Abstract
Background and aims
We studied damage accrual and factors determining development and progression of damage in an international cohort of systemic lupus erythematosus (SLE) patients.
Methods
The Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort recruited patients within 15 months of developing four or more 1997 American College of Rheumatology (ACR) criteria for SLE; the SLICC/ACR damage index (SDI) was measured annually. We assessed relative rates of transition using maximum likelihood estimation in a multistate model. The Kaplan–Meier method estimated the probabilities for time to first increase in SDI score and Cox regression analysis was used to assess mortality.
Results
We recruited 1722 patients; mean (SD) age 35.0 (13.4) years at cohort entry. Patients with damage at enrolment were more likely to have further worsening of SDI (SDI 0 vs ≥1; p<0.001). Age, USA African race/ethnicity, SLEDAI-2K score, steroid use and hypertension were associated with transition from no damage to damage, and increase(s) in pre-existing damage. Male gender (relative transition rates (95% CI) 1.48 (1.06 to 2.08)) and USA Caucasian race/ethnicity (1.63 (1.08 to 2.47)) were associated with SDI 0 to ≥1 transitions; Asian race/ethnicity patients had lower rates of new damage (0.60 (0.39 to 0.93)). Antimalarial use was associated with lower rates of increases in pre-existing damage (0.63 (0.44 to 0.89)). Damage was associated with future mortality (HR (95% CI) 1.46 (1.18 to 1.81) per SDI point).
Conclusions
Damage in SLE predicts future damage accrual and mortality. We identified several potentially modifiable risk factors for damage accrual; an integrated strategy to address these may improve long-term outcomes.
Keywords: Systemic Lupus Erythematosus, Outcomes research, Corticosteroids, Inflammation
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease in which adverse long-term outcomes remain a major challenge. In assessing patients with SLE, three disease dimensions are considered in formal outcomes studies: inflammatory disease activity, organ damage and health related quality of life (HRQOL).1 Damage is principally assessed using the Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI), which has been extensively validated.2 3 SDI items represent irreversible damage that has occurred after the diagnosis of SLE. However, an item does not have to be attributable to lupus.2 As a general rule, items should be present for at least 6 months and once recorded in the SDI they are permanent such that the score cannot decrease.
The mean SDI tends to increase over time,4 and in time the majority of SLE patients will accrue damage. The SDI also predicts future mortality.5–7 It is therefore important to understand factors related to the development of damage. To date, studies have mainly focused on established SLE cohorts from a single centre or region.8–10 A number of factors have been associated with higher SDI scores, including older age at SLE onset,11 12 Hispanic and African ancestry race/ethnicity,8 13 14 chronic disease activity12 15 and major flares.16 Steroid exposure also predicts future damage, especially late-onset damage.8 16 17 There is also accumulating evidence that antimalarials (AMs) exert a protective role even after adjusting for their propensity for use in milder disease.18
We aimed to study damage accrual over the early years of follow-up in patients enrolled into the SLICC Inception Cohort. We focused on the rate of accrual of total damage as well as the impact of demographic, racial/ethnic and geographical variables. We also assessed the contribution of disease-related factors, therapy, co-morbidities and serological biomarkers to damage accrual. Finally, we sought to determine the relationship between damage and HRQOL, as well as future mortality.
Methods
SLICC Inception Cohort study
SLICC comprises 31 centres from 11 countries in North America, Latin America, Europe and Asia. An inception cohort was recruited from 2000 to 2011. Data were submitted to the co-ordinating centre at the University of Toronto at enrolment, and patients were reviewed annually. Laboratory tests necessary to evaluate disease activity and damage parameters were performed locally. The study was approved by the Institutional Research Ethics Boards of participating centres in accordance with the Declaration of Helsinki's guidelines for research in humans.
Patients and clinical assessments
Patients were enrolled within 15 months of recognition of four or more 1997 ACR classification criteria for SLE.19 We included patients who either had two study visits or had died after their first study visit, that is, patients who had two data points to model statistically. There were no specific exclusion criteria other than failing to meet four ACR criteria and it being >15 months since diagnosis. We noted demographic features including age, gender, race/ethnicity, geographical region and years in post-secondary education. We also noted the number of ACR criteria fulfilled by the baseline visit. At each visit we also assessed the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K)20 and the SDI.21 At each visit we recorded whether the patient was taking corticosteroids (yes or no). In addition to steroid use, we also recorded whether patients were taking AMs, immunosuppressives (ISs), or both AMs and ISs. Co-morbidities (recorded at each visit) included in our analysis were diabetes mellitus (physician confirmed diagnosis) and hypertension (systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg and/or taking antihypertensive medications). Baseline serological markers included antibodies to double-stranded DNA and C3 and C4 complement (in local clinical laboratories at each centre). Antibodies to cardiolipin, β-2-glycoprotein I and the lupus anti-coagulant were measured at a central laboratory at the Oklahoma Medical Research Foundation as previously described.22 HRQOL was assessed using the Medical Outcomes Survey Short-Form 36 (SF-36). All patients provided written informed consent.
