Contention
Our contention is that endogenous 5-hydroxytryptamine (5-HT) is not necessary for colonic peristalsis, nor other neurogenic motor patterns in the lower gastrointestinal (GI) tract, such as colonic migrating motor complexes (CMMCs).
Background
Large quantities of 5-hydroxytryptamine (5-HT) are synthesized in the gut, in enterochromaffin (EC) cells of the mucosa (via tryptophan hydroxylase-1) and in a small proportion in enteric neurons (via tryptophan hydroxylase-2). It has been well established that 5-HT can be released from the mucosa by mechanical stimulation, including contractile activity of the gut and compression of the gut wall (Bertrand, 2006). Numerous 5-HT receptors are expressed on enteric neurons and extrinsic afferent endings, and exogenous 5-HT has potent effects on neuronal excitability and transmitter release (Gershon, 2000). Despite this, the evidence that 5-HT is necessary for peristalsis or CMMCs in the lower GI-tract is all circumstantial. Extensive evidence now suggests that these motor patterns occur independent of 5-HT.
Three lines of disputed evidence
High quantities of 5-HT are synthesized and released from the mucosa. Release of endogenous 5-HT can occur during peristalsis and CMMCs and exogenous 5-HT can evoke peristalsis or CMMCs
The observation that mucosal compression causes release of endogenous 5-HT at the same time as the onset of peristalsis or CMMCs does not reflect a causal relationship between these events. Mucosal compression distorts multiple cell types in the gut wall, including the underlying myenteric plexus – which is itself exquisitely sensitive to mechanical deformation (Kunze et al. 2000). Compression applied to the myenteric plexus still evokes peristaltic reflexes, even after the mucosa has been removed (Spencer et al. 2003) and CMMCs also persisted in these preparations (Keating & Spencer, 2010; Zagorodnyuk & Spencer, 2011). Thus, while mucosal stimulation can release large amounts of endogenous 5-HT, we contend that this release is not a necessary step in initiating peristalsis or CMMCs. The observation that exogenous 5-HT (and many other agonists) can initiate peristalsis and CMMCs does not reveal any information about the functional role of endogenous 5-HT.
It has been suggested that endogenous 5-HT release from the mucosa initiates the colonic peristaltic reflex (Foxx-Orenstein et al. 1996; Grider et al. 1996) and is ‘critical’ for spontaneous CMMCs (Heredia et al. 2009). It was reported that ‘removing the mucosa appeared to abolish spontaneous CMMCs, suggesting that the mucosa is normally critical for their generation’ (Heredia et al. 2009) – a conclusion based on four mice. It was further stated that ‘the trigger for the CMMC appears to be spontaneous or evoked (i.e. a fecal pellet) release of 5-HT from EC cells to stimulate AH neurons’. Although, 5-HT release from the mucosa was not measured, nor were recordings made from neurons to confirm this hypothesis. If 5-HT release from the mucosa was essential for peristalsis or CMMCs, then both these motor patterns would be expected to cease when the mucosa is removed. They don’t. With practice it was demonstrated that the entire mucosa can be reliably removed from an isolated specimen of colon and still record peristalsis (Spencer et al. 2011) and spontaneous and evoked CMMCs (Keating & Spencer, 2010). In these mucosa-free preparations, release of 5-HT that is normally associated with peristaltic or CMMC contractions was abolished. Importantly, when removing the mucosa we observed that if the dissection technique was too vigorous, the delicate neural circuitry required for peristalsis or CMMCs is disrupted. This may explain why spontaneous CMMCs were not recorded after removal of the mucosa in the four mice studied in Heredia et al. (2009). We consider it inconceivable that dissection damage in our preparations could have created a whole new motor pattern that mimicked normal peristalsis and CMMCs.
