Abstract
Objective
Hospice/palliative care patients may differ from better studied populations, and data from other populations cannot necessarily be extrapolated into hospice/palliative care clinical practice. Pharmacovigilance studies provide opportunities to understand the harms and benefits of medications in routine practice. Gabapentin, a γ-amino butyric acid analogue antiepileptic drug, is commonly prescribed for neuropathic pain in hospice/palliative care. Most of the evidence however relates to non-malignant, chronic pain syndromes (diabetic neuropathy, postherpetic neuralgia, central pain syndromes, fibromyalgia). The aim of this study was to quantify the immediate and short-term clinical benefits and harms of gabapentin in routine hospice/palliative care practice.
Design
Multisite, prospective, consecutive cohort.
Population
127 patients, 114 of whom had cancer, who started gabapentin for neuropathic pain as part of routine clinical care.
Settings
42 centres from seven countries. Data were collected at three time points—at baseline, at day 7 (and at any time; immediate and short-term harms) and at day 21 (clinical benefits).
Results
At day 21, the average dose of gabapentin for those still using it (n=68) was 653 mg/24 h (range 0–1800 mg) and 54 (42%) reported benefits, of whom 7 (6%) experienced complete pain resolution. Harms were reported in 39/127 (30%) patients at day 7, the most frequent of which were cognitive disturbance, somnolence, nausea and dizziness. Ten patients had their medication ceased due to harms. The presence of significant comorbidities, higher dose and increasing age increased the likelihood of harm.
Conclusions
Overall, 42% of people experienced benefit at a level that resulted in continued use at 21 days.
Keywords: Drug administration, Pain, Terminal care
Background
Pain is a common concern of patients receiving hospice/palliative care. While it is possible for pain in many people to be well managed with simple analgesics and/or opioids,1 a proportion of patients experience neuropathic pain. Neuropathic pain has been defined by the International Association for the Study of Pain (IASP) as “pain caused by a lesion or disease of the somatosensory system.”2 Adjuvant analgesics are commonly recommended for the management of neuropathic pain.1 3–5 A wide array of adjuvant drug choices are available from within several main classes: the ones most commonly used are antidepressants and antiepileptics.
Gabapentin was originally approved in 1993 as an antiepileptic for treatment of complex partial seizures.6 It was developed as an analogue of γ-aminobutyric acid. Its site and mode of action in neuropathic pain are thought to be via modulation of the α and δ calcium channel subunits.6 7 Gabapentin is water soluble with a very high volume of distribution and is not metabolised but rather excreted unchanged in the urine at a rate proportional to creatinine clearance, requiring dose reduction in people with renal impairment.8 Plasma concentrations are proportional to the dose administered at doses up to 1800 mg/day. However, at higher doses, drug absorption becomes saturated, resulting in an effective dose ‘ceiling’ and adding to variability between patients. Its elimination half-life is 5–9 h.
Potential harms documented in the literature include: dizziness (21%), somnolence (16%), peripheral oedema (8%) and gait disturbance (9%), although serious adverse events (4%) were reported to be no more common than placebo.9 Adverse drug reactions reported to the French Pharmacovigilance System most commonly included neuropsychiatric symptoms, but hepatic problems were also noted.10 The evidence collected in such a database is very likely to under-represent the incidence of adverse effects, as in usual clinical practice only the most severe problems are commonly reported.
