Abstract
Conversion of white into brown adipose tissue may have important implications in obesity resistance and treatment. Several browning agents or conditions ignite thermogenesis in white adipose tissue (WAT). To reveal the capacity of WAT to function in a brownish/burning mode over the long term, we investigated the progression of the rat retroperitoneal WAT (rpWAT) browning during 45 days of cold acclimation. During the early stages of cold acclimation, the majority of rpWAT adipocytes underwent multilocularization and thermogenic-profile induction, as demonstrated by the presence of a multitude of uncoupling protein 1 (UCP1)-immunopositive paucilocular adipocytes containing peroxisome proliferator-activated receptor (PPAR) coactivator-1α (PGC-1α) and PR domain-containing 16 (PRDM16) in their nuclei. After 45 days, all adipocytes remained PRDM16 immunopositive, but only a few multilocular adipocytes rich in mitochondria remained UCP1/PGC-1α immunopositive. Molecular evidence showed that thermogenic recruitment of rpWAT occurred following cold exposure, but returned to starting levels after cold acclimation. Compared with controls (22 ± 1°C), levels of UCP1 mRNA increased in parallel with PPARγ (PPARα from days 1 to 7 and PGC-1α on day 1). Transcriptional recruitment of rpWAT was followed by an increase in UCP1 protein content (from days 1 to 21). Results clearly showed that most of the adipocytes within rpWAT underwent transient brown-fat-like thermogenic recruitment upon stimulation, but only a minority of cells retained a brown adipose tissue-like phenotype after the attainment of cold acclimation. Therefore, browning of WAT is dependent on both maintaining the thermogenic response and retaining enough brown-like thermogenically competent adipocytes in the long-term. Both aspects of browning could be important for long-term energy homeostasis and body-weight regulation.
Key points
White to brown adipose tissue conversion and thermogenesis can be ignited by different conditions or agents and its sustainability over the long term is still unclear.
Browning of rat retroperitoneal white adipose tissue (rpWAT) during cold acclimation involves two temporally apparent components: (1) a predominant non-selective browning of most adipocytes and an initial sharp but transient induction of uncoupling protein 1, peroxisome proliferator-activated receptor (PPAR) coactivator-1α, PPARγ and PPARα expression, and (2) the subsistence of relatively few thermogenically competent adipocytes after 45 days of cold acclimation.
The different behaviours of two rpWAT beige/brown adipocyte subsets control temporal aspects of the browning process, and thus regulation of both components may influence body weight and the potential successfulness of anti-obesity therapies.
Introduction
Cold-induced sympathetic stimulation triggers a series of physiological and biochemical changes in adipose tissues that result in increased heat generation and metabolic demand, leading to cold acclimation.
In the brown adipose tissue (BAT), exposure to low temperatures activates a defined set of transcription factors that drive increased thermogenic capacity. Among them, peroxisome proliferator-activated receptors (PPARs) γ and α, PPARγ-coactivator-1α (PGC-1α) and PR domain-containing 16 (PRDM16) are of particular importance because of their role in upregulating uncoupling protein 1 (UCP1) expression and the uncoupling of mitochondrial fat combustion from ATP production (Puigserver et al. 1998). In parallel these transcription factors drive oxidative metabolism, mitochondriogenesis and brown adipocyte differentiation – all prerequisite for complete thermogenic recruitment of BAT (Puigserver et al. 1998; Nedergaard et al. 2005).
In parallel with effects on BAT, sustained cold exposure causes intensive mobilization of stored lipids (Trayhurn et al. 1995) and the appearance of brown-like (beige or brite) UCP1-expressing adipocytes in classic white adipose tissue (WAT) depots (Young et al. 1984; Loncar et al. 1986; Cousin et al. 1992; Guerra et al. 1998; Murano et al. 2005). This phenomenon, the occurrence of BAT-like UCP1-containing adipocytes in WAT, was also observed following exercise and treatments with β-adrenergic and PPARγ agonists (Kajimura & Saito, 2014; Nedergaard & Cannon, 2014). In fact, all white fat depots studied to date possess the ability to darken to some degree, and are classified as white or beige, depending on the relative abundance of different types of adipocytes, which can be differentiated by their characteristic molecular signature (Walden et al. 2012).
Browning of WAT depots and BAT recruitment following cold exposure appear to form part of a concerted adaptive response that takes several weeks to develop (Puerta & Abelenda, 1987; Griggio, 1988). During cold acclimation, euthermy is initially defended by shivering muscles (days 1–7), and over a longer period shivering is replaced by increased energy expenditure in BAT in a process known as non-shivering thermogenesis (Sellers et al. 1954; Peralta et al. 2003; Cannon & Nedergaard, 2004). Our previous studies showed that metabolic remodelling of skeletal muscle (Stancic et al. 2013) and BAT (Petrovic et al. 2010; Vucetic et al. 2011) are phase dependent, and reflect the aforementioned thermogenic recruitment during 45 days of cold acclimation. Additionally, remodelling of WAT during cold acclimation involves a phase-dependent switch towards a more oxidative tissue (Jankovic et al. 2009, 2013), whereas the biggest changes occur between the thermogenic recruitment of BAT (day 3) and completion of cold acclimation (45 days).
The occurrence of brown-like adipocytes in WAT depots has been observed in cold-exposed humans also (van Marken Lichtenbelt et al. 2009; van der Lans et al. 2013), and growing data suggest that browning of white fat depots may facilitate resistance to obesity (Guerra et al. 1998; Cederberg et al. 2001; Leonardsson et al. 2004; Ishibashi & Seale, 2010; Kozak, 2011). Thus, understanding how the browning process progresses over time is an important goal of obesity research. The present study examined the browning process in the retroperitoneal WAT (rpWAT) of rats during the 45-day cold acclimation. To this end, levels of the UCP1, PPARs, PGC-1α and PRDM16 were measured and correlated with morphological changes occurring in the rpWAT.
Methods
Animals and experimental protocol
All animal procedures were approved by the Ethical Committee for the Use of Laboratory Animals of the Institute for Biological Research ‘Siniša Stanković’, University of Belgrade, Serbia, and all experiments conform to the principles of UK regulations, as described in Drummond (2009). Three-month-old male Mill Hill hybrid hooded rats (Rattus norvegicus, Berkenhout 1769) were housed under a 12/12 h reverse light/dark cycle and had free access to standard laboratory rat chow and water. Animals were divided into two main groups, one kept at room temperature (22 ± 1°C) for the entire experimental period and sampled as the control, and the other kept in the cold (4 ± 1°C). The duration of cold exposure was 1, 3, 7, 12, 21 and 45 days, with six animals per experimental group.
Sample collection
Animals were killed by decapitation at the end of the experimental period. Harvested rpWATs were cut into small pieces, snap-frozen in liquid nitrogen and stored at −80°C until subsequent RNA extraction, Western blotting and microscopic examination.
RNA extraction and semi-quantitative RT–PCR
Total RNA was prepared from 100 mg of isolated rpWAT using TRIzol reagent (Invitrogen, Life Technologies Ltd, Carlsbad, CA, USA). Tissue was homogenized in 1 ml TRIzol, and after addition of 1/5 volume chloroform the mixture was incubated at room temperature for 2–3 min and centrifuged (12,000 g, 15 min, 4°C). Supernatants were aspirated, precipitated with 500 μl isopropanol overnight at −80°C and centrifuged (10,000 g, 10 min, 4°C) to yield pellets that were washed with 75% ethanol and centrifuged (7500 g, 5 min, 4°C). The resulting pellets were air-dried and RNA was resuspended in diethyl pyrocarbonate-treated water. The purity and concentration of the total RNA were determined from the absorbance at 260 nm, and 1 μg was reverse-transcribed to cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions.
