Abstract
Objective:
To evaluate whether time to treatment modifies the effect of endovascular reperfusion in stroke patients with evidence of salvageable tissue on MRI.
Methods:
Patients from the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) cohort study with a perfusion-diffusion target mismatch were included. Reperfusion was defined as a decrease in the perfusion lesion volume of at least 50% between baseline and early follow-up. Good functional outcome was defined as a modified Rankin Scale score ≤2 at day 90. Lesion growth was defined as the difference between the baseline and the early follow-up diffusion-weighted imaging lesion volumes.
Results:
Among 78 patients with the target mismatch profile (mean age 66 ± 16 years, 54% women), reperfusion was associated with increased odds of good functional outcome (adjusted odds ratio 3.7, 95% confidence interval 1.2–12, p = 0.03) and attenuation of lesion growth (p = 0.02). Time to treatment did not modify these effects (p value for the time × reperfusion interaction is 0.6 for good functional outcome and 0.3 for lesion growth). Similarly, in the subgroup of patients with reperfusion (n = 46), time to treatment was not associated with good functional outcome (p = 0.2).
Conclusion:
The association between endovascular reperfusion and improved functional and radiologic outcomes is not time-dependent in patients with a perfusion-diffusion mismatch. Proof that patients with mismatch benefit from endovascular therapy in the late time window should come from a randomized placebo-controlled trial.
Recent endovascular trials have demonstrated benefit from endovascular therapy for patients treated within 6 hours after symptom onset.1–4 Whether patients treated outside of this time window also benefit from endovascular therapy remains unknown. Some studies suggest a lack of benefit from endovascular reperfusion beyond 7 hours after symptom onset.5,6 However, this time threshold is likely not uniformly valid7 because the duration of the therapeutic time window depends on the degree to which cerebral blood flow is reduced in an individual patient.8–10 Patients with good collaterals can have substantial volumes of salvageable tissue for a relatively long time and may remain good candidates for endovascular treatment even beyond 12 hours after symptom onset.11,12 Patient selection in the delayed time window (>6 hours after symptom onset) will therefore have to rely on an assessment of salvageable brain tissue. The best-studied biomarker of salvageable brain tissue is the volumetric mismatch between the magnetic resonance perfusion (MRP) lesion, segmented using a validated threshold, and the diffusion-weighted imaging (DWI) lesion.13
The MRP-DWI mismatch hypothesis posits that patients with mismatch benefit from reperfusion regardless of the time at which reperfusion occurs.14–16 In this study, we test this hypothesis and investigate whether the association between reperfusion and good clinical outcome depends on the duration between symptom onset and endovascular therapy in patients with an MRP-DWI mismatch.
METHODS
Study design and participants.
The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) study was a prospective multicenter cohort study of patients treated with endovascular reperfusion therapy up to 12 hours after symptom onset.16 The study enrolled participants between 2008 and 2011. Details of the study protocol, including patient selection criteria, MRI scanning parameters, MRI postprocessing techniques, and the definition of reperfusion, have been reported previously.16–18
This subanalysis of the DEFUSE 2 study focused primarily on patients with the target mismatch. Target mismatch is defined as per DEFUSE 2 criteria.16 Early lesion growth was defined as the difference between the DWI lesion volume at baseline and the DWI lesion volume at early follow-up. Good functional outcome was defined as a 90-day modified Rankin Scale (mRS) score ≤2.
Standard protocol approvals, registrations, and patient consents.
The study complied with the Declaration of Helsinki, the ethics committee at each participating site approved the research protocol, and written informed consent was obtained from all participants or their surrogates.
Statistical analysis.