Prescribing practice in hospice/palliative care has been extrapolated from related areas of clinical practice with populations that are more readily studied. For instance, much of the available data for the efficacy of gabapentin is derived from studies in chronic pain syndromes such as diabetic or postherpetic neuropathic pain.11 The efficacy reported in the literature for gabapentin in the two best studied neuropathic conditions is: (A) to achieve a greater than 50% pain reduction for painful diabetic neuropathy: 33% response rate with a number needed to treat (NNT) of 5.8 (4.3–9.0) and an associated placebo response rate of 23%, at doses of between 600 and 3600 mg/day5; and (B) to achieve a 50% pain reduction for postherpetic neuralgia: a response rate of 33% with an NNT of 7.5 (5.2–14) and a placebo response rate of 20%, at doses of 1800–3600 mg/day.5 On the basis of evidence that includes the available data and the risk/benefit profiles and availability of gabapentin and alternative adjuvants, current guidelines for the management of neuropathic pain suggest gabapentin as one of the first-line treatment options.3 4 12
Owing to the limited available evidence, an international initiative was started in 2011 to improve clinicians’ understanding of the net clinical effects of key medicines used in hospice/palliative care and to further inform prescribing in this important area of patient care.13 This is part of expanding the evidence base for clinical care by the Australian Palliative Care Clinical Studies Collaborative (PaCCSC). The general rationale and methodology for these studies have been reported previously.14
The aim of this study was to describe the net clinical effect (ie, the overall benefits and the immediate and short-term harms) of gabapentin when prescribed for neuropathic pain in a consecutive, prospective cohort of hospice/palliative care patients in a variety of clinical settings where the medication was started as part of routine clinical care. The null hypothesis was that there would be no clinicodemographic factors that predict benefit or separately harms.
Methods
All participating sites received ethical waivers (as the work falls under quality assurance, quality improvement, performance monitoring and/or clinical audit study type) or approval for low-risk research. The study involved only the collection of routine data by treating clinicians already involved in the care of the patient, using data which are, or should be, routinely collected in order to monitor treatment outcomes, and precautions were taken to ensure non-identifiability of all data entered into the RAPID study (for instance, by the use of age rather than date of birth). Patients’ and/or family members’ consent was therefore not required for the study.
The study methods have been described in detail previously.13 14 An expert committee defined prespecified clinical benefit and harm fields based on the available literature relating to the use of gabapentin for neuropathic pain, selecting time points for benefits and harms according to its profile of action. The National Cancer Institute's Common Toxicity Criteria for Adverse Events (NCI CTCAE v4) Likert scales for grading effects were used.15 Non-identifying demographic and clinical data were entered pro forma using a 128-bit encrypted web portal (http://www.caresearch.com.au). Data were recorded on consecutive patients at participating clinical sites.
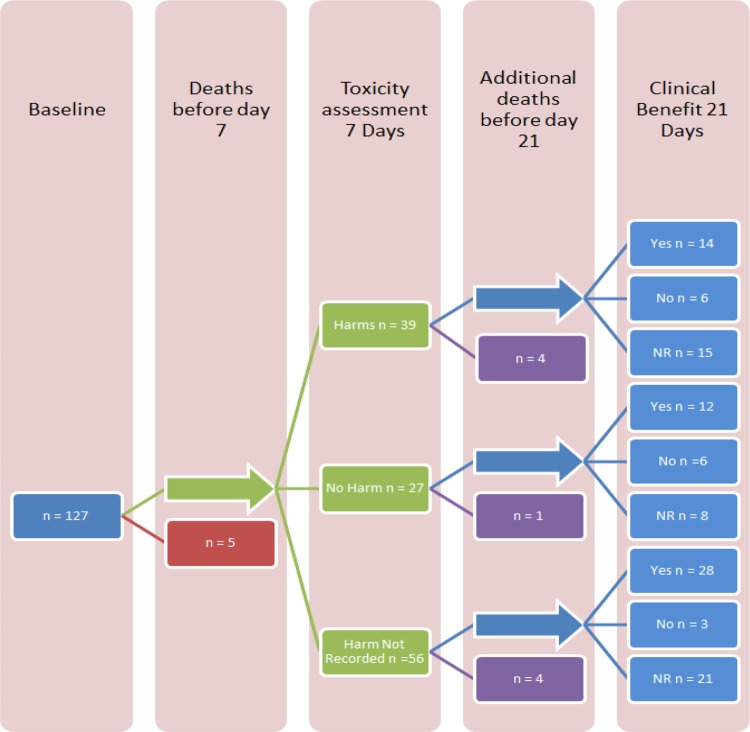
Data were recorded at three set time points: at baseline (the index symptom, and symptoms that could reflect harm subsequently from the medication); at day 7 (immediate and short-term harms); and at day 21 (index symptom (neuropathic pain response) after starting gabapentin; figure 1). The NCI CTCAE for neuropathic pain ask the clinician to rate the overall severity of the symptoms, including also their impact on activities of daily living. This approach was chosen as the intention was to quantify the degree of impact from a symptom perspective, and to align the assessment approaches of benefits and harms.
