Abstract
Objective:
To evaluate the relationship between early relapse recovery and onset of progressive multiple sclerosis (MS).
Methods:
We studied a population-based cohort (105 patients with relapsing-remitting MS, 86 with bout-onset progressive MS) and a clinic-based cohort (415 patients with bout-onset progressive MS), excluding patients with primary progressive MS. Bout-onset progressive MS includes patients with single-attack progressive and secondary progressive MS. “Good recovery” (as opposed to “poor recovery”) was assigned if the peak deficit of the relapse improved completely or almost completely (patient-reported and examination-confirmed outcome measured ≥6 months post relapse). Impact of initial relapse recovery and first 5-year average relapse recovery on cumulative incidence of progressive MS was studied accounting for patients yet to develop progressive MS in the population-based cohort (Kaplan-Meier analyses). Impact of initial relapse recovery on time to progressive MS onset was also studied in the clinic-based cohort with already-established progressive MS (t test).
Results:
In the population-based cohort, 153 patients (80.1%) had on average good recovery from first 5-year relapses, whereas 30 patients (15.7%) had on average poor recovery. Half of the good recoverers developed progressive MS by 30.2 years after MS onset, whereas half of the poor recoverers developed progressive MS by 8.3 years after MS onset (p = 0.001). In the clinic-based cohort, good recovery from the first relapse alone was also associated with a delay in progressive disease onset (p < 0.001). A brainstem, cerebellar, or spinal cord syndrome (p = 0.001) or a fulminant relapse (p < 0.0001) was associated with a poor recovery from the initial relapse.
Conclusions:
Patients with MS with poor recovery from early relapses will develop progressive disease course earlier than those with good recovery.
In multiple sclerosis (MS), the ability of some patients to recover faster and better than others is an important but poorly understood observation. Predictors of incomplete recovery are older patient age, location, and severity or duration of relapse.1–6 Each relapse carries a risk of irreversible myelin and axonal loss determined by the extent and duration of the injury and inherent ability to recover.7,8 Time to maximum recovery is believed to be associated with duration of pathogenic immunologic processes.6 Early remyelination is likely important in determining the extent of recovery.
Progressive disease course is characterized by insidious and irreversible accumulation of neurologic disability, with or without superimposed relapses or ongoing MRI activity.9–14 Patients with relapsing forms of MS often develop progressive MS.15 Secondary progressive MS (SPMS) follows relapsing-remitting MS (RRMS) and single-attack progressive MS (SAPMS) follows clinically isolated syndrome.15,16 Chronic demyelination, absence of remyelination, and progressive axonal loss are the pathologic hallmarks of progressive MS.17,18
Onset of progressive disease course is age-dependent rather than disease duration–dependent and is the strongest determinant of poor long-term prognosis.4,5,9,10,15,16,19–24 The initial syndrome location (brainstem, cerebellar, or spinal cord) and early relapse frequency also correlate with poor long-term prognosis.5,9,10,25,26 Incomplete recovery from individual relapses causes additional cumulative disability associated with poor long-term prognosis seemingly independent of progressive MS.26–28 We hypothesize that complete to almost-complete recovery from early relapses can potentially delay or prevent progressive MS onset.
METHODS
Standard protocol approvals, registrations, and patient consents.
We obtained written informed consent to access medical information from all patients under a protocol approved by the Mayo Clinic Institutional Review Board.
Study populations.
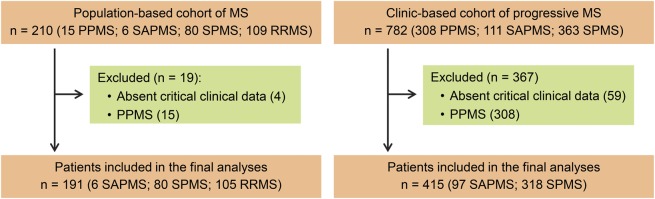
Details of our study populations have been published.14,15 We studied 2 independent populations representing population-based as well as clinic-based ascertainment of patients with MS fulfilling McDonald diagnostic criteria29,30 (figure 1). The population-based cohort was nested within the original MS cohort from Olmsted County, MN, based on availability of relapse recovery information. The original cohort was first studied in 199224 and was reascertained in 200231 and 201015 (109 RRMS, 6 SAPMS, 80 SPMS, 15 primary progressive MS [PPMS]). The population-based cohort captured the full spectrum of MS followed for more than 20 years in the same population. The clinic-based cohort was nested within the previously published clinic-based progressive MS cohort (original cohort size: 106 SAPMS, 341 SPMS, 307 PPMS) seen between 2003 and 2007 (inclusive).14,15 This cohort enriched for the less common SAPMS form of MS. Since our outcome was the onset of progressive MS following relapses, patients with PPMS were excluded from both cohorts.