PCR was performed on a thermal cycler (PerkinElmer Gene Amp PCR System 2004) in a 25 μl reaction mixture containing 5 μl of a 1/10 dilution of cDNA reaction product as template, 12.5 μl of PCR master mix (Fermentas, USA) and 7.5 μl of nuclease-free water. Each thermal cycling protocol began with a denaturing step of 94°C for 5 min and finished with 72°C for 10 min. The following pairs of primers and cycle protocols were used. For UCP1: forward 5′-GTGAAGGTCAGAATGCAAGC-3′ and reverse 5′-AGGGCCCCCTTCATGAGGTC-3′; 45 min at 94°C, 60 min at 60°C, 60 min at 72°C, which yielded a band of 200 bp. For PGC-1α: forward 5′-CGCACAACTCAGCAAGTCCTC-3′ and reverse 5′-CCTTGCTGGCCTCCAAAGTCTC-3′; 15 min at 95°C, 60 min at 60°C, 60 min at 72°C, which yielded a band of 262 bp. For 18S rRNA: forward 5′-CGCAGCTAGGAATAATGGAA-3′ and reverse 5′-TTATGACCCGCACTTACTGG-3′; 30 min at 94°C, 30 min at 56°C, 30 min at 72°C, which yielded a band of 860 bp. Amplified products were separated on a 1.5% agarose gel in Tris–borate–EDTA buffer (89 mm Tris, 89 mm boric acid, 2 mm EDTA, pH 8.0) containing ethidium bromide. Band intensities were quantified using a GelDoc XM System (Bio-Rad Laboratories) equipped with Quantity One software for Bio-Rad Image Analysis system, version 4.6.0. Expression levels were calculated by comparing the intensity of UCP1/PGC-1α transcripts with the 18S rRNA house-keeping gene. Results are expressed as percentages relative to control values that were taken as 100%.
Western blotting
For Western blotting analysis, a portion of rpWAT from each rat weighing 400 mg was homogenized (Ultra/Turrax homogenizer, Janke und Kunkel Ka/Werke, Staufen, Germany, 0–4°C) in 0.25 m sucrose, 0.1 mm EDTA and 50 mm Tris-HCl buffer, pH 7.4, containing 10 μg ml−1 protease inhibitor cocktail (Roche Diagnostic GmbH, Mannheim, Germany). Homogenates were sonicated for 10 s at 40 kHz and centrifuged at 38,000 g for 90 min.
Protein concentrations in the supernatant were estimated as described (Lowry et al. 1951). Extracts were stored at −80°C until needed for Western blot analysis. Ten micrograms of each protein aliquot was boiled, electrophoresed using SDS–PAGE, and transferred to a Hybond-P polyvinylidene fluoride membrane (Amersham, Piscataway, NJ, USA). Non-specific binding was blocked with 5% BSA in TBS (200 mm Tris, 1.5 m NaCl, pH 7.4) for 1 h at room temperature. Blots were then incubated with a specific primary antibody in TBS-T (0.2% Tween-20 and 5% BSA in TBS) as follows: UCP1 (1:1000), PPARγ (1:400), PPARα (1:1000), PPARβ (1:400), PGC-1α (1:1000), cytochrome c (1:1000), UQCRC2 (a constituent of complex III/ubiquinol-cytochrome c reductase) (1:2000) and β-actin (1:1000), which were all purchased from Abcam (Cambridge, UK). Incubation was carried out overnight at 4°C followed by a 2 h incubation period at room temperature with a horseradish peroxidase-conjugated IgG secondary antibody. Protein bands were visualized using an API chemiluminescence detection system (Amersham, Indianapolis, IN, USA). Band intensity was quantified using ImageQuant software. The volume was the sum of all pixel intensities within a given band (1 pixel = 0.007744 mm2). The dots per band for target proteins and the β-actin control were averaged from three independent experiments for each time point, and expressed relative to the room temperature-acclimated control, which was standardized as 100%. Data were subjected to statistical analysis.
Microscopy and immunohistochemistry
Immediately following removal, samples of rpWAT were cut into small pieces, fixed in 2.5% glutaraldehyde in a 0.1 mol l−1 phosphate buffer (pH 7.2), post-fixed in 2% osmium tetroxide in the same buffer, dehydrated through a series of alcohol solutions of increasing concentration, and embedded in Araldite (Sigma-Aldrich Laborchemikalien GmbH, Hamburg, Germany). Blocks were trimmed and cut into 1 μm sections using an LKBIII ultramicrotome. Sections were cut into series (four), mounted on glass slides, stained with toluidine blue and visualized with a Leica DMLB (Leica Microsystems, Vienna, Austria) microscope.
Semi-thin sections were used for UCP1 and PGC-1α detection by routine immunohistochemistry. After removal of Araldite with 1% sodium hydroxide in absolute ethanol (30 min, 37°C), rpWAT sections were rehydrated with decreasing concentrations of ethanol, subsequently incubated in 10 mm citrate buffer for 3 min at 600 W to retrieve antigens and washed in phosphate buffered saline (PBS, pH 7.4). After washing, sections were incubated with 3% H2O2 in methanol for 10 min at room temperature, washed in PBS, and incubated with anti-UCP1 (1:200, Abcam) or anti-PGC-1α (1:200, Abcam) antibodies (overnight at 4°C). Immunodetection was performed using the Dako LSAB Universal Kit (Dako Scientific, Glostrup, Denmark). The sections which were washed three times were incubated with 0.02% hydrogen peroxide and 0.075% 3,3′-diaminobenzidine-tetrahydrochloride (Sigma-Aldrich, Inc. St Louis, MO, USA) in 0.05 m Tris buffer, pH 7.6 for 10 min in the dark. Finally, after rinsing in distilled water, the sections were counterstained with haematoxylin, mounted and examined with a Leica DMLB microscope (Leica Microsystems, Austria).
Araldite-embedded 2 μm-thick serial sections of rpWAT samples were used for immunofluorescence detection of UCP1 (1:500; ab10983; Abcam) and PRDM16 (1:500; ab106410; Abcam). After removal of Araldite, antigen retrieval and washing in PBS, sections were incubated with normal goat serum (1:100; ab7481; Abcam) for 1 h followed by an overnight incubation at 4°C with antibodies against UCP1 or PRDM16. After rinsing in PBS, slides were incubated for 30 min at room temperature with the appropriate fluorescein isothiocyanate-conjugated secondary antibody (1:200; ab6717; Abcam) and counterstained for 10 min with propidium iodide (Sigma-Aldrich). After staining, slides were rinsed in PBS and mounted in Mowiol (Polysciences, Eppelheim, Germany).
For PPARγ (ab19481; Abcam) and PGC-1α (ab54481; Abcam) detection and colocalization, slides were incubated with tetramethyl rhodamine isothiocyanate-conjugated (TRITC) secondary antibody (1:300; ab6718; Abcam) or fluorescein isothiocyanate-conjugated (FITC) secondary antibody (1:200; ab6717; Abcam), respectively. After washing with PBS for 20 min, slides were mounted with Mowiol (Polysciences). The double-stained samples were excited with 547 nm and 495 nm light, respectively. Confocal images were acquired with a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems) in sequential mode to avoid cross-talk between channels. The specificity of immunostaining, for both immunofluorescence and routine immunohistochemistry, was tested by the omission of primary antibodies.
Immunogold labelling
After antigen retrieval in 10 mm citrate buffer, ultra-thin tissue sections (70 nm) mounted on nickel grids were blocked with 1% BSA in TBS for 60 min at room temperature and incubated with primary antibodies for 2 h at 37°C against UCP1 (1:100 Abcam, Cambridge, UK) and PGC-1α (1:40, Abcam), respectively. After washing in TBS, sections were incubated with appropriate 10 nm gold-conjugated secondary antibodies for 1 h at ambient temperature (1:20; Abcam), rinsed in TBS and distilled water, dried and examined with a Philips CM12 transmission electron microscope (Philips/FEI, Amsterdam, the Netherlands) equipped with the digital camera SIS MegaView III (Olympus Soft Imaging Solutions, Münster, Germany). The specificity of the immune reactions was tested by replacing the primary antibody with TBS.
Statistics
One-way analysis of variance (ANOVA) was used for within-group comparison of the data from the molecular analysis. If the F test showed an overall difference, Tukey’s test was used to evaluate the significance of the differences. Statistical significance was accepted at P < 0.05.