Figure 1.
Gabapentin pharmacovigilance study flow chart (NR, not reported).
Overall benefit was defined as a one point reduction in the NCI CTCAE (eg, severe to moderate, moderate to mild, mild to none). Harms were attributed to gabapentin if the criteria for NCI CTCAE were greater than the patient's baseline measurement at or before day 7. For harms rated as 3 or greater on the NCI CTCAE at day 7, data were collected on the modified Naranjo scale.16 The Naranjo questionnaire is designed to aid in determining attribution of an adverse drug reaction to the drug itself. The Naranjo score was modified for the hospice/palliative care setting and to facilitate consistency between patients. Questions 2, 3, 5, 9 and 10 were reported, along with the dose at the time of the toxicity (table 1). Other items, such as rechallenge with the medication, were excluded as the information could not be obtained for the majority of palliative care patients.16 Functional status was recorded using the Australian modified Karnofsky Performance Scale, and comorbidities were assessed using the unweighted Charlson Comorbidity Index.17 18
Table 1.
Modified Naranjo scores for toxicities grade 3 or higher on the NCI CTCAE
| Patient | Dose at time of reporting (mg/day) | Question 2 | Question 3 | Question 5 | Question 9 | Question 10 | Total score | Possibility of ADR* |
|---|---|---|---|---|---|---|---|---|
| A | 200 | 2 | 0 | −1 | 0 | 0 | 1 | Possible |
| B | 200 | 2 | 1 | −1 | 0 | 0 | 2 | Possible |
| C | 100 | 2 | 1 | −1 | 0 | 0 | 2 | Possible |
| D | 300 | 2 | 1 | −1 | 0 | 0 | 2 | Possible |
| E | 200 | 2 | 0 | −1 | 0 | 0 | 1 | Possible |
| F | 200 | 2 | 1 | 2 | 0 | 0 | 5 | Probable |
| G | 1200 | 2 | 0 | −1 | 0 | 0 | 1 | Possible |
Median dose 200 mg/day, with a range of 100–1200 mg/day.
Q2: Did the adverse event occur after the suspected drug was administered?
Q3: Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered?
Q5: Are there alternative causes (other than the drug) that could have on their own caused the reaction?
Q9: Did the patient have a similar reaction to the same or similar drugs in any previous exposure?
Q10: Was the adverse event confirmed by any objective evidence including clinician observation.
*According to modified Naranjo checklist.
ADR, adverse drug reaction; NCI CTCAE, National Cancer Institute's Common Toxicity Criteria for Adverse Events.
Statistical methods
T tests, Mann–Whitney U tests and χ2 tests were used to test for differences between groups for continuous and categorical variables as appropriate. Univariable logistic regression model for each outcome on key clinical and demographic parameters was undertaken, clustering over site to account for correlated readings. Additionally, logistic regression was performed for each outcome on each pair of key parameters and their product term to identify possible subgroups that were associated with the outcomes. We used multiple imputations to account for missing data, with 20 resamples drawn for a sensitivity analysis. Results are reported as ORs with 95% CIs. No adjustment was made for multiple comparisons, as the results are considered to be hypothesis generating. All models were validated using the Hosmer-Lemeshow goodness of fit test. A two-tailed p value less than 0.05 is considered statistically significant. Data were imported into Excel (Microsoft Corp, Seattle, Washington, USA, 2006). All analyses were performed in STATA SE V.12.1 (Statacorp, Texas, USA).
