of mTOR rs1064261 and AKT rs1130233 polymorphisms with the risk of atrophic gastritis and gastric cancer.
| Stratified | SNP | Gastric mucosa status | AG vs. CON | GC vs. AG | GC vs. CON | GC vs. CON+AG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON(%) | AG(%) | GC(%) | OR(95%CI) | P-value | OR(95%CI) | P-value | OR(95%CI) | P-value | OR(95%CI) | P-value | ||
| mTOR rs1064261* | ||||||||||||
| TT | 560(83.2) | 584(85.1) | 402(83.2) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | |||||
| TC | 107(15.9) | 97(14.1) | 76(15.7) | 0.92(0.66–1.28) | 0.626 | 1.22(0.87–1.71) | 0.259 | 0.96(0.67–1.37) | 0.804 | 1.12(0.83–1.51) | 0.478 | |
| CC | 6(0.9) | 5(0.7) | 5(1.0) | 0.56(0.15–2.09) | 0.391 | 1.78(0.50–6.36) | 0.374 | 1.26(0.35–4.51) | 0.722 | 1.38(0.46–4.15) | 0.564 | |
| HWE | 0.724 | |||||||||||
| AKT rs1130233* | ||||||||||||
| GG | 144(21.5) | 129(19.1) | 89(18.5) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | |||||
| GA | 329(49.0) | 362(53.5) | 236(49.0) | 1.21(0.89–1.64) | 0.234 | 1.06(0.76–1.49) | 0.719 | 1.14(0.80–1.62) | 0.481 | 1.16(0.86–1.56) | 0.347 | |
| AA | 198(29.5) | 186(27.5) | 157(32.6) | 1.09(0.77–1.54) | 0.637 | 1.26(0.88–1.82) | 0.214 | 1.25(0.85–1.85) | 0.252 | 1.31(0.95–1.82) | 0.100 | |
| HWE | 0.737 | |||||||||||
| Sex | mTOR rs1064261 § | |||||||||||
| Male | TT | 283(82.7) | 336(87.0) | 260(82.3) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| TC | 55(16.1) | 48(12.4) | 54(17.1) | 0.70(0.44–1.12) | 0.138 | 1.55(1.00–2.38) | 0.049 | 0.97(0.62–1.51) | 0.880 | 1.29(0.89-.186) | 0.184 | |
| CC | 4(1.2) | 2(0.5) | 2(0.6) | 0.28(0.04–1.94) | 0.199 | 1.34(0.18–9.79) | 0.775 | 0.73(0.12–4.30) | 0.724 | 0.87(0.17–0.448) | 0.863 | |
| Sex | AKT rs1130233 § | |||||||||||
| Male | GG | 74(21.7) | 84(22.0) | 53(16.8) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| GA | 170(49.9) | 200(52.5) | 154(48.7) | 0.97(0.64–1.47) | 0.891 | 1.32(0.87–2.00) | 0.192 | 1.30(0.83–2.05) | 0.255 | 1.33(0.91–1.94) | 0.139 | |
| AA | 97(28.4) | 97(25.5) | 109(34.5) | 0.88(0.55–1.42) | 0.609 | 1.76(1.12–2.77) | 0.014 | 1.70(1.03–2.81) | 0.039 | 1.71(1.14–2.57) | 0.009 | |
| H.pylori | AKT rs1130233 # | |||||||||||
| Negative | GG | 118(22.6) | 43(15.9) | 47(20.6) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| GA | 246(47.2) | 149(55.2) | 105(46.1) | 1.69(1.12–2.54) | 0.012 | 0.72(0.43–1.19) | 0.194 | 1.21(0.78–1.87) | 0.400 | 1.02(0.67–1.53) | 0.945 | |
| AA | 157(30.1) | 78(28.9) | 76(33.3) | 1.39(0.89–2.18) | 0.144 | 0.94(0.55–1.62) | 0.830 | 1.29(0.81–2.05) | 0.288 | 1.18(0.76–1.83) | 0.470 | |
Note:
*using Logistic Regession adjusted by sex, age and H.pylori infection status.
§using Logistic Regession adjusted by age and H.pylori infection status.
#using Logistic Regession adjusted by sex and age.
Abbreviations: SNP, single nucleotide polymorphism; CON, control; AG, atrophic gastritis; GC, gastric cancer; OR, odds ratio; CI, confidence interval; Ref, reference; HWE, Hardy-Weinberg Equilibrium in population.
Table 3. Clinical features of gastric cancer patients.
| Variables | GC patients | Death | MST | Log-rank | HR(95%CI) |
|---|---|---|---|---|---|
| N = 205(%) | N = 67 | M | P-value | ||
| Age | |||||
| ≤50 | 54(26.34) | 20 | 55.31 a | 1(Ref) | |
| >50 | 151(73.66) | 47 | 62.23 a | 0.495 | 0.83(0.49–1.41) |
| Sex | |||||
| Female | 63(30.73) | 25 | 43.43 a | 1(Ref) | |
| Male | 142(69.27) | 42 | 64.93 a | 0.130 | 0.66(0.42–1.20) |
| Borrmann type | |||||
| Borrmann I–II | 76(37.07) | 29 | 60.81 a | 1(Ref) | |
| Borrmann III–IV | 101(49.27) | 37 | 44.0 | 0.632 | 1.13(0.69–1.84) |
| Lauren classification | |||||
| Intestinal | 75(36.59) | 18 | 64.55 a | 1(Ref) | |
| Diffuse | 129(62.93) | 49 | 57.88 a | 0.036 | 1.78(1.04–3.06) |
| TNM stage | |||||
| I–II | 120(58.54) | 22 | 73.69 a | 1(Ref) | |
| III–IV | 85(41.46) | 45 | 31.0 | 4.01×10 −7 | 3.76(2.25–6.27) |
| Growth pattern | |||||
| Massive and Nested | 70(34.15) | 12 | 35.07 a | 1(Ref) | |
| Diffused | 64(31.22) | 24 | 27.15 a | 0.005 | 2.73(1.36–5.46) |
| Depth of invasion | |||||
| T1+T2 | 43(20.98) | 1 | 37.63 a | 1(Ref) | |
| T3+T4 | 91(44.39) | 35 | 28.74 a | 0.003 | 20.15(2.76–147.11) |
| Lymphatic metastasis | |||||
| Negative | 77(37.56) | 11 | 71.77 a | 1(Ref) | |
| Positive | 128(62.44) | 56 | 53.01 a | 4.39×10 −5 | 3.86(2.02–7.36) |
| Smoking | |||||
| Never Smoker | 80(39.02) | 21 | 31.79 a | 1(Ref) | |
| Ever Smoker | 54(26.34) | 15 | 31.23 a | 0.795 | 1.09(0.56–2.12) |
| Alcohol drinking | |||||
| Nondrinker | 90(43.90) | 24 | 31.59 a | 1(Ref) | |
| Drinker | 44(21.46) | 12 | 30.76 a | 0.909 | 1.04(0.52–2.08) |
| Family history | |||||
| No | 107(52.20) | 33 | 30.48 a | 1(Ref) | |
| Yes | 27(13.17) | 3 | 35.85 a | 0.052 | 0.31(0.10–1.01) |
| H. pylori-IgG | |||||
| Negative | 86(41.95) | 29 | 60.74 a | 1(Ref) | |
| Positive | 117(57.07) | 37 | 59.02 a | 0.743 | 0.92(0.57–1.50) |
Note:
a, mean survival time was provided when MST could not be calculated.
