Abstract
Objectives
Rheumatoid arthritis (RA) patients are at an increased risk of developing comorbid conditions. A close monitoring of the disease targeting a status of low disease activity is associated with a better outcome. The aim of this trial was to evaluate the impact of a nurse-led programme on comorbidities and the impact of patient self-assessment of disease activity on the management of RA.
Methods
We enrolled 970 patients (mean age 58 years, 79% women) in a prospective, randomised, controlled, open-label, 6-month trial. In the comorbidity group (n=482), the nurse checked comorbidities and sent the programme results to the attending physicians. In the self-assessment group (n=488), the nurse taught the patient how to calculate his/her Disease Activity Score which had to be reported on a booklet to be shared with the treating rheumatologist. The number of measures taken for comorbidities and the percentage of patients recording a change (initiation, switch or increased dose) in disease-modifying antirheumatic drugs (DMARDs) in the 6 months follow-up period of the study defined the outcomes of the trial.
Results
The number of measures taken per patient was statistically higher in the comorbidity group: 4.54±2.08 versus 2.65±1.57 (p<0.001); incidence rate ratio: 1.78 (1.61–1.96) and DMARD therapy was changed more frequently in the self-assessment group: 17.2% versus 10.9% (OR=1.70 (1.17; 2.49), p=0.006).
Conclusions
This study demonstrates the short-term benefit of a nurse-led programme on RA comorbidity management and the impact of patient self-assessment of disease activity on RA treatment intensification.
Trial registration number
NCT #01315652.
Keywords: Cardiovascular Disease, Rheumatoid Arthritis, Nursing
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory rheumatic disease that is most likely to be debilitating. It is associated with increased mortality in comparison with the general population because of the consequences of the disease and treatments, as well as the commonly observed comorbidities.1–3
In RA patients, comorbidities are more common, more severe and less well-managed than in the general population.3–5
Numerous studies have shown that excess mortality in RA patients is due to increased cardiovascular (CV) disease.6–8 CV morbidity is also increased.6 8 Therefore, the European League Against Rheumatism (EULAR) has proposed that all RA patients should undergo annual CV risk assessments.9 The management of CV risk factors, however, is far from optimal in both primary and secondary prevention.10
RA is associated with an increased risk of infection.11 The EULAR has recommended influenza and pneumococcal vaccines in all patients with autoimmune diseases.12 However, influenza and pneumococcal vaccinations appear far from optimal in RA.4 13–15
In RA patients, the prevalence of lung cancer and lymphomas is increased, whereas breast and colon cancer are decreased.16 Skin cancer appears to be more frequent in RA patients treated with antitumour necrosis factor agents.16 It has been suggested that RA patients receive fewer cancer screening tests than subjects without RA.3 4 17–19
Osteoporosis and osteoporotic vertebral fractures are more prevalent in RA patients.20–22 Nevertheless, therapies and measures for preventing osteoporosis in this population are underused, although osteoporosis management has improved in recent years.21 22
Nurse-led programmes have demonstrated their benefit in the cost-effective management of CV risk factors,23–28 improved pneumococcal vaccination coverage in at-risk patients29 30 and the management of osteoporosis with fracture risk in older women.31 Such evidence is not yet achieved in the field of RA.32–34
The prognosis of RA has improved dramatically in recent decades35 due to the availability of new drugs such as methotrexate36 and biologics,37 38 and to increased monitoring of disease activity.39 40
The use of a composite index including the number of swollen joints, number of tender joints, patient’s global assessment and erythrocyte sedimentation rate (ESR; mm/1st hour), such as the Disease Activity Score 28 (DAS28)-ESR, is recommended41 to evaluate the extent of RA.
RA disease status can be classified according to observed DAS28-ESR values from remission to high disease activity.42 Several studies have clearly shown that both high and moderate disease activity affect the long-term outcome of patients.43–45 Therefore, the current recommendation is to monitor patients frequently to check their levels of disease activity and propose a change in therapy (initiation/switch/intensification) if remission (early stage) or low disease activity (established disease) are not observed.46
Two main barriers to applying these recommendations successfully in daily practice have been identified: (1) time constraints mean that treating rheumatologists are unable to monitor the disease on a regular basis47 and (2) rheumatologists are reluctant to change the therapy when the disease activity is moderate.48
Therefore, an intensification of disease-modifying antirheumatic drug (DMARD) therapy can be expected in case of an optimal implementation of these recommendations. Alongside this, the role of nurses in managing chronic conditions, and RA in particular, is increasingly important for the regular assessment of disease activity.49 50 Finally, several studies have recently highlighted the importance of patient self-assessment in rheumatic diseases, which makes it possible to raise awareness,51 make joint decisions on treatment strategy with treating rheumatologists52 and increase the ability to assess RA disease activity.53 54
Incorporating a patient-based DAS into routine care can improve efficiency and quality of care.55
These preliminary remarks prompted us to conduct a prospective, randomised, controlled trial evaluating the impact of nurse-led consultation programmes on the management of comorbidities and the impact of patient self-assessment of disease activity on the management of RA.
Methods
Participants
Patients
Consecutive patients fulfilling the 1987 American College of Rheumatology criteria for the diagnosis of RA56 and visiting one of the rheumatologists working in or in close connection with 20 participating centres (secondary and/or tertiary care French rheumatology departments) were invited to participate if the following criteria were met: aged between 18 and 80, disease considered by the treating rheumatologist to have been stable for at least 3 months, no surgery planned in the 6 months from study baseline, and able to understand and comply with the study treatment. Prior to inclusion in the study, patients were asked to provide various medical documents (recent laboratory test results, vaccination diary, radiological test results, surgical reports, etc). A screening prescription, including blood cell count, ESR, C reactive protein, creatine and vitamin D levels, lipid profile and dipstick urine protein test with microalbuminuria evaluation when the dipstick test was positive, was sent to each patient.
Nurses
Nurses from the 20 participating rheumatology departments were invited to participate. The volunteer nurses attended a 1.5-day training session during which the calculation and interpretation of DAS were explained. The nurses were trained in assessing joint count, enabling them to calculate DAS under the supervision of trained physicians. This session was followed by an additional evaluation of 20 consecutive patients in comparison with the local senior rheumatologist in the regional centre.57 Moreover, a specific programme for patient self-assessment of joint count was explained via video (available at http://www.rhumatismes.net) and demonstration with volunteer patients.