Results
The clinical and demographic data of the study participants are shown in table 2. Data were available for 127 patients from 42 hospice/palliative care sites in seven countries (table 3) collected between September 2012 and August 2013. Clinical sites were drawn from consultative services, ambulatory clinics and specialist inpatient hospice/palliative care units, reflecting the scope of current specialist hospice/palliative care practice in the participating countries. In this sample, the majority (89.7%) of patients had cancer. The mean NCI CTCAE score for neuropathic pain at baseline was 2.33. Baseline symptoms are recorded in table 4. Of note, the rates of fatigue (60%), somnolence (42%), cognitive disturbance (23%) and dizziness (16%) were all high at baseline.
Table 2.
Baseline clinical and demographic data
| n (%) | Median | Range | Mean | SD | |
|---|---|---|---|---|---|
| Age | 127 (100) | 68 | 63 (26–89) | 66.2 | 12.6 |
| Gender (male) | 47 (80) | ||||
| Australian-modified Karnofsky Performance Status Score | 126 (99) | 60 | 70 (20–90) | 56.7 | 15.7 |
| Body mass index | 101 (80) | 23.7 | 20 (13.9–33.9) | 24.1 | 4.1 |
| C reactive protein | 32 (25) | 76.5 | 378 (5–383) | 102.2 | 85.9 |
| Calculated creatinine clearance | 71 (56) | 70 | 239.1 (0.9–240) | 74.5 | 36.4 |
| National Cancer Institute Common Toxicity Grading for Pain | 127 (100) | 2 | 2 (1–3) | 2.33 | 0.64 |
| Primary life-limiting illness | |
| Cancer | 121 (95.3) |
| End-stage renal disease | 2 (1.6) |
| End-stage respiratory disease | 1 (0.8) |
| Other (severe peripheral vascular disease (1), unrecorded (2)) | 3 (2.4) |
Table 3.
Participating service descriptors n (%)
| Ambulatory and community | Inpatient direct | Inpatient consultative | Inpatient direct and consultative | Ambulatory/community and inpatient | Total | |
|---|---|---|---|---|---|---|
| Metro | 5 (12) | 9 (21) | 7 (17) | 4 (10) | 4 (10) | 29 (70) |
| Non-metro | 3 (7) | 3 (7) | 2 (5) | 2 (5) | 3 (7) | 13 (30) |
| Total | 8 (19) | 12 (29) | 9 (21) | 6 (14) | 7 (17) | 42 (100) |
Participating countries—Australia, Canada, Hong Kong, the UK, Italy, India, New Zealand.
Table 4.
Symptoms at baseline—prior to start of gabapentin
| Baseline symptoms* | Total n n (%) |
Severity† | |
|---|---|---|---|
| Median | Range | ||
| Fatigue | 76 (60) | 2 | 1–3 |
| Dizziness | 20 (16) | 1 | 1–3 |
| Somnolence | 53 (42) | 1 | 1–3 |
| Cognitive disturbance | 29 (23) | 2 | 1–3 |
| Ataxia | 11 (9) | 1 | 1–5 |
| Tremors | 5 (4) | 1 | 1–3 |
| Nystagmus | 0 (0) | 0 | 0 |
| Headache | 7 (6) | 2 | 1–3 |
| Nausea | 28 (22) | 1.5 | 1–3 |
| Suicidal ideation | 4 (3) | 2 | 1–3 |
| Other | 25 (20) | 2 | 1–3 |
*Patients could have more than one symptom.
†National Cancer Institute Common Toxicity Criteria (NCI CTC).
At day 21 (the time point for assessment of clinical benefits), 14 (11%) patients had died, 44 (35%) had no data recorded (usually because they were community-based patients with no clinical contact around that time point), and an overall symptom score was documented in 69/127 patients (table 5). Of these, 54 (78% of patients where data were available) experienced an improvement in their pain. At day 21, 68 were still on regular gabapentin, and were receiving an average of 653 mg of gabapentin/24 h (SD 402; median 600 mg; range 100–1800 mg).
Table 5.