In other studies, electrochemical detection of 5-HT release from the mucosa showed that 5-HT release was only associated with some, but not all, contractions underlying peristalsis (Bertrand, 2006) or CMMCs (Keating & Spencer, 2010), again arguing against a causal link between 5-HT release and these colonic motor patterns. If 5-HT release from the mucosa was critical for peristalsis and CMMCs, then deleting the gene responsible for synthesizing mucosal 5-HT (TPH-1) should block CMMCs and affect transit. It doesn’t. The laboratory that concluded 5-HT release from the mucosa was critical for CMMCs (Heredia et al. 2009) also showed that CMMCs still occurred when mucosal 5-HT synthesis had been prevented (Heredia et al. 2013). Furthermore, conscious mice lacking mucosal 5-HT synthesis have no inhibitory deficits in GI-transit (Yadav et al. 2010; Li et al. 2011).
Endogenous 5-HT is synthesized in enteric neurons and numerous 5-HT receptors are expressed in the gut
There is some evidence that 5-HT may be a neurotransmitter in a small population of myenteric (Zhou & Galligan, 1999; Galligan et al. 2000) and submucosal neurons (Monro et al. 2004) of the small intestine, despite only ∼1% of enteric neurons expressing 5-HT (Costa et al. 1982; Wardell et al. 1994; Costa et al. 1996). For a substance to be a neurotransmitter, it must be ‘present in the presynaptic terminal and is released in amounts sufficient to exert a defined action on the postsynaptic neuron or effector organ’ (Gardner et al. 2000). Based on this criterion, we are not aware of any evidence that endogenous 5-HT participates in any synaptic potential in the colon of the mouse (Furukawa et al. 1986; Nurgali et al. 2004) and rarely (Nurgali et al. 2003) or never (Wade & Wood, 1988; Spencer & Smith, 2004) in the colon of the guinea-pig, which are the species we have been studying CMMCs and peristalsis with. In our experience in guinea-pig colon (Spencer & Smith, 2004) and in mouse (Furukawa et al. 1986; Nurgali et al. 2004), human (Brookes et al. 1987) and rat colon (Brookes et al. 1988), all fast synaptic potentials are blocked by hexamethonium.
5-HT antagonists can block peristalsis and CMMCs and retard transit
Perhaps the strongest evidence that endogenous 5-HT is necessary for peristalsis is based on the finding that 5-HT antagonists can substantially inhibit or block colonic peristalsis (Foxx-Orenstein et al. 1996; Grider et al. 1996; Kadowaki et al. 1996). However, when these experiments were repeated with the same antagonists (SDZ-205-557 and ondansetron) they were only found to cause a temporary blockade of peristalsis. In the continued presence of both antagonists peristalsis reappeared (Sia et al. 2013a, b). Importantly, these antagonists also blocked peristalsis in colonic preparations from mucosa-free, reserpine-treated animals, where endogenous 5-HT had been depleted. This showed that endogenous 5-HT may not be necessary for activation of 5-HT3 and 5-HT4 receptors. We suggest that 5-HT3 and 5-HT4 antagonists bind to 5-HT3 and 5-HT4 receptors which are constitutively active even in the absence of any 5-HT. Indeed, ligand-gated 5-HT3 receptors (Hu & Peoples, 2008) and G-protein coupled 5-HT4 receptors (Berthouze et al. 2005) both show constitutive activity. These antagonists could work as inverse agonists, reducing the constitutive activity of the receptors, rather than blocking the effects of endogenous 5-HT.
At present, we are unsure of the role of 5-HT in the lower GI tract. We do know that 5-HT can have many effects and it may modulate enteric circuits and motility. However, recent evidence from a number of laboratories, using a number of techniques, shows that neither mucosal nor neuronal 5-HT is necessary for colonic peristalsis or CMMCs.
Biographies
Nick Spencer completed his PhD in 1998 in the field of electrophysiology of intestinal smooth muscle, at Monash University. Since then, his primary interests involve the mechanisms that activate intrinsic and extrinsic neural circuits in the gastrointestinal tract. After his PhD, he spent 10 years at the University of Nevada School of Medicine, investigating neuronal mechanisms underlying gastrointestinal motility. The past 7 years, he has been at Flinders University, where he is currently an Associate Professor.