Treating rheumatologists
Treating rheumatologists invited their patients to participate in the study and were responsible for managing patients with no specific instructions given (in particular, they were not aware of the primary objective of the trial).
Trial design
This was a prospective, multicentre, randomised, 6-month, parallel-group, open-label trial (NCT01315652). Such a trial comprised two arms (eg, comorbidity and disease activity self-assessment), one being the control of the other. It was conducted in accordance with the ethical principles of the Declaration of Helsinki, ICH-EG Good Clinical Practice guidelines and French regulations. All participating patients provided written informed consent. The study protocol and informed consent form were approved by the institutional review board (Ile-de-France III Ethics Committee, file #4-11 (B110057-30)).
Interventions
Intervention allocation
Once the inclusion criteria and written informed consent (data entered into an electronic-Case Report Form (e-CRF) by the nurse) had been checked, the study treatment was randomly allocated via an electronic system. Randomisation was centralised for all centres. Information on each patient’s allocated treatment was recorded in the e-CRF and also entered into the patient’s local medical file by the nurse. After the inclusion of the patient has been accepted and the randomisation code has been allocated by the electronic system, the nurses collected the additional baseline information and provided the allocated intervention.
Intervention in the comorbidity arm
This programme included three parts: (1) the report of the presence of pre-existing comorbidities (eg, stroke); (2) the detection of the presence of risk factors (eg, hypertension for CV disease (CVD)); and (3) the implementation of the recommendation for the detection (eg, yearly evaluation of CV risk factors) and/or management (eg, lipid-lowering therapy in case of hypercholesterolemia) of such comorbidities.
Nurses were given a booklet to be used for the systematic identification and assessment of the comorbidities associated with RA (http://www.rhumatismes.net/fichiers/Brochure_SFR_V7.pdf) (see online supplementary material). In case of a detected risk factor (eg, hypertension) and/or a non-optimally managed comorbidity (eg, lack of vaccination against pneumococcus), the nurse reminds the patient the interest of the management of such comorbidity and advises the patient to visit her/his general practitioner and/or rheumatologist to take care of it. In parallel, a report of this visit was sent to the general practitioner and the rheumatologist of each evaluated patient.
Intervention in the self-assessment arm
This nurse-led programme included: (1) a video presentation (available at http://www.rhumatismes.net) explaining the purpose, calculation and interpretation of the DAS28-ESR to the patient; (2) training on joint self-assessment by the participating nurse using the video given to each participating patient; and (3) a booklet and DAS calculator asking the patient to report the results of his/her ‘self-DAS28-ESR’ (using the last available ESR value) on a regular basis (at least monthly) and discuss the results with her/his treating rheumatologist.
Data collected
At baseline, the following information was recorded on patients who met the inclusion criteria: age, sex, Body Mass Index, occupation group and previous/current RA treatments (non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, RA-related surgical interventions and DMARDs (conventional and biological)). At both the baseline and 6-month visit, disease activity using the DAS28-ESR was collected by the nurse; functional impairment using the modified Health Assessment Questionnaire (mHAQ)58 and disease impact using the Rheumatoid Arthritis Impact of Disease (RAID) score59 were collected by the patient. At the 6-month visit, comorbidities measures taken against comorbidities and any changes in the RA therapies (eg, analgesics, corticosteroids, NSAIDs and DMARDs) since baseline were recorded. The following measures against comorbidities were considered for the definition of the primary objective of the trial: (1) for CVD: blood pressure measurement by the general practitioner or the rheumatologist, purchase of blood pressure self-measurement devices, initiation of a diet because of overweight, smoking cessation, initiation of lipid-lowering therapy, initiation of antiplatelet therapy, serum creatine measurement, or visit to a nephrologist because of renal deficiency; (2) for infectious diseases: vaccination against influenza, pneumococcus, hepatitis or meningococcus; and (3) for cancers: mammography (breast), smear (uterus), blood-in-stool screening and/or colonoscopy (colon), visit to a dermatologist (skin), digital rectal examination and/or visit to an urologist (prostate), bone densitometry and/or initiation of osteoporosis therapy and/or vitamin D/calcium supplementation and/or increase in calcium intake and/or increase in physical activity and/or alcohol cessation (osteoporosis). Moreover, specific information was collected in each study group at baseline: the time the nurse spent to monitor comorbidities (comorbidity arm) and the one to train the patients (self-assessment arm). Finally, in the self-assessment arm at the 6-month visit, patients were asked to show their booklet to the nurse, and both their level of satisfaction and willingness to continue the proposed self-assessment programme were noted at the 6-month visit.
Outcome measures
Because of the design of the trial, we defined two primary endpoints (1) the number of measures taken against comorbidities in order to evaluate the comorbidity programme and (2) the percentage of patients in whom DMARDs was intensified, initiated and/or changed in order to evaluate the self-assessment programme. As secondary endpoints, in the comorbidity programme, we evaluated the number of actions taken in the four subgroups (eg, CV, infection, cancer and osteoporosis); in the self-assessment programme, we evaluated the changes in DAS28-ESR and RAID.
Sample size calculation
The calculation had to take into account the two endpoints. Bilateral tests were conducted at a 2.5% threshold (and not 5%) to address the multiplicity caused by the two main endpoints. The power required was 80%. The minimum clinically relevant difference was 10%, irrespective of the endpoint. In the literature, there were no precise hypotheses on proportions in the reference groups. Therefore, it was decided to adopt the worst-case scenario, meaning a reference proportion of 50%; for example, we anticipated a change in DMARD be observed in 50% of patients in the control group and in 60% in the ‘active group’. With these hypotheses, the number of subjects to be enrolled in the study was 470 per arm.
Statistical analysis
The analysis was based on intention-to-treat. In the event of missing data on a measure taken for comorbidities in the comorbidity group, this action was considered as not taken while missing data in the self-assessment group were handled with multiple imputations. Qualitative variables were expressed as numbers and percentages. Quantitative variables with symmetrical distribution were summarised as mean±SD and as median and IQRs (25th percentile; 75th percentile) for the variables with a non-normal distribution.