Statistical analysis
Simple descriptive statistics were used to summarise enrolment data. The SDI scores are discrete values that are observed over time for each patient. Thus, we used a multistate model for transitions among damage states, defined by the SDI scores. Specifically, at each visit a patient is assigned to one of the damage states according to their current SDI score. Since there are relatively few transitions to states 5–11, we merged these states into one state indicating an SDI score ≥5. We employed a multistate model with seven states, shown pictorially below:

Patients may only show deterioration in the SDI damage index over time and patients may die at any time during observation.
Multistate models allow estimation of the transition rates between these observed states, and these transition rates can be modelled as a function of explanatory variables (both time-independent and time-dependent). If λij(t) denotes the transition intensity from state i to state j at time t, then λij(t) may be modelled as follows:
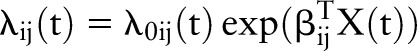 |
where λ0ij(t) denotes a baseline state i to state j transition intensity at time t, T denotes matrix transpose, and X(t) is a vector of explanatory variables with associated explanatory variable effects on the state i to state j intensity denoted by βij.
In this work, we assume constant baseline transition intensities for the relevant transitions. That is, λ0ij(t)=λ0ij for all t, for the state i to state j transitions. Maximum likelihood estimation is used to estimate the unknown model parameters (λ0ij and βij) for each transition in the model together with their associated SEs.
The correlation among the states of a patient at the different assessment visits is directly modelled through the Markov assumption that the future evolution of a patient's damage process depends only on his/her current state and not on his/her previous history. Initial modelling was based on a proportional hazards assumption and assumed common explanatory variable effects across selected transition rates. Notably we assumed that transition rates and explanatory variable effects between damage states where SDI ≥1 were the same and also that transitions to death from these higher SDI states were equal. Explanatory variable effects are reported as relative rates of transition, together with corresponding 95% CIs, obtained using maximum likelihood estimation. Age at diagnosis was standardised as (age in years−34.5)/13.4. For disease activity we report effects corresponding to 3-point increments in SLEDAI-2K.The Kaplan–Meier method was used to estimate the probabilities relating to time until first worsening of SDI score.
For the modelling of HRQOL outcomes, we fitted linear models using generalised estimating equations (GEEs) to account for the correlation among observations over time within each patient. Standard Cox regression analysis was performed with patient survival as the outcome and functions of damage scores over time as explanatory variables.
Results
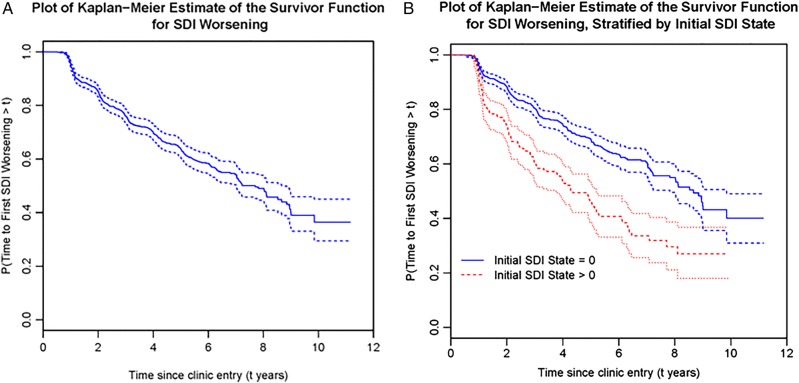
There were a total of 1722 patients in the SLICC Inception Cohort up to September 2011 and the mean (SD) number of follow-up visits was 4.25 (2.72). Demographic and disease-related factors at clinic entry are summarised in table 1. At baseline, 671 patients had their SDI scores recorded as they had more than 6 months of disease; of these 671 patients, 130 (19.4%) had an SDI score of 1 or more at the baseline visit. For this group (figure 1A), the overall estimate of the probability of SDI first worsening at a time greater than 6 years since clinic entry is approximately 0.58 (figure 1A). Put another way, of the 348 patients who were observed 6 years after clinic entry, 178 (51.1%) had at least one item of damage by that time point. When we stratified these 671 patients by baseline SDI score, those with initial damage were significantly more likely to have further worsening of the SDI at each follow-up visit (p<0.001) (figure 1B).
Table 1.