Abbreviations: MST, median survival time (months); HR, hazard rate; CI, confidence interval; GC, gastric cancer; N, number of patients; M, months.
Association of SNPs with GC and AG risk
Multivariable logistic regression was used to investigate the association of MTOR rs1064261and AKT rs1130233 with GC and AG risk. There was no significant difference of MTOR rs1064261 and AKT rs1130233 on the risk of CON→AG, AG→GC, CON→GC, or CON+AG→GC progression (Table 2).
In the stratification analysis of males (Table 2), carriers of the TC genotype of MTOR rs1064261 had a 1.55-fold increased risk of GC compared with AG (P = 0.049, 95% CI 1.00–2.38); the A allele of AKT rs1130233 conferred 1.32-fold, 1.29-fold, and 1.30-fold increases in AG→GC, CON→GC, and (CON+AG)→GC progression, respectively, over the G allele (P = 0.013, 95% CI 1.06–1.64; P = 0.038, 95% CI 1.01–1.64; and P = 0.008, 95% CI 1.07–1.58, S2 Table). In the H. pylori-negative group, the AKT rs1130233 GA genotype was associated with a 1.69-fold increased risk of AG compared with CON (P = 0.012, 95% CI 1.12–2.54, Table 2). In addition, no significant association was found between these two polymorphisms and intestinal-type or diffuse-type GC in the overall and stratified analyses (S3 and S4 Tables).
We then performed an interaction analysis for MTOR rs1064261 and AKT rs1130233 with H. pylori infection. The results indicated that the AKT rs1130233 (GA+AA) genotype had a significant interaction with H. pylori infection in CON→AG and AG→GC progression (P = 0.013 and P = 0.049; Table 4). However, no significant interaction of MTOR rs1064261 with AKT rs1130233 was observed for CON→AG→GC progression in the overall and stratified analyses (S5 Table). Additionally, there was no significant interaction between these two polymorphisms and H. pylori infection in CON→AG→GC progression (Table 4).
Table 4. The interaction of MTOR rs1064261 and AKT rs1130233 polymorphisms in the risk of atrophic gastritis and gastric cancer*.
| Genotypes | AG vs. CON | GC vs. AG | GC vs. CON | GC vs. CON+AG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H.pylori(-) | H.pylori(+) | H.pylori(-) | H.pylori(+) | H.pylori(-) | H.pylori(+) | H.pylori(-) | H.pylori(+) | |||
| MTOR rs1064261 | ||||||||||
| TT | Controls/Cases | 432/229 | 124/348 | 229/196 | 348/202 | 432/196 | 124/202 | 661/196 | 472/202 | |
| OR(95%CI) | 1(Ref) | 5.33(4.10–6.91) | 1(Ref) | 0.68(0.53–0.89) | 1(Ref) | 3.64(2.74–4.83) | 1(Ref) | 1.46(1.16–1.84) | ||
| TC+CC | Controls/Cases | 91/44 | 22/55 | 44/33 | 55/47 | 91/33 | 22/47 | 135/33 | 77/47 | |
| OR(95%CI) | 0.92(0.62–1.37) | 4.76(2.83–8.00) | 0.92(0.56–1.50) | 1.00(0.65–1.56) | 0.85(0.55–1.31) | 4.78(2.79–8.18) | 0.87(0.58–1.32) | 2.08(1.39–3.11) | ||
| P interaction = 0.926 | P interaction = 0.195 | P interaction = 0.270 | P interaction = 0.093 | |||||||
| AKT rs1130233 | ||||||||||
| GG | Controls/Cases | 118/43 | 24/86 | 43/47 | 86/41 | 118/47 | 24/41 | 161/47 | 110/41 | |
| OR(95%CI) | 1(Ref) | 9.83(5.55–17.41) | 1(Ref) | 0.43(0.24–0.76) | 1(Ref) | 4.23(2.27–7.87) | 1(Ref) | 1.26(0.76–2.08) | ||
| GA+AA | Controls/Cases | 403/227 | 122/313 | 227/181 | 313/208 | 403/181 | 122/208 | 630/181 | 435/208 | |
| OR(95%CI) | 1.54(1.05–2.26) | 7.02(4.67–10.54) | 0.77(0.49–1.24) | 0.64(0.41–1.02) | 1.19(0.81–1.76) | 4.52(2.99–6.85) | 1.04(0.72–1.52) | 1.73(1.19–2.53) | ||
| P interaction = 0.013 | P interaction = 0.049 | P interaction = 0.702 | P interaction = 0.366 | |||||||
| MTOR rs1064261 | AKT rs1130233 | |||||||||
| TT | GG | Controls/Cases | 35/92 | 75/20 | 5/8 | 9/11 | 42/92 | 32/20 | 42/127 | 32/95 |
| OR(95%CI) | 1(Ref) | 9.86(5.26–18.48) | 1(Ref) | 0.36(0.19–0.67) | 1(Ref) | 3.51(1.77–6.96) | 1(Ref) | 1.02(0.59–1.77) | ||
| TT | GA+AA | Controls/Cases | 192/339 | 269/104 | 28/153 | 38/170 | 153/339 | 170/104 | 153/531 | 170/373 |
| OR(95%CI) | 1.48(0.97–2.27) | 6.77(4.32–10.63) | 0.71(0.43–1.18) | 0.56(0.34–0.93) | 1.05(0.69–1.61) | 3.82(2.43–5.99) | 0.93(0.62–1.39) | 1.47(0.98–2.21) | ||
| TC+CC | GG | Controls/Cases | 8/26 | 11/4 | 8/35 | 11/75 | 5/26 | 9/4 | 5/34 | 9/15 |
| OR(95%CI) | 0.81(0.34–1.96) | 7.23(2.16–24.21) | 0.58(0.17–1.93) | 0.67(0.24–1.86) | 0.47(0.17–1.30) | 4.84(1.38–17.04) | 0.49(0.18–1.34) | 1.78(0.70–4.52) | ||
| TC+CC | GA+AA | Controls/Cases | 35/64 | 44/18 | 35/192 | 44/269 | 28/64 | 38/18 | 28/99 | 38/62 |
| OR(95%CI) | 1.44(0.82–2.53) | 6.43(3.28–12.59) | 0.74(0.38–1.45) | 0.78(0.41–1.46) | 1.06(0.59–1.90) | 4.98(2.53–9.81) | 0.95(0.54–1.65) | 1.99(1.16–3.44) | ||
| P interaction = 0.809 | P interaction = 0.258 | P interaction = 0.432 | P interaction = 0.260 | |||||||
Note:
*using Logistic Regession adjusted by sex and age.
