Abstract
Objectives:
Determine potential risk factors for progressive visual field loss in the Idiopathic Intracranial Hypertension Treatment Trial, a randomized placebo-controlled trial of acetazolamide in patients with idiopathic intracranial hypertension and mild visual loss concurrently receiving a low sodium, weight reduction diet.
Methods:
Logistic regression and classification tree analyses were used to evaluate potential risk factors for protocol-defined treatment failure (>2 dB perimetric mean deviation [PMD] change in patients with baseline PMD −2 to −3.5 dB or >3 dB PMD change with baseline PMD −3.5 to −7 dB).
Results:
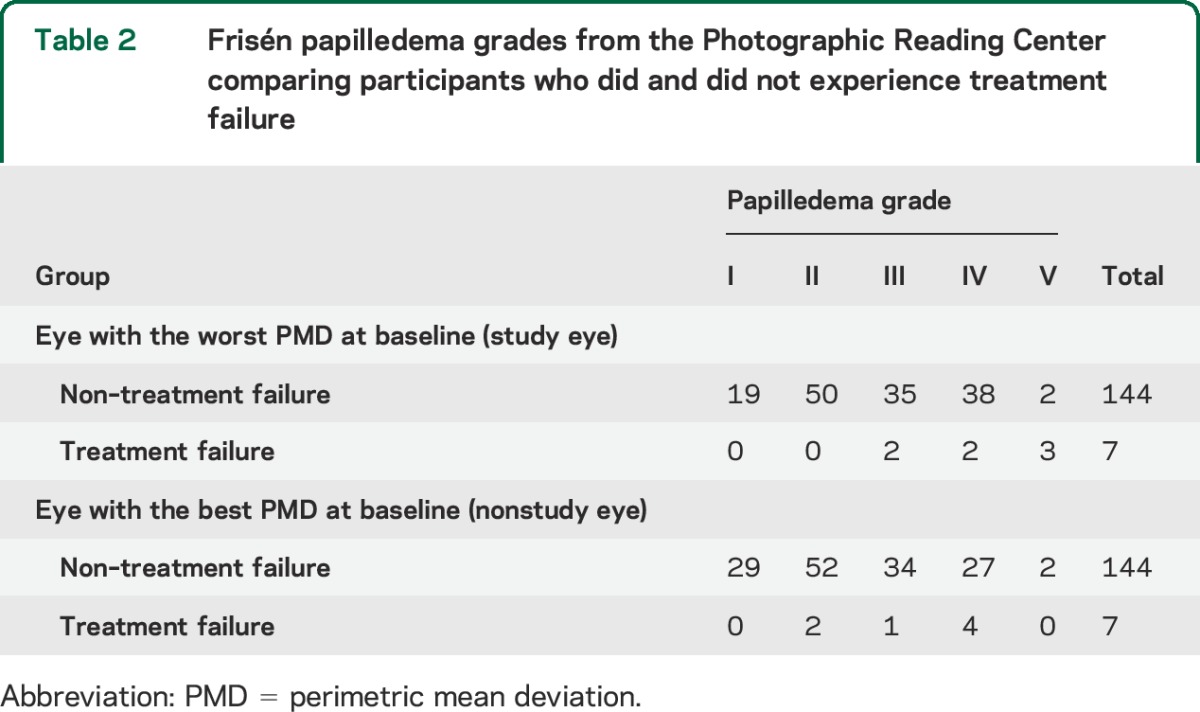
Seven participants (6 on diet plus placebo) met criteria for treatment failure. The odds ratio for patients with grades III to V papilledema vs those with grades I and II was 8.66 (95% confidence interval [CI] 1.65–∞, p = 0.025). A 1-unit decrease in the number of letters correct on the ETDRS (Early Treatment Diabetic Retinopathy Study) chart at baseline was associated with an increase in the odds of treatment failure by a factor of 1.16 (95% CI 1.04–1.30, p = 0.005). Compared with female participants, the odds ratio for male participants was 26.21 (95% CI 1.61–433.00, p = 0.02). The odds of treatment failure were 10.59 times higher (95% CI 1.63–116.83, p = 0.010) for patients with >30 transient visual obscurations per month vs those with ≤30 per month.
Conclusions:
Male patients, those with high-grade papilledema, and those with decreased visual acuity at baseline were more likely to experience treatment failure. All but one of these patients were treated with diet alone. These patients should be monitored closely and be considered for aggressive treatment of their idiopathic intracranial hypertension.
Idiopathic intracranial hypertension (IIH) is a disorder primarily of overweight young women characterized by increased intracranial pressure with its associated signs and symptoms. Neuroimaging and CSF analysis are normal except for findings related to increased intracranial pressure, and no secondary cause of intracranial hypertension is apparent. The above features comprise the modified Dandy criteria for IIH.1
We have completed the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), a multicenter, randomized, double-masked, placebo-controlled clinical trial of acetazolamide in participants with IIH and mild visual loss. All participants received a weight reduction program including a low sodium diet. Participants were randomized to receive either acetazolamide or placebo and were followed for 6 months. We found that the acetazolamide group had significantly improved visual field function, papilledema grade, quality-of-life measures, and CSF pressure reduction relative to the placebo group.2
The major morbidity of IIH is visual loss. Studies estimating the lifetime incidence of blindness in patients with IIH vary with studies from academic centers giving estimates that are in the 5% to 10% range.3,4 During the 6-month period, 7 participants (4.2%) had substantial worsening of their IIH and met study criteria for treatment failure; 6 were in the placebo group. Herein, we report these cases and investigate potential risk factors for progressive visual field loss.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board at each participating site, and individual written informed consent was obtained. The study was conducted in accordance with the Declaration of Helsinki. Participants were enrolled at 38 sites in North America from March 2010 to November 2012, with follow-up ending in June 2013. Trial registration at clinicaltrials.gov (identifier: NCT01003639).