Univariate associations between categorical variables were investigated using χ2 or Fisher exact tests. Continuous and ordinal variables were compared using the Student t test and Mann-Whitney U test. All tests were 2-tailed and considered significant at α <0.05. The effects of reperfusion and time to endovascular treatment (defined by sheath insertion) on clinical and radiologic outcomes were assessed with multivariable regression analyses. First, using logistic regression, we assessed the effects of reperfusion and time to endovascular treatment on good functional outcome. Using data from all included patients, we built a model with good functional outcome as the dependent variable and with the following independent variables: time to endovascular treatment, reperfusion, and the interaction between these 2 variables. Second, we assessed the effect of time to endovascular treatment on the probability of good functional outcome in the subset of patients with reperfusion. Third, we investigated the effects of time to endovascular treatment, reperfusion, and their interaction on early lesion growth with generalized linear regression analysis. Adjusted effect sizes were calculated by including age and baseline DWI lesion volume as covariates in the models. All analyses were conducted in IBM SPSS 22.0 and SAS 9.4.
RESULTS
Of the 138 patients who consented to participate in the DEFUSE 2 study, the 78 patients who had the target mismatch profile on baseline imaging and underwent endovascular treatment were included in the primary analyses of this study. Baseline characteristics, listed in the table, were well-matched between the early and late treatment groups with the exception of “IV tissue plasminogen activator (tPA) treatment,” which was more frequent in the early than in the late endovascular treatment group (66% vs 28%, p = 0.01). Patients treated early had their baseline MRI a median of 2.8 hours (interquartile range [IQR] 2.1–4.2) after symptom onset, started endovascular treatment 4.8 hours (IQR 3.4–5.4) after symptom onset, and ended the procedure (defined as time of removal of the femoral sheath) 6.5 hours (IQR 5.7–7.2) after symptom onset. Patients in the late treatment group had their baseline MRI a median of 6.2 hours (IQR 5.0–8.2) after symptom onset, started endovascular treatment 7.9 hours (IQR 6.5–9.4) after symptom onset, and ended the procedure 9.3 hours (IQR 8.4–11.0) after symptom onset. The rate of reperfusion was 61% (22 of 36) in the early treatment group and 57% (24 of 42) in the late treatment group (p = 0.72).