Results
Expression and transcriptional regulation and subcellular distribution of UCP1
Immunohistochemistry revealed a faint UCP1 immunopositive reaction in unilocular rpWAT adipocytes in rats kept at room temperature (Fig.1B). In contrast, UCP1 mRNA levels were markedly induced on the first day (P < 0.001) in cold-exposed rats, and remained increased throughout the first 7 days of cold acclimation, before expression returned to the initial levels after 12 days (Fig.1A). Similarly, UCP1 protein levels were also increased in cold-exposed rats by day 1 (P < 0.05) and remained elevated at days 3, 7, 12 (P < 0.001) and 21 (P < 0.05).
Figure 1.
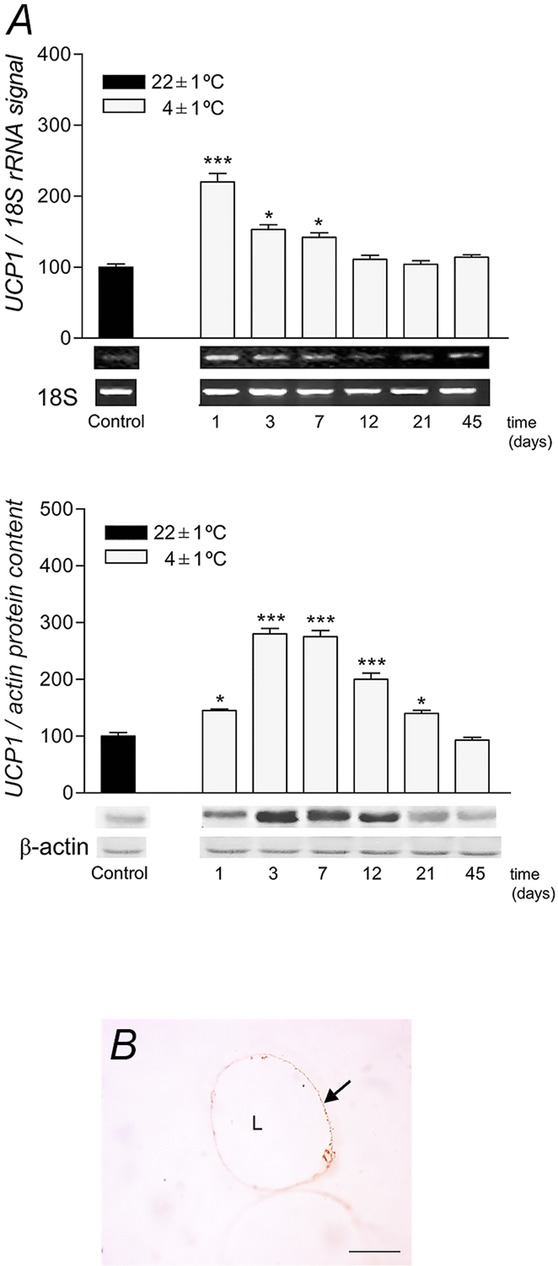
Expression levels of UCP1 in rpWAT and UCP1 presence in unilocular rpWAT adipocyte
A, time course of the changes in UCP1 mRNA and protein levels in rat rpWAT during cold acclimation. mRNA was quantified using Quantity One software, version 4.6.0. UCP1 transcripts were normalized against the 18S rRNA house-keeping gene. Protein content and mRNA levels were expressed relative to a control acclimated to room temperature, which was standardized as 100%. The results of the representative example from three observations are shown. Data were quantified as described in Methods. The values represent the means ± SEM. Compared to control, *P < 0.05 and ***P < 0.001. B, immunohistochemical localization of UCP1 in rpWAT unilocular adipocytes from control rats maintained at 22°C. Low granular immunoreactivity for UCP1 is visible in the cytoplasm surrounding the unilocular lipid droplet (L). Magnification 100×, orig. Scale bar, 20 μm.
Immunogold labelling of UCP1 protein clearly shows a different pattern of subcellular distribution in rpWAT adipocytes depending on the environmental temperature and the duration of cold exposure (Fig.2). A scant, sporadic UCP1 reaction is evident in the unilocular, typically rpWAT white adipocytes of rats maintained at room temperature and in rats exposed to cold for 45 days. However, in the former rats UCP1 is localized sporadicaly in the cytoplasm of rpWAT adipocytes, while in rats exposed to cold for 45 days there was both cytoplasmic and mitochondrial reaction. An abundance of UCP1-labelled immunogold particles was detected in the mitochondria of unilocular rpWAT adipocytes after 3 days of cold exposure. These UCP1-positive mitochondria, had irregular, random mitochondrial cristae. The expression levels of PPAR isoforms were also investigated, and both PPARα and PPARγ protein levels were increased on days 1, 3 and 7, but returned to initial levels thereafter (Fig.3). PPARδ was not affected significantly by cold exposure.
Figure 2.
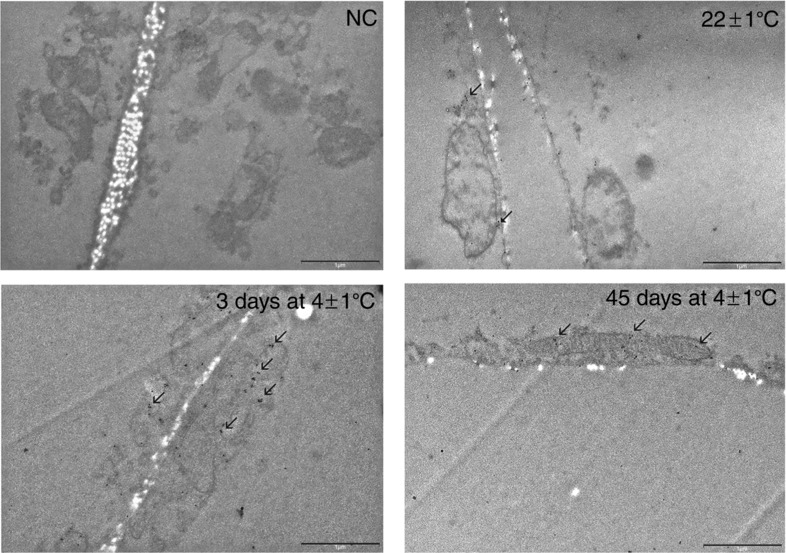
Ultrastructural immunolocalization of UCP1 protein on ultrathin sections of rpWAT of rats maintained at room temperature, following 3 or 45 days of cold exposure
A scant UCP1 reaction is evident in the rpWAT of rats maintained at room temperature and in rats exposed to cold for 45 days. Subcellular distribution of UCP1 clearly shows different pattern in rpWAT adipocytes depending on the environmental temperature and the duration of cold exposure. Abundance of UCP1-labelled immunogold particles were detected in the mitochondria of unilocular rpWAT adipocytes after 3 days of cold exposure. These UCP1-positive mitochondria, however, had irregular, random mitochondrial cristae. NC, negative control; L, lipid droplet. Transmission electron microscopy; Immunogold technique. Scale bar, 1 μm.
Figure 3.