Participants.
Participants aged 18 to 60 years were eligible if they met the modified Dandy criteria1 and had reproducible mild visual loss (−2 to −7 dB perimetric mean deviation [PMD]) in the most affected eye (study eye). Participants needed to have bilateral papilledema, have an elevated CSF opening pressure, be untreated for IIH, and have no secondary cause of increased intracranial pressure present; other entry criteria are found in our methods article.5
Intervention.
A specific dietary plan and lifestyle modification program was offered to all study participants through the New York Obesity Nutrition Research Center. The study drug was acetazolamide (250 mg) or matching placebo tablets. The initial dosage of study drug was 4 tablets daily in 2 divided doses followed by dosage increases of 1 tablet every week up to a maximum dosage of 4 g daily for those receiving acetazolamide. Participants who were unable to tolerate the study drug could gradually decrease the dosage to a minimum of one-half tablet daily.
Treatment failure criteria.
Possible treatment failure was considered to be the classification when a participant with baseline PMD up to −3.5 dB had visual function worsen by more than 2 dB PMD from baseline in either eye or when a participant with baseline PMD between −3.5 and −7 dB had visual function worsen by more than 3 dB PMD from baseline in either eye. The worsening in PMD needed confirmation by a second perimetric examination. To be classified as a treatment failure, an adjudication committee, using all available clinical information, decided whether the worsening of PMD was most likely due to uncontrolled intracranial pressure and worsening of IIH or was due to another cause (such as poor perimetric performance).5 Participants who experienced treatment failure were withdrawn from further participation in the trial and referred to their physicians for additional treatment.
Evaluations.
Participants had visits at screening, baseline, and 1, 2, 3, 4.5, and 6 months after baseline and had unscheduled visits if symptoms worsened. Participants had automated perimetry in both eyes using Humphrey Field Analyzer SITA Standard program 24-2. The testing was performed by a technician certified by the Visual Field Reading Center. Each participant had at least 2 initial visual field examinations that were reviewed by the Visual Field Reading Center and met study criteria.5
The papilledema grade (Frisén Scale)6,7 was documented by the Photographic Reading Center using fundus photographs and by the site investigator; values range from 0 (normal) to 5 (severe papilledema). A best corrected visual acuity using trial lenses mounted in spectacles was measured using Early Treatment Diabetic Retinopathy Study (ETDRS) charts. Details of other evaluations can be found in the primary trial report.2
Schedule of evaluations.
The vital signs, ophthalmologic examination, visual acuity testing, perimetry, and papilledema evaluation were performed at each planned follow-up and unscheduled visit.
Statistical analysis.
Analyses were conducted to investigate potential risk factors for treatment failure. Among the 165 participants in the study sample, 7 participants experienced treatment failure. To allow enough time for observing a treatment failure, 14 participants who withdrew from follow-up before the 2-month visit were excluded, leaving 151 participants for analysis. These 14 participants (9 on placebo and 5 on acetazolamide) did not remain in the trial for a sufficiently long period to allow determination of treatment failure. Their baseline features were not unusual, with mean (SD) values as follows: PMD −3.3 (0.9), papilledema grade 2.9 (1.0), and visual acuity 57.6 (3.5) in the study eye. Of note, 5 of these participants did not return for any follow-up visits after baseline (lost to follow-up). Of the other 9 participants who were evaluated at month 1, 5 were lost to follow-up, 3 withdrew because of adverse events and/or the time commitment, and 1 (on placebo) was initially declared to have experienced treatment failure but was later deemed by the adjudication committee to have poor perimetric performance. None of these 9 participants had clinically significant changes at month 1.
We investigated 18 potential risk factors based on previous reports and our clinical experience, all measured at the baseline visit: age, race, sex, body mass index (BMI), papilledema grade in the study eye and in the nonstudy eye, weight change in the 6 months before entry, PMD in the study eye and in the nonstudy eye, visual acuity in the study eye and in the nonstudy eye, CSF pressure, number of episodes of transient visual obscurations (TVOs) per month, headache severity (0–10 scale), days of headache per month, days of pulsatile tinnitus per month, and days of nonpulsatile tinnitus per month.
Separate logistic regression models were used to examine the associations between each of the potential risk factors and treatment failure. Because of the small number of treatment failures, we used the Firth penalized likelihood approach8 for continuous variables and exact (conditional) logistic regression for categorical variables to estimate odds ratios (ORs) and associated confidence intervals (CIs).
We also performed exploratory classification tree analyses to predict treatment failures based on the given potential risk factors. The χ2 automatic interaction detection technique9–11 was used to construct a classification tree using both categorical and continuous risk factors. For continuous risk factors, the variable was divided into 10 categories of approximately equal size in implementing the procedure. Significance levels of 5% and 10% were used for both splitting nodes and merging categories based on the results of χ2 tests examining the association between the risk factor and the outcome of treatment failure. Each final node of the tree results in a classification of participants as experiencing either treatment failure or nonfailure and misclassification probabilities are readily estimated.