Table.
Baseline characteristics and treatment characteristics
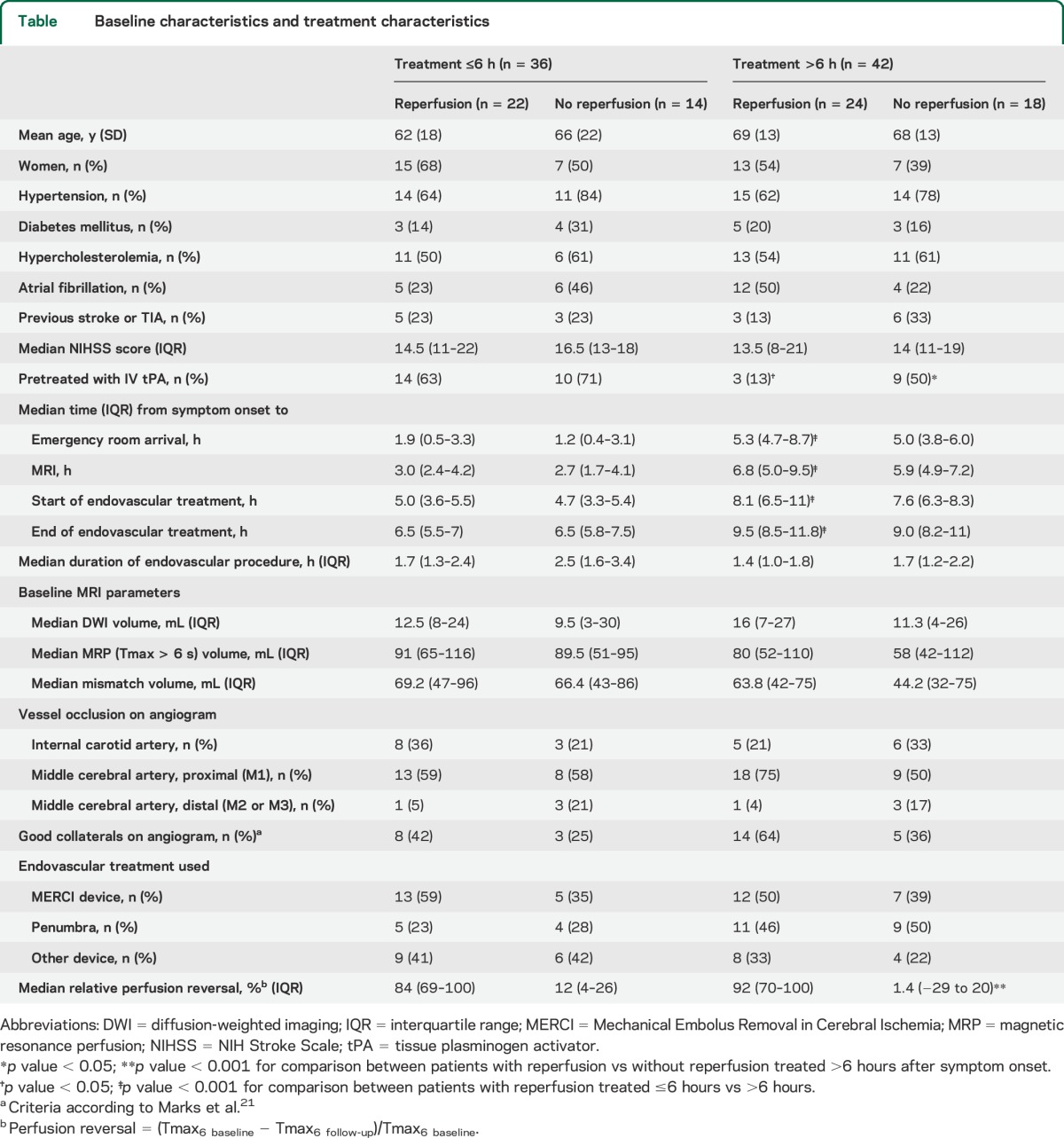
Three predictive models were constructed to assess whether time to treatment alters the association between reperfusion and clinical or radiographic outcomes. First, we constructed models with time to treatment, reperfusion, and the interaction between time to treatment and reperfusion as independent variables. Among target mismatch patients (n = 78), reperfusion was associated with good functional outcome (odds ratio [OR] adjusted for age, baseline DWI lesion volume, and time to treatment 3.7, 95% confidence interval [CI] 1.2–12, p = 0.03), but time to treatment (p = 0.13) and the interaction between time to treatment and reperfusion were not (p = 0.57) (figure 1). Similarly, among all patients (n = 105), reperfusion was associated with good functional outcome (adjusted OR 3.5, 95% CI 1.3–9.4, p = 0.02), whereas time to treatment (p = 0.27) and the interaction between time to treatment and reperfusion (p = 0.50) were not. In contrast, among non–target mismatch patients (n = 21), neither reperfusion (adjusted OR 2.7, 95% CI 0.2–75, p = 0.47) nor time to treatment (p = 0.23) nor the interaction between time to treatment and reperfusion (p = 0.27) was associated with good functional outcome.
Figure 1. Effect of time to treatment on the association between reperfusion and good functional outcome.
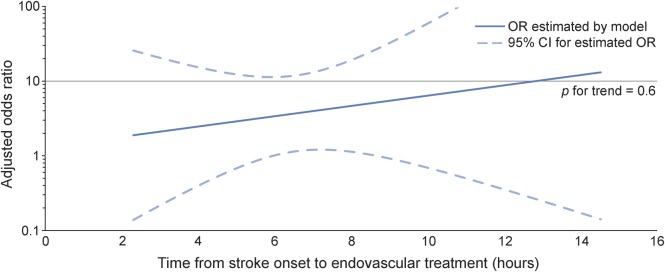
The adjusted odds ratio (OR) for good functional outcome with reperfusion in patients with the target mismatch (n = 78) is displayed as a function of time from stroke onset to the start of the endovascular procedure. The 95% confidence interval (CI) is indicated by dashed lines. The association between reperfusion and good functional outcome is not modified by time to treatment in patients with target mismatch (adjusted p value for the interaction between reperfusion and time to treatment = 0.6). Values are derived from a multivariate logistic regression model adjusted for age and baseline diffusion-weighted imaging lesion volume.
