Abstract
Congenital diaphragmatic hernia (CDH) is characterised by a spectrum of lung hypoplasia and consequent pulmonary hypertension, leading to high morbidity and mortality rates. Moreover, CDH has been associated with an increase in the levels of pulmonary neuroendocrine factors, such as bombesin and ghrelin, and a decrease in the action of retinoic acid (RA). The present study aimed to elucidate the interaction between neuroendocrine factors and RA. In vitro analyses were performed on Sprague–Dawley rat embryos. Normal lung explants were treated with bombesin, ghrelin, a bombesin antagonist, a ghrelin antagonist, dimethylsulfoxide (DMSO), RA dissolved in DMSO, bombesin plus RA and ghrelin plus RA. Hypoplastic lung explants (nitrofen model) were cultured with bombesin, ghrelin, bombesin antagonist or ghrelin antagonist. The lung explants were analysed morphometrically, and retinoic acid receptor (RAR) α, β and γ expression levels were assessed via Western blotting. Immunohistochemistry analysis of RAR was performed in normal and hypoplastic lungs 17.5 days post-conception (dpc). Compared with the controls, hypoplastic lungs exhibited significantly higher RARα/γ expression levels. Furthermore considering hypoplastic lungs, bombesin and ghrelin antagonists decreased RARα/γ expression. Normal lung explants (13.5 dpc) treated with RA, bombesin plus RA, ghrelin plus RA, bombesin or ghrelin exhibited increased lung growth. Moreover, bombesin and ghrelin increased RARα/γ expression levels, whereas the bombesin and ghrelin antagonists decreased RARα/γ expression. This study demonstrates for the first time that neuroendocrine factors function as lung growth regulators, sensitising the lung to the action of RA through up-regulation of RARα and RARγ.
Key points
Retinoic acid (RA) and ghrelin levels are altered in human hypoplastic lungs when compared to healthy lungs. Although considerable data have been obtained about RA, ghrelin and bombesin in the congenital diaphragmatic hernia (CDH) rat model, neuroendocrine factors have never been associated with the RA signalling pathway in this animal model.
In this study, the interaction between neuroendocrine factors and RA was explored in the CDH rat model.
The authors found that normal fetal lung explants treated with RA, bombesin and ghrelin showed an increase in lung growth. Hypoplastic lungs presented higher expression levels of the RA receptors α and γ. Moreover bombesin and ghrelin supplementation, in vitro, to normal lungs increased RA receptor α/γ expression whereas administration of bombesin and ghrelin antagonists to normal and hypoplastic lungs decreased it.
These data reveal for the first time that there is a link between neuroendocrine factors and RA, and that neuroendocrine factors sensitise the lung to the RA action through RA receptor modulation.
Introduction
Congenital diaphragmatic hernia (CDH) is a severe developmental anomaly with a mean incidence of 1:3000 live births and a poorly understood aetiology (van den Hout et al. 2009; Keller et al. 2010; Nogueira-Silva et al. 2011). This congenital anomaly is characterised by a diaphragmatic defect that allows intra-thoracic herniation of abdominal organs and maldevelopment of the alveoli and pulmonary vessels (van Loenhout et al. 2009). Thus, newborns with CDH develop pulmonary hypoplasia and pulmonary hypertension and, consequently, severe respiratory failure. In fact, among the known causes of severe respiratory failure in newborns, CDH remains the most life threatening (Goshe et al. 2005).
Despite the increased understanding of the pathophysiology of CDH and recent advances in neonatal care, CDH remains a challenging condition that is associated with high morbidity and mortality rates (Pereira-Terra et al. 2015a; Pereira-Terra et al. 2015b). Thus, the development of a less invasive, safer and cheaper approach with minimum risk to the mother and the fetus is crucial (Leeuwen & Fitzgerald, 2014). To this end, a search for less invasive antenatal approaches that promote fetal lung growth has emerged.
Retinoic acid (RA), an active metabolite of vitamin A, binds to specific RA receptors (RARs and RXRs) and functions as an essential signal for lung growth and differentiation (Thébaud et al. 1999). Indeed, RA is involved in various phenomena related to lung morphogenesis, including the formation of the lung primordium, distal bud outgrowth, differentiation of epithelial cells and the distal mesenchyme, and promotion of alveolisation by inducing the formation of secondary septae. Additionally, the retinoid signalling pathway has been shown to be involved in CDH pathophysiology. For example, decreased plasma levels of retinol and retinol-binding protein are observed in individuals with CDH (Major et al. 1998). Furthermore, CDH has been observed in patients with deletions within chromosome 15q, which contains the gene encoding cellular retinoic acid binding protein (CRABP1) (Pober, 2007). In animal studies, double RAR knockouts (RARα and RARβ knockouts) were shown to exhibit unilateral lung agenesis and contralateral lung hypoplasia (Mendelsohn et al. 1994; Beurskens et al. 2009). Moreover, in a nitrofen-induced CDH rat model, which is the best animal model mimicking CDH, inhibition of the RA signalling pathway was reported to occur, and rescue of nitrofen-induced pulmonary hypertension in fetal rat lungs was observed upon maternal administration of RA (Antipatis et al. 1998; Asabe et al. 1999; Thébaud et al. 1999; Baptista et al. 2005; Sugimoto et al. 2008; Doi et al. 2010; Ruttenstock et al. 2011).