RESULTS
Overall, 147 women and 4 men were included in the analyses, and of the 7 participants who met criteria for treatment failure, 5 were women and 2 were men (table 1). Six of the 7 participants who experienced treatment failure were in the placebo group. All 7 participants who experienced treatment failure (100%) had grade III to V papilledema in the study eye, compared with 75 of 144 participants (52%) who did not experience treatment failure (table 2). Participants who experienced treatment failure had a higher median (interquartile range [IQR]) number of TVO episodes per month (90 [0–126] vs 10 [0–30]), a higher median (IQR) number of days of pulsatile tinnitus per month (2 [0–20] vs 1 [0–20]), a higher median (IQR) number of days of headache per month (30 [8–30] vs 17 [4–30]), and lower visual acuity in the study eye (mean [SD] number of correct letters 49.7 [9.7] vs 56.4 [5.3], with 55 correct letters indicating 20/20 vision) than those who did not experience treatment failure (table 1).
Table 1.
Baseline characteristics of treatment failure and nonfailure groups and results from logistic regression analyses
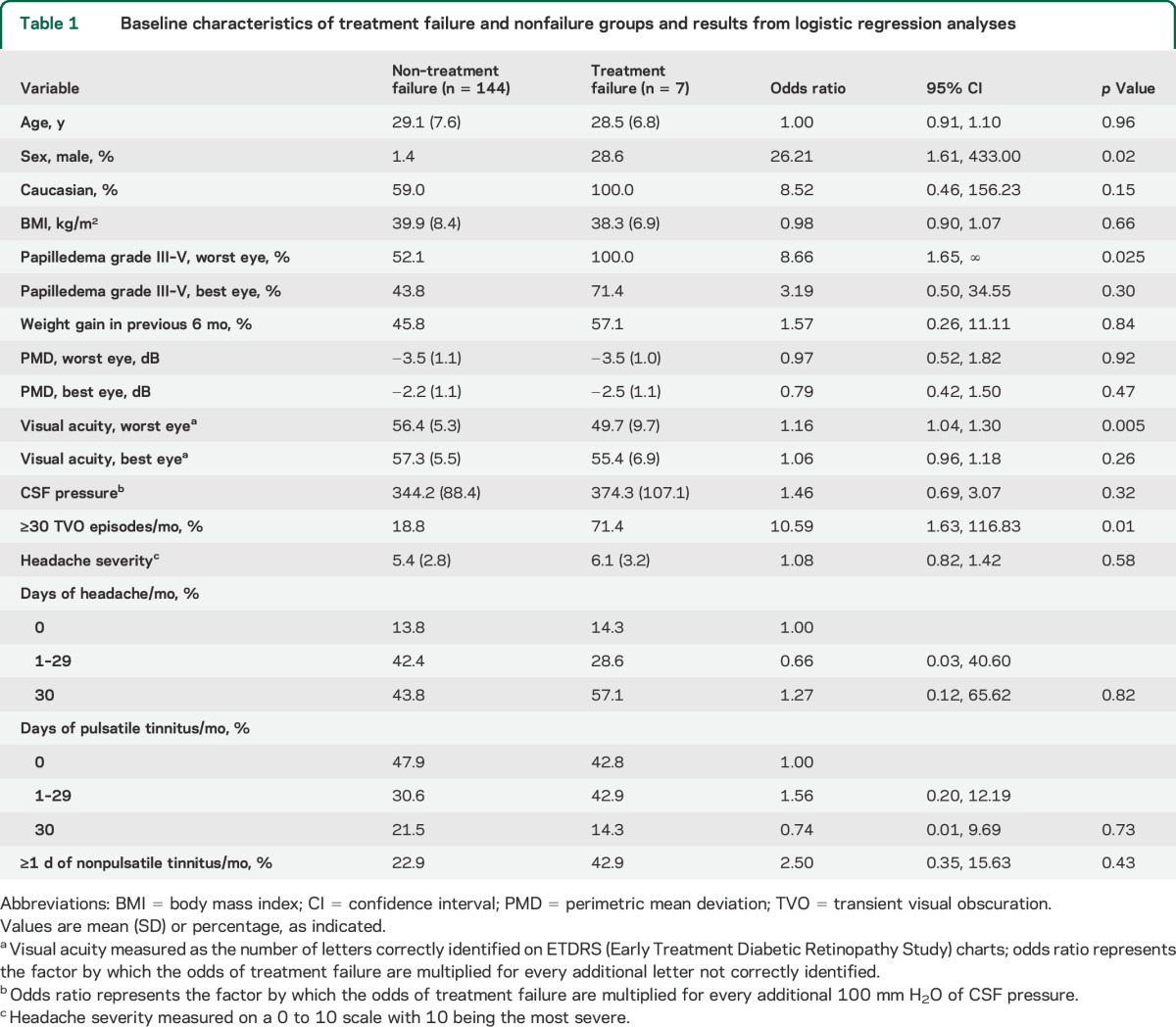
Table 2.