No statistical test was planned to compare the two groups at baseline after randomisation. Depending on the distribution of the number of measures taken as a result of comorbidities, a mixed Poisson model with a random effect for the centre was used to compare the two groups before and after adjusting for corticosteroid intake and biological therapy. The Hochberg procedure was used to adjust for multiple testing of secondary outcomes.60 The percentage of patients in whom a change (initiation/switch/intensification) in any DMARD (conventional or biological) was made during the 6-month study was analysed using a mixed logistic-regression model with a random effect for the centre before and after adjusting for corticosteroid intake and biological therapy. The changes in the secondary outcome variables (ie, changes in DAS28-ESR, RAID and mHAQ) were compared in the two groups using a mixed linear-regression model with a random effect for the patient and centre. Statistical analyses were carried out using R-2.5.1 and SAS V.9.3 software.
Results
Patients and study course
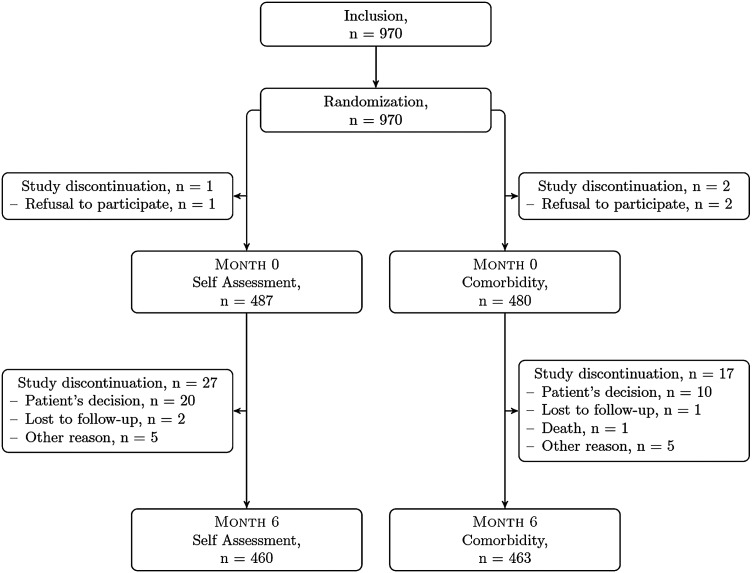
Figure 1 summarises the flow of patients enrolled in the study.
Figure 1.
Study of flowchart.
Eligible patients were recruited by 19 of the 20 participating centres from March 2011 to December 2011. One centre did not recruit any patient because there was no dedicated nurse for this study. In the other 19 centres, the study was managed by 37 nurses: 25 specific, dedicated research nurses and 12 practicing nurses working in the department of rheumatology. The total is greater than 20 because more than one nurse participated in the study in most centres.
After giving their informed written consent and being randomised into an intervention arm (eg, comorbidity vs self-disease activity assessment) three patients refused to participate (two in the comorbidity arm and one in the self-assessment arm). Of the 967 randomised patients who attended the first visit, 923 (95.4%) returned at the 6-month follow-up visit. The reasons for not completing the study among the remaining 44 patients (n=17 and n=27 in the comorbidity and self-assessment groups, respectively) were death (n=1), patient's refusal to return (n=30), lost to follow-up (n=3) and other reasons (n=10).
The patients’ baseline characteristics are summarised in table 1.
Table 1.
Patient characteristics
| Total population N=970 |
Comorbidity group N=482 |
Self-assessment group N=488 |
|
|---|---|---|---|
| Age, years; mean±SD | 58±11 | 58±11 | 57±11 |
| Sex, female; n (%) | 767±(79.1) | 383±(79.5) | 384±(78.7) |
| Body Mass Index; mean±SD | 25.2±4.8 | 25.2±4.8 | 25.2±4.9 |
| Educational level, % university | 285±(29.4) | 153±(31.7) | 132±(27.0) |
| Disease duration, years; median (Q1; Q3) | 11.10 (6.22; 19.08) | 11.04 (6.42; 18.47) | 11.21 (5.74; 19.38) |
| Positive RF or anti-CCP; n (%) | 806 (83.7) | 408 (85.4) | 398 (82.1) |
| Erosive RA; n (%) | 703 (73.5) | 353 (74.3) | 350 (72.6) |
| DAS28 score; mean±SD | 3.09±1.28 | 2.98±1.23 | 3.20±1.32 |
| mHAQ score; mean±SD | 0.4±0.5 | 0.4±0.4 | 0.4±0.5 |
| RAID mean±SD | 3.0±2.0 | 2.9±2.0 | 3.1±2.1 |
| History of RA-related definite surgeries, n (%)† | 290.0 (30) | 148 (30.8) | 142 (29.2) |
| Number of prior DMARD therapies; mean±SD | 2.62±1.58 | 2.62±1.53 | 2.61±1.62 |
| Current MTX; n (%) | 677 (70.5) | 326 (68.3) | 351 (72.7) |
| Number of biotherapies; mean±SD | 1.50±1.39 | 1.55±1.39 | 1.46±1.39 |
| Current biotherapy, n (%) | 725 (74.7) | 364 (75.5) | 361 (73.9) |
| Current corticosteroid intake | |||
| Patients, n (%) | 367 (38) | 170 (35) | 197 (40) |
| Dose, mg/kg; mean±SD | 2.15±4.39 | 2.11±5.34 | 2.18±3.19 |
| Myocardial infarction; n (%) | 14 (1.5) | 9 (1.9) | 5 (1.1) |
| Angina pectoris; n (%) | 11 (1.2) | 3 (0.6) | 8 (1.7) |
| Stroke; n (%) | 18 (1.9) | 9 (1.9) | 9 (2.0) |
| Peripheral arterial disease; n (%) | 11 (1.2) | 5 (1.0) | 6 (1.3) |
| Current smoking; n (%) | 157 (16.7) | 66 (13.8) | 91 (19.8) |
| Diabetes; n (%) | 56 (6.0) | 29 (6.0) | 27 (5.9) |
| Hypertension; n (%) | 284 (30.2) | 147 (30.6) | 137 (29.8) |
| Lipid-lowering therapy; n (%) | 186 (19.8) | 94 (19.6) | 92 (20.0) |
| Prior breast cancer; n (%) | 29 (3.9) | 16 (4.2) | 13 (3.6) |
| Prior skin cancer; n (%) | 34 (3.6) | 18 (3.8) | 16 (3.5) |
| Prior fracture; n (%) | 299/939 (31.8) | 152/480 (31.7) | 147/459 (32.0) |
| Osteoporosis treatment; n (%) | 155/939 (16.5) | 78/480 (16.3) | 77/459 (16.8) |
Data presented are either mean±SD, number and (percentage), median and (tertile Q1–Q3).