Figure 1. Study design and patient populations.
MS = multiple sclerosis; PPMS = primary progressive MS; RRMS = relapsing-remitting MS; SAPMS = single-attack progressive MS; SPMS = secondary progressive MS.
Definition of relapse and recovery.
Relapse was defined as a sudden-onset CNS syndrome suggestive of inflammatory demyelination (appropriate time course of onset, stabilization, and recovery) in the absence of another explanation (e.g., fever, infection) with objective findings on examination, MRI, or evoked potentials. Symptoms occurring within a month after previous symptoms of an MS attack were considered to be part of the same episode, and pseudoexacerbations were excluded. Peak deficit of almost-complete to complete loss of function in a system was considered a fulminant relapse. This could have been patient reported and confirmed by later examinations (if the patient did not recover) or documented by a physician at the time of the relapse.
Recovery was defined as the maximum improvement from the peak deficit associated with the relapse. Amount of relapse recovery was assessed based on the change between the peak deficit and the residual deficit at least 6 months after the relapse (in many cases years, given the availability of data). We chose 6 months as the minimum period of observation because earlier studies and clinical experience suggest that most, if not all, recovery happens within 3 months and rarely beyond 6 months.32 Deficit was defined as the patient-reported and/or examination-confirmed amount of neurologic impact of the individual relapse. Whereas this definition of a deficit reflects the real-world clinical practice, it also introduced physicians' judgment and patient recall into the definition of amount of recovery.
Good recovery was defined as complete to almost-complete recovery. Irrefutable good recovery therefore includes the following types of assessments: (1) complete recovery with no subjective (historical) or objective (examination) deficit, (2) almost-complete recovery without objective (examination) deficit but with subjective deficit per patient (e.g., ongoing paresthesia), and (3) almost-complete recovery with minimal objective (examination) deficit compared to original examination. Some borderline patients therefore could have been classified as poor recoverers when with prospective data collection they might have been classified as good recoverers. However, the reverse was unlikely. Some patients lacked sufficient data to make a judgment and therefore were unclassifiable (table 1).
Table 1.
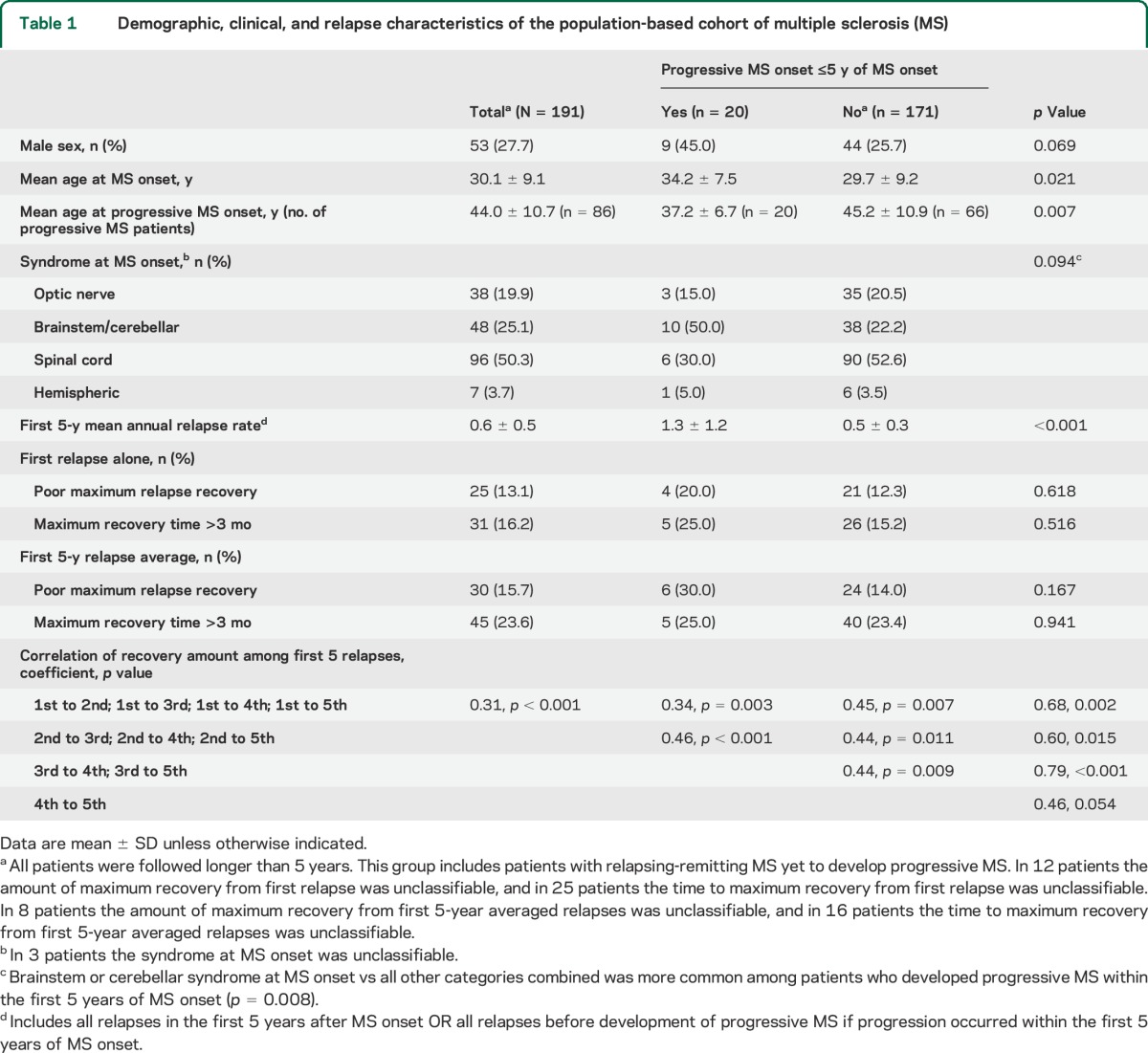
Demographic, clinical, and relapse characteristics of the population-based cohort of multiple sclerosis (MS)
