Abstract
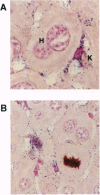
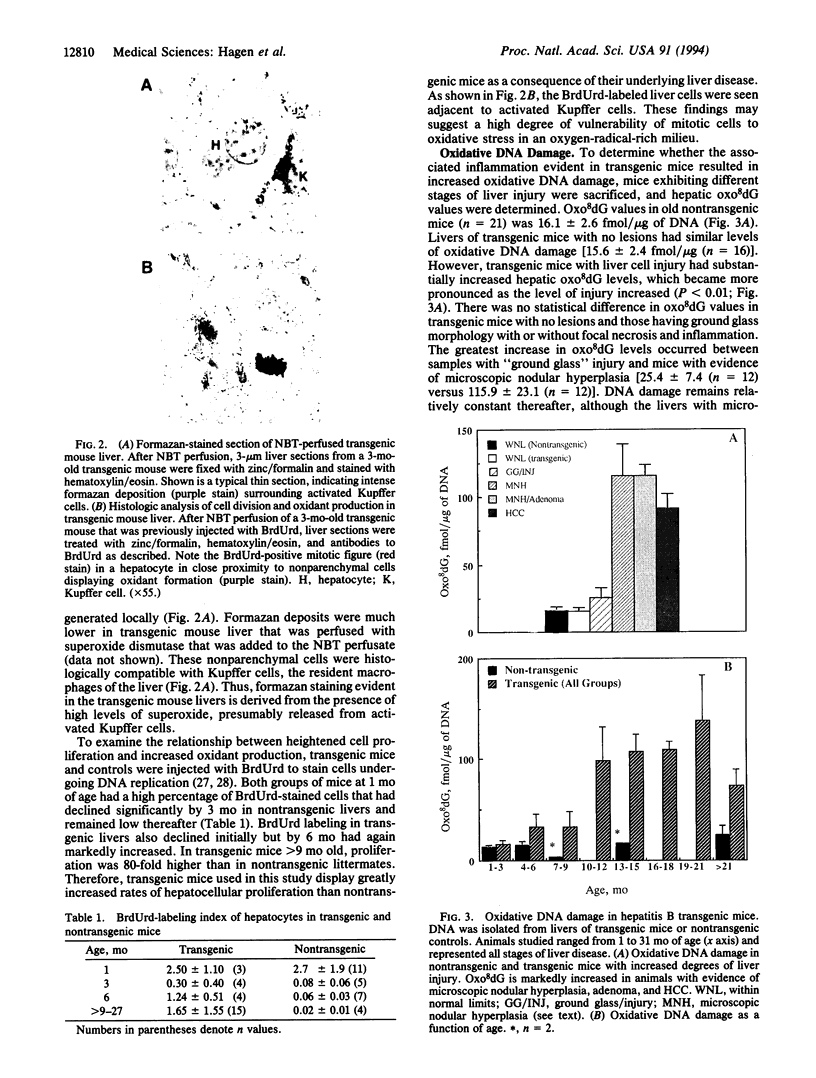
A transgenic mouse strain that expresses the hepatitis B virus (HBV) large envelope protein in the liver was used to determine the extent of oxidative DNA damage that occurs during chronic HBV infection. This mouse strain develops a chronic necroinflammatory liver disease that mimics the inflammation, cellular hyperplasia, and increased risk for cancer that is evident in human chronic active hepatitis. When perfused in situ with nitroblue tetrazolium, an indicator for superoxide formation, the liver of transgenic mice displayed intense formazan deposition in Kupffer cells, indicating oxygen radical production, and S-phase hepatocytes were commonly seen adjacent to the stained Kupffer cells. Similar changes were not observed in nontransgenic control livers. To determine whether these events were associated with oxidative DNA damage, genomic DNA from the livers of transgenic mice and nontransgenic controls was isolated and examined for 8-oxo-2'-deoxyguanosine, an oxidatively modified adduct of deoxyguanosine. Results showed a significant, sustained accumulation in steady-state 8-oxo-2'-deoxyguanosine that started early in life exclusively in the transgenic mice and increased progressively with advancing disease. The most pronounced increase occurred in livers exhibiting microscopic nodular hyperplasia, adenomas, and hepatocellular carcinoma. Thus, HBV transgenic mice with chronic active hepatitis display greatly increased hepatic oxidative DNA damage. Moreover, the DNA damage occurs in the presence of heightened hepatocellular proliferation, increasing the probability of fixation of the attendant genetic and chromosomal abnormalities and the development of hepatocellular carcinoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Filippi P., Buras J., McLachlan A., Popper H., Pinkert C. A., Palmiter R. D., Brinster R. L. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6909–6913. doi: 10.1073/pnas.84.19.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Filippi P., McLachlan A., Milich D. R., Riggs M., Lee S., Palmiter R. D., Pinkert C. A., Brinster R. L. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol. 1986 Dec;60(3):880–887. doi: 10.1128/jvi.60.3.880-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Klopchin K., Moriyama T., Pasquinelli C., Dunsford H. A., Sell S., Pinkert C. A., Brinster R. L., Palmiter R. D. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989 Dec 22;59(6):1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Pinkert C. A., Milich D. R., Filippi P., McLachlan A., Palmiter R. D., Brinster R. L. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science. 1985 Dec 6;230(4730):1157–1160. doi: 10.1126/science.3865369. [DOI] [PubMed] [Google Scholar]
- Colombo M., Kuo G., Choo Q. L., Donato M. F., Del Ninno E., Tommasini M. A., Dioguardi N., Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989 Oct 28;2(8670):1006–1008. doi: 10.1016/s0140-6736(89)91016-7. [DOI] [PubMed] [Google Scholar]
- Columbano A., Rajalakshmi S., Sarma D. S. Requirement of cell proliferation for the initiation of liver carcinogenesis as assayed by three different procedures. Cancer Res. 1981 Jun;41(6):2079–2083. [PubMed] [Google Scholar]