Damien Keating is Associate Professor in the Discipline of Human Physiology at Flinders University. He received his PhD in cell physiology from the University of Adelaide in 2003 and is currently an R. D. Wright Biomedical Research Fellow with the NHMRC. His research focuses on the mechanisms controlling cell signalling and the release of hormones and neurotransmitters. He currently undertakes a large amount of this work using primary cultures of serotonin-secreting human enterochromaffin cells.
Figure 1.
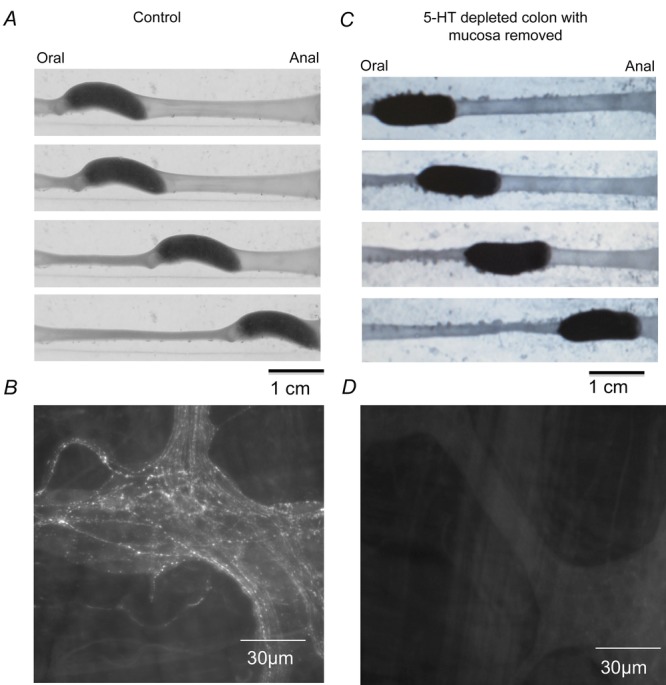
Colonic Paristalsis occurs without endogenous 5-HTA, peristalsis in the distal colon. B, 5-HT immunoreactivity. C, in mucosa-free colon also depleted of neuronal 5-HT, peristalsis still occurs. D, confirmation of depletion of neuronal 5-HT after reserpine.
Additional information
Competing interests
None declared.
Funding
The experiments carried out in this study were funded by a grant to N.J.S. (grant no. 1067335) from the National Health and Medical Research Council (NH & MRC) of Australia and to D.J.K. (grant no. FT0990901) from the Australian Research Council.
References
- Berthouze M, Ayoub M, Russo O, Rivail L, Sicsic S, Fischmeister R, Berque-Bestel I, Jockers R. Lezoualc’h F. Constitutive dimerization of human serotonin 5-HT4 receptors in living cells. FEBS Lett. 2005;579:2973–2980. doi: 10.1016/j.febslet.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol. 2006;577:689–704. doi: 10.1113/jphysiol.2006.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Ewart WR. Wingate DL. Intracellular recordings from myenteric neurones in the human colon. J Physiol. 1987;390:305–318. doi: 10.1113/jphysiol.1987.sp016702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Ewart WR. Wingate DL. Intracellular recordings from cells in the myenteric plexus of the rat duodenum. Neuroscience. 1988;24:297–307. doi: 10.1016/0306-4522(88)90332-6. [DOI] [PubMed] [Google Scholar]
- Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E. Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB, Cuello AC, Verhofstad AA, Steinbusch HW. Elde RP. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience. 1982;7:351–363. doi: 10.1016/0306-4522(82)90272-x. [DOI] [PubMed] [Google Scholar]
- Foxx-Orenstein AE, Kuemmerle JF. Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Taylor GS. Bywater RA. An intracellular study of myenteric neurons in the mouse colon. J Neurophysiol. 1986;55:1395–1406. doi: 10.1152/jn.1986.55.6.1395. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, LePard KJ, Schneider DA. Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Martin JH. Jessell TM. Kandel ER, Shwartz JH. Jessell TM. Principles of Neural Science. 4th edn. 2000. The bodily senses; pp. 430–450. McGraw-Hill. [Google Scholar]
- Gershon MD. 5-HT (serotonin) physiology and related drugs. Curr Opin Gastroenterol. 2000;16:113–120. doi: 10.1097/00001574-200003000-00004. [DOI] [PubMed] [Google Scholar]
- Grider JR, Kuemmerle JF. Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J PhysiolGastrointestLiver Physiol. 1996;270:G778–782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW. Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T. Smith TK. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol. 2013;591:5939–5957. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ. Peoples RW. The 5-HT3B subunit confers spontaneous channel opening and altered ligand properties of the 5-HT3 receptor. J Biol Chem. 2008;283:6826–6831. doi: 10.1074/jbc.M707571200. [DOI] [PubMed] [Google Scholar]