Frisén papilledema grades from the Photographic Reading Center comparing participants who did and did not experience treatment failure
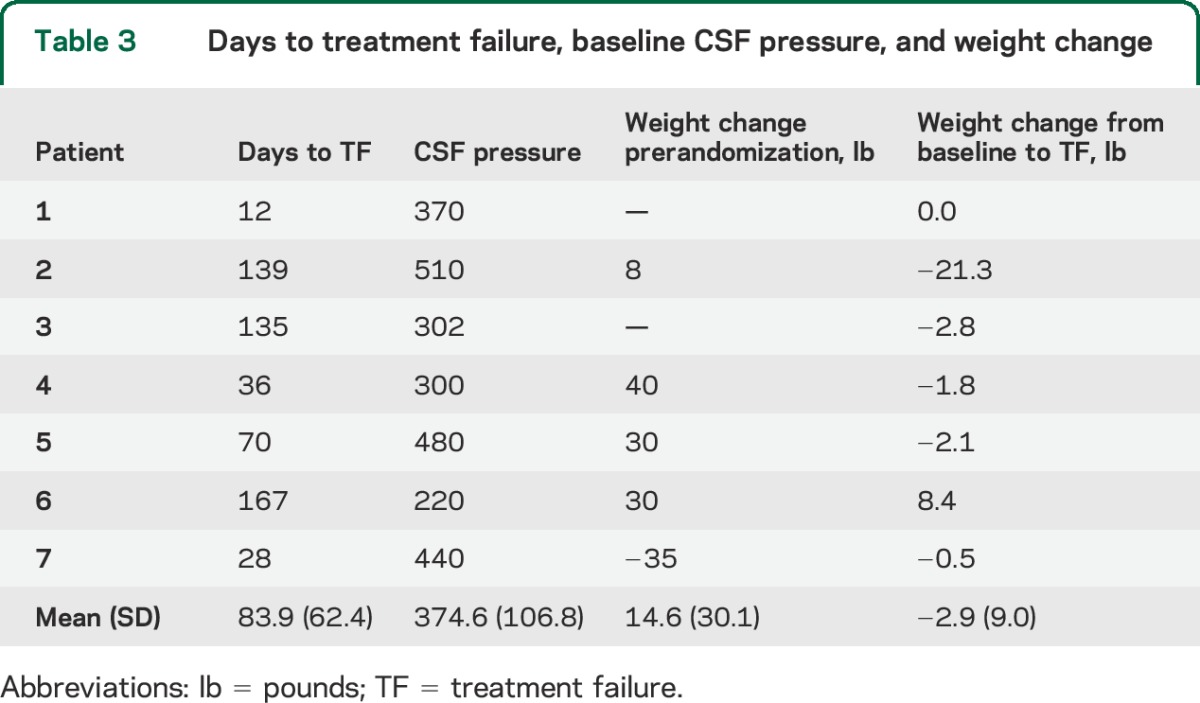
Criteria for treatment failure were met by the study eye in all cases and by both eyes in 4 cases. All 7 treatment failures occurred in Caucasians (table 1); this race group represented 92 of the 151 participants (64%) who reached the 6-month outcome. The time from study enrollment to treatment failure ranged from 12 to 167 days (table 3). The mean baseline CSF pressure of the treatment failure group was 374.6 with a range of 220–510 mm H2O (table 3). Case histories of the 7 patients can be found in the supplemental data on the Neurology® Web site at Neurology.org (case reports).
Table 3.
Days to treatment failure, baseline CSF pressure, and weight change
Individual logistic regression analyses (table 1) showed that male sex, high papilledema grade, and ETDRS chart acuity loss in the study eye at enrollment were significant risk factors for treatment failure. Male participants (OR 26.21, 95% CI 1.61–433.00; p = 0.02), patients with grade III to V papilledema (OR 8.66, 95% CI 1.65–∞; p = 0.025), patients with worse ETDRS acuity in the study eye (OR 1.16 for every letter not correctly identified; 95% CI 1.04–1.30; p = 0.005), and patients with >30 TVO episodes per month (OR 10.50, 95% CI 1.63–116.83; p = 0.010) had a higher odds of treatment failure. Other baseline factors such as BMI, age, weight gain in the 6 months before randomization, PMD, headache severity or number of headache days per month, pulse synchronous tinnitus, and CSF pressure were not significant risk factors for treatment failure.