The primary analysis was repeated with time to treatment dichotomized into early (≤6 hours) vs late (>6 hours) start of endovascular treatment. In target mismatch patients, reperfusion was again associated with good functional outcome (OR adjusted for age, baseline DWI lesion volume, and the dichotomized time-to-treatment variable 4.0, 95% CI 1.3–13, p = 0.02), whereas time to treatment (p = 0.56) and the interaction between reperfusion and time to treatment (p = 0.62) were not. The 90-day mRS outcomes, stratified by reperfusion status and time to treatment (early vs late), are shown in figure 2.
Figure 2. Day 90 modified Rankin Scale scores among patients with the target mismatch stratified by treatment time and reperfusion status.
The distribution of the 90-day modified Rankin Scale (mRS) scores is shown for target mismatch patients stratified by treatment time (≤6 hours vs >6 hours) and reperfusion status. Based on multivariate logistic regression, reperfusion is associated with an increased chance of good functional outcome (odds ratio adjusted for age and baseline diffusion-weighted imaging lesion volume 4.0, 95% confidence interval 1.3–13, p = 0.02), whereas time to treatment (p = 0.6) and the interaction between reperfusion and time to treatment are not (p = 0.6).
Second, we limited the sample to target mismatch patients with reperfusion and constructed a model with time to treatment as the independent variable and good functional outcome as the dependent variable. This model showed no significant association between time to treatment and good functional outcome in unadjusted (p = 0.17) or adjusted analyses (p = 0.19) (figure 3).
Figure 3. Effect of time to treatment on the probability of good functional outcome with reperfusion.
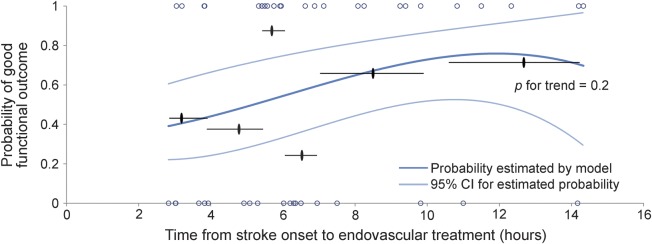
The probability of good functional outcome in patients with target mismatch who achieved reperfusion (n = 46) is displayed as a function of time from stroke onset to the start of the endovascular procedure. The 2 curved light blue lines indicate the 95% confidence interval (CI). Short horizontal black lines indicate the mean observed probability of good functional outcome in patients grouped (n = 7 or 8) according to their consecutive time from symptom onset to start of procedure. Black ovals indicate the mean time from symptom onset to start of procedure for each of the 6 subgroups. Blue circles indicate the observed outcome for each individual patient in the cohort, where 0 corresponds to a modified Rankin Scale (mRS) score >2 and 1 to an mRS score ≤2 at day 90. “p for trend” is the adjusted p value for the association between time to treatment and the probability of good functional outcome (p = 0.2). Values are derived from a logistic regression model with time from stroke onset to start of the endovascular procedure as the predictor variable. Probabilities are adjusted for age and baseline diffusion-weighted imaging lesion volume.
Third, we assessed predictors of lesion growth in target mismatch patients. Within the early (≤6 hours) treated cohort, median lesion growth was 12.8 mL (IQR 1.1–49.7) among nonreperfusers (n = 12) vs 3.2 mL (IQR −1.7 to 11.9) among reperfusers (n = 22) (p = 0.07). In the late (>6 hours) treated cohort, median lesion growth was 37.8 mL (IQR 15.3–116.2) among nonreperfusers (n = 16) vs 1.3 mL (IQR −1.9 to 12.5) among reperfusers (n = 24) (p < 0.001). In multivariate analysis, reperfusion was associated with attenuation of lesion growth (p < 0.001), whereas time to treatment and the interaction between reperfusion and time to treatment were not (p = 0.3 for both). After adjusting for baseline DWI lesion volume, reperfusion remained the only significant predictor of lesion growth (p = 0.02).