Baseline characteristics of the Systemic Lupus International Collaborating Clinics cohort at entry to the study
| Number of patients | 1722 |
| Age, years | 35.0 (S.D. 13.4) |
| Gender | |
| Female | 1536 (89.2) |
| Male | 186 (10.8) |
| Enrolment location | |
| Canada | 398 (23.1) |
| USA | 497 (28.9) |
| Mexico | 212 (12.3) |
| Europe | 446 (25.9) |
| Asia | 169 (9.8) |
| Race/ethnicity | |
| Caucasian | 830 (48.2) |
| Hispanic | 268 (15.6) |
| Asian | 271 (15.7) |
| African origin | 280 (16.3) |
| Other | 71 (4.1) |
| Disease phenotype at baseline | |
| SLEDAI-2K | 5.3 (5.3)* |
| Active renal disease | 467 (27.1) |
| Thrombocytopenia (platelet count <100 000) | 47 (2.7) |
| Medication use | |
| Oral corticosteroids (CS) | 1199 (69.6) |
| Average CS dose (mg/day) | 24.1 (16.7)* |
| Highest CS dose (mg/day) | 43.5 (74.3)* |
| Immunosuppressants | 684 (39.7) |
| Antimalarials | 1153 (67) |
| Co-morbidities | |
| Systolic blood pressure (mm Hg) | 119.5 (16.8)* |
| Diastolic blood pressure (mm Hg) | 75.1 (11.1)* |
| Taking antihypertensives | 505 (29.3) |
| Diabetes mellitus | 60 (3.5) |
| Current smoker | 263 (15.3) |
| Post-menopausal | 162 (9.4) |
| Body mass index (kg/m2) | 25.2 (5.9)* |
| Baseline serology | |
| Anti-dsDNA positive | 663 (38.5) |
| Low C3 and/or C4 complement | 586 (34.0) |
| Anti-B2-GPI positive | 241 (14) |
| Anti-cardiolipin positive | 133 (7.7) |
| Lupus anticoagulant positive | 208 (12.1) |
*All data are n (%) of patients or mean (SD) where indicated.
SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
Figure 1.
(A) and (B) Kaplan–Meier plots demonstrating the estimate of the proportions of patients who remain free of damage progression/SDI worsening for the patients within the SLICC cohort who had their SDI reported at their baseline visit (n=671) (A) and in this cohort stratified by whether or not they had an SDI score 0 (n=541) or SDI >0 (n=130) at baseline (B). SDI, Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index.
Transitions
There were 1502 (1337 female and 165 male) patients who had at least two clinic visits or who had one clinic visit and subsequently died. In this group the predicted probability of remaining with the same damage score over a 5-year period was conditional on the pre-existing SDI score. The probability of death also increased with higher SDI scores (table 2).
Table 2.
The predicted probability of a patients’ SDI state or mortality in 5 years’ time, conditional on their current SDI score
| Current SDI state | Estimated probability of being in SDI state in 5 years’ time | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ≥5 | Death | |
| 0 | 0.655 | 0.198 | 0.086 | 0.030 | 0.006 | 0.002 | 0.022 |
| 1 | 0.360 | 0.341 | 0.192 | 0.055 | 0.026 | 0.027 | |
| 2 | 0.323 | 0.375 | 0.155 | 0.107 | 0.040 | ||
| 3 | 0.354 | 0.264 | 0.310 | 0.072 | |||
| 4 | 0.204 | 0.705 | 0.092 | ||||
| ≥5 | 0.880 | 0.120 | |||||
SDI, Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index.
Influence of age, gender, race/ethnicity and geographical region
Increasing age and male gender both had a significant influence on the probability of damage accrual. Higher standardised age increased the risk of future damage, especially in those with no current damage, and the influence of age was non-linear. The significant effect of (standardised age)2 suggests that the effect of increases in age is most marked in older patients. Therefore, assuming all other covariates have the same values for each age at diagnosis compared to a patient aged 35.4 years (the mean age of diagnosis), the relative transition rate was 1.58 for a patient aged 50 years. Compared to a 50-year-old, the relative transition rate was 2.51 for a 60-year-old, and for patients between 60 and 70 years of age the transition rate increased by a factor of 4.52. We also found that the effects of race/ethnicity and location of study sites were not independent (Pearson's χ2=2096.775, 16 df; p<0.001) (data on file) and that both had a significant impact on damage accrual. We therefore combined these into new variables (tables 3 and 4). Compared to Caucasians in Europe or Canada, USA patients of African ancestry had a higher risk of moving from no damage to damage and also of progressing from baseline damage to higher damage (relative transition rates (RTR) (95% CI) 1.99 (1.33 to 2.96) and 2.55 (1.92, 3.40), respectively), while Asians had lower transition rates (0.66 (0.43, 0.99)). Hispanic patients in Mexico also had a higher risk of progressing from baseline damage to higher damage (RTR (95% CI) 1.36 (1.02 to 1.83)) (tables 3 and 4).
Table 3.