Abbreviations: CON, control; AG, atrophic gastritis; GC, gastric cancer; OR, odds ratio; CI, confidence interval; Ref, reference.
Associations of SNPs with clinicopathological parameters of GC patients
Analysis of the relation of MTOR rs1064261 and AKT rs1130233 with clinicopathological parameters in GC patients suggested that the AKT rs1130233 GA and (GA+AA) variant genotypes occur more frequently in GC patients without lymph node metastasis than in those with lymph node metastasis (GA 89.1% vs. 71.1%, P = 0.012; GA+AA 92.2% vs. 81.1%, P = 0.030). The GA, AA, and (GA+AA) genotypes were more frequent in drinkers than in nondrinkers (GA 61.0% vs. 95.8%, P = 0.047; AA 63.6% vs. 88.9%, P = 0.047; GA+AA 76.8% vs. 96.9%, P = 0.038; Table 5). However, no significant association was observed between MTOR rs1064261 variants and clinicopathological parameters in GC patients.
Table 5. Correlation between MTOR rs1064261 or AKT rs1130233 polymorphisms and clinicopathological parameters in gastric cancer.
| Variables | Wild | Heterozygous | P | Mutation | P | Dominance model | Recessive model |
|---|---|---|---|---|---|---|---|
| MTOR rs1064261 | |||||||
| Age | 0.772 | 1.000 a | 0.845 | 1.000 a | |||
| ≤50 | 44 | 10 | 0 | ||||
| >50 | 124 | 25 | 1 | ||||
| Sex | 0.278 | 0.325 a | 0.400 | 0.309 a | |||
| Male | 114 | 27 | 0 | ||||
| Female | 54 | 8 | 1 | ||||
| Borrmann type | 0.814 | 0.429 a | 0.672 | 0.432 a | |||
| Borrmann I–II | 62 | 13 | 1 | ||||
| Borrmann III–IV | 84 | 16 | 0 | ||||
| Lauren classification | 0.443 | 1.000 a | 0.381 | 1.000 a | |||
| Intestinal | 64 | 11 | 0 | ||||
| Diffuse | 105 | 24 | 1 | ||||
| TNM stage | 0.855 | 1.000 a | 0.759 | 1.000 a | |||
| I–II | 98 | 21 | 1 | ||||
| III–IV | 70 | 14 | 0 | ||||
| Growth pattern | 0.787 | NA | 0.787 | NA | |||
| Massive and Nested | 59 | 11 | 0 | ||||
| Diffused | 52 | 11 | 0 | ||||
| Lymphatic metastasis | 0.266 | 0.361 a | 0.196 | 0.377 a | |||
| Negative | 60 | 16 | 1 | ||||
| Positive | 108 | 19 | 0 | ||||
| Depth of invasion | 0.955 | NA | 0.955 | NA | |||
| T1+T2 | 36 | 7 | 0 | ||||
| T3+T4 | 75 | 15 | 0 | ||||
| Smoking | 0.557 | NA | 0.557 | NA | |||
| Nonsmoker | 68 | 12 | 0 | ||||
| Smoker | 43 | 10 | 0 | ||||
| Acohol drinking | 0.150 | NA | 0.150 | NA | |||
| Nondrinker | 78 | 12 | 0 | ||||
| Drinker | 33 | 10 | 0 | ||||
| Family history | 0.774 a | NA | 0.774 a | NA | |||
| No | 89 | 17 | 0 | ||||
| Yes | 22 | 5 | 0 | ||||
| H. pylori-IgG | 0.171 | 0.443 a | 0.241 | 0.421 a | |||
| Negative | 73 | 11 | 1 | ||||
| Positive | 93 | 24 | 0 | ||||
| AKT rs1130233 | |||||||
| Age | 0.627 | 0.799 | 0.673 | 0.873 | |||
| ≤50 | 7 | 30 | 17 | ||||
| >50 | 23 | 78 | 49 | ||||
| Sex | 0.733 | 0.209 | 0.458 | 0.156 | |||
| Male | 19 | 72 | 50 | ||||
| Female | 11 | 36 | 16 | ||||
| Borrmann type | 0.960 | 0.880 | 0.922 | 0.866 | |||
| Borrmann I–II | 11 | 39 | 26 | ||||
| Borrmann III–IV | 15 | 52 | 33 | ||||
| Lauren classification | 0.758 | 0.594 | 0.973 | 0.262 | |||
| Intestinal | 11 | 36 | 28 | ||||
| Diffuse | 19 | 71 | 38 | ||||
| TNM stage | 0.912 | 0.618 | 0.887 | 0.391 | |||
| I–I | 18 | 66 | 36 | ||||
| III–IV | 12 | 42 | 30 | ||||
| Growth pattern | 0.957 | 0.624 | 0.810 | 0.497 | |||
| Massive and Nested | 9 | 36 | 25 | ||||
| Diffused | 9 | 35 | 19 | ||||
| Lymphatic metastasis | 0.012 | 0.183 | 0.030 | 0.369 | |||
| Negative | 6 | 49 | 22 | ||||
| Positive | 24 | 59 | 44 | ||||
| Depth of invasion | 0.970 | 0.769 | 0.922 | 0.629 | |||
| T1+T2 | 6 | 24 | 13 | ||||
| T3+T4 | 12 | 47 | 31 | ||||
| Smoking | 0.560 | 0.578 | 0.544 | 0.861 | |||
| Nonsmoker | 12 | 42 | 26 | ||||
| Smoker | 6 | 29 | 18 | ||||
| Alcohol drinking | 0.047 | 0.047 | 0.038 | 0.484 | |||
| Nondrinker | 16 | 25 | 28 | ||||
| Drinker | 2 | 46 | 16 | ||||
| Family history | 0.083 a | 0.541 a | 0.203 a | 0.343 | |||
| No | 12 | 61 | 33 | ||||
| Yes | 6 | 10 | 11 | ||||
| H. pylori-IgG | 0.973 | 0.759 | 0.880 | 0.680 | |||
| Negative | 13 | 46 | 26 | ||||
| Positive | 17 | 61 | 39 |
Note:
a, when the theoretical frequency of less than 5, using the Fisher's exact test; Wild, heterozygous, mutation, dominance model and recessive model of MTOR rs1064261 polymorphisms are TT, TC, CC, TC+CC vs. TT and CC vs. TC+TT, respectively. Wild, heterozygous, mutation, dominance model and recessive model of AKT rs1130233 polymorphisms are GG, GA, AA, GA+AA vs. GG, AA vs. GA+GG, respectively.