When the above analyses were repeated with “time to end of procedure” as the time measure instead of “time to start of procedure,” the overall results were unchanged.
DISCUSSION
This study suggests that the association between endovascular reperfusion and good functional outcome is not time-dependent in patients with evidence of salvageable brain tissue on MRI (i.e., patients with the target mismatch pattern on MRI) who are treated up to 12 hours after symptom onset. Similarly, the association between reperfusion and attenuation of lesion growth is not time-dependent in this patient population.
Several analyses were performed to address whether these findings may be due, in part, to a bias toward more favorable characteristics among patients who were treated late. Prior studies have shown that baseline DWI lesion volume and the degree of reperfusion are associated with clinical outcome.16,19–21 In our study, these variables did not differ between early and late treated patients. Other characteristics were also well-matched, except for an expected lower rate of IV tPA treatment in the late treatment group. Among patients with reperfusion, patients treated late had a slightly lower median baseline NIH Stroke Scale score (13.5 vs 14.5) and had an occlusion of the internal carotid artery less frequently (21% vs 36%). While these differences could have biased our secondary analysis (the effect of time to treatment on good functional outcome in the subgroup of patients with reperfusion), this is unlikely because adjusting for these variables did not alter the results of the secondary analysis. Similarly, patient characteristics were well-matched between patients with and without reperfusion, arguing against bias affecting our primary analysis. Consequently, the unadjusted and adjusted primary analyses yielded similar results: both showed that reperfusion is significantly associated with good functional and radiologic outcomes and that these associations are independent of the time to treatment.
The current study differs in 3 important ways from earlier analyses that have assessed the effect of time to endovascular treatment on patient outcomes. The first difference is that the primary analysis of the current study focused on patients with evidence of salvageable tissue on MRI, whereas prior studies included patients regardless of their imaging pattern. In a recent subanalysis of the Interventional Management of Stroke (IMS) III study, time to angiographic reperfusion was an independent predictor of good functional outcome following endovascular therapy (adjusted relative risk for every 30-minute delay 0.88).6 Our study demonstrates that this association is lost when imaging criteria are applied that limit the population to patients with salvageable tissue (figure 1). Together, these studies support the notion that time to treatment is a valid surrogate for tissue at risk but that the MRP-DWI mismatch is a more direct measure of tissue at risk that allows for a more individualized approach to patient selection. A second difference is that prior studies have focused on the probability of good outcome in patients who reperfuse, whereas our primary analysis focused on the effect of reperfusion in patients who reperfused vs those who did not reperfuse. However, in order to compare our results directly with those of previous studies, we also report an analysis that is limited to patients who reperfuse (figure 3). In this subanalysis, we again showed no effect of time to treatment on good functional outcome among patients with salvageable tissue on MRI. A third difference lies in the availability of early follow-up MRI data in our study. These data allowed us to assess the effect of time to treatment on both clinical and radiologic outcomes. Earlier studies have shown that reperfusion is associated with attenuation of lesion growth.16,21,22 Here we confirm those results and also show that the effect of reperfusion on the attenuation of lesion growth is not dependent on time to treatment in patients with salvageable tissue. These results are in line with a recent study that showed attenuation of infarct growth with recanalization in a select cohort of patients who were treated with endovascular therapy more than 8 hours after symptom onset.23