Factors associated with the development of new damage that is, transition from SDI 0 to ≥1 in a multivariate, multistate model
| Variable | Univariate model relative transition rate (95% CI) | Multivariate model relative transition rate (95% CI) |
|---|---|---|
| Gender: Female | 1 | 1 |
| Male | 1.96 (1.42 to 2.71) | 1.48 (1.06 to 2.08) |
| Standardised age at diagnosis (years) | 1.21 (1.09 to 1.34) | 1.30 (1.12 to 1.52) |
| (Standardised age at diagnosis (years))2 | 1.14 (1.08 to 1.20) | 1.12 (1.03 to 1.23) |
| Ethnicity/location | ||
| Caucasian (Canada/Europe) | 1 | 1 |
| Caucasian (USA) | 1.47 (0.98 to 2.20) | 1.63 (1.08 to 2.47) |
| Hispanic (Mexico) | 1.44 (0.98 to 2.12) | 1.17 (0.75 to 1.82) |
| Hispanic (elsewhere) | 1.74 (0.88 to 3.44) | 1.71 (0.85 to 3.43) |
| African (USA) | 1.99 (1.33 to 2.96) | 1.58 (1.03 to 2.44) |
| African (elsewhere) | 1.47 (0.95 to 2.27) | 1.30 (0.83 to 2.03) |
| Asian | 0.66 (0.43 to 0.99) | 0.60 (0.39 to 0.93) |
| Other | 1.76 (0.98 to 3.14) | 1.51 (0.83 to 2.73) |
| Post-secondary education*: No | 1 | 1 |
| Yes | 0.76 (0.59 to 0.97) | 0.80 (0.61 to 1.04) |
| No. of ACR criteria fulfilled at enrolment | 1.19 (1.06 to 1.34) | 1.12 (0.99 to 1.27) |
| (SLEDAI-2K)/3 | 1.25 (1.15 to 1.35) | 1.17 (1.07 to 1.27) |
| Corticosteroid use: No | 1 | 1 |
| Yes | 1.81 (1.40 to 2.34) | 1.64 (1.21 to 2.21) |
| Additional SLE therapy (with or without corticosteroids) | ||
| Antimalarials (AMs) only: No | 1 | 1 |
| Yes | 0.86 (0.57 to 1.29) | 0.81 (0.53 to 1.22) |
| Immunosuppressants (ISs) only: No | 1 | 1 |
| Yes | 1.69 (1.08 2.63) | 1.13 (0.71 to 1.80) |
| AMs and ISs: No | 1 | 1 |
| Yes | 1.28 (0.85 to 1.92) | 1.06 (0.69 to 1.62) |
| Diabetes: No | NA | NA |
| Yes | NA | NA |
| Hypertension: No | 1 | 1 |
| Yes | 2.61 (1.97 to 3.46) | 1.71 (1.27 to 2.31) |
| Anti-ds-DNA at baseline: No | 1 | |
| Yes | 0.98 (0.76 to 1.28) | |
| Hypocomplementaemia at baseline: No | 1 | |
| Yes | 1.08 (0.83 to 1.39) | |
| Anti-B2-GPI at baseline: No | 1 | |
| Yes | 0.86 (0.58 to 1.28) | |
| Anticardiolipin at baseline: No | 1 | |
| Yes | 0.90 (0.61 to 1.35) | |
| Lupus anticoagulant at baseline: No | 1 | |
| Yes | 1.24 (0.90 to 1.70) | |
*A ‘missing’ indicator was included for the 6.1% of patients for whom this information was lacking.
SDI, Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
Table 4.
Factors associated with progression of damage in patients with present damage that is, transition from SDI ≥1 to a higher score in a multivariate, multistate model
| Variable | Univariate model relative transition rate (95% CI) | Multivariate model relative transition rate (95% CI) |
|---|---|---|
| Gender: Female | 1 | 1 |
| Male | 1.19 (0.93 to 1.52) | 1.12 (0.86 to 1.46) |
| Standardised age at diagnosis (years) | 0.93 (0.86 to 1.81) | 1.00 (0.87 to 1.14) |
| (Standardised age at diagnosis (years))2 | 1.06 (1.02 to 1.09) | 1.07 (1.00 to 1.14) |
| Ethnicity/location | ||
| Caucasian (Canada/Europe) | 1 | 1 |
| Caucasian (USA) | 1.25 (0.90 to 1.74) | 1.26 (0.89 to 1.78) |
| Hispanic (Mexico) | 1.36 (1.02 to 1.83) | 1.08 (0.76 to 1.54) |
| Hispanic (elsewhere) | 0.37 (0.09 to 1.52) | 0.37 (0.09 to 1.52) |
| African (USA) | 2.55 (1.92 to 3.40) | 2.39 (1.75 to 3.27) |
| African (elsewhere) | 1.07 (0.72 to 1.57) | 0.99 (0.65 to 1.50) |
| Asian | 1.10 (0.77 to 1.57) | 0.95 (0.65 to 1.38) |
| Other | 1.08 (0.58 to 2.01) | 1.00 (0.53 to 1.89) |
| Post-secondary education*: No | 1 | 1 |
| Yes | 0.98 (0.80 to 1.19) | 1.00 (0.81 to 1.24) |
| No. of ACR criteria fulfilled at enrolment | 1.04 (0.95 to 1.13) | 1.01 (0.92 to 1.11) |
| (SLEDAI-2k)/3 | 1.11 (1.05 to 1.17) | 1.10 (1.03 to 1.16) |
| Corticosteroid use: No | 1 | 1 |