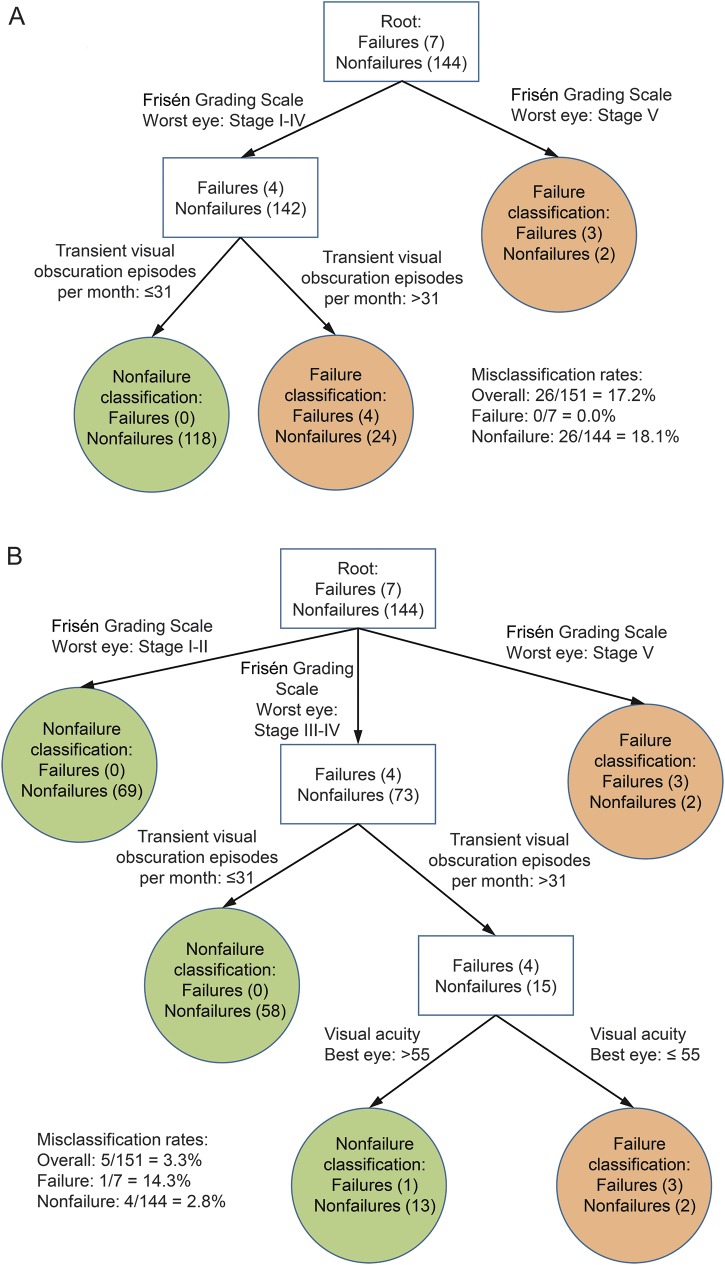
The results of the exploratory classification tree analyses are provided in the figure, A and B. Frisén papilledema grade and the number of TVO episodes were selected as classification variables for the analysis that used a more stringent significance level (5%) for identifying a new node (figure, A). When the significance level was less stringent (10%), visual acuity in the best eye was also identified as a classification variable. The tree based on a 5% significance level for splitting had the first split based on Frisén grade for the worst eye at stage V, and the second split based on the number of TVO episodes per month greater than 31 for patients with a Frisén grade of I to IV. A classification rule that classifies patients with Frisén grade for the worst eye at stage V, or patients with grade of I to IV but with >31 TVO episodes per month as experiencing treatment failure, and others as nonfailure, has an overall misclassification rate of 17.2%, a misclassification rate of 0.0% for treatment failure (i.e., 0.0% false-negatives), and a misclassification rate of 18.1% for nonfailure (i.e., 18.1% false-positives).
Figure. Classification tree analysis.
(A) Classification tree analysis with 0.05 significance level for splitting shows high-Frisén-grade papilledema and daily transient visual obscurations as risk factors for poor outcome. (B) Classification tree analysis with 0.1 significance level for splitting shows high-grade papilledema, frequent transient visual obscurations, and reduced visual acuity as risk factors for poor visual outcome.
The tree based on a 10% significance level for splitting had the first split based on Frisén grade for the worst eye at stage V, stages I–II, and stages III–IV, and the second split based on the number of TVO episodes per month >31 for patients with a Frisén grade of III–IV. The third split is based on the visual acuity in the best eye ≤55 letters correct for those with a Frisén grade of III–IV and number of TVO episodes per month >31. A classification rule that classifies patients with Frisén grade for the worst eye at stage V, or patients with grade of III–IV but with the number of TVO episodes per month >31 and visual acuity in the best eye ≤55 letters correct as experiencing treatment failure, and others as nonfailure, has an overall misclassification rate of 3.3%, a misclassification rate of 14.3% for treatment failure (i.e., 14.3% false-negatives), and a misclassification rate of 2.8% for nonfailure (i.e., 2.8% false-positives). Compared with the classification rule in the figure, A, this classification rule yields slightly fewer false-positives and slightly more false-negatives.
DISCUSSION
We found high-grade papilledema to be a significant risk factor for treatment failure in our cohort of IIHTT participants with mild visual loss. Other significant risk factors in this group included the number of TVO episodes per month and decreased visual acuity in the study eye, the eye with the most visual field loss at baseline. Participants experiencing treatment failure were also more likely to be male, although the number of male participants in our study was very small (n = 4).
While it has been shown that there is a significant relationship between papilledema grade and average amount of visual field loss, the reported relationship was relatively weak.12 Others have reported worsening vision in patients with IIH and high-grade or atrophic papilledema or peripapillary subretinal hemorrhage.13 The IIHTT participants on acetazolamide had lower risk of treatment failure (1 of 86 in the acetazolamide-plus-diet group vs 6 of 79 in the placebo-plus-diet group; p = 0.06) suggesting a protective effect of acetazolamide.2 This may be attributable to the finding in the IIHTT that acetazolamide had a large and significant effect on reduction of papilledema, with much of the effect occurring in the first month.2 Given the potential role of acetazolamide in reducing the risk of treatment failure, the identified risk factors may be more relevant for participants with mild visual loss not treated with acetazolamide.