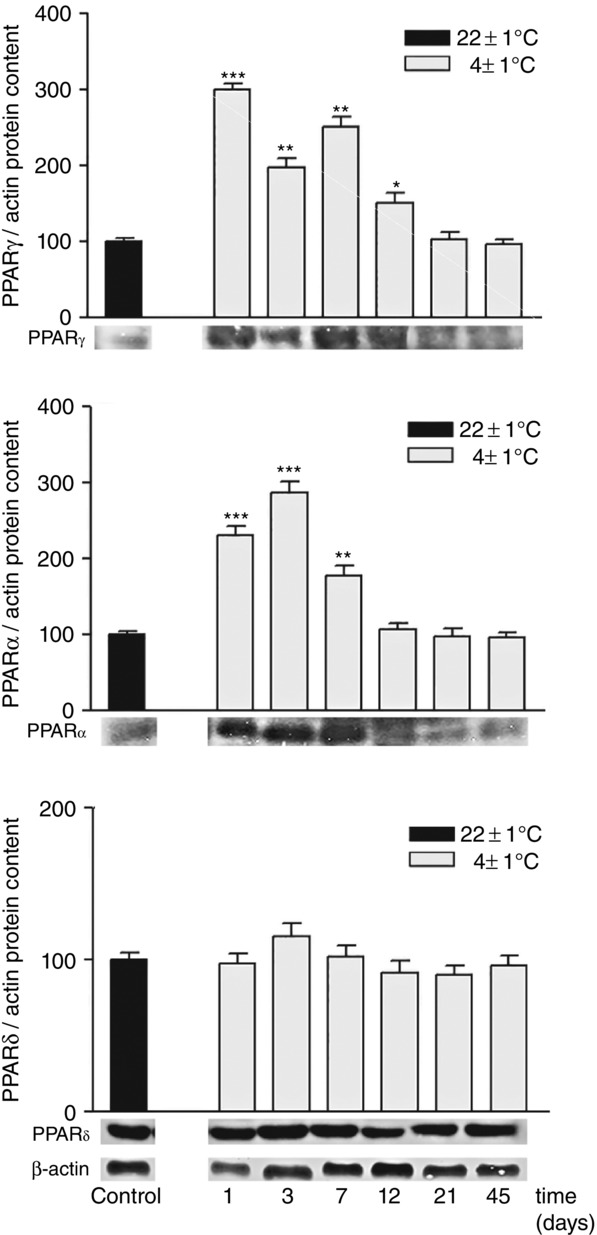
Time-dependent changes in expression of PPARγ, PPARα and PPARδ in rat rpWAT during 45-day cold acclimation
Protein content is expressed relative to control rats acclimated to room temperature (100%). Experiments were repeated in triplicate. Data were quantified as described in Methods. The values represent the means ± SEM. Compared to control, *P < 0.05, **P < 0.01 and ***P < 0.001.
PGC-1α mRNA and PGC-1α protein levels were measured in rpWAT, and the subcellular distribution of PGC-1α protein was investigated by immunomicroscopic analyses. PGC-1α mRNA was almost absent in the rpWAT of control rats, but there was a noticeable protein expression of PGC-1α. PGC-1α transcripts were sharply increased after 1 day of cold exposure (P < 0.001), but decreased below control values thereafter (Fig.4A). However, protein expression of PGC-1α was increased above the control value in two time periods, initially following cold exposure (after 1 and 3 days) and after the cold acclimation. Immunohistochemistry shown in Fig.4B, and immunoelectron microscopy revealed the presence of PGC-1α protein in the flattened nuclei of unilocular adipocytes after 3 days of cold exposure. Only rounded nuclei of sporadic multilocular adipocytes in rpWAT were immunopositive for PGC-1α after the cold-acclimation period (Fig.4B).
Figure 4.
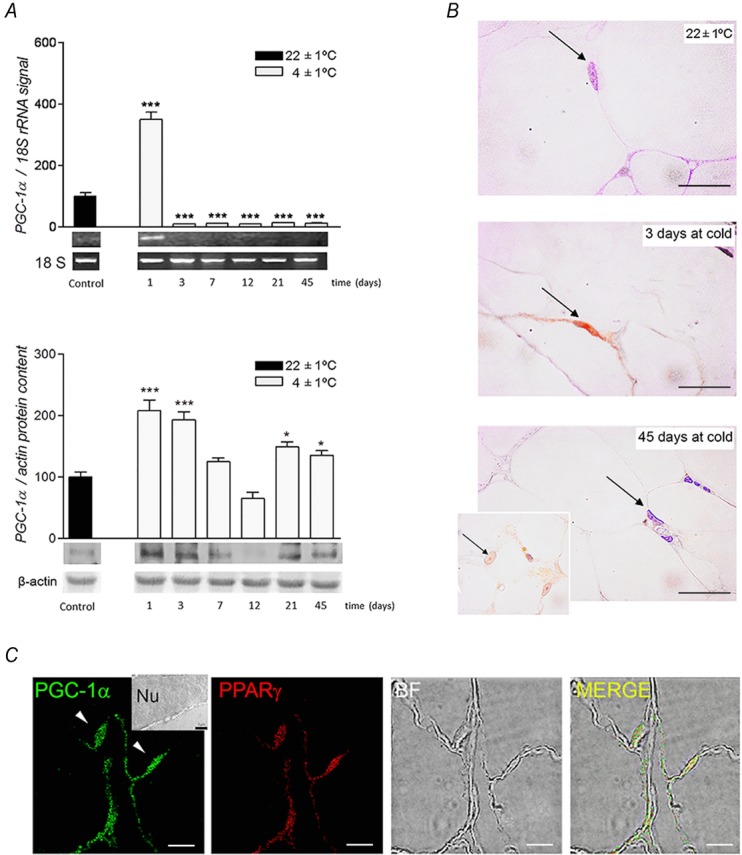
Expression levels, cellular and subcellular distribution of PGC-1α in rpWAT
A, time-dependent changes in PGC-1α mRNA and protein expression levels in rat rpWAT during cold acclimation. Samples were collected on day 1, 3, 7, 12, 21 and 45 of cold exposure. mRNA was quantified using Quantity One software, version 4.6.0. PGC-1α transcripts were normalized against the 18S rRNA house-keeping gene, while PGC-1α protein was normalized against the β-actin. Relative mRNA/protein levels are presented as the percentage compared with samples from control room temperature-acclimated rats (100%). Data are expressed as means ± SEM and represent three independent experiments with triplicate observations for each. Representative ethidium-stained agarose gels are shown. Compared to control, *P < 0.05 and ***P < 0.001. B, after 3 days of cold exposure, flattened nuclei of unilocular adipocytes were immunopositive for PGC-1α. After a 45-day cold acclimation, only round nuclei of multilocular brown/beige adipocytes were immunopositive for PGC-1α. Magnification 100×, oil immersion. Scale bars, 20 μm. C, co-localization of PGC-1α and PPARγ by double immunofluorescence in rpWAT adipocytes of rats exposed to cold for 3 days. RpWAT sections were double-labelled with anti-PGC-1α and anti-PPARγ antibodies, and fluorescence analysed by a dual-channel confocal microscope. PGC-1α and PPARγ are stained green and red, respectively. The merged image reveals superposition of the PGC-1α FITC signal (excitation/emission: 495 nm/519 nm) and PPARγ TRITC signal (excitation/emission: 547 nm/572 nm) inside the nuclei (yellow). Magnification 63×, oil immersion. Scale bar, 7.5 μm. Ultrastructural immunolocalization of PGC-1α protein on ultrathin sections (inset) revealed presence of gold particles in flattened nucleus of unilocular white adipocyte. Magnification 15,000×, orig.
Double-immunofluorescence staining revealed colocalization of PGC-1α and PPARγ, the master regulator of adipogenic/thermogenic programmes, in the flattened nuclei of rpWAT adipocytes after 3 days of cold exposure (Fig.4C).
Signs of adaptive thermogenesis at the tissue level
Samples from control rpWAT contained unilocular large adipocytes with a single large central lipid vacuole and a very narrow layer of cytoplasm with a flattened nucleus at the cell periphery. On day 3 of the cold-acclimation period, rpWAT exhibited an increased number of paucilocular adipocytes that contained a large central lipid droplet surrounded by several smaller lipid droplets and a thicker layer of cytoplasm with a greater number of electron dense, rounded mitochondria (Fig.5A). After 45 days of cold acclimation, unilocular/paucilocular cells were the predominant adipocyte type. However, sporadic polygonal adipocytes with multilocular organization (many small lipid droplets), central rounded nuclei and a high number of large mitochondria were observed (Fig.5A and B). Additional magnification of this region (Fig.5B) revealed a granular cytoplasm containing mitochondria in all rpWAT adipocytes regardless of the lipid vacuole organization. There was, however, a clear gradation in the abundance and size of mitochondria present in adipocytes of different morphological appearance, with the number and size increasing with multilocularization of the adipocytes (Fig.5B, insets).