- Daniel L. N., Mao Y., Saffiotti U. Oxidative DNA damage by crystalline silica. Free Radic Biol Med. 1993 May;14(5):463–472. doi: 10.1016/0891-5849(93)90103-2. [DOI] [PubMed] [Google Scholar]
- De Mitri M. S., Pisi E., Bréchot C., Paterlini P. Low frequency of allelic loss in the cyclin A gene in human hepatocellular carcinomas: a study based on PCR. Liver. 1993 Oct;13(5):259–261. doi: 10.1111/j.1600-0676.1993.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie A. M., Rustgi V. K., Hoofnagle J. H., Dusheiko G. M., Lotze M. T. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988 Mar;108(3):390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- Dunsford H. A., Sell S., Chisari F. V. Hepatocarcinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res. 1990 Jun 1;50(11):3400–3407. [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry J. R., Jones C. A., Wiebkin P., Bellemann P., Bridges J. W. The enzymic isolation of adult rat hepatocytes in a functional and viable state. Anal Biochem. 1976 Apr;71(2):341–350. doi: 10.1016/s0003-2697(76)80003-6. [DOI] [PubMed] [Google Scholar]
- Himeno Y., Fukuda Y., Hatanaka M., Imura H. Expression of oncogenes in human liver disease. Liver. 1988 Aug;8(4):208–212. doi: 10.1111/j.1600-0676.1988.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Tokiwa T., Bennett W., Metcalf R. A., Welsh J. A., Sun T., Harris C. C. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis. 1993 May;14(5):987–992. doi: 10.1093/carcin/14.5.987. [DOI] [PubMed] [Google Scholar]
- Jezequel A. M., Paolucci F., Benedetti A., Mancini R., Orlandi F. Enumeration of S-phase cells in normal rat liver by immunohistochemistry using bromodeoxyuridine-antibromodeoxyuridine system. Dig Dis Sci. 1991 Apr;36(4):482–484. doi: 10.1007/BF01298879. [DOI] [PubMed] [Google Scholar]
- Kasai H., Chung M. H., Yamamoto F., Ohtsuka E., Laval J., Grollman A. P., Nishimura S. Formation, inhibition of formation, and repair of oxidative 8-hydroxyguanine DNA damage. Basic Life Sci. 1993;61:257–262. doi: 10.1007/978-1-4615-2984-2_23. [DOI] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nishimura S., Kurokawa Y., Hayashi Y. Oral administration of the renal carcinogen, potassium bromate, specifically produces 8-hydroxydeoxyguanosine in rat target organ DNA. Carcinogenesis. 1987 Dec;8(12):1959–1961. doi: 10.1093/carcin/8.12.1959. [DOI] [PubMed] [Google Scholar]
- Kew M. C., Popper H. Relationship between hepatocellular carcinoma and cirrhosis. Semin Liver Dis. 1984 May;4(2):136–146. doi: 10.1055/s-2008-1040653. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Kurathong S., Lerdverasirikul P., Wongpaitoon V., Pramoolsinsap C., Kanjanapitak A., Varavithya W., Phuapradit P., Bunyaratvej S., Upatham E. S., Brockelman W. Y. Opisthorchis viverrini infection and cholangiocarcinoma. A prospective, case-controlled study. Gastroenterology. 1985 Jul;89(1):151–156. doi: 10.1016/0016-5085(85)90755-3. [DOI] [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Induction of 5,6-ring-saturated thymine bases in NIH-3T3 cells by phorbol ester-stimulated macrophages: role of reactive oxygen intermediates. Cancer Res. 1985 Mar;45(3):1270–1275. [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Inflammation, oxidative DNA damage, and carcinogenesis. Environ Health Perspect. 1987 Dec;76:19–27. doi: 10.1289/ehp.877619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D. M., Srivatanakul P., Khlat M., Chenvidhya D., Chotiwan P., Insiripong S., L'Abbé K. A., Wild C. P. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991 May 30;48(3):323–328. doi: 10.1002/ijc.2910480302. [DOI] [PubMed] [Google Scholar]