†‘Definite’ RA-related surgery was defined as either total articular replacement or resection arthrodesis of the metatarsophalangeal joint.
There was no difference between the whole and the completer populations. As expected, most patients were women and had disease onset during the fifth decade with relatively long disease duration (ie, 11 years) at the time of the study. The percentage of patients who had been treated with at least one biological was very high in this population (75%), perhaps reflecting the particular context of the study (ie, secondary and/or tertiary care). As expected in this population, and in accordance with the protocol, the majority of patients had mild or moderate active disease (the percentages of patients with a high, moderate and low activity score or in remission were 6.5%, 38.2%, 17.5% and 37.8%, respectively).
A history of myocardial infarction was reported in 1.5% of patients and 1.9% had had a stroke, 30% of patients were hypertensive, 19.8% were receiving lipid-lowering therapy and 6% were diabetic. Current smoking was recorded in 16.7% of patients and 15.4% were obese. Breast (3.9%) and skin (3.6%) tumours were the most frequently recorded cancers (10 cervical cancers, six colon cancers, two lung cancers and three lymphomas). Overall, 31.8% of patients had a history of prior osteoporotic fractures, mainly involving the upper extremities of the radius and ulna (19.3%), and 16.5% had osteoporosis medication, which was a bisphosphonate in 85% of cases.
Outcome of the comorbidities management programme
Measures taken against comorbidities during the study
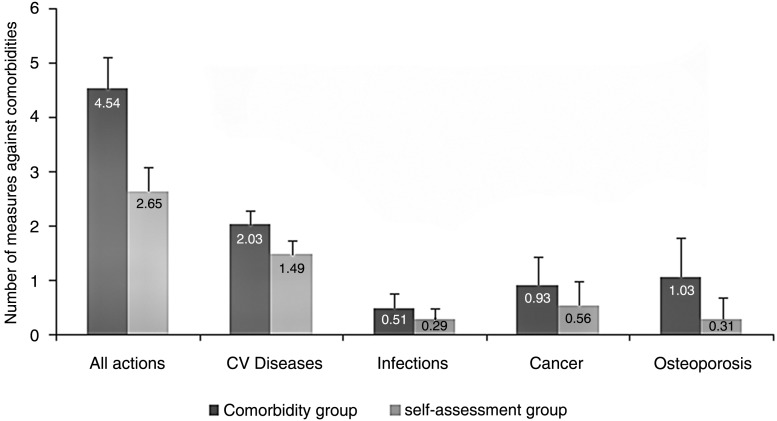
The number of measures taken per patient during the study which lasted for a mean (±SD) duration of 76 (±41.5) min was significantly higher in the comorbidity group than in the self-assessment group (4.54±2.08 vs 2.65±1.57, p<0.001) (figure 2). The incidence rate ratio (IRR) was 1.78 (1.61–1.96) and the IRR was 1.72 (1.57–1.88) after adjusting for corticosteroid therapy and biotherapy.
Figure 2.
Number of measures against comorbidities.
Cardiovascular diseases
The mean (±SD) number of measures taken for CVD was 2.03 (±0.88) in the comorbidity group versus 1.49 (±0.85) in the self-assessment group. The differences were statistically significant (p<0.001) with an IRR of 1.44 (1.6130–1.61) and 1.40 (1.26–1.56), after adjusting for corticosteroid therapy and biotherapy (table 2 and figure 2). This increase in measures involved blood pressure management, with more frequent measurements and purchases of self-measurement devices. While the application of dietetic measures was significantly more common in the comorbidity group than in the self-assessment group, nurse consultations had no impact on smoking cessation attempts. Initiation of lipid-lowering therapy was more frequent in the comorbidity group, but not the initiation of antiplatelet therapy. Serum creatine measurements were taken more often in the comorbidity group.
Table 2.
Measures taken during the study
| Comorbidity group; n (%) N=482 | Self-assessment group; n (%) N=488 | Adjusted p value | |
|---|---|---|---|
| Cardiovascular diseases | |||
| Blood pressure measurement | 405 (84.0) | 365 (74.8) | 0.006 |
| Purchasing of blood pressure self-measurement devices | 29 (6.0) | 5 (1.0) | 0.006 |
| Diet | 48 (10.0) | 28 (5.7) | 0.04 |
| Smoking cessation | 29 (6.0) | 23 (4.7) | 0.41 |
| Initiation of lipid-lowering therapy | 29 (6.0) | 10 (2.0) | 0.01 |
| Initiation of antiplatelet therapy | 13 (2.7) | 6 (1.2) | 0.30 |
| Serum creatine measurement | 417 (86.5) | 278 (57.0) | 0.006 |
| Nephrology consultation | 9 (1.9) | 13 (2.7) | 0.41 |
| Infection | |||
| Influenza vaccine | 188 (39.0) | 109 (22.3) | 0.005 |
| Pneumococcal vaccine | 54 (11.2) | 28 (5.7) | 0.008 |
| Hepatitis A vaccination | 1 (0.2) | 2 (0.4) | 1.00 |
| Hepatitis B vaccination | 1 (0.2) | 1 (0.2) | 1.00 |
| Meningococcal vaccination | 2 (0.4) | 0 (0) | 0.75 |
| Cancer | |||
| Mammography | 100 (20.7) | 68 (13.9) | 0.02 |
| Smear | 87 (18.0) | 68 (13.9) | 0.32 |
| Blood-in-stool screening | 89 (18.5) | 30 (6.1) | 0.006 |
| Colonoscopy | 26 (5.4) | 20 (4.1) | 0.81 |
| Dermatological consultation | 129 (26.8) | 69 (14.1) | 0.006 |
| Digital rectal examination | 7 (1.5) | 8 (1.6) | 0.81 |
| Urological consultation | 8 (1.7) | 11 (2.3) | 0.81 |
| Osteoporosis | |||
| DEXA scan | 67 (13.9) | 34 (7.0) | 0.006 |
| Initiation of osteoporosis therapy, vitamin D supplementation or calcium supplementation | 169 (35.1) | 72 (14.8) | 0.002 |
| Increased calcium intake | 153 (31.7) | 6 (1.2) | 0.002 |
| Increased physical activity | 126 (26.1) | 39 (8.0) | 0.002 |
| Alcohol discontinuation | 4 (0.8) | 2 (0.4) | 0.40 |
DEXA, Dual Energy-X-Ray Absorptiometry.