| Yes | 1.69 (1.35 to 2.11) | 1.43 (1.12 to 1.84) |
| Additional SLE therapy (with or without corticosteroids) | ||
| Antimalarials (AMs) only: No | 1 | 1 |
| Yes | 0.60 (0.42 to 0.84) | 0.63 (0.44 to 0.89) |
| Immunosuppressants (ISs) only: No | 1 | 1 |
| Yes | 1.05 (0.76 to 1.45) | 0.94 (0.66 to 1.33) |
| AMs and ISs: No | 1 | 1 |
| Yes | 0.95 (0.69 to 1.29) | 0.83 (0.60 to 1.16) |
| Diabetes: No | 1 | 1 |
| Yes | 1.59 (0.93 to 2.73) | 0.96 (0.54 to 1.70) |
| Hypertension: No | 1 | 1 |
| Yes | 1.97 (1.59 to 2.45) | 1.61 (1.28 to 2.03) |
| Anti-ds-DNA at baseline: No | 1 | |
| Yes | 0.97 (0.77 to 1.21) | |
| Hypocomplementaemia at baseline: No | 1 | |
| Yes | 1.14 (0.92 to 1.41) | |
| Anti-B2-GPI at baseline: No | 1 | |
| Yes | 1.14 (0.82 to 1.61) | |
| Anticardiolipin at baseline: No | 1 | |
| Yes | 1.35 (0.98 to 1.86) | |
| Lupus anticoagulant at baseline: No | 1 | |
| Yes | 1.14 (0.87 to 1.48) | |
*A ‘missing’ indicator was included for the 6.1% of patients for whom this information was lacking.
ACR, American College of Rheumatology; SLE, systemic lupus erythematosus; SLEDAI-2k, Systemic Lupus Erythematosus Disease Activity Index 2000.
Clinical, therapeutic and serological factors associated with development and/or progression of damage
Corticosteroid use, immunosuppressive use, SLEDAI-2K score and hypertension were all significantly associated with both the development of damage in patients free of damage at baseline, as well as progression of damage in patients with baseline damage (tables 3 and 4). The number of ACR criteria at enrolment was also associated with higher transitions from SDI 0 to ≥1 (RTR (95% CI) 1.19 (1.06 to 1.34)) as was IS use (1.69 (1.08, 2.63)). In addition, AM use was associated with a reduced transition rate to higher damage (0.60 (0.42, 0.84)) (tables 3 and 4).
Multivariate models
Multivariate, multistate models for both transitions confirmed that age, USA patients of African ancestry, SLEDAI-2K score, steroid use and hypertension were predictive in both models of damage accrual (tables 3 and 4). For transition from SDI 0 to ≥1, male gender (RTR (95% CI) 1.48 (1.06 to 2.08)) and USA Caucasian race/ethnicity (RTR (95% CI) 1.63 (1.08 to 2.47)) were also associated with damage, while patients of Asian ethnicity had lower rates of transition (RTR (95% CI) 0.60 (0.39 to 0.93)). For transitions from SDI ≥1 to higher damage, patients taking AMs also had lower rates of transition (RTR (95% CI) 0.63 (0.44 to 0.89)). We found no evidence that baseline autoantibody status influenced damage accrual. We also noted a significant interaction between SLEDAI-2K and steroid use for transitions from SDI 0 to ≥1 (SLEDAI/3×(Corticosteroids = Yes); RTR (95% CI) 1.33 (1.02 to 1.74)). This suggests that the association between disease activity and transition to damage is greater for those patients taking corticosteroids.
In a secondary analysis we also assessed the influence of having ‘active renal disease’ during follow-up. We found that transition to higher damage states was greater in those with active renal disease (RTR (95% CI) SDI 0 to ≥1: 1.62 (1.10 to 2.38) and SDI ≥1 to higher damage: 1.66 (1.28 to 2.15), respectively).
Influence of SDI on HRQOL
The physical component domains of the SF-36 were more influenced by damage than the mental health components (figure 2). In a regression analysis with GEEs, the Physical Component Summary score (PCS) declined steadily with increased damage (table 5). We also found that PCS values were most influenced by recent changes in damage (coefficient (95% CI) −1.36 (−1.99 to −0.73) per SDI unit) and to a lesser extent by pre-existing damage (coefficient (95% CI) −0.39 (−0.36 to −0.58)).
Figure 2.