- Parsonnet J. Helicobacter pylori and gastric cancer. Gastroenterol Clin North Am. 1993 Mar;22(1):89–104. [PubMed] [Google Scholar]
- Pasquinelli C., Bhavani K., Chisari F. V. Multiple oncogenes and tumor suppressor genes are structurally and functionally intact during hepatocarcinogenesis in hepatitis B virus transgenic mice. Cancer Res. 1992 May 15;52(10):2823–2829. [PubMed] [Google Scholar]
- Purtilo D. T. Clonorchiasis and hepatic neoplasms. Trop Geogr Med. 1976 Mar;28(1):21–27. [PubMed] [Google Scholar]
- Rom W. N., Travis W. D., Brody A. R. Cellular and molecular basis of the asbestos-related diseases. Am Rev Respir Dis. 1991 Feb;143(2):408–422. doi: 10.1164/ajrccm/143.2.408. [DOI] [PubMed] [Google Scholar]
- Rosin M. P., Saad el Din Zaki S., Ward A. J., Anwar W. A. Involvement of inflammatory reactions and elevated cell proliferation in the development of bladder cancer in schistosomiasis patients. Mutat Res. 1994 Mar 1;305(2):283–292. doi: 10.1016/0027-5107(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Sakuma K., Saitoh N., Kasai M., Jitsukawa H., Yoshino I., Yamaguchi M., Nobutomo K., Yamumi M., Tsuda F., Komazawa T. Relative risks of death due to liver disease among Japanese male adults having various statuses for hepatitis B s and e antigen/antibody in serum: a prospective study. Hepatology. 1988 Nov-Dec;8(6):1642–1646. doi: 10.1002/hep.1840080628. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Aboujaoude E. N., Chen Q., Ames B. N. Assays of oxidative DNA damage biomarkers 8-oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- Shimoda R., Nagashima M., Sakamoto M., Yamaguchi N., Hirohashi S., Yokota J., Kasai H. Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res. 1994 Jun 15;54(12):3171–3172. [PubMed] [Google Scholar]
- Shindo K. Significance of Schistosomiasis japonica in the development of cancer of the large intestine: report of a case and review of the literature. Dis Colon Rectum. 1976 Jul-Aug;19(5):460–469. doi: 10.1007/BF02590835. [DOI] [PubMed] [Google Scholar]
- Thomas H. C. The hepatitis B virus and the host response. J Hepatol. 1990;11 (Suppl 1):S83–S89. doi: 10.1016/0168-8278(90)90170-v. [DOI] [PubMed] [Google Scholar]
- Thoolen B. BrdUrd labeling of S-phase cells in testes and small intestine of mice, using microwave irradiation for immunogold-silver staining: an immunocytochemical study. J Histochem Cytochem. 1990 Feb;38(2):267–273. doi: 10.1177/38.2.1688900. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Buendia M. A. Hepatitis B virus. Sci Am. 1991 Apr;264(4):116–123. doi: 10.1038/scientificamerican0491-116. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D., Day N. E., Tzonou A., Hadziyannis S., Kaklamani E., Sparos L., Muñoz N., Hatzakis A. Delta agent and the etiology of hepatocellular carcinoma. Int J Cancer. 1987 Mar 15;39(3):283–286. doi: 10.1002/ijc.2910390303. [DOI] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Gordon L. I. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990 Aug 15;76(4):655–663. [PubMed] [Google Scholar]