Application of this relapse-impact model on Functional System Scores (FSS) from the Kurtzke scale33 would be like in figure 2. We assumed that patients with minimal peak deficits would be more likely to return to baseline than patients with severe relapses. Accordingly, patients with minimal peak deficit in the first place (FSS 1) or patients with marked improvement in FSS compared to peak deficit were classified as good recoverers. Although the subjective nature of some symptoms (paresthesia) and patient-reported peak deficit in some cases prevented us from exactly applying the model in figure 2, we still used it as a guideline.
Figure 2. Application of the relapse-impact model on Functional System Scores from the Kurtzke scale.
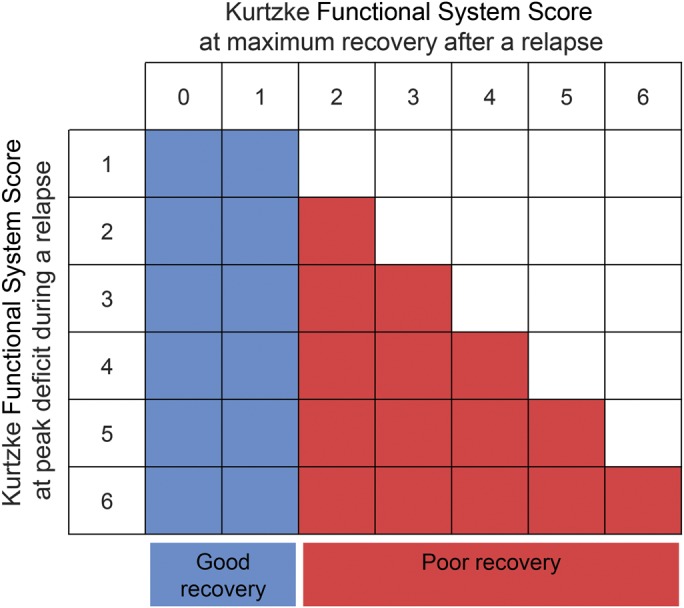
Patients with minimal baseline deficit (Functional System Score [FSS] 1) or marked improvement in FSS compared to peak deficit were classified as good recoverers. Others were classified as poor recoverers.
Definition of progressive disease course.
Progressive disease course is defined as an insidious and irreversible worsening of CNS syndrome most commonly characterized by progressive weakness, ataxia, or bladder dysfunction lasting for ≥1 year11 and unrelated to relapse-related worsening.15 Disability progression, a confusing term used in some studies, results from progressive disease course leading to insidious worsening, insufficient recovery from relapses leading to stepwise worsening, or both.13 In our study, we focused on the onset of a progressive disease course as our outcome measure and not on disability worsening. Patients with SAPMS and SPMS were analyzed separately and were also grouped together as patients with bout-onset progressive MS. Typical of a standard clinical practice setting, the most rigorous historical documentation and clinical examination closest in time to progressive MS onset was used to establish the date of progressive MS onset.14,15
Study variables.
We extracted the following data: sex, age at MS onset, syndrome at MS onset, whether a relapse was fulminant, number of relapses occurring within the first 5 years of MS onset or until last follow-up or until progressive MS onset (whichever came first), amount of recovery and time to maximum recovery from all individual relapses within the first 5 years, disease-modifying drug (DMD) use duration (as percent duration of the time to progressive MS onset), number of DMDs used until progressive MS onset (as a secondary measure of disease activity level), and age at progressive MS onset. In each cohort, data extracted from the medical records were validated independently by 2 MS specialists, and any discrepancies were arbitrated by the principal investigator. The final analyses were conducted by statisticians and interpreted by a neurologist who was not involved in the original data extraction.