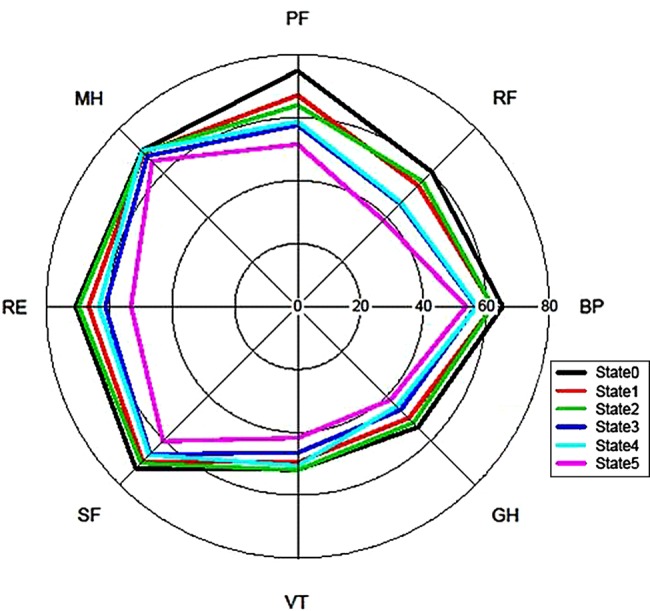
Spider plot of how each component score of the SF-36 varies according to the damage state. BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, role emotional; RF, role physical; SF, social functioning; VT, vitality. State 0: SDI 0; State 1: SDI 1; State 2: SDI 2; State 3: SDI 3; State 4: SDI 4; State 5: SDI 5 or more. SF-36, Medical Outcomes Survey Short-Form 36.
Table 5.
Influence of damage state on the Physical Component Summary Score (PCS) of the SF-36 in SLE patients
| Variables | Coefficient | SE | p Value | Mean | Lower CI | Upper CI |
|---|---|---|---|---|---|---|
| Intercept | 43.55 | 0.34 | – | – | – | – |
| Damage 0 | – | – | – | 43.55 | 42.88 | 44.22 |
| Damage 1 | −2.64 | 0.69 | <0.001 | 40.91 | 39.65 | 42.16 |
| Damage 2 | −2.86 | 0.96 | 0.003 | 40.69 | 38.91 | 42.46 |
| Damage 3 | −5.94 | 1.21 | <0.001 | 37.61 | 35.33 | 39.89 |
| Damage 4 | −6.35 | 1.84 | <0.001 | 37.20 | 33.66 | 40.74 |
| Damage 5+ | −8.11 | 1.69 | <0.001 | 35.44 | 32.19 | 38.69 |
SF-36, Medical Outcomes Survey Short-Form 36; SLE, systemic lupus erythematosus.
Influence of SDI on mortality risk
To date there have been 41 deaths in the cohort. Using a Cox proportional hazards model with SDI score classed as a numerical variable rather than a factor, the SDI score was associated with an increased HR of 1.46 (95% CI 1.18 to 1.81) for mortality. A generalised likelihood ratio test of this model against a model with SDI score stratified by factors produced a test statistic of 14.25 (p=0.007 when compared to the quantiles of a χ2 distribution on 4 df). This suggests that the level of damage has a significant effect on mortality but there is not a simple relationship with SDI score (i.e. log-linear).
Discussion
In a large international SLE inception cohort we have observed a steady accrual of damage over time and as has been reported by others, patients with damage are more likely to develop further damage over time and are also at higher risk of future mortality.5 7 9 14 We also found that damage has a significant effect on physical functioning. This steady accrual of damage has also been reported by other groups.10 14 23 24 We found no evidence of a plateau effect in our early cohort; studies that have suggested a plateau effect have tended to follow a cohort for more than 10 years.12 These observations are of clinical importance as the SDI is relatively easy to administer with some training in routine clinical settings and clearly identifies lupus patients at particularly increased risk of future adverse health outcomes.
Damage, especially a recent increase in the SDI, had a significant influence on physical functioning. Patients with recent damage may experience the maximal physical disability soon after acquiring the damage item. Over time, this may be ameliorated by physical adaptation or by corrective interventions. For example, patients with cataracts may later have lens replacement, patients with osteonecrosis may have joint arthroplasty, and patients with stroke are likely to rehabilitate over time.
Our data also allowed us to estimate the probabilities of developing future damage based on the patient's current SDI score. Such data allow us to consider how the SDI may be used as a clinical trial endpoint. For example, the estimated probability of remaining damage-free at 2 years is 0.844 if a patient has no damage at baseline. If there is one unit of damage, the estimated probability is 0.664 (similar for more than one). If we want to detect a doubling of the odds of remaining damage-free with an intervention, then in the first case the sample size needed (test at 5% and 80% power) would be approximately 670 (335 per group) and 349 (175 per group) in the second scenario.
We found a non-linear effect of age, with the effect of increases in age being most marked in older patients. Certain damage items such as cataracts, stroke and osteoporosis are, of course, more common with increasing age in the general population. Therefore, there may be a greater sensitivity to the additional effects of SLE and drug adverse effects with increasing age due to reduced organ reserve. There were also important differences among subsets of patients according to race/ethnicity and location. USA patients of African ancestry have an increased risk of damage accrual and USA Caucasians were also more likely to develop new damage. Of note, Asians had a lower risk of developing damage. There are a number of explanations for these findings, including differences in the clinical phenotype and/or its severity across different racial/ethnic groups. Response to therapy may also vary in different racial/ethnic groups25 26 and socio-economic factors and access to healthcare may also contribute. We used post-secondary education as a surrogate for socio-economic status, which was not significant in our models. Other more direct measures of socio-economic status were not collected but may have helped address this question more fully.