Abbreviations: NA, not available; T1+T2, mucosa, submucosa, muscularis propria; T3+T4, subserosa, serosa.
No association between SNPs and GC patient survival
Univariable and multivariable Cox proportional hazards models were performed to assess the effect of MTOR rs1064261 and AKT rs1130233 polymorphisms on GC survival (Table 6). Because Lauren’s classification, TNM stage, growth pattern, invasion depth, and lymph node metastasis were significantly associated with survival (P<0.05, shown in Table 3), they were considered as adjusted covariables in the Cox proportional hazards regression model. The results showed no significant association between the two SNPs and GC prognosis (Table 6). Similarly, a stratified analysis did not demonstrate any significant association between SNP genotype and GC prognosis (S6 Table).
Table 6. Univariable and multivariable cox proportional hazard analysis for MTOR rs1064261 and AKT rs1130233 polymorphisms.
| Variables | All GC | Death | MST | Univariable | Multivariable b | ||
|---|---|---|---|---|---|---|---|
| N | N | M | P-value | HR(95%CI) | P-value | HR(95%CI) | |
| MTOR rs1064261 | |||||||
| TT | 168 | 57 | NA | 1(Ref) | 1(Ref) | ||
| TC | 35 | 10 | NA | 0.593 | 0.83(0.43–1.63) | 0.224 | 0.52(0.18–1.49) |
| CC | 1 | 0 | NA | 0.655 | 0.05(0–27266.94) | NA | NA |
| TC+CC vs. TT | 0.523 | 0.80(0.41–1.57) | 0.224 | 0.52(0.18–1.49) | |||
| CC vs. TC+TT | 0.663 | 0.05(0–36725.40) | NA | NA | |||
| AKT rs1130233 | |||||||
| GG | 30 | 10 | 62.81 a | 1(Ref) | 1(Ref) | ||
| GA | 108 | 33 | 59.47 a | 0.893 | 0.95(0.47–1.93) | 0.517 | 1.44(0.48–4.35) |
| AA | 66 | 24 | 54.30 a | 0.790 | 1.11(0.53–2.31) | 0.434 | 1.57(0.51–4.86) |
| GA+AA vs. GG | 0.991 | 1.00(0.51–1.97) | 0.483 | 1.45(0.51–4.15) | |||
| AA vs. GG+GA | 0.546 | 1.17(0.71–1.92) | 0.882 | 1.05(0.54–2.07) | |||
Note:
a, mean survival time was provided when MST could not be calculated;
b, using multivariable COX proportional hazards model adjusted by Lauren classification, TNM stage, growth pattern, depth of invasion, lymphatic metastasis, and multivariate survival analysis was carried out by adding the SNP variable to the clinicopathological parameters with P<0.05.
Abbreviations: MST, median survival time (months); HR, hazard rate; CI, confidence interval; Ref, reference; GC, gastric cancer; NA, not available; N, number; M, months.
SNP genotype correlates with total and phosphorylated proteins expression
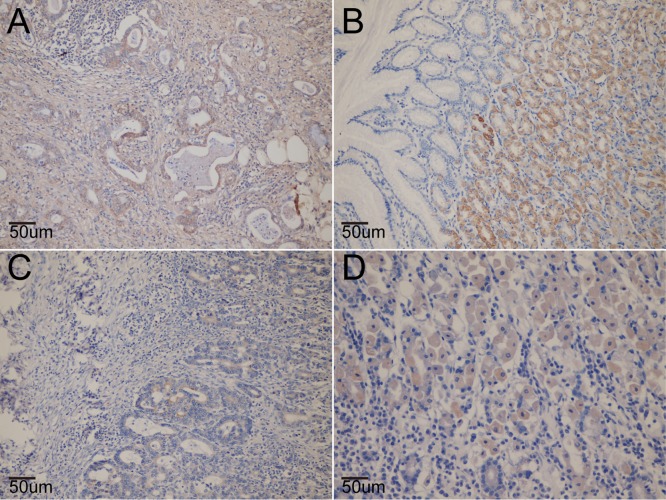
The expression of total and phosphorylated MTOR and AKT proteins was analyzed in tissues from the different groups of participants (Fig 1). Overall, there was no significant association between the MTOR rs1064261 or AKT rs1130233 polymorphism and total or phosphorylated MTOR and AKT proteins. In the H. pylori-positive subgroup, the proportion of p-AKT-positive cells was significantly higher in the variant genotype group than in the wild-type group (P = 0.045; Table 7).
Fig 1. The expression of mTOR and AKT protein in tissue in situ.
A. the expression of mTOR protein in gastric cancer mucosa (100×); B. the expression of p-mTOR protein in gastric mucosa (100×); C. the expression of AKT protein in gastric cancer mucosa (100×); D. the expression of p-AKT protein in gastric mucosa (100×).