Data analyses in the population-based cohort.
The first 5-year average relapse recovery and the recovery from the first relapse alone were studied. Cumulative rate of developing progressive MS (accounting for censoring) was summarized using Kaplan-Meier curves beginning at 5 years from MS onset for first 5-year average relapse recovery and beginning at 3 months from MS onset for the initial relapse recovery alone. Patients who would have already progressed within the first 5 years therefore were left out of the Kaplan-Meier analyses for the first 5-year average relapse recovery. The log-rank test was used to compare those with poor vs good relapse recovery. To capture the impact of this early progression group, we compared the patient and recovery characteristics between those who developed progressive MS within the first 5 years and those who did not (table 1). Continuous data are shown as means with SD and categorical data are reported as percent frequencies. We used the t test for continuous data and χ2 analysis for categorical data. Logistic regression models took into account multiple relapses observed per patient to explore the relationship of relapse recovery with relapse age.
Data analyses in the clinic-based cohort.
The clinic-based cohort consisted of patients with established progressive MS only. Therefore, time from MS onset to progressive MS onset and other relapse characteristics were compared for good vs poor recovery from the initial relapse with means and percentages. We used the t test for continuous data and χ2 analysis (or Fisher exact test when appropriate) for categorical data. A multivariate logistic regression model was generated and included interactions between initial relapse recovery, other relapse characteristics, progressive MS subtype, DMD use duration, number of DMD switches, and time from MS onset to progressive MS onset. All probability tests were 2-tailed and p values <0.05 were considered statistically significant.
RESULTS
Figure 1 shows the number of patients included in our nested study. All patients with PPMS (n = 322) and patients with incomplete data (n = 63) were excluded. The final analyses included 191 patients (105 RRMS, 6 SAPMS, 80 SPMS) in the population-based cohort and 415 patients (97 SAPMS, 318 SPMS) in the clinic-based cohort.
Population-based cohort.
Patients who developed progressive MS within the first 5 years of MS onset (compared to those who did not) were commonly men, were older at MS onset, were more likely to have a brainstem or cerebellar syndrome at MS onset, and were younger at progressive MS onset (table 1). Annual relapse rate before progressive MS onset was higher in patients who progressed within the first 5 years than in those who did not progress within this period (table 1). Relapse recovery metrics did not differ between the 2 groups.
Overall, 438 (191 1st relapses, 119 2nd relapses, 76 3rd relapses, 34 4th relapses and 18 5th relapses) of 453 relapses could be classified for relapse recovery. The relapse recovery amount correlated between each relapse and the following relapses (correlation coefficient range 0.31–0.79, table 1), suggesting that good recoverers tended to remain good recoverers (and vice versa).
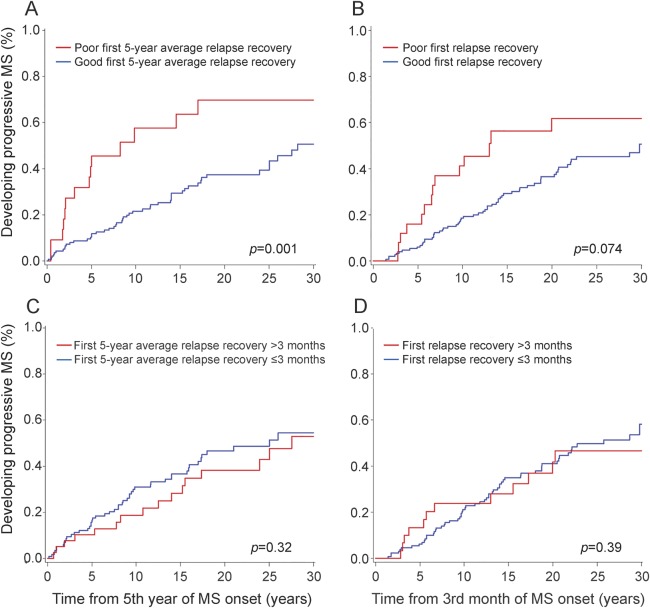
In the first 5-year averaged relapse recovery model, patients with good recovery reached progressive MS later (50% by 30.2 years) than those with poor recovery (50% by 8.3 years) (p = 0.001) (figure 3A). Time to maximum recovery did not have an impact (figure 3C). Initial relapse recovery alone had a similar but weaker impact on time to progressive MS onset than the first 5-year averaged relapse recovery (figure 3, B and D).
Figure 3. Kaplan-Meier analyses of time from MS onset to progressive MS onset.