A variety of risk factors have been suggested for poor visual outcome in IIH. Marked recent weight gain has been shown to be significantly associated with poor visual outcome in a prospective study.4 Patients with BMI more than 40 kg/m2 have been reported to be more likely to have severe papilledema and to have a trend toward more visual loss.14 Similar to our findings, others have reported that men are at higher risk of poor visual outcome.15,16
We found TVOs with an average of more than one per day to be useful in a classification tree analysis to predict treatment failure. TVOs are episodes of visual loss that usually last less than 30 seconds and are followed by restoration of baseline sensory visual function. They are reported by about two-thirds of patients with IIH.4,17 The cause of these episodes is thought to be transient ischemia of the anterior optic nerve or disc coupled with the optic nerve head being under elevated pressure.18,19 Although this symptom has been suggested as a risk factor for visual loss13 and might be used as an indication for surgical intervention,20 other authors have not found it to be associated with poor visual outcome.3,18,21–23 One study has reported a significant association between TVOs and visual loss.13 They found TVOs occurred in 88% of eyes with severe visual loss and only 50% of all eyes (p = 0.05). They also reported presence of optociliary collaterals, older age, anemia, and high myopia as other risk factors for visual loss.
Two of the risk factors for poor outcome are optic disc–related. As discussed above, TVOs are related to high optic disc tissue pressure. Their mechanism is likely brief optic disc ischemia since they are of acute onset, rapidly resolve, and often occur upon standing. In another IIHTT-related study, we found a significant association between CSF pressure and papilledema grade at baseline.24 Therefore, participants with high-grade papilledema have higher CSF pressures that may lead to high optic nerve tissue pressure and TVOs. We speculate that visual acuity is a risk factor for poor outcome since grade II papilledema or greater must be present to have the related papillomacular retinal nerve fiber bundle involved by optic disc swelling; the presence of visual acuity loss may be from its association with high-grade papilledema.
The time from the baseline visit to treatment failure ranged from 12 to 167 days with a mean of 84 days. The patient might do poorly at the time of diagnosis or much later. This finding supports frequent follow-up of recently diagnosed patients with IIH, at least until it is clear their papilledema is low grade. The main weakness of our study is the small number of treatment failures along with the restrictive entry criteria, particular regarding mild visual loss. Therefore, the results should be interpreted with caution and may not be generalizable to all patients with IIH. Also, the small number of treatment failures precluded examination of multivariable logistic regression models that adjust for confounding; for example, the increased odds of treatment failure in men may be explained, at least in part, by their increased likelihood of presentation with visual acuity loss, as has been suggested in previous studies.16 Finally, the analyses, including the classification tree analyses, require cross-validation in other cohorts of patients with IIH and mild visual loss; the stated misclassification rates, for example, are optimistic given that the classification rules were derived from the same dataset. As noted above, the results may be most relevant to patients with IIH treated with diet alone (without acetazolamide).
Risk factors for treatment failure appear to be high-grade papilledema, decreased visual acuity in the study eye, and male sex. TVOs in those with grade III or IV papilledema may be useful in identifying patients with IIH who are at risk of poor outcome. Patients with IIH who have these risk factors should be monitored closely for progressive visual loss and intensive treatment should be considered.
Supplementary Material
ACKNOWLEDGMENT
Steering Committee: Michael Wall, MD (principal investigator) (University of Iowa), James Corbett, MD (University of Mississippi Medical Center), Steven Feldon, MD, MBA (David & Ilene Flaum Eye Institute, University of Rochester School of Medicine & Dentistry), Deborah Friedman, MD (UT Southwestern Medical Center), John Keltner, MD (UC Davis Medical Center), Karl Kieburtz, MD, MPH (David & Ilene Flaum Eye Institute, University of Rochester School of Medicine & Dentistry), Mark Kupersmith, MD (network chair) (Roosevelt Hospital), Michael P. McDermott, PhD (David & Ilene Flaum Eye Institute, University of Rochester School of Medicine & Dentistry), Eleanor B. Schron, PhD, RN (project officer, National Eye Institute), David Katz, MD (Bethesda Neurology LLC), Tippi Hales (Raleigh Neurology Associates PA); Cindy Casaceli, MBA (David & Ilene Flaum Eye Institute, University of Rochester School of Medicine & Dentistry).
GLOSSARY
- BMI
body mass index
- CI
confidence interval
- ETDRS
Early Treatment Diabetic Retinopathy Study
- IIH
idiopathic intracranial hypertension
- IIHTT
Idiopathic Intracranial Hypertension Treatment Trial
- IQR
interquartile range
- OR
odds ratio
- PMD
perimetric mean deviation
- TVO
transient visual obscuration