Figure 5.
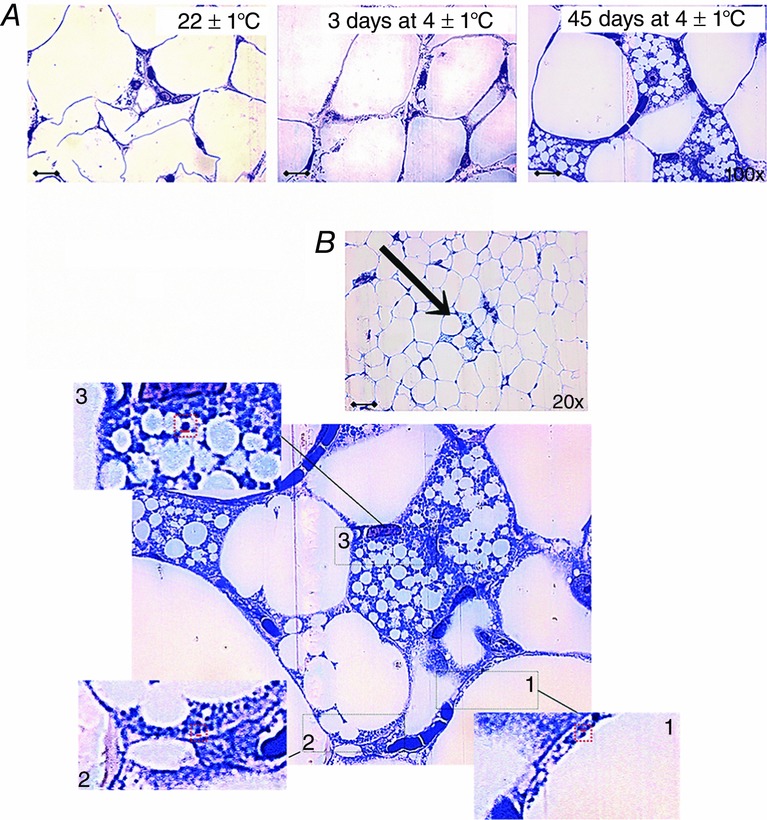
Signs of browning in rpWAT at the cellular level after 3 and 45 days of cold acclimation
A, multiloculation of lipid droplets and the appearance of the paucilocular adipocytes in rpWAT after 3 days of cold exposure. The majority of adipocytes exhibited paucilocular lipid organization (one dominant lipid droplet), but some individual polygonal cells contained multilocular lipid droplets after 45-day cold acclimation. Scale bars = 20 μm. B, enlargement of the area of rpWAT from rats exposed to cold for 45 days (indicated by an arrow). A large number of mitochondria are evident in all rpWAT adipocytes after 45 days of cold acclimation, whereas a clear gradation in the number and size of mitochondria is evident among neighbouring adipocytes of different morphology: (1) unilocular; (2) paucilocular and (3) multilocular. Multilocular adipocytes (3) are characterized by the largest and most densely packed mitochondria. Insets: enlargement of the framed areas showing differences in mitochondrial size. Magnification 100×, oil immersion.
Significant immunostaining for UCP1 (green fluorescence) was found only in a few scattered multilocular adipocytes of brown-like appearance in 45-day cold-exposed rats (Fig.6B). The vast majority of paucilocular/unilocular adipocytes were therefore negative for UCP1 in rpWAT after 45 days of cold acclimation. This was in contrast to the non-selective UCP1 staining in rpWAT after 3 days of cold exposure (Fig.6A).
Figure 6.
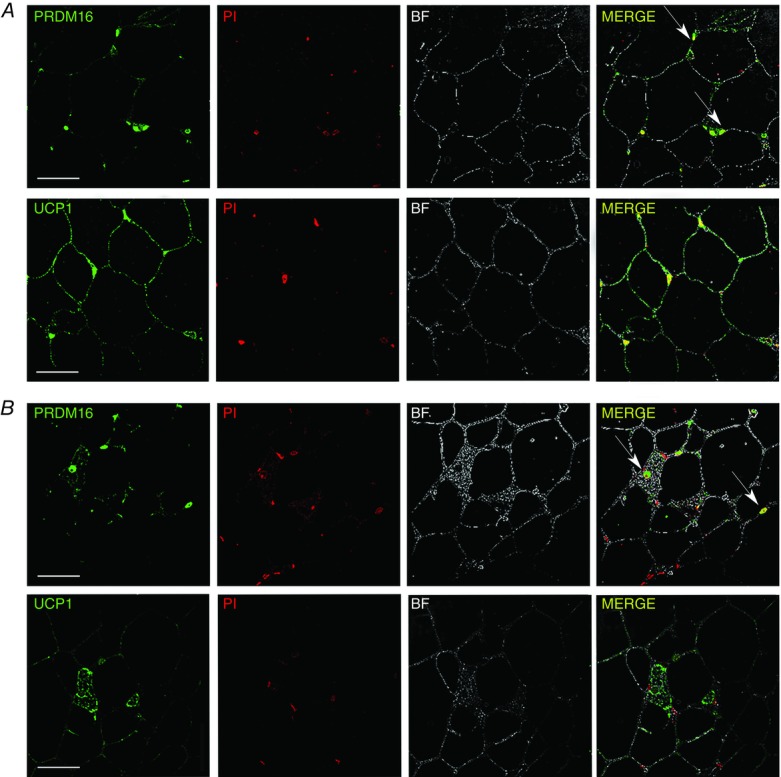
Immunofluorescence of UCP1 and PRDM16 in rpWAT of rats maintained at 4 ± 1°C for 3 (A) or 45 days (B)
Immunofluorescence labelling of unilocular/paucilocular and multilocular adipocytes for UCP1 (green) showing all adipocytes in rpWAT pass through the brown-adipocyte-like molecular stage during the early stages of cold acclimation, as demonstrated by the elevated UCP1 levels (A). After cold acclimation, however (B), only a small number of multilocular adipocytes exhibit brown-like appearance and were immunopositive for UCP1. PRDM16 protein levels do not directly correlate with the recruitment status of rpWAT adipocytes. PRDM16-immunopositive nuclei are visible in rpWAT adipocytes after 3 (A) and 45 (B) days of cold acclimation regardless of adipocyte appearance. Furthermore, the PRDM16 signal was diminished in multilocular (UCP1-expressing) adipocytes compared with adjacent unilocular/paucilocular (UCP1-negative) adipocytes after a 45-day cold acclimation (B). PRDM16 immunofluorescence (green) and staining of cell nuclei with propidium iodide (red) resulted in PRDM16 appearing yellow in the merged image. Nuclei of PRDM16-positive adipocytes are marked with arrows. UCP1 and PRDM16 were not stained and therefore absent in control samples (not shown). Scale bars = 50 μm.
To further characterize the relationship between PRDM16 and UCP1 expression in rpWAT adipocytes following cold acclimation, immunohistochemical analysis was performed. PRDM16 staining was in all rpWAT adipocytes tested, irrespective of the recruitment state, UCP1 expression and cell appearance (Fig.6A and B). This led us to assume that PRDM16 is only needed for thermogenic activation of rpWAT adipocytes, whereas it is not involved in the maintenance of the thermogenic capacity of rpWAT cells following 45 days of cold acclimation.
ETC components in rpWAT
Compared to room-temperature acclimated controls, protein expressions of the UQCRC2 (a constituent of complex III/ubiquinol-cytochrome c reductase) and cytochrome c were increased from the beginning of cold acclimation in rpWAT of cold exposed rats (Fig.7).
Figure 7.

Time-dependent changes in protein expression of complex III (UQCRC2) and cytochrome c in rat rpWAT during 45-day cold acclimation
Protein content is expressed relative to control rats acclimated to room temperature (100%). Experiments were repeated in triplicate. Data were quantified as described in Methods. The values represent the means ± SEM. Compared to control, *P < 0.05, **P < 0.01 and ***P < 0.001.