Patients with good recovery from early relapses reached progressive multiple sclerosis (MS) >20 years later than poor recoverers (A). Time to maximum recovery from early relapses did not have an impact on time from MS onset to progressive MS onset (C). (B, D) Results of recovery from the first relapse alone.
Clinic-based cohort.
Mean time from MS onset to progressive MS onset was delayed by almost 5 years in patients with good recovery from the first relapse compared with patients with poor recovery. This effect was driven mainly by the SAPMS group (table 2).
Table 2.
Relapse characteristics, recovery from averaged early relapses, and time to progressive MS onset in the clinic-based cohort of patients with progressive MS
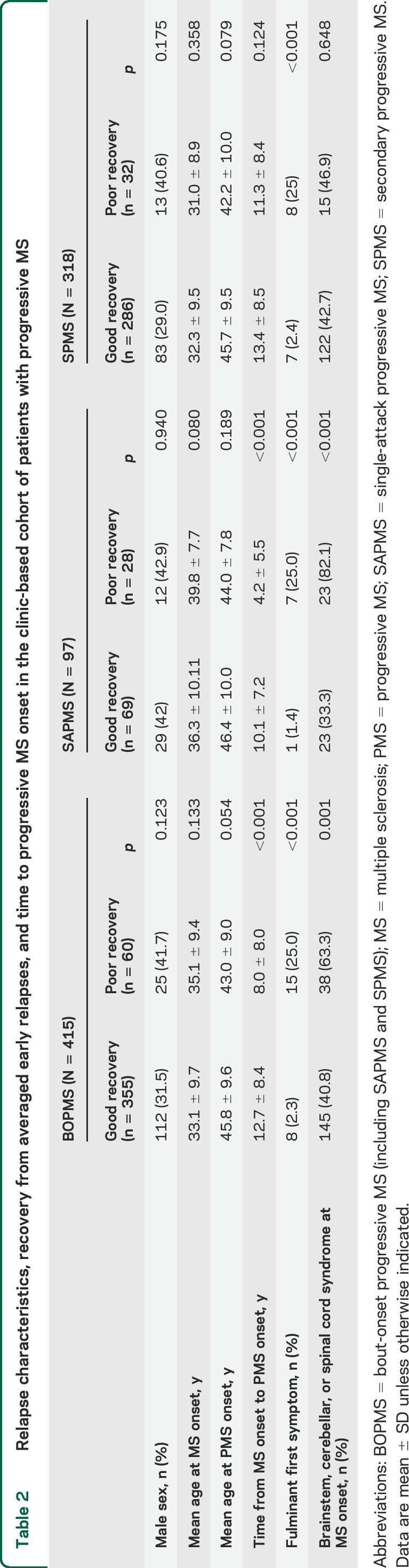
Age at MS onset was older in SAPMS patients with poor recovery from the initial relapse than in SAPMS patients with good recovery, SPMS patients with good recovery, or SPMS patients with poor recovery (table 2).
Overall, fulminant initial relapse was the strongest determinant of poor recovery in all patient groups, while the poor prognostic syndromes of brainstem, cerebellar, or spinal cord involvement at MS onset correlated with poor recovery from initial relapse, especially in the SAPMS group (table 2). The multivariate analyses did not contribute any additional insight (data not shown).
DISCUSSION
We showed that poor recovery from early relapses led to considerably earlier onset of progressive MS than good recovery from early relapses in a population-based cohort accounting for patients yet to develop progressive MS. We confirmed this finding in an independent clinic-based cohort including only patients with progressive MS, strengthening the conclusion of our study.
Prior studies have documented that early relapses contribute to development of long-term disability, but none of them explicitly studied the impact of early relapse recovery on the onset of progressive disease course.2,3,9,10,19,26–28,34–40,e1,e2 The London Ontario group studied the predictive effect of relapse characteristics and the predictive effect of latency to progression on time to attain high disability levels (Expanded Disability Status Scale scores 6, 8, and 10), with variable outcomes.e1 In that study, relapse recovery was mentioned but no data were provided; however, age at relapses seemed to play an important role in disability attainment.
The chance of complete recovery from a relapse drops by approximately 1% per annum after the index event.e3 Animal model studies of CNS demyelination also demonstrated that capacity for remyelination declines with age as a result of poor progenitor oligodendrocyte recruitment and differentiation.e4–e6 In our study, patients who developed progressive MS within the first 5 years of MS onset had remarkably worse early relapse recovery, and they were 5 years older at the time of MS onset and 8 years younger at the time of progressive MS onset than those who did not progress within the same period. Our results together with previous studies suggest an inherent inverse relationship between age and relapse recovery.
The detrimental effect of poor relapse recovery was more obvious when averaged from all relapses within the first 5 years of MS onset than when considering only the initial relapse alone. Furthermore, poor recovery from each relapse correlated with poor recovery from the following relapses within the first 5 years, strongly suggesting that relapse recovery is an intrinsically determined process.