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Rudrani Banik, Sanjay Kedhar, Flora Levin, Jonathan Feistmann, Katy Tai, Alex Yang, Karen Tobias, Melissa Rivas, Lorena Dominguez, Violete Perez, Reid Longmuir, Matthew Thurtell, Trina Eden, Randy Kardon, Robert Lesser, Yanina O’Neil, Sue Heaton, Nathalie Gintowt, Danielle Rudich, Kathleen Digre, Judith Warner, Barbara Hart, Kimberley Wegner, Bonnie Carlstrom, Susan Allman, Bradley Katz, Anne Haroldsen, Byron L. Lam, Joshua Pasol, Potyra R. Rosa, Alexis Morante, Jennifer Verriotto, David Katz, Tracy Asbury, Robert Gerwin, Mary Barnett, Steven Hamilton, Caryl Tongco, Beena Gangadharan, Eugene May, Anil Patel, Bradley Farris, R. Michael Siatkowsk, Heather Miller, Vanessa Bergman, Kammerin White, Steven O’Dell, Joseph Andrezik, Timothy Tytle, Kenneth Shindler, Joan Dupont, Rebecca Salvo, Sheri Drossner, Susan Ward, Jonathan Lo, Stephanie Engelhard, Elizabeth Windsor, Sami Khella, Madhura Tamhankar, Gregory Van Stavern, Jamie Kambarian, Renee Van Stavern, Karen Civitelli, J. Banks Shepherd, Beau B. Bruce, Valérie Biousse, Nancy J. Newman, Judy Brower, Linda Curtis, Michael Vaphiades, Karen Searcey, Lanning Kline, Roy McDonald, Syndee J. Givre, Tippi Hales, Penni Bye, Keisha Fuller, Kenneth M. Carnes, Kimberly James, Marisol Ragland, Sophia M. Chung, Dawn M. Govreau, John T. Lind, Zoe Williams, George O’Gara, Kari Steinmetz, Mare Perevich, Karen Skrine, Elisabeth Carter, Rajeev Ramchandran, Steven Katz, Marc Criden, Gina Coman, John McGregor, Andrea Inman, Prem S. Subramanian, Paul N. Hoffman, Marianne Medura, M. Michaele Hartnett, Madiha Siddiqui, Diane Brown, Ellen Arnold, Jeff Boring, Neil R. Miller, Peter Quiros, Sylvia Ramos, Margaret Padilla, Lupe Cisneros, Anne Kao, Carlos Filipe Chicani, Kevin Na, Rosa Tang, Laura Frishman, Priscilla Cajavilca, Sheree Newland, Liat Gantz, Maria Guillermo Prieto, Anastas Pass, Nicky R. Holdeman, Michael S. Lee, Helen Roemhild, Wendy Elasky, Anne Holleschau, Jody Fissgus, Jamie Walski, Andrew Harrison, Julie Falardeau, William Hills, Cristi Bryant, Donna Kim, Rebecca Armour, Lori Higginbotham, Steven A. Newman, Kristina Holbrook, Laura D. Cook, Holly Bacon, Janis Beall, Thomas Goddard, William Hall Technician, Debbie Hamilton, Alan Lyon, William Fletcher, Suresh Subramaniam, Jeannie Reimer, Jeri Nickerson, Fiona Costello, Vivian Rismondo-Stankovich, Maureen Flanagan, Allison Jensen, Patrick Sibony, Ann Marie Lavorna, Mary Mladek, Ruth Tenzler, Robert Honkanen, Jill Miller-Horn, Lauren Krupp, Joseph Rizzo, Dean Cestari, Neal Snebold, Brian Vatcher, Christine Matera, Edward Miretsky, Judith Oakley, Josyane Dumser, Tim Alperen, Sandra Baptista-Pires, Ursula Bator, Barbara Barrett, Charlene Callahan, Sarah Brett, Kamella Zimmerman, Marcia Grillo, Karen Capaccioli, M. Tariq Bhatti, LaToya Greene, Maria Cecilia Santiago-Turla, Noreen McClain, Mays El-Dairi, Martha Schatz, John E. Carter, Patrick O’Connor, Daniel Mojica, Joan Smith, Yolanda Trigo, Sherry Slayman Kellogg, Alexandra Martinez, Paul Comeau, Andres Sanchez, Nathan McCarthy, Erika Perez COT, Carlos Bazan, Charles Maitland, H. Logan Brooks, Jr, Ronda Gorsica, Brian Sherman, Joel Kramer, Larry Frohman, Ribeiro Amanda, Kathryn Boschert, Yu fei Tu, Susan Rivera, Roger Turbin, Martin ten Hove, Adriana Breen, Craig Simms, Mary Kemp, Jim Farmer, Robert Granadier, Tammy Osentoski, Kristi Cumming, Bobbie Lewis, Lori Stec, Jorge C. Kattah, John Pula, Mary Rose Buttice, Kimberly DuPage, Kimberly Cooley, Judith Beck, Lynn Bannon Technician, Cynthia Guede, Luis Mejico, Melissa Ko, Burk Jubelt, Megan Grosso, Mark Chilton, Mary Lou Watson, Jennifer Moore, Tim Martin, Cara Everhart, Joan Fish, Lori Cooke, J. Paul Dickinson, Marie D. Acierno, Rachelle Watts, Amy Thomassie, Aravinda Rao, Trisha Mary Chiasson, Janet C. Rucker, Christine Hannigan, Ilana Katz-Sand, Deepali Rajguru, Sachin Kedar, Nubia Vega, Stephanie Morris, Andrew Pearson, Mike Hanson, Betty Kovacs, Richard Weil, Xavier Pi-Sunyer Steven Feldon, William Fisher, Dorothea Castillo, Valerie Davis, Lourdes Fagan, Rachel Hollar, Tammy Keenan, Peter MacDowell, John Keltner, Kim Plumb, Laura Leming, John S. Werner, Danielle Harvey, Chris Johnson, John Keltner, John S. Werner, Kim Plumb, Laura Leming, Jan Bausch, Shan Gao, Xin Tu, Hua He, Arthur Watts, Debbie Baker, Radu Constantinescu, Karen Helles, Nichole McMullen, Bev Olsen, Larry Preston, Victoria Snively, Ann Stoutenburg, Deborah Friedman, O. Iyore Ayanru, Elizabeth-Ann Moss, Pravin Patel, Richard Mills, Maureen Maguire, William Hart, Jr, Joanne Katz, David Kaufman, Cynthia McCarthy, John Selhorst, Kathleen Digre, James Corbett, Neil R. Miller, and Richard Mills
AUTHOR CONTRIBUTIONS
Michael Wall: study design, concept and supervision, data collection and analysis, manuscript writing. Julie Falardeau: data collection and analysis, manuscript writing. William A. Fletcher: data collection and analysis, manuscript writing. Robert J. Granadier: data collection and analysis, manuscript writing. Byron L. Lam: data collection and analysis, manuscript writing. Reid A. Longmuir: data collection and analysis, manuscript writing. Anil D. Patel: data collection and analysis, manuscript writing. Beau B. Bruce: data collection and analysis, manuscript writing. Hua He: statistical analysis, manuscript writing. Michael P. McDermott: study design and concept, statistical analysis, manuscript writing.