This study has some limitations. First, the DEFUSE 2 study was not powered for this post hoc analysis. Therefore, a small modifying effect of time to treatment on the association between reperfusion and favorable outcome could have been missed. However, there was not even a trend toward a decline in the benefit from reperfusion with later treatment, making it unlikely that a strong association would have been present in a larger study. Second, we primarily assessed the effect of time to treatment in the subset of patients with a target mismatch because patients without a target mismatch are underrepresented in the DEFUSE 2 cohort, potentially because of selection bias.12 Other patient groups may also have been underrepresented because they were judged to be poor candidates for endovascular therapy by DEFUSE 2 investigators. This could include patients who were older or who had other unfavorable clinical characteristics. The proportion of patients that was excluded from DEFUSE 2 because they were deemed poor candidates for endovascular therapy and the baseline characteristics of these patients are unknown. A comparison with the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) study demonstrates that in DEFUSE 2 the proportion of patients with penumbral tissue is higher (79% in DEFUSE 2 vs 55% in MR RESCUE) and the infarct core volumes of penumbral patients is smaller (median 13 mL in DEFUSE 2 vs 36 mL in MR RESCUE).16,20 Third, patients enrolled in DEFUSE 2 were treated between 2.3 and 14.5 hours after symptom onset. The results of this analysis therefore apply only to this time window and should not be extrapolated to treatment at later times. Finally, DEFUSE 2 was a cohort study and not a randomized controlled trial, so the results should be interpreted in that context. Specifically, the results suggest that patients with evidence of salvageable tissue may benefit from reperfusion within a fairly wide time window, but this should not be interpreted as evidence that endovascular therapy is beneficial. That evidence should come from a randomized controlled trial of endovascular therapy vs best medical therapy. The results of this study suggest that a randomized controlled trial that enrolls patients with the target mismatch profile could be designed with a relatively wide time window.
There is convincing evidence from prior studies that earlier stroke treatment is associated with better outcomes. An ongoing effort to expedite the treatment of acute stroke patients, both in clinical practice and in clinical trials, should therefore be strongly encouraged. However, because time is an imprecise surrogate for the presence of salvageable brain tissue, it is also an imperfect criterion for selecting patients who are likely to benefit from reperfusion. Selecting patients with evidence of salvageable tissue on brain imaging is an approach that can personalize the time window for individual patients and potentially widen the time window for the overall population of stroke patients.
Supplementary Material
ACKNOWLEDGMENT
Data used in preparation of this article were obtained from the DEFUSE 2 study. The authors are grateful to the patients, families, and clinical staff for their cooperation in the investigation of this study. The authors acknowledge the investigators who contributed to the design and implementation of DEFUSE 2 and/or provided data but did not participate in analysis or writing of this report.
GLOSSARY
- CI
confidence interval
- DEFUSE 2
Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2
- DWI
diffusion-weighted imaging
- IQR
interquartile range
- MRP
magnetic resonance perfusion
- MR RESCUE
Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy
- mRS
modified Rankin Scale
- OR
odds ratio
- tPA
tissue plasminogen activator
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: J. Saver, P. Fayad, G. Howard, T. Tomsick., T. Jovin, L. Wechsler, S. DeCesare, D. Thai, A. Sherr, M. Wilder, A. Tricot, H. Lutsep, L. McDaneld, D. Larsen, T. Czartoski, B. Keogh, A.M. Malik, A. Brown, R. Bernstein, K. Muskovich, C. Chang, T. Stern, S. Warach, L. Davis, F. Fazekas, and T. Seifert-Held
AUTHOR CONTRIBUTIONS
The study was designed by Dr. Lansberg, Dr. Cereda, Dr. Mlynash, Ms. Kemp, and Dr. Albers. Data were collected by Dr. Lansberg and Dr. Albers. Data were analyzed and interpreted by Dr. Lansberg, Dr. Cereda, Dr. Mlynash, Dr. Mishra, Dr. Inoue, Dr. Christensen, and Dr. Albers. The manuscript was drafted by Dr. Lansberg, Dr. Cereda, and Dr. Albers. Critical revisions were made by all authors.
STUDY FUNDING
The study was funded by grants from the National Institute for Neurological Disorders and Stroke (NINDS) R01 NS03932505 (Dr. Albers), R01 NS075209 (Dr. Lansberg), and 1ZIANS00.