Outcomes at 21 days after starting gabapentin for neuropathic pain in palliative care patients (n=127)
| NCI CTCAE* neuropathic pain score at baseline (T0) before starting gabapentin | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Subtotals (n) | 12 | 62 | 53 | ||
| NCI CTCAE* neuropathic pain score at 21 days (T1) after starting gabapentin | 0 | 7 | 1 improved | 6 improved | 0 improved |
| 1 | 32 | 2 unchanged | 20 improved | 10 improved | |
| 2 | 28 | 2 worsened | 9 unchanged | 17 improved | |
| 3 | 2 | 0 worsened | 0 worsened | 2 unchanged | |
| 5 | 14† | 3 | 6 | 5 | |
| NR | 44 | 4 | 21 | 19 | |
Within 21 days of starting gabapentin, pain scores
Improved n=54 (42.2%) of whom 7 (5.5%) had a total pain resolution.
Doses at day 21 (mg): mean 590.6, SD 380.8, median 600, range 0–1800.
Unchanged n=13 (10.2%).
Doses at day 21 (mg): mean 707.7, SD 499.9, median 600, range 0–1800.
Worsened n=2 (1.6%), and 14† (11%) died.
Doses at day 21 (mg): mean 600, SD 300, median 600, range 300–900.
*NCI CTCAE v3 National Cancer Institute's Common Toxicity Criteria for Adverse Events—pain scale.
0. None.
1. Mild pain.
2. Moderate pain; limiting instrumental activities of daily living (ADL).
3. Severe pain; limiting self-care ADL.
5. Death.
†The 14 deaths were excluded from the statistics.
NR, not recorded.
A total of 39/122 surviving patients had harms recorded (32%; CI 23.6% to 40.4%; table 6) and they recorded 11 different harms, but information about harms was not recorded for almost half of our sample (n=56). The most frequently encountered harms were somnolence (n=24, 26% of harms), cognitive disturbance (n=19, 20% of harms) and fatigue (n=14, 15% of harms). Ten patients had their medication ceased for harms which included cognitive disturbance, somnolence, nausea and dizziness, while for 19 patients the gabapentin dose was reduced (table 7). Eleven patients were prescribed a medication to treat toxicity, while nine experienced a harm of grade 3 or higher. Of these, seven were assessed using the modified version of the Naranjo scale, and two were excluded because the harm was recorded as not occurring after the administration of gabapentin. The total number of patients who received benefit from gabapentin without experiencing any harms was 12 (9.4%).
Table 6.
Overall effect
| New harm N, % (95% CI) |
Action following harm | Benefit N (%) |
Benefit/s (1 point NCI* reduction) N, % (95% CI) |
|---|---|---|---|
| Yes n=39 32.0 (23.6 to 40.4) |
Dose reduction | 1 (0.8) | Yes n=14/39 40.0 (22.9 to 57.1) |
| No change in med | 7 (5.7) | ||
| Toxicity treat—other | 2 (1.6) | ||
| Other medication | 3 (2.4) | ||
| NR | 1 (0.8) | ||
| Dose reduction | 1 (0.8) | No n=6/39 17.1 (4.05 to 30.3) |
|
| No change in med | 1 (0.8) | ||
| Toxicity treat—other | 1 (0.8) | ||
| NR | 3 (2.4) | ||
| Medication cessation | 4 (3.1) | NR n=15/39 42.9 (25.6 to 60.1) |
|
| Dose reduction | 2 (1.6) | ||
| No change in med | 3 (2.4) | ||
| Toxicity treat—other | 2 (1.6) | ||
| Other medication | 1 (0.8) | ||
| NR | 3 (2.4) | ||
| Dose reduction | 1 (0.8) | Died n=4/39 | |
| No change in med | 1 (0.8) | ||
| Toxicity treat—other | 1 (0.8) | ||
| Other medication | 1 (0.8) | ||
| No n=27 22.1 (14.7 to 29.6) |
Dose reduction | 1 (0.8) | Yes n=12/27 46.2 (25.6 to 66.7) |
| Toxicity treat—other | 1 (0.8) | ||
| NR | 10 (8.2) | ||
| NR | 6 (4.9) | No n=6/27 23.1 (5.7 to 40.4) |
|
| No change in med | 1 (0.8) | NR n=8/27 30.1 (11.8 to 50.0) |
|
| NR | 7 (5.7) | ||
| No change in med | 1 (0.8) | Died n=1/27 | |
| NR n=56 45.9% (36.9% to 54.9%) |
28 (23) | Yes n=28/56 53.8 (39.8 to 67.9) |
|
| 3 (2.4) | No n=3/56 5.8 (−0.8 to 12.3) |
||
| 21 (17.2) | NR n=21/56 40.4 (26.6 to 54.2) |
||
| 4 (3.3) | Died n=4/56 | ||
| Died prior to assessment of harms n=5† |
Harms at 7 days and benefits at 21 days.