Discussion
The present study showed that browning of rpWAT during a 45-day cold acclimation involves two temporally apparent components: (1) a predominant non-selective browning of most adipocytes and an initial sharp but transient induction of PGC-1α, PPARγ and PPARα expression, and (2) the subsistence of relatively few thermogenically competent adipocytes after completion of the cold-acclimation period. Although all adipocytes within rpWAT share aspects of thermogenic induction, the differences in behaviour of the different rpWAT beige adipocyte subsets over long-term cold acclimation reflect their distinct adaptive capacity, discrete functions and development. Therefore, browning of WAT is dependent on both maintaining the thermogenic response and retaining enough brown-like thermogenically competent adipocytes in the long term to facilitate body-weight regulation and avoid obesity.
The substantial increase in energy expenditure induced by chronic sympathetic stimulation is associated with thermogenic activation in UCP1 expressing adipocytes, both in BAT and WAT depots (Himms-Hagen et al. 1994; Ghorbani et al. 1997).
WAT depots are principally devoid of UCP1 at thermoneutrality, and cold exposure leads to molecular browning, with some UCP1 expression in animals kept at room temperature (∼20°C), but much more in animals maintained at cold temperatures (∼4°C) (Walden et al. 2012; Nedergaard & Cannon, 2014). These findings are consistent with those of previous studies (Loncar, 1991; Giordano et al. 1998; Himms-Hagen et al. 2000; Frontini & Cinti, 2010) showing increased expression of UCP1 in WAT depots after 7–10 days of cold exposure. Our results showed increased UCP1 mRNA and protein levels in rpWAT from 1 to 7, i.e. from 1 to 21 days of cold exposure, respectively. The UCP1 gene is regulated at the transcription level (Ricquer et al. 1986), but (post)translational mechanisms (protein stability/turnover) of regulation play important role in the regulation of the final UCP1 protein amount (Jacobsson et al. 1987; Patel et al. 1987). Usually, there is an obligatory time delay of ∼10 days in the UCP1 mRNA and protein levels during the different transition phases including during control-to-cold transitions (Nedergaard et al. 2001), reflecting a significantly longer UCP1 protein half-life (2–20 days, usually 5 days), as compared with its mRNA half-life (3 h) in vivo (Jacobsson et al. 1987; Patel et al. 1987). Accordingly, the delay in the maximal increase and the return to the control values of UCP1 protein, compared to faster time course changes in its mRNA levels in the rpWAT, on cold acclimation agrees with data in the literature. Lastly, UCP1 incorporation into mitochondria, as observed in rpWAT mitochondria after 3 days of cold, significantly stabilizes UCP1 protein half-life (Puigserver et al. 1992) and may explain the time discrepancy/delay in the UCP1 protein vs. mRNA profile in rpWAT.
Transcriptional regulation of UCP1 is controlled by members of the PPAR family, primarily PPARγ (Kozak & Anunciado-Koza, 2008). Essential to this regulation is a peroxisome proliferator response element (PPRE) site in the distal UCP1 enhancer that forms a complex with the coactivator PGC-1α (Tontonoz et al. 1994; Sears et al. 1996; Puigserver et al. 1998). PPARγ controls the expression of the WAT-characteristic genes also (Rosen & Spiegelman, 2001), and its tissue- and depot-distinct effects of PPARγ towards brown, energy dissipating, or white, energy saving, adipocytes is determined by the tissue-specific presence of PGC-1α (Tiraby & Langin, 2003; Laplante et al. 2006). Compared to BAT, muscle and some others tissues with a high oxidative metabolism, WAT, characterized by a lower oxidative metabolism capacity, expresses only minute levels of PGC-1α. However, forced expression of PGC-1α (for example in primary culture of adipocytes by PGC-1α adenovirus) accompanies the induction of UCP1 mRNA expression in these adipocytes (Tiraby et al. 2003). Besides, among agonists for different PPARs, rosiglitazone, a PPARγ agonist, was the most potent stimulator of UCP1 in white adipocytes (Tiraby et al. 2003). Therefore, we investigated the association between PPARs and PGC-1α expression and browning in rpWAT during cold acclimation, and revealed the following. (i) UCP1 mRNA levels increased in parallel with PPARγ and PPARα during days 1–7 of cold exposure. (ii) PGC-1α mRNA increased dramatically on the first day of cold exposure, and by day 3 the flattened nuclei of the unilocular adipocytes remained PGC-1α immunopositive. (iii) PGC-1α colocalized with PPARγ at the time of the most significant increase of UCP1 (after 3 days of cold exposure) in typically unilocular white adipocytes of rpWAT. All this strongly indicates that the PGC-1α–PPARγ signalling cascade plays an important role in the induction and control of UCP1 expression in rpWAT on cold exposure. UCP1 protein levels in rpWAT are therefore regulated at the transcriptional level by induction of transcriptional regulators that are characteristic of genuine BAT.
In parallel with these molecular changes, signs of delipidization and transdifferentiation of uni- into paucilocular adipocytes were revealed in rpWAT during the early stages of cold exposure. Thus, it seems that beigeing/browning of white UCP1-expressing adipocytes is dependent on the prevailing metabolic conditions and these cells may appear as uni- or paucilocular (expressing UCP1). Notably, browning is not simply due to a single class of adipocytes (beige), but rather all the rpWAT adipocytes possess some capacity for UCP1 expression under acute cold exposure.
The total level of UCP1 protein in BAT on cold acclimation increases due to BAT proliferation, i.e. due to the total amount of protein in the BAT increase (Nedergaard & Cannon, 2013). When expressed per mg tissue protein (thus annulling the effect of total tissue protein increase), UCP1 protein content also increases throughout 1 month in the cold, then decreases ∼50% from this maximal level (Petrovic et al. 2010; Nedergaard & Cannon, 2013; Gospodarska et al. 2015), but still remains increased significantly (∼2-fold) above the room-temperature maintained control during the whole 45-day acclimation time (Petrovic et al. 2005, 2010). A similar trend in UCP1 protein level seems to exist in the brite/beige cells appearing after the cold acclimation (Shabalina et al. 2013), and the results of this study showing specifically UCP1 immunopositive brown-like adipocytes after 45 days of cold support this. This is, however, in contrast to UCP1 protein level in whole rpWAT and typically white adipocytes on cold acclimation where the level of UCP1 per mg protein sharply, but transiently, increases, and on day 45 of cold returns to the initial level. Thus, in contrast to genuine BAT depots such as interscapular BAT, where the upregulation of UCP1 and the resultant non-shivering thermogenesis enables cold acclimation (Cannon & Nedergaard, 2004), a short-lived UCP1 protein level increase in rpWAT homogenates suggests that the browning is much less sustainable in most WAT.
The number of multilocular brown-like adipocytes appearing after some weeks upon cold exposure in other WAT depots, like the inguinal depot, may be higher (Walden et al. 2012), but in rpWAT, only sporadic UCP1 immunopositive adipocytes were detected. These cells quite resembled classic brown adipocytes (multilocular lipid droplet organization, oval nuclei in a central position, polygonal cell shape and numerous swollen mitochondria) and had numerous adjacent capillaries. To investigate the temporal mismatch of the cold-induced browning of rpWAT at the molecular and structural level, the brown-like adipocytes were assessed after 45 days of cold acclimation. PRDM16 acts as a transcriptional coregulator that controls the development of brown adipocytes in brown adipose tissue. Previously, this coregulator was believed to be present only in BAT, but more recent studies have shown that PRDM16 is also highly expressed in subcutaneous WAT (Seale et al. 2011). The activity of PRDM16 in white adipose tissue leads to the production of brown fat-like adipocytes and significant up-regulation of UCP1. We therefore expected that PRDM16 would be a marker of brown adipocytes present only in the nuclei of brown-like UCP1-expressing adipocytes that appeared after 45 days of cold acclimation. Unexpectedly, immunohistochemical analysis revealed the presence of PRDM16 in most rpWAT adipocytes, which suggested that PRDM16 may be involved in the thermogenic phenotype activation, but not in its maintenance in rpWAT adipocytes. These results, along with others confirming the presence in PRDM16 in paucilocular adipocytes expressing UCP1 during early cold exposure, indicated that all rpWAT adipocytes may switch molecular phenotype from white to beige/brown cells. Indeed, it appears that all rpWAT adipocytes can undergo beigeing, but cannot brown.