DISCLOSURE
M. Lansberg, C. Cereda, M. Mlynash, N. Mishra, M. Inoue, S. Kemp, S. Christensen, and M. Straka report no disclosures relevant to the manuscript. G. Zaharchuk is a member of the Neuroradiology Advisory Board for GE Healthcare and receives research funding support from GE Healthcare. M. Marks reports no disclosures relevant to the manuscript. R. Bammer is an equity shareholder in iSchemaView. G. Albers has received consulting fees and expenses from Lundbeck for Steering Committee work and consulting fees from Concentric for serving on a Data Safety and Monitoring Board. G. Albers is an equity shareholder in iSchemaView. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PSS, Beumer D, et al. ; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 4.Saver J. Solitaire FR with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke: preliminary results. Presented at the International Stroke Conference; February 11, 2015; Nashville, TN.
- 5.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA; IMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009;73:1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatri P, Yeatts SD, Mazighi M, et al. ; IMS III Trialists. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol 2014;13:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron JC, von Kummer R, del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. Stroke 1995;26:2219–2221. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez RG, Hakimelahi R, Schaefer PW, Roccatagliata L, Sorensen AG, Singhal AB. Stability of large diffusion/perfusion mismatch in anterior circulation strokes for 4 or more hours. BMC Neurol 2010;10:10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakimelahi R, González RG. Neuroimaging of ischemic stroke with CT and MRI: advancing towards physiology-based diagnosis and therapy. Expert Rev Cardiovasc Ther 2009;7:29–48. [DOI] [PubMed] [Google Scholar]
- 10.Hakimelahi R, Vachha BA, Copen WA, et al. Time and diffusion lesion size in major anterior circulation ischemic strokes. Stroke 2014;45:2936–2941. [DOI] [PubMed] [Google Scholar]
- 11.Marchal G, Beaudouin V, Rioux P, et al. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke 1996;27:599–606. [DOI] [PubMed] [Google Scholar]
- 12.Baron JC, Macrae IM, Adams HP, Jr, Dirnagl U. ESC-BRAIN: experimental and clinical stroke research—do they connect? Meeting report of the ESC-BRAIN joint symposium held in London and Shanghai in May 2013 Cerebrovasc Dis 2013;36:306–321. [DOI] [PubMed] [Google Scholar]
- 13.Davis S, Campbell B, Christensen S, et al. Perfusion/Diffusion mismatch is valid and should be used for selecting delayed interventions. Transl Stroke Res 2012;3:188–197. [DOI] [PubMed] [Google Scholar]
- 14.Donnan GA, Baron JC, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol 2009;8:261–269. [DOI] [PubMed] [Google Scholar]
- 15.Fisher M, Albers GW. Advanced imaging to extend the therapeutic time window of acute ischemic stroke. Ann Neurol 2013;73:4–9. [DOI] [PubMed] [Google Scholar]
- 16.Lansberg MG, Straka M, Kemp S, et al. ; DEFUSE 2 study investigators. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012;11:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010;32:1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–e137. [DOI] [PubMed] [Google Scholar]
- 19.Albers GW. Endovascular treatment for stroke: when does the window for good outcome close? Lancet Neurol 2014;13:529–531. [DOI] [PubMed] [Google Scholar]
- 20.Kidwell CS, Jahan R, Gornbein J, et al. ; MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks MP, Lansberg MG, Mlynash M, et al. ; DEFUSE Investigators. Angiographic outcome of endovascular stroke therapy correlated with MR findings, infarct growth, and clinical outcome in the DEFUSE 2 trial. Int J Stroke 2014;9:860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picanço MR, Christensen S, Campbell BCV, et al. Reperfusion after 4.5 hours reduces infarct growth and improves clinical outcomes. Int J Stroke 2014;9:266–269. [DOI] [PubMed] [Google Scholar]
- 23.Leiva-Salinas C, Aghaebrahim A, Zhu G, et al. Tissue at risk in acute stroke patients treated beyond 8 h after symptom onset. Neuroradiology 2013;55:807–812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