*Anon. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, V4.0, DCTD, NCI, NIH, DHHS. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed 28 May 2009).
†Excluded from statistical analysis.
NR, not reported.
Table 7.
New harms and the response to those harms
| Harms—yes* n=39 |
n (% of harms) |
Severity median (range) | Clinical responses | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benefits | Died n=4† | Med ceased | Dose reduced | No change in med | Med to treat toxicity | ||||||
| Yes n=14 | No n=6 |
NR n=15 |
|||||||||
| Fatigue | 6 | 2 | 3 | 3 | 14 (15) | 2 (1–3) | 0 | 2 | 7 | 1 | 3 |
| Dizziness | 3 | 1 | 5 | 0 | 9 (10) | 1 (1–3) | 1 | 3 | 2 | 1 | 2 |
| Somnolence | 8 | 2 | 10 | 4 | 24 (26) | 1 (1–3) | 3 | 4 | 11 | 1 | 3 |
| Cognitive disturbance | 5 | 2 | 8 | 4 | 19 (20) | 2 (1–3) | 4 | 4 | 4 | 1 | 4 |
| Ataxia | 1 | 0 | 3 | 0 | 4 (4) | 1 (1–5) | 0 | 2 | 1 | 0 | 1 |
| Tremors | 0 | 1 | 2 | 1 | 4 (4) | 1 (1–3) | 0 | 2 | 1 | 1 | 0 |
| Nystagmus | 0 | 0 | 0 | 0 | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 |
| Nausea | 2 | 1 | 4 | 0 | 7 (8) | 1.5 (1–3) | 1 | 1 | 2 | 2 | 1 |
| Suicidal ideation | 0 | 1 | 0 | 1 | 2 (2) | 2 (1–3) | 0 | 0 | 0 | 1 | 1 |
| Other | 3 | 2 | 4 | 1 | 10 (11) | 2 (1–3) | 1 | 1 | 4 | 3 | 1 |
| Total harms experienced | 93* (100) | ||||||||||
*Patients may experience more than one harm.
†Died before day 7.
NR, not reported.
Multiple imputation on the outcome ‘harm’ revealed that a higher Charlson Comorbidity Index was associated with an increase in the likelihood of experiencing a harm (OR=1.97; CI 1.16 to 3.36; p=0.013). In the logistic regression analyses, there was a significant interaction between daily gabapentin dose (in increments of 100 mg/day) and each additional year of age on those experiencing harm (OR=1.20 (1.03 to 1.39), p=0.017), but this effect attenuated (OR=1.02 (p=0.22)) when the outcomes for harm were imputed for missing data. Subgroup analysis on the crude data indicated that among those experiencing a higher dose (300 mg/day or greater), 10/14 of those greater than mean age (of 65 years) experienced harm versus 4/13 of those less than mean age who experienced harm (p=0.035).