Table 7. The effect on the polymorphisms to its protein expression in the group of gastric cancer * .
| Protein Expression | P-value | ||
|---|---|---|---|
| Variable | Negative | Positive | |
| MTOR | |||
| MTOR rs1064261 TT | 14(82.4) | 9(75.0) | |
| MTOR rs1064261 TC | 3(17.6) | 3(25.0) | 0.645 |
| MTOR rs1064261 CC | 0 | 0 | NA |
| Phosphoryltion MTOR | |||
| MTOR rs1064261 TT | 30(83.3) | 11(73.3) | |
| MTOR rs1064261 TC | 6(16.7) | 4(26.7) | 0.126 |
| MTOR rs1064261 CC | 0 | 0 | NA |
| AKT(PAN) | |||
| AKT rs1130233 GG | 1(5.9) | 1(12.5) | |
| AKT rs1130233 GA | 8(47.1) | 5(62.5) | 0.475 |
| AKT rs1130233 AA | 8(47.1) | 2(25.0) | 0.266 |
| GA+AA vs. GG | 0.638 | ||
| AA vs. GA+GG | 0.236 | ||
| Phosphoryltion AKT | |||
| AKT rs1130233 GG | 8(17.0) | 3(37.5) | |
| AKT rs1130233 GA | 23(48.9) | 3(37.5) | 0.311 |
| AKT rs1130233 AA | 16(34.0) | 2(25.0) | 0.251 |
| GA+AA vs. GG | 0.233 | ||
| AA vs. GA+GG | 0.654 | ||
| Male | |||
| MTOR | |||
| MTOR rs1064261 TT | 8(72.7) | 5(71.4) | |
| MTOR rs1064261 TC | 3(27.3) | 2(28.6) | 1.000 |
| MTOR rs1064261 CC | 0 | 0 | |
| Phosphoryltion MTOR | |||
| MTOR rs1064261 TT | 21(77.8) | 4(50.0) | |
| MTOR rs1064261 TC | 6(22.2) | 4(50.0) | 0.186 |
| MTOR rs1064261 CC | 0 | 0 | |
| AKT(PAN) | |||
| AKT rs1130233 GG | 1(8.3) | 1(16.7) | |
| AKT rs1130233 GA | 4(33.3) | 4(66.7) | 0.282 |
| AKT rs1130233 AA | 7(58.3) | 1(16.7) | |
| Phosphoryltion AKT | |||
| AKT rs1130233 GG | 5(16.7) | 2(40.0) | |
| AKT rs1130233 GA | 15(50.0) | 2(40.0) | 0.562 |
| AKT rs1130233 AA | 10(33.3) | 1(20.0) | |
| Female | |||
| MTOR | |||
| MTOR rs1064261 TT | 6(100.0) | 4(80.0) | |
| MTOR rs1064261 TC | 0 | 1(20.0) | 0.455 |
| MTOR rs1064261 CC | 0 | 0 | |
| Phosphoryltion MTOR | |||
| MTOR rs1064261 TT | 9 | 7 | |
| MTOR rs1064261 TC | 0 | 0 | NA |
| MTOR rs1064261 CC | 0 | 0 | |
| AKT(PAN) | |||
| AKT rs1130233 GG | 0 | 0 | |
| AKT rs1130233 GA | 4(80.0) | 1(50.0) | |
| AKT rs1130233 AA | 1(20.0) | 1(50.0) | 1.000 |
| Phosphoryltion AKT | |||
| AKT rs1130233 GG | 3(17.6) | 1(33.3) | |
| AKT rs1130233 GA | 8(47.1) | 1(33.3) | 1.000 |
| AKT rs1130233 AA | 6(35.3) | 1(33.3) | |
| H.pylori (+) | |||
| MTOR | |||
| MTOR rs1064261 TT | 2(66.7) | 3(75.0) | |
| MTOR rs1064261 TC | 1(33.3) | 1(25.0) | 1.000 |
| MTOR rs1064261 CC | 0 | 0 | |
| Phosphoryltion MTOR | |||
| MTOR rs1064261 TT | 14(82.4) | 4(66.7) | |
| MTOR rs1064261 TC | 3(17.6) | 2(33.3) | 0.576 |
| MTOR rs1064261 CC | 0 | 0 | |
| AKT(PAN) | |||
| AKT rs1130233 GG | 0 | 1(20.0) | |
| AKT rs1130233 GA | 3(42.9) | 3(60.0) | 0.369 |
| AKT rs1130233 AA | 4(57.1) | 1(20.0) | |
| Phosphoryltion AKT | |||
| AKT rs1130233 GG | 2(66.7) | 2(11.1) | |
| AKT rs1130233 GA | 0 | 11(61.1) | 0.045 |
| AKT rs1130233 AA | 1(33.3) | 5(27.8) | |
| H.pylori (-) | |||
| MTOR | |||
| MTOR rs1064261 TT | 7(77.8) | 11(84.6) | |
| MTOR rs1064261 TC | 2(22.2) | 2(15.4) | 1.000 |
| MTOR rs1064261 CC | 0 | 0 | |
| Phosphoryltion MTOR | |||
| MTOR rs1064261 TT | 16(84.2) | 7(77.8) | |
| MTOR rs1064261 TC | 3(15.8) | 2(22.2) | 1.000 |
| MTOR rs1064261 CC | 0 | 0 | |
| AKT(PAN) | |||
| AKT rs1130233 GG | 1(10.0) | 0 | |
| AKT rs1130233 GA | 5(50.0) | 2(66.7) | 1.000 |
| AKT rs1130233 AA | 4(40.0) | 1(33.3) | |
| Phosphoryltion AKT | |||
| AKT rs1130233 GG | 1(20.0) | 6(20.7) | |
| AKT rs1130233 GA | 3(60.0) | 12(41.4) | 0.826 |
| AKT rs1130233 AA | 1(20.0) | 11(37.9) | |
Note:
*using Logistic Regession adjusted by sex, age and H.pylori infection status.
Discussion
In this study, we report for the first time an association of the MTOR rs1064261 and AKT rs1130233 polymorphisms with GC risk and prognosis. In males, the MTOR rs1064261 (TC+CC) genotype and the A allele of AKT rs1130233 were associated with an increased GC risk. In H. pylori-negative males, the AKT rs1130233 (GA+AA) genotype was associated with an increased AG risk. In addition, the AKT rs1130233 (GA+AA) genotype showed a significant interaction with H. pylori infection in CON→AG→GC progression. The variant genotype of AKT rs1130233 was associated with increased p-AKT protein expression. The AKT rs1130233 polymorphism was also associated with lymph node metastasis and alcohol drinking. These findings provide experimental evidence to support MTOR and AKT as potential biomarkers of specific types of GC and also provide clues to the interaction between H. pylori infection and MTOR/AKT signaling.