In accordance with previous studies,10,e2 we confirmed that a high frequency of early relapses leads to a high risk of developing early progressive MS. Although multiple early relapses portend a worse prognosis than a single relapse, we found that even a single relapse associated with poor recovery may lead to rapid onset of progressive MS. We were able to test this hypothesis because we had access to a large number of patients with SAPMS in whom only a single relapse could be identified before the onset of progressive MS.
While brainstem-cerebellar syndrome at MS onset is a known poor long-term prognostic indicator,8 we also showed that a brainstem-cerebellar syndrome at MS onset predicts a rapid (within 5 years) conversion to progressive MS. Fulminance of a relapse, which was the strongest predictor of recovery in our study, seems to interact with location of neurologic involvement to precondition the patient early for the limited remyelination and later progressive axonal degeneration observed in pathology studies.8,17,18,e7–e9
Our conclusions are based on our definition of poor vs good recovery. In our study, classification was done using a combination of objective (examination confirmed) and subjective (patient recall) assessments, which introduces a notable limitation: we were not able to get absolute quantification for certain functional system improvements. We followed the model identified in figure 2 for judging recovery. However, as in real-world clinical practice, physicians' judgment and patient recall were at times inherent in the definition of a fulminant relapse or recovery from a relapse.
To overcome the potential recall bias, we applied a 2-tiered approach from 2 separate populations: one rather small in size but population-based and largely prospectively assessed and the other clinic-based and enriched for progressive MS but largely retrospectively assessed. Similar findings regarding the first relapse's impact on recovery as well as onset of progressive MS (extensively discussed in a previous publication)15 in these 2 distinct populations suggest that patient recall and follow-up bias were not strong factors in our study. Our study therefore forms a model and can help power calculations for future prospective studies recruiting from clinic-based populations, especially in remyelination-repair trials using progressive MS onset as an outcome measure.
An additional bias could have been the impact of age at MS onset on time to progressive MS onset. In the multivariable model that we were able to generate in the larger clinic-based cohort, age at MS onset was not an independent determinant of progressive MS onset.
There are several additional weaknesses inherent in our study design that can potentially be overcome in prospective studies. Contribution of back-to-back relapses and subclinical relapses to later examination findings (e.g., silent optic neuritis with color plate changes not evident on the first examination) could have affected assigning recovery retrospectively. The amount of peak deficit determines the amount of recovery, so results could be biased toward good recovery in cases of minimal peak deficits and toward poor recovery in cases of fulminant relapse; thus, good recovery may imply lesser severity of attack, better recovery, or both.
We generally follow a standard protocol of high-dose IV methylprednisolone followed by plasma exchange when indicated in our patients, as published elsewhere.e10 However, our clinic-based referral cohort would have had most of their early treatments elsewhere. Differences do exist among neurologists and centers regarding early treatment of relapses as well as use of disease-modifying drugs (DMDs), which could not be controlled prospectively in a study of this type. We therefore initially did not include the effect of medications as a confounding variable. However, the similarity of findings between the population-based and clinic-based cohorts suggested absence of systematic impact of different treatment practices. Therefore, following our initial conclusion, we ran the models with inclusion of both the number of DMD switches and the duration of total DMD exposure as confounders. We found no independent effect of DMD use on our results, but in the absence of a better controlled trial, this finding must be interpreted with caution. Also, patients from our original population-based cohort and the majority of patients from the clinic-based cohort had their first attacks before the era of DMDs. Therefore, an impact of early aggressive DMD use on outcome was not possible to study.
In clinical practice, physicians start prognostic inferences after the first relapse. The considerable delay in developing progressive MS in patients with good recovery has the following treatment implications that need to be studied prospectively: (1) an early and aggressive recovery strategy such as plasma exchange could be adopted in patients with a fulminant relapse or a history of poor recovery from relapses; (2) in patients with one or more relapses annually, a rapid escalation to stronger second-line DMDs is already being practiced, and a similar strategy can be considered in patients with poor recovery from relapses, especially those that involve brainstem, cerebellum, or spinal cord, without waiting to establish the high frequency of relapses; and (3) future remyelination and axonal repair strategies will likely work the best if applied for early relapses to delay or ultimately prevent later progressive disease course development.