A number of similar factors drove development of new damage and/or progression of existing damage. Levels of disease activity, use of corticosteroids and hypertension all significantly influenced damage accrual. The significant interaction between disease activity and steroid therapy on new damage suggests that both act together to enhance the development of irreversible organ changes. Conversely, AMs were associated with reduced progression of damage, particularly in patients with baseline damage. These are all potentially modifiable risk factors. A multidimensional approach to damage prevention may therefore be needed and components of this would include better suppression of disease activity, minimising/avoiding corticosteroid use, use of AMs from an early stage and close control of hypertension. Also, if a novel therapy for SLE could achieve better disease control and steroid-sparing/avoidance, this ‘double benefit’ may translate to significant effects on damage accrual; indeed the interaction of inflammation and steroid therapy we found suggests there may be major gains in reducing future damage by such an approach.
Our study has a number of strengths. This is a large international inception cohort from diverse racial/ethnic backgrounds and geographical locations which has helped us understand how these factors influence damage development. We could also estimate probabilities of damage progression to help inform the use of the SDI as a clinical trial endpoint.
There are some limitations to our study. Patients were followed annually so it is difficult to fully model disease activity and therapeutic exposures. We also lack data on psychosocial factors which may influence damage progression. Sundaramurthy et al previously demonstrated that locus of control and time orientation were strong predictors of future damage.27 Our cohort, followed at a number of major teaching centres, may represent a lower estimate of damage accrual rates than that seen in general rheumatology practice; conversely, the tertiary referral case mix in many centres may act in the opposite way to influence our estimates. Finally, while our multivariate modelling suggests an independent effect of steroids and AMs on certain outcomes, we cannot exclude the possibility of residual confounding and that unmeasured factors may also influence the use of these agents in SLE patients.
In conclusion, we describe a steady increase in damage over time in SLE patients, with pre-existing damage being an important predictor of future damage accrual. We have also identified a number of modifiable risk factors that can be targeted as an integrated strategy. Overall, the SDI may therefore act in a way analogous to an erosion score in rheumatoid arthritis and could also act as a valid intermediate surrogate outcome for future mortality in SLE clinical trials.
Acknowledgments
We are grateful for the generous donation of our patients’ time and the dedication of all the fellows, research coordinators and research assistants in the SLICC network to the completion of this work.
Correction notice: This article has been corrected since it was published Online First. Figure 1 has been corrected.
Funding: INB is a National Institute for Health Research (NIHR) Senior Investigator and is supported by Arthritis Research UK, The Manchester Academic Health Science Centre, the NIHR Biomedical Research Unit Funding Scheme, the NIHR Manchester Wellcome Trust Clinical Research Facility and the Manchester Biomedical Research Centre. AGO'K and VF were supported by MRC (UK) funding U105261167. MBU was supported by the Canadian Institutes of Health Research (grant MOP-49529), the Lupus Foundation of Ontario, the Ontario Lupus Association, Lupus UK, the Lupus Foundation of America, the Lupus Alliance of Western New York, the Conn Smythe Foundation, the Lupus Flare Foundation, and the Tolfo family of Toronto, Ontario, Canada. S-CB was supported by the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A120404). CG was supported by Lupus UK, the Arthritis Research Campaign, and the NIHR/Wellcome Trust Clinical Research Facility, Birmingham, UK. GSA was supported by the University of Alabama at Birmingham (NIH/NIAMS grant P60-AR-48095). AEC was supported by the Singer Family Fund for Lupus Research; she is also a Fonds de la Recherche en Sante du Quebec National Scholar. SB was supported by the Fonds de la Recherche en Sante du Quebec Jeune Chercheure and the McGill University Health Centre Research Institute; she is also a recipient of a Canadian Institutes of Health Research Junior Investigator Award and a Canadian Arthritis Network Scholar Award. DAI and AR were supported by the National Institute for Health Research, University College London Hospitals Biomedical Research Centre. PRF is a Distinguished Senior Investigator of The Arthritis Society with additional support from the Arthritis Centre of Excellence, University of Toronto. JGH was supported by a grant from the Canadian Institutes of Health Research (grant MOP-86526). DDG was supported by the Canadian Institutes of Health Research. MP was supported by the Hopkins Lupus Cohort (grant AR-43727) and the Johns Hopkins University General Clinical Research Center (grant M01-RR-00052). MAD was supported by the University of North Carolina Chapel Hill General Clinical Research Center (RR00046). ON and GKS were supported by grants from the Medical Faculty at Lund University, Swedish Combine Projects, the Crafoord Foundation and Lund University Hospital. RRG was supported by the NIH (grants UL 1RR 025741, K24-AR-02318 and P60-AR-48098). DLK was supported by the NIH (grants UL1 RR029882 and P60 AR062755). We acknowledge funding support to SLICC for this study from GlaxoSmithKline and Human Genome Sciences. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Competing interests: None.