Infections
The mean (±SD) total number of measures taken was 0.51 (±0.64) in the comorbidity group versus 0.29 (±0.52) (p<0.001) in the self-assessment group (table 2 and figure 2). The IRR was 1.81 (1.43–2.30) and 1.77 (1.40–2.23) after adjusting for corticosteroid therapy and biotherapy. The measures taken primarily involved influenza and pneumococcal vaccines. Influenza vaccines were given to 39% versus 22.3% and pneumococcal vaccines to 11.2% versus 5.7% of patients of the comorbidity versus the self-assessment groups, respectively.
Cancer
The mean (±SD) number of measures taken in relation to cancer was significantly higher in the comorbidity group than in the self-assessment group (0.93 (±1.00) vs 0.56 (±0.84); p<0.001), with an IRR of 1.65 (1.40–1.94) and 1.60 (1.36–2.88) after adjusting for corticosteroid therapy and biotherapy (table 2 and figure 2). In total, 129 (26.8%) patients underwent dermatological consultations in the comorbidity group compared with only 69 (14.1%) in the self-assessment group. Breast cancer screening was more frequent in the comorbidity group, with mammograms performed in 100 (20.7%) patients compared with only 68 (13.9%) patients in the self-assessment group. However, the nursing consultation did not affect screening for cervical cancer. Faecal blood screening was also conducted more often in the comorbidity group. There were no significant differences between the two groups with respect to colonoscopy examinations, digital rectal examinations or urological consultations.
Osteoporosis
Dual Energy-X-Ray Absorptiometry (DEXA) scans were performed more often in the comorbidity group than in the self-assessment group, and osteoporosis management was more common (table 2 and figure 2). The initiation of osteoporosis management, vitamin D supplementation and increased dietary calcium intake were therefore more frequent in the comorbidity group, as was increased physical activity. Overall, the mean (±SD) number of measures taken in the comorbidity group was 1.08 (±0.99) versus 0.31 (±0.55) (p<0.001) in the self-assessment group, with an IRR of 3.45 (2.91–4.09) and 3.34 (2.83–3.96) after adjusting for corticosteroid therapy and biotherapy.
Outcome of the self-disease activity programme
Study programme burden, compliance and satisfaction
The time spent by the nurse to explain the programme and train the patient was close to 1 h (mean 56±SD: 22 min).
Only 27 patients (5.5%) refused to return at the 6-month visit (see figure 1). The percentage of patients returning their booklet at the 6-month visit was relatively high (89.1%). The percentage of self-DAS reported in the booklet at the requested frequency (ie, at least monthly) was 72% (39%, 33%, 20% and 8% of patients calculated and reported their self-DAS more than once a month, exactly once a month, at least every 2 months or less than once every 2 months, respectively).
While visiting their treating rheumatologist during the 6-month study follow-up, 138 (30%) patients showed and discussed their results with him/her. These patients judged this discussion completely useless, useless, useful or very useful in 4%, 9%, 57% or 30% of cases, respectively. Among the remaining patients who met their treating rheumatologist, the reasons for not showing their booklet to him/her were as follows: 30% had forgotten their booklet, 14% did not dare show and 56% cited other reasons.
At the end of the study, the proportion of patients claiming that they would continue with self-assessment was ‘definitely not’: 12%; ‘probably not’: 10%; ‘yes, occasionally’: 23%; and ‘yes, regularly’: 55%.
Changes in therapies and disease activity/severity
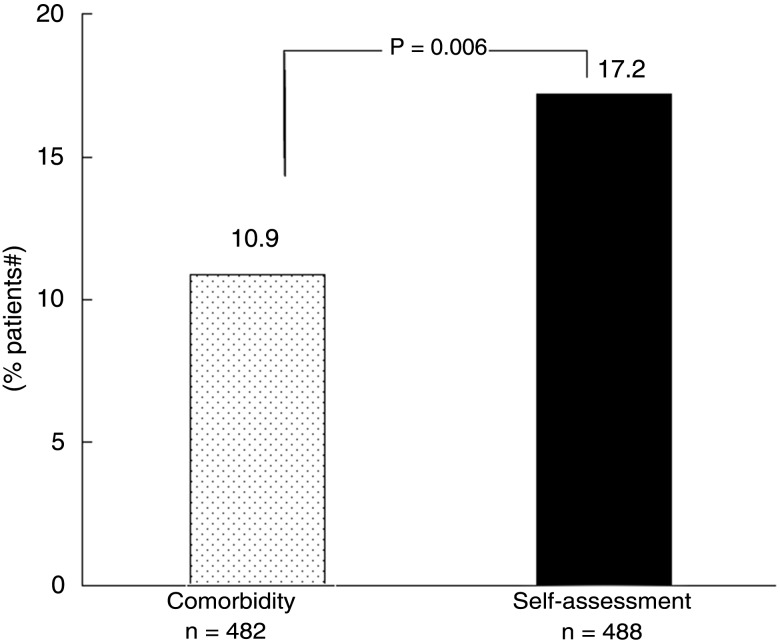
Figure 3 illustrates the results observed in the primary outcome variable: in the 6 months of follow-up, an intensification in DMARD therapy was proposed by the treating rheumatologist and accepted by the patient in 84 (17.2%) versus 53 (10.9%) of cases in the self-assessment versus the comorbidity groups, respectively (p=0.0006; OR=1.70 (1.17; 2.49)). Similar results were found with analysis after adjustment on corticosteroid and/or biological intake (p=0.014; OR=1.63 (1.10; 240)) or with no adjustment and random effect on the centre (p=0.007; OR=1.69 (1.16; 2.47)).