Discussion
Gabapentin is a common adjuvant pain medication, which has an indication for neuropathic pain. The reported NNTs in the literature range from 5.8 to 7.5, and it has been highlighted previously that in cancer pain, as experienced by the majority of our sample, the number needed to harm (NNH) is lower and the NNT higher than in other pain populations.19 In comparison, in this unselected hospice/palliative care population with significant pain, benefit was reported in 42% of people at 21 days. However, the rates of harm were also higher than those reported in the literature, with 30% of the hospice/palliative care population experiencing one or more harms, 9 (7%) of which were rated as ‘severe’. The most common harms in the hospice/palliative care population—somnolence, cognitive disturbance and fatigue—appear to be more frequent than those reported in the literature. The contribution of disease progression, gabapentin or both to the experience of fatigue that had worsened since the start of medication cannot be separated in a cohort study and instead requires the use of controlled studies. In previous pharmacovigilance reports in this hospice/palliative care population (eg, for haloperidol in delirium20), the presence of significant comorbidities appears to increase the risk that a patient will experience a harm. Only 9% of patients benefited without also experiencing any harm. As in previous studies, baseline data (table 3) reveal a pattern of high rates of relevant symptomatology in this patient group which, if not documented prior to starting gabapentin, could be misattributed to the medication under study.
Finally, it is unclear to what extent the speed of titration of gabapentin in this population may also contribute to its adverse effect profile, and whether this medication is titrated differently in a population with neuropathic cancer pain and a shorter life expectancy than it is in the better-studied chronic non-malignant pain population.
Limitations
The primary outcome is a clinician-rated tool for neuropathic pain used most frequently for the evaluation of chemotherapy-induced symptoms. Patient-defined tools can be used in formal studies where consent is sought, but are not feasible for pharmacovigilance using data from routine clinical practice across multiple settings and countries. The substantial number of patients (35%) for whom no score was reported at day 21—a time point that was selected as being clinically meaningful—and the death of 11% of patients within the period of the data collection reflect the great difficulties of studying this population. Ten people died by day 21 in this cohort and, given that key studies in neuropathic pain in settings such as diabetic neuropathy titrated the dose over a 4-week period, one question is whether prognostication should be considered before selecting gabapentin over alternative analgesics.21 Although prognostication is difficult and has a large subjective component,22 it is important when using medications that take longer periods of time to deliver clinical benefit. The balance of timeliness and efficacy is an ongoing question in palliative care.
No information on harms was given on almost half the sample. Given the rate of attrition of patients from this study, the use of intention-to-treat analysis may well underestimate both the clinical benefits and harms in this population, while the ability to identify longer term benefits and harms is also limited.
Data on changes in doses of opioids and other concurrent medications were not collected. A recent systematic review17 on coprescribing of opioids and adjuvants has suggested that harms are less if opioid doses are reduced at the time of initiation of an adjuvant medication. It is therefore not possible to consider whether changes in opioid dosage may have contributed to these findings. As with all pharmacovigilance studies, this study sought to capture accurately current clinical practice and therefore not to influence current practice by trying to standardise the clinical approach taken by participating clinical sites in areas including a titration schedule. Likewise, the way that gabapentin doses were titrated was not captured in this study, accepting that the speed of titration is influenced by both adverse effects and efficacy in this heterogeneous group. Finally, the definition of ‘neuropathic pain’ was also dealt with by using the standard clinical practice of participating sites.
The NCI CTCAE only uses a three-point rating scale without psychometric validation. This scale anchors to the values of mild, moderate and severe, which are in widespread clinical use and are meaningful.
Generalisability
This study has recruited patients from across a large number of sites, the work of which can be considered representative of palliative care across different institutional settings, communities and countries. The population, which mainly includes patients with cancer, is consistent with that served by many hospice/palliative care organisations.
Conclusions
Gabapentin is widely used in hospice/palliative care clinical practice. This study of its use outside the setting of randomised controlled trials suggests lower response rates and higher levels of harms than in highly selected populations. This study again highlights the need to understand the actual performance of medications as prescribed rather than simply relying on literature derived from trials in selected populations.