Uncoupling of ATP synthesis in unilocular cells exerts strong effects on their metabolic profile, including stimulation of catabolic activity and inhibition of lipogenesis that together ensure that ATP is restored/spared for essential functions (Kopecky et al. 2001). Consistent with this, our previous study showed a continuous upregulation of AMP-activated protein kinase (AMPK) in rpWAT, starting from day 3 of cold exposure (Jankovic et al. 2013). Thus, an energy-saving adaptation may be responsible for the observed reversion to initial UCP1 mRNA and protein levels in most rpWAT adipocytes after 7 and 21 days of cold acclimation, respectively. Retention of elevated UCP1 levels in a few adipocytes after cold acclimation may be associated with complete thermogenic recruitment of a limited number of cells, which may be connected, at least in part, with PGC-1α-mediated mitochondrial biogenesis/function. Thermogenic reprogramming is rather costly, even for brown adipocytes, and elevated AMPKα is observed in BAT also, but only on the first day of cold exposure (Vucetic et al. 2011), which is entirely correlated with the fat-burning capacity that is determined by increased beta-oxidation and higher mitochondrial number. Consistent with this, the presence of PGC-1α in the nuclei of a small number of multilocular cells in rpWAT that contain many mitochondria and retain elevated UCP1 expression after cold acclimation indicated that PGC-1α may be important for determining the phenotype of different rpWAT adipocyte subsets.
Thermogenesis in BAT, in addition to the large increase in UCP1, is dependent on the complex morphological recruitment of BAT for mitochondrial biogenesis, swelling, reorganization and neovascularization (Cannon & Nedergaard, 2004; Petrovic et al. 2010). All of these processes are needed for the production and distribution of heat, and are partially stimulated in rpWAT during the early stages of cold acclimation. Besides, the mitochondrial respiratory machinery needs to be upregulated with UCP1 to substantially increase the thermogenic activity and/or capacity of the tissue. Some ETC components in rpWAT (complex III-UQCRC2, cytochrome c) were indeed increased from the beginning of cold acclimation. Also, increased levels of enzymes involved in substrate oxidation (glucose and subsequently fatty acids) observed in our previous study indicate a higher respiratory potential/capacity of rpWAT in this time (from 3 to 21 days) of cold exposure. This may be related with the BAT-like phenotype of rpWAT early on cold exposure. Indeed the random cristae organization of UCP1-containing mitochondria in unilocular adipocytes of rpWAT after 3 days of cold exposure does not support their powerful activation. Besides, the results of our previous study revealed delayed increase in subunit IV of cytochrome c oxidase and ATP synthase (from day 21 of cold acclimation) as compared to UCP1, observed previously on cold exposure (Jankovic et al. 2013).
A burst of molecular activity accompanied by incomplete morphological recruitment appears to be triggered in rpWAT during early cold acclimation as part of the complex metabolic remodelling, expansion of catabolic capacity, and excessive fatty acid mobilization (Li et al. 2005). This paradoxical increase of UCPs in the muscle in energy turnover-attenuating conditions (Millet et al. 1997; Weigle et al. 1998; Samec et al. 1999), or after high fat feeding (Schrauwen et al. 2001) seems to be associated with a fat-metabolism-mediated effect, and argues against their role in energy dissipation (Schrauwen et al. 2002). In a similar way, transient increase of UCP1 in rpWAT may also be an effect of increased lipolytic activity that is most intensive during early cold exposure, and not of the cold stimulus (adrenergic stimulation) per se. Besides, recent data suggest that UCP1 induction in WAT primarily prevents a strong increase of electrochemical potential and consequent increment of reactive oxygen species production in response to increased substrate oxidation (Carriere et al. 2014). Accordingly, UCP1 increases in rpWAT in parallel with indications of substrate oxidation (glucose, and subsequently fatty acids) and antioxidant defence (Jankovic et al. 2009, 2013) indicating that thermogenic/antioxidant effects of UCP1 in rpWAT are in fact a part of the same phenomenon (Skulachev, 1998). The experiments investigating mitochondrial bioenergetics (mitochondrial respiration before and after the addition of different metabolic substrates) in detail in rpWAT samples of rats exposed to cold for different time periods would further reveal the thermogenic capacity of UCP1 expressing adipocytes in rpWAT. The results of Shabalina et al. have shown that recruited beige/brite adipocytes occurring after cold acclimation are as thermogenically competent as genuine brown adipocytes (Shabalina et al. 2013). Still, the thermogenic potential of unilocular white adipocytes in WAT remains to be determined. Our efforts along these lines are in progress.
In summary, the amount of UCP1 protein in rpWAT is regulated at the transcriptional level by induction of transcriptional regulators that are characteristic of genuine BAT. Cold-induced browning and the molecular changes involved appear to occur to some degree in all classes of adipocytes in rpWAT, not just in beige adipocytes as previously believed. However, only relatively few cells that undergo these changes early in cold exposure retain the full brown-like phenotype, while most revert to the pre-cold-exposed state. There are therefore two key temporally distinguishable components of WAT browning: (1) the initial, sharp, but transient non-selective browning of most of adipocytes, and (2) the maintenance of a few brown-like adipocytes in the long-term. Regulation of both components may influence body weight and the potential success of anti-obesity therapies.
Glossary
- BAT
brown adipose tissue
- PGC-1α
PPARγ-coactivator-1α
- PPARs
peroxisome proliferator-activated receptors
- PRDM16
PR domain-containing 16
- rpWAT
rat retroperitoneal white adipose tissue
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
Additional information
Competing interests
The authors declare no conflict of interest.
Author contributions
A.J. performed experiments, interpreted data and drafted the paper. I.G. and M.M. interpreted data. A.S., V.O. and B.B. critically revised the manuscript. A.K. designed experiments, interpreted data and critically revised the manuscript. B.K. designed experiments, interpreted data and critically revised the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions. All experiments were done in Department of Physiology, Institute for Biological Research ‘Sinisa Stankovic’ and the Centre for Electron Microscopy, Faculty of Biology, University of Belgrade.
Funding
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grants No 173054 and 173055.