Supplementary Material
GLOSSARY
- DMD
disease-modifying drug
- FSS
Functional System Scores
- MS
multiple sclerosis
- PPMS
primary progressive MS
- RRMS
relapsing-remitting MS
- SAPMS
single-attack progressive MS
- SPMS
secondary progressive MS
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M. Novotna contributed to study design; extracted, analysed, and interpreted data; and wrote the manuscript. M.M. Paz Soldán contributed to study design and participated in data extraction, interpretation, and manuscript revision. N. Abou Zeid, N. Kale, and M. Tutuncu extracted data and participated in data interpretation and manuscript revision. D.J. Crusan and E.J. Atkinson completed statistical analysis. A. Siva, B.M. Keegan, I. Pirko, S.J. Pittock, C.F. Lucchinetti, J.H. Noseworthy, B.G. Weinshenker, and M. Rodriguez evaluated patients and participated in manuscript revision. O.H. Kantarci conceived of and designed the study, evaluated patients, analyzed and interpreted data, and participated in manuscript preparation.
STUDY FUNDING
Supported by the Mayo Clinic Department of Neurology, the European Regional Development Fund (FNUSA-ICRC), and a pilot grant from the National Multiple Sclerosis Society.
DISCLOSURE
M. Novotna receives support from the European Regional Development Fund—Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123), European Social Fund, and the State Budget of the Czech Republic. M.M. Paz Soldán, N. Abou Zeid, N. Kale, M. Tutuncu, D.J. Crusan, E.J. Atkinson, and A. Siva report no disclosures relevant to the manuscript. B.M. Keegan has served as a consultant to Novartis, Bionest, and Bristol Meyers Squibb and receives research support from Terumo BCT. I. Pirko is deceased. S.J. Pittock is a named inventor on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; receives research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation, and National Institute of Neurological Disorders and Stroke (NS065829); and has provided consultation to Alexion Pharmaceuticals, Medimmune, and Chugai Pharma USA but has received no personal fees or personal compensation for these consulting activities (all compensation for consulting activities paid directly to Mayo Clinic). C.F. Lucchinetti receives research support from National Institute of Neurological Disorders and Stroke (R01 NS049577 and 2P50NS038667), the Department of Defense (W81XWH-13-1-0098), the Guthy-Jackson Charitable Foundation, and Koltan Pharmaceuticals. J.H. Noseworthy reports no disclosures relevant to the manuscript. B.G. Weinshenker serves on data safety monitoring boards for Novartis, Biogen Idec, and Mitsubishi Pharmaceuticals and on an adjudication panel for Medimmune Pharmaceuticals; serves on the editorial boards of Neurology, Canadian Journal of Neurological Sciences, and the Turkish Journal of Neurology; receives license royalties from RSR Ltd. and University of Oxford for the detection of AQP4 antibodies as a diagnostic aid for neuromyelitis optica; has received consulting fees from Asahi Kasei Medical Company, GlaxoSmithKline, Ono Pharmaceuticals, CHORD Pharmaceuticals, Elan, and Novartis Pharmaceuticals; and receives research support from the Guthy-Jackson Charitable Foundation. M. Rodriguez receives research support from NIH (R01 GM092993, R01 NS024180, R01 NS032129, R01 NS048357, and R21 NS073684), NIH CTSA (UL1 RR024150 and UL1 TR000135), the National Multiple Sclerosis Society (CA 1060A11), Novartis Pharmaceuticals (CFTY720DUSNC18T), the Minnesota Partnership Award for Biotechnology and Medical Genomics, the European Regional Development Fund (FNUSA-ICRC CZ.1.05/1.1.00/02.0123), and the Applebaum, Hilton, Peterson, and McNeilus Foundations. O.H. Kantarci receives research support from the European Regional Development Fund (FNUSA-ICRC CZ.1.05/1.1.00/02.0123) and the National Multiple Sclerosis Society and has given scientific presentations at meetings supported by Teva and Novartis Pharmaceuticals but has received no personal fees or personal compensation for this activity (all compensation for consulting activities paid directly to Mayo Clinic) and has not spoken about the specific medications involving these companies. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Leone MA, Bonissoni S, Collimedaglia L, et al. Factors predicting incomplete recovery from relapses in multiple sclerosis: a prospective study. Mult Scler 2008;14:485–493. [DOI] [PubMed] [Google Scholar]
- 2.Vercellino M, Romagnolo A, Mattioda A, et al. Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand 2009;119:126–130. [DOI] [PubMed] [Google Scholar]
- 3.Mowry EM, Pesic M, Grimes B, Deen S, Bacchetti P, Waubant E. Demyelinating events in early multiple sclerosis have inherent severity and recovery. Neurology 2009;72:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cossburn M, Ingram G, Hirst C, Ben-Shlomo Y, Pickersgill TP, Robertson NP. Age at onset as a determinant of presenting phenotype and initial relapse recovery in multiple sclerosis. Mult Scler 2012;18:45–54. [DOI] [PubMed] [Google Scholar]
- 5.Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 1993;116:117–134. [DOI] [PubMed] [Google Scholar]