Figure 3.
Percentage of patients with an intensification of their disease-modifying antirheumatic drug (DMARD) therapy during the 6 months of the COmorbidities, EDucation in Rheumatoid Arthritis (COMEDRA) trial.
Table 3 summarises the changes observed in the secondary outcome variables during the study. There was no statistically significant difference in the various outcomes evaluating RA disease activity (DAS28-ESR), functional impairment (mHAQ) or disease impact on patients’ daily lives (RAID score). A subanalysis evaluating the changes in each of the seven areas of the RAID scale (pain, functional capacity, fatigue, physical and emotional wellbeing, quality of sleep and coping) did not show any statistically significant inter-group difference (data not shown).
Table 3.
Changes in the secondary outcome variables during the 6 months of the COMEDRA trial by treatment group
| Outcome | Baseline | 6 months | Inter-group difference | p Value | ||
|---|---|---|---|---|---|---|
| Self-assessment | Control | Self-assessment | Control | |||
| DAS28-ESR | 3.2±1.3 | 3.0±1.2 | 3.1±1.4 | 2.9±1.2 | −0.1 (−0.2; 0.0) | 0.141 |
| mHAQ | 0.4±0.5 | 0.4±0.4 | 0.4±0.5 | 0.4±0.4 | 0.0 (−0.1; 0.0) | 0.806 |
| RAID (total) | 3.1±2.1 | 2.9±2.0 | 2.9±2.1 | 2.8±2.0 | 0.0 (−0.3; 0.2) | 0.761 |
Intention-to-treat analysis: 488 patients in the self-assessment group and 482 in the control group.
Discussion
The study findings highlight the benefits of implementing a nurse-led programme both for comorbidity detection and management and for training RA patients permitting a disease activity self-assessment. Within the 6 months follow-up after the nurse consultation, the number of actions taken by the patient's general practitioner or rheumatologist for treating or detecting comorbidities was shown to increase by 78%; moreover, the disease activity self-assessment programme resulted in an increase in intensification of DMARDS in 17% of the patients (versus only 10% in the comorbidity group).
This study has some limitations but also several strengths which might have clinical implications in the management of RA patients. The design of the study (prospective, randomised, controlled) in a large number of patients can be seen as a strength of this study.
The participating centres (secondary and/or tertiary care settings) have facilitated the recruitment of this study but, at the same time, prevent generalisability of the results observed in this trial since the patients enrolled had a relatively long disease duration and most of them had received at least one biological therapy. Moreover, we are not in a position to know exactly the number of patients who declined the invitation by their rheumatologist to participate in this study.
The enrolment of this study was relatively easy with active participation by 19 of the 20 selected centres. The main difficulty in the centre that was unable to recruit any patient as well as in other centres was for nurses to have specific, allocated time to optimally manage these patients. Originally, we planned to run this study with practice nurses working in different rheumatology departments. However, because of difficulties, we also invited research nurses working in the same rheumatology departments to participate. This difficulty highlights the importance of recognising the role of nurses in the management of chronic rheumatisms and musculoskeletal diseases,49 in conducting research studies and/or educational programmes and in monitoring and even treating the patients.50
Concerning the comorbidity programme of our study, the improvement in patient care concerned CVD, infection, cancer and osteoporosis management. The usefulness of a nurse-led programme for decreasing CV risk has already been shown in two studies.23 24 In both studies, however, the nurse intervention was of long duration, between 6 and 12 h per patient. Our study suggests that a single visit with a nurse might facilitate the management of risk factors of CVD by the general practitioner and/or the rheumatologist.
In the scientific literature, there are seemingly little data on the usefulness of a nurse-led programme in cancer screening.
It seems that not a single study has been focused on the effectiveness of a nurse-led programme in increasing influenza vaccination in a high-risk population.29 However, a telephone intervention with a nurse has been shown to be instrumental in doubling vaccination rates in chronic disease patients over the age of 18.30 Our study confirms these results.
Likewise, there are little data on the value of nurse-led management in osteoporosis prevention. Nonetheless, the study conducted by Clark and colleagues, albeit with a different design from ours, provided positive indices in favour of such an approach.31
Concerning the disease activity self-assessment programme of our study, apart from its impact on the changes in therapies, the observed results suggest also the feasibility of such a programme and its acceptability by both the patients and their treating rheumatologists.
The definition of the primary objective of this trial (ie, the impact on changes in therapy) could be seen as a weakness. Because of the typically relatively short duration of studies evaluating the impact of a self-management strategy and/or an educational programme, the primary objective of these studies is rarely a hard endpoint (eg, retinopathy in diabetes, CV events for hypertension, loss of functional impairment or mortality in RA) but rather either a surrogate marker of such a hard endpoint (eg, glycaemia for diabetes,61 blood pressure for hypertension62 or disease activity for RA63) or a domain known to be particularly sensitive to the study treatment (eg, coping, level of knowledge51 64 65). We have not chosen such endpoints in our study because of its short-term duration and the anticipated low level of disease activity of the disease at baseline. In RA, a persistent disease activity even at a moderate level has been shown to be deleterious in terms of structural damage and/or reduction in work capacity.43–45 Moreover, in this condition, DMARD therapies can be of benefit but are frequently not used or underused.48 This is why we anticipated that, in the event of positive impact of the self-assessment programme in this trial, the frequency of the DMARD therapy should be increased and therefore we chose this parameter as the primary endpoint of our trial. This was indeed the case with a relevant difference in comparison with the control group (eg, 17.2% vs 10.9%).
One of the issues of such a programme is related to the competence of the nurses. Obviously, the quality of the patient-training is dependent on the competence of the nurse. In our study, we have improved such competence by a specific comprehensive nurse training programme focused on joint count.57 This characteristic can be seen as a strength in terms of the quality of our study but also as a weakness when considering the potential generalisability of the observed results.
The number of patients refusing the trial (n=1) or refusing to return at the 6-month visit (n=27), or claiming that they would not continue their RA disease activity self-assessment (22%), suggests that a proportion of patients are reluctant to collaborate. It might be of interest to further evaluate the reasons of such non-acceptability/adherence.
Another important aspect which has not been evaluated in this study (eg, the benefit to cost ratio regarding screening and prevention) should be further investigated.