References
- Cannon B. Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F. Casteilla L. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63:3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P. Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L. Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E. Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A. Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Ghorbani M, Claus TH. Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a β3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- Giordano A, Morroni M, Carle F, Gesuita R, Marchesi GF. Cinti S. Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation. J Cell Sci. 1998;111:2587–2594. doi: 10.1242/jcs.111.17.2587. [DOI] [PubMed] [Google Scholar]
- Gospodarska E, Nowialis P. Kozak LP. Mitochondrial turnover: A phenotype distinguishing brown adipocytes from interscapular brown adipose tissue and white adipose tissue. J Biol Chem. 2015;290:8243–8255. doi: 10.1074/jbc.M115.637785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggio MA. Thermogenic mechanisms in cold-acclimated animals. Braz J Med Biol Res. 1988;21:171–176. [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K. Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes D, Jang SS, Waters BL. Claus TH. Effect of CL-316243 a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G. Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Ishibashi J. Seale P. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson A, Cannon B. Nedergaard J. Physiological activation of brown adipose tissue destabilizes thermogenin mRNA. FEBS Lett. 1987;224:353–356. doi: 10.1016/0014-5793(87)80483-0. [DOI] [PubMed] [Google Scholar]
- Janković A, Buzadžić B, Korać A, Petrović V, Vasilijević A. Korać B. Antioxidative defense organization in retroperitoneal white adipose tissue during acclimation to cold – the involvement of L-arginine/NO pathway. J Therm Biol. 2009;34:358–365. [Google Scholar]
- Jankovic A, Korac A, Buzadzic B, Otasevic V, Stancic A, Vucetic M, Markelic M, Velickovic K, Golic I. Korac B. Endocrine and metabolic signaling in retroperitoneal white adipose tissue remodeling during cold acclimation. J Obes. 2013;2013:937572. doi: 10.1155/2013/937572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S. Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014;76:225–249. doi: 10.1146/annurev-physiol-021113-170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecký J, Rossmeisl M, Flachs P, Bardová K. Brauner P. Mitochondrial uncoupling and lipid metabolism in adipocytes. Biochem Soc Trans. 2001;29:791–797. doi: 10.1042/bst0290791. [DOI] [PubMed] [Google Scholar]
- Kozak LP. Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond) 2008;32:S32–38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak LP. The genetics of brown adipocyte induction in white fat depots. Front Endocrinol (Lausanne) 2011;2:64. doi: 10.3389/fendo.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Festuccia WT, Soucy G, Gélinas Y, Lalonde J, Berger JP. Deshaies Y. Mechanisms of the depot specificity of peroxisome proliferator-activated receptor γ action on adipose tissue metabolism. Diabetes. 2006;55:2771–2778. doi: 10.2337/db06-0551. [DOI] [PubMed] [Google Scholar]
- Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R. Parker MG. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci USA. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhu Z, Lu Y. Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-α. Am J Physiol Endocrinol Metab. 2005;289:E617–626. doi: 10.1152/ajpendo.00010.2005. [DOI] [PubMed] [Google Scholar]
- Loncar D, Bedrica L, Mayer J, Cannon B, Nedergaard J, Afzelius BA. Svajger A. The effect of intermittent cold treatment on the adipose tissue of the cat. Apparent transformation from white to brown adipose tissue. J Ultrastruct Mol Struct Res. 1986;97:119–129. doi: 10.1016/s0889-1605(86)80012-x. [DOI] [PubMed] [Google Scholar]
- Loncar D. Convertible adipose tissue in mice. Cell Tissue Res. 1991;266:149–161. doi: 10.1007/BF00678721. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL. Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Millet L, Vidal H, Andreelli F, Larrouy D, Riou JP, Ricquier D, Laville M. Langin D. Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. J Clin Invest. 1997;100:2665–2670. doi: 10.1172/JCI119811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano I, Zingaretti CM. Cinti S. The adipose organ of Sv129 mice contains a prevalence of brown adipocytes and shows plasticity after cold exposure. Adipocytes. 2005;1:121–130. [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A. Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Petrović N, Lindgren EM, Jacobsson A. Cannon B. PPARγ in the control of brown adipocyte differentiation. Biochim Biophys Acta. 2005;1740:293–304. doi: 10.1016/j.bbadis.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Nedergaard J. Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta. 2013;1831:943–949. doi: 10.1016/j.bbalip.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Nedergaard J. Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Patel HV, Freeman KB. Desautels M. Selective loss of uncoupling protein mRNA in brown adipose tissue on deacclimation of cold-acclimated mice. Biochem Cell Biol. 1987;65:955–959. doi: 10.1139/o87-124. [DOI] [PubMed] [Google Scholar]
- Peralta JG, Finocchietto PV, Converso D, Schöpfer F, Carreras MC. Poderoso JJ. Modulation of mitochondrial nitric oxide synthase and energy expenditure in rats during cold acclimation. Am J Physiol Heart Circ Physiol. 2003;284:H2375–H2383. doi: 10.1152/ajpheart.00785.2002. [DOI] [PubMed] [Google Scholar]
- Petrović V, Korać A, Buzadzić B. Korać B. The effects of L-arginine and L-NAME supplementation on redox-regulation and thermogenesis in interscapular brown adipose tissue. J Exp Biol. 2005;208:4263–4271. doi: 10.1242/jeb.01895. [DOI] [PubMed] [Google Scholar]
- Petrović V, Buzadzić B, Korać A, Vasilijević A, Janković A. Korać B. NO modulates the molecular basis of rat interscapular brown adipose tissue thermogenesis. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152:147–159. doi: 10.1016/j.cbpc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Puerta ML. Abelenda M. Cold acclimation in food-restricted rats. Comp Biochem Physiol A Comp Physiol. 1987;87:31–33. doi: 10.1016/0300-9629(87)90420-8. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Herron D, Gianotti M, Palou A, Cannon B. Nedergaard J. Induction and degradation of the uncoupling protein thermogenin in brown adipocytes in vitro and in vivo. Evidence for a rapidly degradable pool. Biochem J. 1992;284:393–398. doi: 10.1042/bj2840393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M. Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Ricquier D, Bouillaud F, Toumelin P, Mory G, Bazin R, Arch J. Pénicaud L. Expression of uncoupling protein mRNA in thermogenic or weakly thermogenic brown adipose tissue. Evidence for a rapid β-adrenoreceptor-mediated and transcriptionally regulated step during activation of thermogenesis. J Biol Chem. 1986;261:13905–13910. [PubMed] [Google Scholar]
- Rosen ED. Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- Samec S, Seydoux J. Dulloo AG. Skeletal muscle UCP3 and UCP2 gene expression in response to inhibition of free fatty acid flux through mitochondrial β-oxidation. Pflugers Arch. 1999;438:452–457. doi: 10.1007/s004249900080. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hoppeler H, Billeter R, Bakker AH. Pendergast DR. Fiber type dependent upregulation of human skeletal muscle UCP2 and UCP3 mRNA expression by high-fat diet. Int J Obes Relat Metab Disord. 2001;25:449–456. doi: 10.1038/sj.ijo.0801566. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hesselink MK, Vaartjes I, Kornips E, Saris WH, Giacobino JP. Russell A. Effect of acute exercise on uncoupling protein 3 is a fat metabolism-mediated effect. Am J Physiol Endocrinol Metab. 2002;282:E11–E17. doi: 10.1152/ajpendo.2002.282.1.E11. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S. Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears IB, MacGinnitie MA, Kovacs LG. Graves RA. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor γ. Mol Cell Biol. 1996;16:3410–3419. doi: 10.1128/mcb.16.7.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EA, Scott JW. Thomas N. Electrical activity of skeletal muscle of normal and acclimatized rats on exposure to cold. Am J Physiol. 1954;177:372–376. doi: 10.1152/ajplegacy.1954.177.3.372. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B. Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Stancic A, Buzadzic B, Korac A, Otasevic V, Jankovic A, Vucetic M, Markelic M, Velickovic K, Golic I. Korac B. Regulatory role of PGC-1α/PPAR signaling in skeletal muscle metabolic recruitment during cold acclimation. J Exp Biol. 2013;216:4233–4241. doi: 10.1242/jeb.089334. [DOI] [PubMed] [Google Scholar]
- Tiraby C. Langin D. Conversion from white to brown adipocytes: a strategy for the control of fat mass? Trends Endocrinol Metab. 2003;14:439–441. doi: 10.1016/j.tem.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D. Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem. 2003;278:33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E. Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Duncan JS. Rayner DV. Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice: mediation by the sympathetic system. Biochem J. 1995;311:729–733. doi: 10.1042/bj3110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, Schrauwen P. van Marken Lichtenbelt WD. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P. Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vucetic M, Otasevic V, Korac A, Stancic A, Jankovic A, Markelic M, Golic I, Velickovic K, Buzadzic B. Korac B. Interscapular brown adipose tissue metabolic reprogramming during cold acclimation: Interplay of HIF-1α and AMPKα. Biochim Biophys Acta. 2011;1810:1252–1261. doi: 10.1016/j.bbagen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Walden TB, Hansen IR, Timmons JA, Cannon B. Nedergaard J. Recruited versus nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–E31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- Weigle DS, Selfridge LE, Schwartz MW, Seeley RJ, Cummings DE, Havel PJ, Kuijper JL. BeltrandelRio H. Elevated free fatty acids induce uncoupling protein 3 expression in muscle: a potential explanation for the effect of fasting. Diabetes. 1998;47:298–302. doi: 10.2337/diab.47.2.298. [DOI] [PubMed] [Google Scholar]
- Young P, Arch JR. Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]