The MTOR gene is located on human chromosome 1p36.2 and encodes the 289 kDa MTOR protein, consisting of 2549 amino acids. MTOR is a member of phosphatidyl inositol kinase (PIK) family and has serine/threonine kinase activities. We found that the MTOR gene (TC+CC) variant genotype was associated with an increased GC risk in males, suggesting that it is involved in carcinogenesis. MTOR mainly exists in the cytoplasm under normal conditions and enters the nucleus after activation to regulate the downstream targets eukaryotic initiation factor 4E (eIF-4E) binding protein 1 (4E-BP1) and ribosome 40S small subunit S6 protein kinase (p70S6K) [22–24]. The latter is an essential member of the PI3K/AKT/MTOR pathway and a central metabolic signaling molecule. MTOR integrates a variety of cellular signals from growth factors and nutritional and energy status; thus, it has important biological functions in angiogenesis and cell growth, proliferation, metabolism, migration, differentiation, and apoptosis [25]. Its function is closely linked to the transformation of normal to cancer cells and to cancer cell proliferation. Genetic variations in genes of the MTOR signaling pathway (PI3K, AKT, and PTEN) can promote carcinogenesis [26–28]. MTOR SNPs are reported to be associated with susceptibility to GC [7, 9], renal cell carcinoma [6], prostate cancer [8], and esophageal squamous cancer [10]. These SNPs might affect the levels of MTOR expression and transcriptional activity, thereby altering the protein function. The G allele of MTOR rs2295080 polymorphism is associated with a reduced GC risk, possibly resulting from reduced promoter activity and mRNA expression [7]. Another MTOR polymorphism located in the promoter region, rs1883965 G→A, confers increased risks of GC [9] and esophageal squamous cancer [10]. Our findings suggest that the (TC+CC) genotype is associated with an increased GC risk in males, although no effect on protein expression was observed. MTOR can promote cell proliferation and somewhat “oncogenic” characteristics. The variant genotype might have even more oncogenic activity. As men are more likely to be exposed to multiple risk exposure factors (smoking, drinking and unhealthy living habits), carriers of certain genotype may be susceptible to an increased risk of GC.
AKT, the v-AKT murine thymoma viral oncogene homolog, maps to human chromosome 14q32.32 and encodes a 56 kda protein, consisting of 480 amino acids [15]. AKT is an important effector of the PI3K/AKT/MTOR signal pathway, and genetic mutation or abnormal protein expression can alter a variety of cellular process including migration, proliferation, growth, and survival. Additionally, AKT activation is involved in cell proliferation and apoptosis, which are related to cancer initiation and progression [29]. AKT SNPs are reported to be associated with susceptibility to and/or the prognosis of various cancer types including nasopharyngeal carcinoma [12], oral squamous cell carcinoma [13, 30], non-small cell lung cancer [14, 15], pancreatic ductal adenocarcinoma [31], and GC [32] via effects on protein expression and transcriptional activity. Wang et al. studied four SNPs including rs1130233 in a Chinese population [13], and found that three polymorphisms were associated with susceptibility to oral squamous cell carcinoma or DFS, but with no significant relation for rs1130233. Zhang et al. reported that AA haplotypes of AKT rs1130233 and rs2494732 conferred an increased nasopharyngeal carcinoma risk [12] and that some AKT haplotypes cause increased AKT protein expression [33, 34], leading to altered cellular migration and proliferation. Therefore, the AKT rs1130233 A allele might have increased proliferative activity. In this study, we found that the AKT rs1130233 A allele conferred an increased risk of GC in males. Generally, males have a higher GC incidence and a higher GC mortality rate than adult women [35]. The high level of exposure of males to environmental risk factors (smoking, drinking and unhealthy living habits) increases their susceptibility to GC [36]. As the A allele might have stronger proliferative activity, the combined effect of AKT polymorphism and gender could partially explain the observed high GC risk associated with the AKT rs1130233 polymorphism in males.
In addition, we found that in the H. pylori-negative subgroup, the AKT rs1130233 (GA+AA) genotype was associated with increased AG risk. The AKT rs1130233 polymorphism showed a significant interaction with H. pylori infection in CON→AG→GC progression. It is widely accepted that H. pylori is a major cause of GC. H. pylori virulent factors may induce abnormal cell proliferation and apoptosis through regulating signaling pathways (including PI3K/AKT); this is a recent research hotspot [37–39]. Tabassam et al. suggested that the AKT phosphorylation induced by the H. pylori virulence factors cag PAI and OipA regulates intracellular signals responsible for a series of cellular functions involved in gastric carcinogenesis. The specific promotion of AKT serine 473 or threonine 308 phosphorylation by cag PAI and OipA may disrupt downstream proliferation and apoptosis signals. A combination of cag PAI and OipA is sufficient to activate the PI3K/PDK1 pathway and AKT/ERK/ downstream signaling [39]. Nakayama et al. reported that H. pylori virulence factor VacA induces β-catenin function by activating the PI3K/AKT pathway and inactivating GSK33β [38], thus regulating cell proliferation, differentiation and apoptosis. These data suggest that the interaction of H. pylori with activated AKT has a biological function. However, neither of the two SNPs included in this study nor H. pylori infection in the context of these SNPs showed a significant interaction with GC. Overall, these data emphasize the important role of the AKT rs1130233 polymorphism in the PI3K/MTOR/AKT pathway. In addition, in the H. pylori-positive subgroup, those with the AKT rs1130233 variant genotype had increased p-AKT expression. As this polymorphism showed an interaction with H. pylori infection, the effect of this polymorphism on protein expression may only emerge in the presence of H. pylori infection. A genetic mutation leading to abnormal AKT expression is reported to promote cell migration and proliferation [40, 41], which might explain why this polymorphism increased the risk of AG. Future large-scale studies are required to confirm these results.