STUDY FUNDING
This trial was funded by the National Eye Institute. This work was supported by NORDIC 1U10EY017281-01A1, DCBC 1U10EY017387-01A1, ARRA for NORDIC 3U10EY017281-01A1S1 DCBC 1U10EY017387-01A1S1, supplements for NORDIC 3U10EY017281-01A1S2 and K23 EY019341 (B.B.B.). Role of the sponsor: A representative of the sponsor (NEI), Eleanor Schron, PhD, served on the steering committee. Also, the NEI appointed a data and safety monitoring board (DSMB) to monitor participant safety and oversee study conduct and progress. DSMB members were independent academic leaders who received funding from NEI for their DSMB service. The NEI had no other role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript and decision to submit the manuscript for publication. The authors and steering committee members contributed to management, analysis, and interpretation of the data and review of the manuscript. The sites contributed to data collection and compensation was received for patient care. Others contributed to the study design, methods, conduct, and procedures; their efforts were supported by NIH U10 EY017281. Michael Wall, Hua He, and Michael McDermott had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author (Michael Wall) has obtained written permission from all persons named in the acknowledgment section.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol 1985;5:55–56. [PubMed] [Google Scholar]
- 2.Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA 2014;311:1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri: follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol 1982;39:461–474. [DOI] [PubMed] [Google Scholar]
- 4.Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain 1991;114:155–180. [PubMed] [Google Scholar]
- 5.Friedman DI, McDermott MP, Kieburtz K, et al. The idiopathic intracranial hypertension treatment trial: design considerations and methods. J Neuroophthalmol 2014;34:107–117. [DOI] [PubMed] [Google Scholar]
- 6.Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982;45:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott CJ, Kardon RH, Lee AG, et al. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography (OCT) compared to clinical expert assessment using a clinical staging scale. Arch Ophthalmol 2010;128:705–711. [DOI] [PubMed] [Google Scholar]
- 8.Firth D. Bias reduction of maximum likelihood estimates. Biometricka 1993;80:27–38. [Google Scholar]
- 9.Magidson J. The CHAID approach to segmentation modeling: chi-squared automatic interaction detection. In: Bagozzi R, editor. Advanced Methods of Marketing Research. Oxford, UK: Blackwell; 1994:118–159. [Google Scholar]
- 10.Biggs D, DeVille B, Suen E. A method of choosing multi-way partitions for classification and decision trees. J Appl Stat 1991;18:49–62. [Google Scholar]
- 11.Kass G. An exploratory technique for investigating large quantities of categorical data. Appl Stat 1980;29:119–127. [Google Scholar]
- 12.Wall M. The morphology of visual field damage in idiopathic intracranial hypertension: an anatomic region analysis. In: Mills RP, Heijl A, editors. Perimetry Update 1990/1991. Amsterdam: Kugler Publications; 1991:20–27. [Google Scholar]
- 13.Orcutt JC, Page NG, Sanders MD. Factors affecting visual loss in benign intracranial hypertension. Ophthalmology 1984;91:1303–1312. [DOI] [PubMed] [Google Scholar]
- 14.Szewka AJ, Bruce BB, Newman NJ, et al. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. J Neuroophthalmol 2013;33:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Digre KB, Corbett JJ. Pseudotumor cerebri in men. Arch Neurol 1988;45:866–872. [DOI] [PubMed] [Google Scholar]
- 16.Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology 2009;72:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wall M, Kupersmith MJ, Kieburtz KD, et al. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol 2014;71:693–701. 10.1001/jamaneurol.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogan DG. Blackouts not obviously due to carotid occlusion. Arch Ophthalmol 1961;66:180–189. [DOI] [PubMed] [Google Scholar]
- 19.Sadun A, Currie J, Lessell S. Transient visual obscurations with elevated optic discs. Ann Neurol 1984;16:489–494. [DOI] [PubMed] [Google Scholar]
- 20.Smith JL. Pseudotumor cerebri. Trans Am Acad Ophthalmol Otolaryngol 1958;62:432–440. [PubMed] [Google Scholar]
- 21.Rush JA. Pseudotumor cerebri: clinical profile and visual outcome in 63 patients. Mayo Clin Proc 1980;55:541–546. [PubMed] [Google Scholar]
- 22.Bulens C, De Vries WA, van Crevel H. Benign intracranial hypertension: a retrospective and follow-up study. J Neurol Sci 1979;40:147–157. [DOI] [PubMed] [Google Scholar]
- 23.Wall M, Hart WM, Jr, Burde RM. Visual field defects in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol 1983;96:654–669. [DOI] [PubMed] [Google Scholar]
- 24.Kattah JC, Pula JH, Mejico LJ, McDermott MP, Kupersmith MJ, Wall M. CSF pressure, papilledema grade, and response to acetazolamide in the Idiopathic Intracranial Hypertension Treatment Trial. J Neurol Epub 2015 July 10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.