Longer term follow-up of these patients will be of interest to evaluate the sustainability of the observed results, in particular with regard to the management of comorbidities but also to check the potential impact of the disease activity self-assessment programme on other outcomes such as disease activity and/or functional impairment.
Supplementary Material
Footnotes
Contributors: MD, MS, EP and PR: contributed to this study by substantially participating in the study conception and design, by drafting the article and revising it critically for important intellectual content, by giving a final approval of the version of the article to be published. LG, FF, MG, M-HC, SP, R-MF, LC, GM, LE-Z, TS, BF, AS, IC-V, GC, ED, PR, XM, FB and JS: contributed to this study by substantially participating in the study conception and design, by substantially contributing to acquisition of data, by drafting the article and revising it critically for important intellectual content, by giving a final approval of the version of the article to be published.
Funding: This study has been conducted thanks to a grant from the French Minister of health (PHRC 00-41).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ile-de-France III Ethics Committee, file #4-11 (B110057-30).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gabriel SE. Why do people with rheumatoid arthritis still die prematurely? Ann Rheum Dis 2008;67:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2011;25:469–83. [DOI] [PubMed] [Google Scholar]
- 3.Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international,cross-sectional study (COMORA). Ann Rheum Dis 2014;73:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLean CH, Louie R, Leake B, et al. Quality of care for patients with rheumatoid arthritis. JAMA 2000;284:984–92. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Arora T, Narongroeknawin P, et al. The delivery of evidence-based preventive care for older Americans with arthritis. Arthritis Res Ther 2010;12:R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 2009;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- 8.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol 2011;7:399–408. [DOI] [PubMed] [Google Scholar]
- 9.Peters MJL, Symmons DPM, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–31. [DOI] [PubMed] [Google Scholar]
- 10.Primdahl J, Clausen J, Hørslev-Petersen K. Results from systematic screening for cardiovascular risk in outpatients with rheumatoid arthritis in accordance with the EULAR recommendations. Ann Rheum Dis 2013;72:1771–6. [DOI] [PubMed] [Google Scholar]
- 11.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53–61. [DOI] [PubMed] [Google Scholar]
- 12.van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011;70:414–22. [DOI] [PubMed] [Google Scholar]
- 13.Sowden E, Mitchell WS. An audit of influenza and pneumococcal vaccination in rheumatology outpatients. BMC Musculoskelet Disord 2007;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuchtenberger M, Kleinert S, Schwab S, et al. Vaccination survey in patients with rheumatoid arthritis: a cross-sectional study. Rheumatol Int 2012;32:1533–9. [DOI] [PubMed] [Google Scholar]
- 15.Desai SP, Lu B, Szent-Gyorgyi LE, et al. Increasing pneumococcal vaccination for immunosuppressed patients: A cluster quality improvement trial. Arthritis Rheum 2013;65:39–47. [DOI] [PubMed] [Google Scholar]
- 16.Turesson C, Matteson EL. Malignancy as a comorbidity in rheumatic diseases. Rheumatology (Oxford) 2013;52:5–14. [DOI] [PubMed] [Google Scholar]
- 17.Solomon DH, Karlson EW, Curhan GC. Cardiovascular care and cancer screening in female nurses with and without rheumatoid arthritis. Arthritis Rheum 2004;51:429–32. [DOI] [PubMed] [Google Scholar]
- 18.Kiefe CI, Funkhouser E, Fouad MN, et al. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med 1998;13:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SC, Schneeweiss S, Myers JA, et al. No differences in cancer screening rates in patients with rheumatoid arthritis compared to the general population. Arthritis Rheum 2012;64:3076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugeberg G, Uhlig T, Falch JA, et al. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum 2000;43:522–30. [DOI] [PubMed] [Google Scholar]
- 21.Coulson KA, Reed G, Gilliam BE, et al. Factors influencing fracture risk, T score, and management of osteoporosis in patients with rheumatoid arthritis in the Consortium of Rheumatology Researchers of North America (CORRONA) registry. J Clin Rheumatol 2009;15:155–60. [DOI] [PubMed] [Google Scholar]
- 22.McKeown E, Bykerk VP, De Leon F, et al. ; CATCH Investigators. Quality assurance study of the use of preventative therapies in glucocorticoid-induced osteoporosis in early inflammatory arthritis: results from the CATCH cohort. Rheumatology (Oxford) 2012;51:1662–9. [DOI] [PubMed] [Google Scholar]
- 23.Haskell WL, Berra K, Arias E, et al. Multifactor cardiovascular disease risk reduction in medically underserved, high-risk patients. Am J Cardiol 2006;98:1472–9. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Berra K, Haskell WL, et al. Case management to reduce risk of cardiovascular disease in a county health care system . Arch Intern Med 2009;169:1988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall T, Westerby P, Chen J, et al. The Sandwell Project: a controlled evaluation of a programme of targeted screening for prevention of cardiovascular disease in primary care. BMC Public Health 2008;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammed MA, El Sayed C, Marshall T. Patient and other factors influencing the prescribing of cardiovascular prevention therapy in the general practice setting with and without nurse assessment . Med Decis Making 2012;32:498–506. [DOI] [PubMed] [Google Scholar]
- 27.Hebert PL, Sisk JE, Tuzzio L, et al. Nurse-led disease management for hypertension control in a diverse urban community: a randomized trial. J Gen Intern Med 2012;27:630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raftery JP, Yao GL, Murchie P, et al. Cost effectiveness of nurse led secondary prevention clinics for coronary heart disease in primary care: follow up of a randomised controlled trial. BMJ 2005;330:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ompad DC, Galea S, Vlahov D. Distribution of influenza vaccine to high-risk groups. Epidemiol Rev 2006;28:54–70. [DOI] [PubMed] [Google Scholar]
- 30.Winston CA, Mims AD, Leatherwood KA. Increasing pneumococcal vaccination in managed care through telephone outreach. Am J Manag Care 2007;13:581–8. [PubMed] [Google Scholar]