Ethics approval: IRBs and Ethics Committees at all participating centres approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The study data have been archived and are available on request. No additional unpublished data are available.
References
- 1.Strand V, Gladman D, Isenberg D, et al. Outcome measures to be used in clinical trials in systemic lupus erythematosus. J Rheumatol 1999;26:490–7. [PubMed] [Google Scholar]
- 2.Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 1997;40:809–13. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Goldsmith CH, Urowitz MB, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol 2000;27:373–6. [PubMed] [Google Scholar]
- 4.Sutton EJ, Davidson JE, Bruce IN. The Systemic Lupus International Collaborating Clinics (SLICC) damage index: a systematic literature review. Semin Arthritis Rheum 2013;43:352–61. [DOI] [PubMed] [Google Scholar]
- 5.Rahman P, Gladman DD, Urowitz MB, et al. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 2001;10:93–6. [DOI] [PubMed] [Google Scholar]
- 6.Nived O, Jonsen A, Bengtsson AA, et al. High predictive value of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol 2002;29:1398–400. [PubMed] [Google Scholar]
- 7.Cardoso CR, Signorelli FV, Papi JA, et al. Initial and accrued damage as predictors of mortality in Brazilian patients with systemic lupus erythematosus: a cohort study. Lupus 2008;17:1042–8. [DOI] [PubMed] [Google Scholar]
- 8.Gladman DD, Urowitz MB, Rahman P, et al. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955–9. [PubMed] [Google Scholar]
- 9.Alarcon GS, Roseman JM, McGwin G, et al. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology 2004;43:202–5. [DOI] [PubMed] [Google Scholar]
- 10.Chambers SA, Allen E, Rahman A, et al. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology 2009;48:673–5. [DOI] [PubMed] [Google Scholar]
- 11.Maddison P, Farewell V, Isenberg D, et al. The rate and pattern of organ damage in late onset systemic lupus erythematosus. J Rheumatol 2002;29:913–17. [PubMed] [Google Scholar]
- 12.Becker-Merok A, Nossent HC. Damage accumulation in systemic lupus erythematosus and its relation to disease activity and mortality. J Rheumatol 2006;33:1570–7. [PubMed] [Google Scholar]
- 13.Nossent JC. SLICC/ACR Damage Index in Afro-Caribbean patients with systemic lupus erythematosus: changes in and relationship to disease activity, corticosteroid therapy, and prognosis. J Rheumatol 1998;25:654–9. [PubMed] [Google Scholar]
- 14.Stoll T, Seifert B, Isenberg DA. SLICC/ACR Damage Index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol 1996;35:248–54. [DOI] [PubMed] [Google Scholar]
- 15.Stoll T, Sutcliffe N, Mach J, et al. Analysis of the relationship between disease activity and damage in patients with systemic lupus erythematosus–a 5-yr prospective study. Rheumatology 2004;43:1039–44. [DOI] [PubMed] [Google Scholar]
- 16.Mok CC, Ho LY, Cheung MY, et al. Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: a 2-year prospective study. Scand J Rheumatol 2009;38:121–7. [DOI] [PubMed] [Google Scholar]
- 17.Prasad R, Ibanez D, Gladman D, et al. Anti-dsDNA and anti-Sm antibodies do not predict damage in systemic lupus erythematosus. Lupus 2006;15:285–91. [DOI] [PubMed] [Google Scholar]
- 18.Fessler BJ, Alarcon GS, McGwin G, Jr., et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005;52:1473–80. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40: 1725. [DOI] [PubMed] [Google Scholar]
- 20.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 21.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 22.Hanly JG, Urowitz MB, Siannis F, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum 2008;58:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassano G, Roverano S, Paira S, et al. Accrual of organ damage over time in Argentine patients with systemic lupus erythematosus: a multi-centre study. Clin Rheumatol 2007;26:2017–22. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Irastorza G, Egurbide MV, Martinez-Berriotxoa A, et al. Antiphospholipid antibodies predict early damage in patients with systemic lupus erythematosus. Lupus 2004;13:900–5. [DOI] [PubMed] [Google Scholar]
- 25.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009;20:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in patients with moderately to severely active systemic lupus erythematosus (SLE): results from the randomized, double-blind phase II/III study EXPLORER. Arthritis Rheum 2008;58:4029–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundaramurthy S, Bush TM, Neuwelt CM, et al. Time perspective predicts the progression of permanent organ damage in patients with systemic lupus erythematosus. Lupus 2003;12:443–8. [DOI] [PubMed] [Google Scholar]