We also compared the genotype distribution of these two polymorphisms in groups with different clinicopathological parameters and assessed their relation with GC prognosis. We found that the AKT rs1130233 GA, AA, and (GA+AA) genotypes were more frequent in drinkers than in nondrinkers. We therefore assume that drinkers with AKT rs1130233 GA, AA, and (GA+AA) genotypes are more susceptible to GC. Avan et al. [31] reported that the AKT rs1130233 A allele is associated with reduced survival of pancreatic ductal adenocarcinoma patients, which might be attributed to reduced AKT1 mRNA and protein expression and reduced apoptosis efficiency [33, 42]. The AKT rs1130233 A allele is a risk genotype for cancer, possibly in association with alcohol drinking as consuming alcohol was one of the increased risk factors of GC[43–46]. However, no significant association of these two SNPs with GC survival was found by Cox regression analysis. Due to the relatively small sample size, the recall bias might exist in the case-control study, and outcome status may have caused cases to alter their exposure profile. Therefore, further experiments and large-scale studies were needed to confirm our results.
The present study has some limitations. First, the sample size was relatively small, especially for survival and protein expression analyses, both of which need further confirmation in large populations. Second, only OS was analyzed in the survival analysis and other prognostic parameters such as progression-free survival are also warranted. Third, the survival analysis was only performed in a subset of the whole GC patients which could make the probability of a selective bias, and a large sample size for the prognostic study should be performed in the near future. Fourth, functional experiments are required to elucidate the underlying disease mechanism.
Conclusion
In summary, the relation of MTOR rs1064261 and AKT rs1130233 polymorphisms with GC susceptibility and OS has been shown for the first time. MTOR rs1064261 and AKT rs1130233 polymorphisms were significantly associated with GC risk in males. The AKT rs1130233 polymorphism and H. pylori infection showed a significant interaction in CON→AG→GC progression. The AKT rs1130233 polymorphism was associated with increased p-AKT protein expression in H. pylori-positive individuals. The AKT rs1130233 polymorphism was associated with variations in clinicopathological parameters including lymph node metastasis and alcohol drinking. Future large-scale investigations and functional studies are needed to confirm these results.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Key Basic Research Program of China (973 Program ref no. 2010CB529304), the National Natural Science Foundation of China (Ref No.31200968).
References
- 1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011;61(4):212–36. 10.3322/caac.20121 . [DOI] [PubMed] [Google Scholar]
- 2. Lochhead P, El-Omar EM. Gastric cancer. British medical bulletin. 2008;85:87–100. 10.1093/bmb/ldn007 . [DOI] [PubMed] [Google Scholar]
- 3. Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Archiv: an international journal of pathology. 2014;465(1):25–33. Epub 2014/05/23. 10.1007/s00428-014-1588-4 . [DOI] [PubMed] [Google Scholar]
- 4. Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Molecular and cellular neurosciences. 2011;47(2):145–53. 10.1016/j.mcn.2011.03.010 . [DOI] [PubMed] [Google Scholar]
- 5. Yu J, Yaba A, Kasiman C, Thomson T, Johnson J. mTOR controls ovarian follicle growth by regulating granulosa cell proliferation. PloS one. 2011;6(7):e21415 10.1371/journal.pone.0021415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao Q, Ju X, Li P, Meng X, Shao P, Cai H, et al. A functional variant in the MTOR promoter modulates its expression and is associated with renal cell cancer risk. PloS one. 2012;7(11):e50302 Epub 2012/12/05. 10.1371/journal.pone.0050302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu M, Tao G, Kang M, Gao Y, Zhu H, Gong W, et al. A polymorphism (rs2295080) in mTOR promoter region and its association with gastric cancer in a Chinese population. PloS one. 2013;8(3):e60080 Epub 2013/04/05. 10.1371/journal.pone.0060080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Q, Gu C, Zhu Y, Wang M, Yang Y, Wang J, et al. Polymorphisms in the mTOR gene and risk of sporadic prostate cancer in an Eastern Chinese population. PloS one. 2013;8(8):e71968 Epub 2013/08/14. 10.1371/journal.pone.0071968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He J, Wang MY, Qiu LX, Zhu ML, Shi TY, Zhou XY, et al. Genetic variations of mTORC1 genes and risk of gastric cancer in an Eastern Chinese population. Molecular carcinogenesis. 2013;52 Suppl 1:E70–9. Epub 2013/02/21. 10.1002/mc.22013 . [DOI] [PubMed] [Google Scholar]
- 10. Zhu ML, Yu H, Shi TY, He J, Wang MY, Li QX, et al. Polymorphisms in mTORC1 genes modulate risk of esophageal squamous cell carcinoma in eastern Chinese populations. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8(6):788–95. Epub 2013/03/26. . [DOI] [PubMed] [Google Scholar]
- 11. Shao J, Li Y, Zhao P, Yue X, Jiang J, Liang X, et al. Association of mTOR polymorphisms with cancer risk and clinical outcomes: a meta-analysis. PloS one. 2014;9(5):e97085 Epub 2014/05/13. 10.1371/journal.pone.0097085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Chen X, Zhai Y, Cui Y, Cao P, Zhang H, et al. Combined effects of genetic variants of the PTEN, AKT1, MDM2 and p53 genes on the risk of nasopharyngeal carcinoma. PloS one. 2014;9(3):e92135 Epub 2014/03/19. 10.1371/journal.pone.0092135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Lin L, Xu H, Li T, Zhou Y, Dan H, et al. Genetic variants in AKT1 gene were associated with risk and survival of OSCC in Chinese Han Population. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2014. Epub 2014/07/26. 10.1111/jop.12211 . [DOI] [PubMed] [Google Scholar]
- 14. Li Q, Yang J, Yu Q, Wu H, Liu B, Xiong H, et al. Associations between single-nucleotide polymorphisms in the PI3K-PTEN-AKT-mTOR pathway and increased risk of brain metastasis in patients with non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(22):6252–60. Epub 2013/10/01. 10.1158/1078-0432.ccr-13-1093 . [DOI] [PubMed] [Google Scholar]
- 15. Kim MJ, Kang HG, Lee SY, Jeon HS, Lee WK, Park JY, et al. AKT1 polymorphisms and survival of early stage non-small cell lung cancer. Journal of surgical oncology. 2012;105(2):167–74. Epub 2011/08/16. 10.1002/jso.22071 . [DOI] [PubMed] [Google Scholar]