- 31.Clark EM, Gould V, Morrison L, et al. Randomized controlled trial of a primary care-based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA) . J Bone Miner Res 2012;27:664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer D, El Miedany Y. From guidelines to clinical practice: cardiovascular risk management in inflammatory arthritis patients. Br J Community Nurs 2013;18:424–8. [DOI] [PubMed] [Google Scholar]
- 33.Koksvik HS, Hagen KB, Rodevand E, et al. Patient satisfaction with nursing consultations in a rheumatology outpatient clinic: a 21-month randomized controlled trial in patients with inflammatory arthritides. Ann Rheum Dis 2013;72:836–43. [DOI] [PubMed] [Google Scholar]
- 34.Ndosi M, Lewis M, Hale C, et al. The outcome and cost-effectiveness of nurse-led care in people with rheumatoid arthritis : a multicentre randomised controlled trial. Ann Rheum Dis 2014;73:1975–82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Nies JA, de Jong Z, van der Helm-van Mil AH, et al. Improved treatment strategies reduce the increased mortality risk in early RA patients. Rheumatology (Oxford) 2010;49:2210–16. [DOI] [PubMed] [Google Scholar]
- 36.Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 2009;68:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh JA, Christensen R, Wells GA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev 2009;(4):CD007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moots RJ, Naisbett-Groet B. The efficacy of biologic agents in patients with rheumatoid arthritis and an inadequate response to tumour necrosis factor inhibitors: a systematic review. Rheumatology (Oxford) 2012;51:2252–61. [DOI] [PubMed] [Google Scholar]
- 39.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoels M, Knevel R, Aletaha D, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis 2010;69:638–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prevoo ML, van't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 42.van Gestel AM, Prevoo ML, van't Hof MA, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34–40. [DOI] [PubMed] [Google Scholar]
- 43.Conaghan PG, Hensor EM, Keenan AM, et al. Persistently moderate DAS-28 is not benign: loss of function occurs in early RA despite step-up DMARD therapy. Rheumatology (Oxford) 2010;49:1894–9. [DOI] [PubMed] [Google Scholar]
- 44.Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918–29. [DOI] [PubMed] [Google Scholar]
- 45.Combe B, Logeart I, Belkacemi MC, et al. Comparison of the long-term outcome for rheumatoid arthritis patients with persistent moderate disease activity or disease remission during the first year after diagnosis: data from the ESPOIR cohort. Ann Rheum Dis 2015;74:724–9.. [DOI] [PubMed] [Google Scholar]
- 46.Smolen JS, Landewé R, Breedveld FC. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2013;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haraoui B, Bensen W, Bessette L, et al. Treating rheumatoid arthritis to target: a Canadian physician survey. J Rheumatol 2012;39:949–53. [DOI] [PubMed] [Google Scholar]
- 48.Dougados M, Nataf H, Steinberg G, et al. Relative importance of doctor-reported outcomes vs patient-reported outcomes in DMARD intensification for rheumatoid arthritis: the DUO study. Rheumatology (Oxford) 2013;52:391–9. [DOI] [PubMed] [Google Scholar]
- 49.van Eijk-Hustings Y, van Tubergen A, Boström C, et al. EULAR recommendations for the role of the nurse in the management of chronic inflammatory arthritis. Ann Rheum Di 2012;71:13–19. [DOI] [PubMed] [Google Scholar]
- 50.van Hulst LT, Fransen J, den Broeder AA, et al. Development of quality indicators for monitoring of the disease course in rheumatoid arthritis. Ann Rheum Dis 2009;68:1805–10. [DOI] [PubMed] [Google Scholar]
- 51.Iversen MD, Hammond A, Betteridge N. Self-management of rheumatic diseases: state of the art and future perspectives. Ann Rheum Dis 2010;69:955–63. [DOI] [PubMed] [Google Scholar]
- 52.Shoor S, Lorig KR. Self-care and the doctor-patient relationship. Med Care 2002;40(4 Suppl):II40–4. [DOI] [PubMed] [Google Scholar]
- 53.Cheung PP, Ruyssen-Witrand A, Gossec L, et al. Reliability of patient self-evaluation of swollen and tender joints in rheumatoid arthritis: A comparison study with ultrasonography, physician, and nurse assessments. Arthritis Care Res 2010;62:1112–19. [DOI] [PubMed] [Google Scholar]
- 54.Cheung PP, Gossec L, Ruyssen-Witrand A, et al. The relationship of patient-reported joints with active synovitis detected by power Doppler ultrasonography in rheumatoid arthritis. Clin Exp Rheumatol 2013;31:490–7. [PubMed] [Google Scholar]
- 55.Choy EH, Khoshaba B, Cooper D, et al. Development and validation of a patient-based disease activity score in rheumatoid arthritis that can be used in clinical trials and routine practice. Arthritis Rheum 2008;59:192–9. [DOI] [PubMed] [Google Scholar]
- 56.Arnett FC, Edworthy SM, Bloch DA, et al. The Americain Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 57.Cheung PP, Dougados M, Andre V, et al. The learning curve of nurses for the assessment of swollen and tender joints in rheumatoid arthritis. Joint Bone Spine 2014;81:154–9. [DOI] [PubMed] [Google Scholar]
- 58.Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. J Rheumatol 2005;32:1432–9. [PubMed] [Google Scholar]
- 59.Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis 2011;70:935–42. [DOI] [PubMed] [Google Scholar]
- 60.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodological) 1995;57:289–300. [Google Scholar]
- 61.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2011;34:e61–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Institute for Health and Clinical Excellence. Hypertension: clinical management of primary hypertension in adults. August 2011. http://www.nice.org.uk/nicemedia/live/13561/56008/56008.pdf (accessed 6 Apr 2012).
- 63.Giraudet-Le Quintrec JS, Mayoux-Benhamou A, Ravaud P, et al. Effect of a collective educational program for patients with rheumatoid arthritis: a prospective 12-month randomized controlled trial. J Rheumatol 2007;34:1684–91. [PubMed] [Google Scholar]
- 64.Kirwan JR, Hewlett S, Cockshott Z, et al. Clinical and psychological outcomes of patient education in rheumatoid arthritis. Musculoskeletal Care 2005;3:1–16. [DOI] [PubMed] [Google Scholar]
- 65.Niedermann K, Fransen J, Knols R, et al. Gap between short- and long-term effects of patient education in rheumatoid arthritis patients: a systematic review. Arthritis Rheum 2004;51:388–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.