- Kadowaki M, Wade PR. Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J PhysiolGastrointestLiver Physiol. 1996;271:G849–857. doi: 10.1152/ajpgi.1996.271.5.G849. [DOI] [PubMed] [Google Scholar]
- Keating DJ. Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138:659–670. doi: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB. Gola M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol. 2000;526:375–385. doi: 10.1111/j.1469-7793.2000.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J. Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP. Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol. 2004;556:571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurgali K, Furness JB. Stebbing MJ. Analysis of purinergic and cholinergic fast synaptic transmission to identified myenteric neurons. Neuroscience. 2003;116:335–347. doi: 10.1016/s0306-4522(02)00749-2. [DOI] [PubMed] [Google Scholar]
- Nurgali K, Stebbing MJ. Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol. 2004;468:112–124. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- Sia TC, Flack N, Robinson L, Kyloh M, Nicholas SJ, Brookes SJ, Wattchow DA, Dinning P, Oliver J. Spencer NJ. Is serotonin in enteric nerves required for distension-evoked peristalsis and propulsion of content in guinea-pig distal colon? Neuroscience. 2013;240:325–335. doi: 10.1016/j.neuroscience.2013.02.061. [DOI] [PubMed] [Google Scholar]
- Sia TC, Whiting M, Kyloh M, Nicholas SJ, Oliver J, Brookes SJ, Dinning PG, Wattchow DA. Spencer NJ. 5-HT3 and 5-HT4 antagonists inhibit peristaltic contractions in guinea-pig distal colon by mechanisms independent of endogenous 5-HT. Front Neurosci. 2013;7:136. doi: 10.3389/fnins.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW. Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2003;284:G231–241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk VP. Keating DJ. Mechanisms underlying distension-evoked peristalsis in guinea-pig distal colon: is there a role for enterochromaffin (EC) cells? Am J Physiol Gastrointest Liver Physiol. 2011;301:G519–527. doi: 10.1152/ajpgi.00101.2011. [DOI] [PubMed] [Google Scholar]
- Spencer NJ. Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558:577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PR. Wood JD. Synaptic behavior of myenteric neurons in guinea pig distal colon. Am J PhysiolGastrointestLiver Physiol. 1988;255:G184–190. doi: 10.1152/ajpgi.1988.255.2.G184. [DOI] [PubMed] [Google Scholar]
- Wardell CF, Bornstein JC. Furness JB. Projections of 5-hydroxytryptamine-immunoreactive neurons in guinea-pig distal colon. Cell Tissue Res. 1994;278:379–387. doi: 10.1007/BF00414180. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, Medhamurthy R, Vidal M, Karsenty G. Ducy P. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP. Spencer NJ. Localization of the sensory neurons and mechanoreceptors required for stretch-evoked colonic migrating motor complexes in mouse colon. Front Physiol. 2011;2:98. doi: 10.3389/fphys.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. Galligan JJ. Synaptic activation and properties of 5-hydroxytryptamine3 receptors in myenteric neurons of guinea pig intestine. J PharmacolExpTher. 1999;290:803–810. [PubMed] [Google Scholar]