Acknowledgments
Thanks go to all the clinicians and the clinical units who have contributed to this programme. This includes: Sunshine Coast Hospital and Health Service QLD, Braeside Hospital NSW, Calvary Mater Newcastle NSW, Calvary Healthcare NSW, Greenwich Hospital NSW, Wolper Private Hospital NSW, Peter MacCallum Cancer Institute VIC, St Vincent's Hospital Melbourne VIC, Barwon Health VIC, Royal Melbourne Hospital VIC, Sir Charles Gardiner Hospital WA, Fraser Health British Columbia Canada, Hamilton Health Sciences Ontario Canada, Capital Health Integrated Palliative Care Service Canada, Maria Teresa Chiantore Seràgnoli Hospice Italy, Arohanui Hospice New Zealand, Humber NHS Foundation Trust UK, Leeds Teaching Hospitals NHS Trust UK, Haven of Hope Hospital Hong Kong.
Footnotes
Contributors: All authors have contributed to the planning, writing and execution of this article and all have read and approved the final version of the article being submitted.
Funding: The Australian Palliative Care Clinical Studies Collaborative is supported by funding from the Palliative Care Branch of the Australian Government's Department of Health and Ageing.
Competing interests: None.
Ethics approval: Multiple centres.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available to other researchers by contacting the corresponding author.
References
- 1.Dy SM. Evidence-based approaches to pain in advanced cancer. Cancer J 2010;16:500–6. [DOI] [PubMed] [Google Scholar]
- 2.Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd edn Seattle, WA: IASP Press, 1994:212. [Google Scholar]
- 3.Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;118:289–305. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85(3 Suppl):S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev 2013;11:CD010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose MA, Kam PCA. Gabapentin: pharmacology and its use in pain management. Anaesthesia 2002;57:451–62. [DOI] [PubMed] [Google Scholar]
- 7.Bennett MI, Simpson KH. Gabapentin in the treatment of neuropathic pain. Palliat Med 2004;18:5–11. [DOI] [PubMed] [Google Scholar]
- 8.McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology 1994;44(6 Suppl 5):S17–22; discussion S31–2. [PubMed] [Google Scholar]
- 9.Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2011;(3):CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuzier R, Serres J, Guitton E, et al. Adverse drug reactions to gabapentin and pregabalin: a review of the French pharmacovigilance database. Drug Saf 2013;36:55–62. [DOI] [PubMed] [Google Scholar]
- 11.Goodyear-Smith F, Halliwell J. Anticonvulsants for neuropathic pain: gaps in the evidence. Clin J Pain 2009;25:528–36. [DOI] [PubMed] [Google Scholar]
- 12.Australian Adult Cancer Pain Management Working Group. Cancer pain management in adults. Evidence-based clinical practice guidelines adapted for use in Australia. Sydney: Cancer Council Australia; http://wiki.cancer.org.au/australia/Guidelines:Cancer_pain_management [Google Scholar]
- 13.Currow DC, Rowett D, Doogue M, et al. An international initiative to create a collaborative for pharmacovigilance in hospice and palliative care clinical practice. J Palliat Med 2012;15:282–6. [DOI] [PubMed] [Google Scholar]
- 14.Currow DC, Vella-Brincat J, Clark K, et al. Pharmacovigilance in hospice/palliative care. Rapid report of net clinical effect of metoclopramide. J Palliat Med 2012;15:1071–5. [DOI] [PubMed] [Google Scholar]
- 15.Anon. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, V4.0, DCTD, NCI, NIH, DHHS. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed 28 May 2009).
- 16.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. [DOI] [PubMed] [Google Scholar]
- 17.Abernethy AP, Shelby-James TM, Fazekas BS, et al. The Australian-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice. BMC Palliat Care 2005;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 19.Bennett MI. Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliat Med 2011;25:553–9. [DOI] [PubMed] [Google Scholar]
- 20.Crawford GB, Agar MA, Quinn SJ, et al. Pharmacovigilance in hospice/palliative care. Net effect of haloperidol for delirium. J Palliat Med 2012;15:1071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998;280:1831–6. [DOI] [PubMed] [Google Scholar]
- 22.Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003;327:195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]