- 6.Fay AJ, Mowry EM, Strober J, Waubant E. Relapse severity and recovery in early pediatric multiple sclerosis. Mult Scler 2012;18:1008–1012. [DOI] [PubMed] [Google Scholar]
- 7.Traboulsee A. MRI relapses have significant pathologic and clinical implications in multiple sclerosis. J Neurol Sci 2007;256:S19–S22. [DOI] [PubMed] [Google Scholar]
- 8.De Stefano N, Narayanan S, Francis GS, et al. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol 2001;58:65–70. [DOI] [PubMed] [Google Scholar]
- 9.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989;112:133–146. [DOI] [PubMed] [Google Scholar]
- 10.Kantarci O, Siva A, Eraksoy M, et al. Survival and predictors of disability in Turkish MS patients. Turkish Multiple Sclerosis Study Group (TUMSSG). Neurology 1998;51:765–772. [DOI] [PubMed] [Google Scholar]
- 11.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907–911. [DOI] [PubMed] [Google Scholar]
- 12.Young PJ, Lederer C, Eder K, et al. Relapses and subsequent worsening of disability in relapsing-remitting multiple sclerosis. Neurology 2006;67:804–808. [DOI] [PubMed] [Google Scholar]
- 13.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paz Soldan MM, Novotna M, Abou Zeid N, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology 2015;84:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler 2013;19:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremenchutzky M, Cottrell D, Rice G, et al. The natural history of multiple sclerosis: a geographically based study. 7. Progressive-relapsing and relapsing-progressive multiple sclerosis: a re-evaluation. Brain 1999;122:1941–1950. [DOI] [PubMed] [Google Scholar]
- 17.Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol 1996;6:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285. [DOI] [PubMed] [Google Scholar]
- 19.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003;126:770–782. [DOI] [PubMed] [Google Scholar]
- 20.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain 2006;129:595–605. [DOI] [PubMed] [Google Scholar]
- 21.Koch M, Mostert J, Heersema D, De Keyser J. Progression in multiple sclerosis: further evidence of an age dependent process. J Neurol Sci 2007;255:35–41. [DOI] [PubMed] [Google Scholar]
- 22.Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology 2011;77:1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Confavreux C, Aimard G, Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain 1980;103:281–300. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez M, Siva A, Ward J, Stolp-Smith K, O'Brien P, Kurland L. Impairment, disability, and handicap in multiple sclerosis: a population-based study in Olmsted County, Minnesota. Neurology 1994;44:28–33. [DOI] [PubMed] [Google Scholar]
- 25.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–1438. [DOI] [PubMed] [Google Scholar]
- 26.Binquet C, Quantin C, Le Teuff G, Pagliano JF, Abrahamowicz M, Moreau T. The prognostic value of initial relapses on the evolution of disability in patients with relapsing-remitting multiple sclerosis. Neuroepidemiology 2006;27:45–54. [DOI] [PubMed] [Google Scholar]
- 27.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003;61:1528–1532. [DOI] [PubMed] [Google Scholar]
- 28.Scott TF, Schramke CJ. Poor recovery after the first two attacks of multiple sclerosis is associated with poor outcome five years later. J Neurol Sci 2010;292:52–56. [DOI] [PubMed] [Google Scholar]
- 29.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 30.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 31.Pittock SJ, Mayr WT, McClelland RL, et al. Change in MS-related disability in a population-based cohort: a 10-year follow-up study. Neurology 2004;62:51–59. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer T. The natural history of relapses in multiple sclerosis. J Neurol Sci 2007;256(suppl 1):S5–S13. [DOI] [PubMed] [Google Scholar]
- 33.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 34.Tremlett H, Yousefi M, Devonshire V, Rieckmann P, Zhao Y. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology 2009;73:1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennetto L, Burrow J, Sakai H, Cobby J, Robertson NP, Scolding N. The relationship between relapse, impairment and disability in multiple sclerosis. Mult Scler 2011;17:1218–1224. [DOI] [PubMed] [Google Scholar]
- 36.Healy BC, Degano IR, Schreck A, et al. The impact of a recent relapse on patient-reported outcomes in subjects with multiple sclerosis. Qual Life Res 2012;21:1677–1684. [DOI] [PubMed] [Google Scholar]
- 37.Hirst C, Ingram G, Pearson O, Pickersgill T, Scolding N, Robertson N. Contribution of relapses to disability in multiple sclerosis. J Neurol 2008;255:280–287. [DOI] [PubMed] [Google Scholar]
- 38.Menard DA. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2004;63:599; author reply. [DOI] [PubMed] [Google Scholar]
- 39.Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disability. Brain 2010;133:1914–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bethoux F, Miller DM, Kinkel RP. Recovery following acute exacerbations of multiple sclerosis: from impairment to quality of life. Mult Scler 2001;7:137–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.