- 16. Lauren P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta pathologica et microbiologica Scandinavica. 1965;64:31–49. . [DOI] [PubMed] [Google Scholar]
- 17. Xu Q, Chen MY, He CY, Sun LP, Yuan Y. Promoter polymorphisms in trefoil factor 2 and trefoil factor 3 genes and susceptibility to gastric cancer and atrophic gastritis among Chinese population. Gene. 2013;529(1):104–12. 10.1016/j.gene.2013.07.070 . [DOI] [PubMed] [Google Scholar]
- 18. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. The American journal of surgical pathology. 1996;20(10):1161–81. . [DOI] [PubMed] [Google Scholar]
- 19. Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2001;15(9):591–8. . [DOI] [PubMed] [Google Scholar]
- 20. Xu Q, Yuan Y, Sun LP, Gong YH, Xu Y, Yu XW, et al. Risk of gastric cancer is associated with the MUC1 568 A/G polymorphism. International journal of oncology. 2009;35(6):1313–20. . [DOI] [PubMed] [Google Scholar]
- 21. Gong YH, Sun LP, Jin SG, Yuan Y. Comparative study of serology and histology based detection of Helicobacter pylori infections: a large population-based study of 7,241 subjects from China. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2010;29(7):907–11. 10.1007/s10096-010-0944-9 . [DOI] [PubMed] [Google Scholar]
- 22. Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes & development. 2001;15(7):807–26. 10.1101/gad.887201 . [DOI] [PubMed] [Google Scholar]
- 23. Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. European journal of biochemistry / FEBS. 2002;269(22):5360–8. . [DOI] [PubMed] [Google Scholar]
- 24. Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Advances in cancer research. 2002;86:1–39. . [DOI] [PubMed] [Google Scholar]
- 25. Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Advances in cancer research. 2009;102:19–65. 10.1016/S0065-230X(09)02002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–64. 10.1038/sj.onc.1209085 . [DOI] [PubMed] [Google Scholar]
- 27. Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Current genomics. 2007;8(5):271–306. 10.2174/138920207782446160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stiles B, Gilman V, Khanzenzon N, Lesche R, Li A, Qiao R, et al. Essential role of AKT-1/protein kinase B alpha in PTEN-controlled tumorigenesis. Molecular and cellular biology. 2002;22(11):3842–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Marco C, Rinaldo N, Bruni P, Malzoni C, Zullo F, Fabiani F, et al. Multiple genetic alterations within the PI3K pathway are responsible for AKT activation in patients with ovarian carcinoma. PloS one. 2013;8(2):e55362 10.1371/journal.pone.0055362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Lin L, Xu H, Li T, Zhou Y, Dan H, et al. Genetic variants in AKT1 gene were associated with risk and survival of OSCC in Chinese Han Population. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2015;44(1):45–50. Epub 2014/07/26. 10.1111/jop.12211 . [DOI] [PubMed] [Google Scholar]
- 31. Avan A, Avan A, Le Large TY, Mambrini A, Funel N, Maftouh M, et al. AKT1 and SELP polymorphisms predict the risk of developing cachexia in pancreatic cancer patients. PloS one. 2014;9(9):e108057 Epub 2014/09/23. 10.1371/journal.pone.0108057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Lin Y, Lan F, Yu Y, Ouyang X, Wang X, et al. A GG allele of 3'-side AKT1 SNP is associated with decreased AKT1 activation and better prognosis of gastric cancer. Journal of cancer research and clinical oncology. 2014;140(8):1399–411. Epub 2014/04/17. 10.1007/s00432-014-1663-x . [DOI] [PubMed] [Google Scholar]
- 33. Harris SL, Gil G, Robins H, Hu W, Hirshfield K, Bond E, et al. Detection of functional single-nucleotide polymorphisms that affect apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(45):16297–302. 10.1073/pnas.0508390102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nature genetics. 2004;36(2):131–7. 10.1038/ng1296 . [DOI] [PubMed] [Google Scholar]
- 35. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. 10.3322/caac.20107 . [DOI] [PubMed] [Google Scholar]
- 36. Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods in molecular biology. 2009;472:467–77. 10.1007/978-1-60327-492-0_23 . [DOI] [PubMed] [Google Scholar]
- 37. Isomoto H, Moss J, Hirayama T. Pleiotropic actions of Helicobacter pylori vacuolating cytotoxin, VacA. The Tohoku journal of experimental medicine. 2010;220(1):3–14. Epub 2010/01/05. . [DOI] [PubMed] [Google Scholar]
- 38. Nakayama M, Hisatsune J, Yamasaki E, Isomoto H, Kurazono H, Hatakeyama M, et al. Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. The Journal of biological chemistry. 2009;284(3):1612–9. Epub 2008/11/11. 10.1074/jbc.M806981200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tabassam FH, Graham DY, Yamaoka Y. Helicobacter pylori activate epidermal growth factor receptor- and phosphatidylinositol 3-OH kinase-dependent Akt and glycogen synthase kinase 3beta phosphorylation. Cellular microbiology. 2009;11(1):70–82. Epub 2008/09/11. 10.1111/j.1462-5822.2008.01237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science (New York, NY). 1997;275(5300):628–30. . [DOI] [PubMed] [Google Scholar]
- 41. Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Current opinion in cell biology. 1998;10(2):262–7. . [DOI] [PubMed] [Google Scholar]
- 42. Giovannetti E, Zucali PA, Peters GJ, Cortesi F, D'Incecco A, Smit EF, et al. Association of polymorphisms in AKT1 and EGFR with clinical outcome and toxicity in non-small cell lung cancer patients treated with gefitinib. Molecular cancer therapeutics. 2010;9(3):581–93. 10.1158/1535-7163.MCT-09-0665 . [DOI] [PubMed] [Google Scholar]
- 43. Ali Z, Deng Y, Ma C. Progress of research in gastric cancer. Journal of nanoscience and nanotechnology. 2012;12(11):8241–8. . [DOI] [PubMed] [Google Scholar]
- 44. Moy KA, Fan Y, Wang R, Gao YT, Yu MC, Yuan JM. Alcohol and tobacco use in relation to gastric cancer: a prospective study of men in Shanghai, China. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(9):2287–97. 10.1158/1055-9965.EPI-10-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duell EJ, Travier N, Lujan-Barroso L, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, et al. Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. The American journal of clinical nutrition. 2011;94(5):1266–75. 10.3945/ajcn.111.012351 . [DOI] [PubMed] [Google Scholar]
- 46. Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2012;23(1):28–36. 10.1093/annonc/mdr135 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.