The neuroendocrine factors bombesin and ghrelin are produced by pulmonary neuroendocrine cells (PNECs) and appear to play important roles in lung development and maturation from intrauterine life to the postnatal period (Asabe et al. 1999). Furthermore, CDH has been associated with increased bombesin and ghrelin levels. Moreover early maternal administration of ghrelin during gestation was shown to attenuate pulmonary hypoplasia in a CDH animal model (Santos et al. 2006; Cutz et al. 2007). Therefore, the aim of this study was to evaluate the putative association between neuroendocrine factors (bombesin and ghrelin) and RA during normal and hypoplastic fetal lung development.
Methods
Ethical approval
Animal experiments were performed according to the Portuguese law for animal welfare (Diário da República, Portaria 1005/92). Animals were housed in an accredited animal facility and treated as specified by the recommendations of the NIH guidelines and the Guide for the Care and Use of Laboratory Animals, published by the National Academy Press (NIH publication No 85-23, revised 1996).
Animal model and experimental design
Female Sprague–Dawley rats (225 g; Charles River, Barcelona, Spain) were maintained in appropriate cages under controlled conditions and fed with commercial solid food. The rats were mated and checked daily for vaginal plugs. The day of plugging was defined as gestational day 0.5 for the purpose of time dating. According to the nitrofen-induced CDH rat model, randomly selected pregnant rats received 100 mg of nitrofen (2,4-dichlorophenyl-p-nitrophenylether) dissolved in 1 ml of olive oil via gavage on 9.5 dpc (days postconception) (hypoplastic group) (Nogueira-Silva et al. 2011). Control animals only received only olive oil (control group). The dams were anaesthetised with a pentobarbital overdose (150 mg kg−1i.p.) followed by decapitation. The fetuses then were removed through Caesarean section and killed by decapitation. For lung explant cultures, the fetuses were harvested at 13.5 dpc and their lungs dissected and incubated with different compounds or inhibitors (Table1). Lungs from 17.5 dpc fetuses were processed for immunohistochemistry.
Table 1.
Schematic representation of the study design
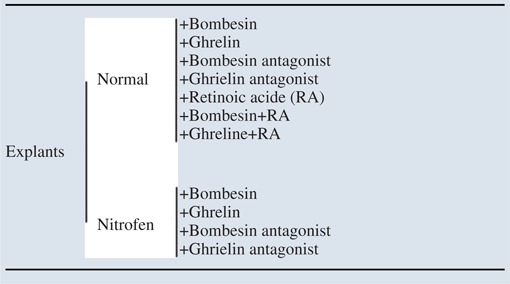 |
Immunohistochemistry
Immunostaining was performed in formalin-fixed and paraffin-embedded control and hypoplastic lungs at 17.5 dpc, as previously described (Nogueira-Silva et al. 2012). All the slides were stained at the same time. The RARα antibody (Santa Cruz Biotechnology Inc., Heidelberg, Germany) was used at a 1:200 dilution, the RARβ antibody (Santa Cruz Biotechnology Inc.) at a 1:50 dilution, and the RARγ antibody (Abcam, Cambridge, UK) at a 1:100 dilution. Incubation with UltraVision detection system anti-polyvalent horseradish peroxidase (Lab Vision Corporation, Fremont, CA, USA) was carried out according to the manufacturer’s instructions. Negative controls were performed by omitting the primary antibodies. The slides were photographed with an Olympus BX61 microscope (Olympus, Tokyo, Japan). At least three independent experiments were performed.
Fetal lung explant culture
Control and hypoplastic lungs were removed from 13.5 dpc embryos, harvested and dissected in DPBS (Lonza, Basel, Switzerland) under a dissection microscope (SZX16, Olympus). The lungs were then transferred to nucleopore membranes (Isopore membrane filters, Millipore, Darmstadt, Germany) and cultured for 4 days as previously described (Piairo et al. 2011).
The control and hypoplastic lung cultures were incubated daily with 1 μm bombesin (Tocris Bioscience, Bristol, UK) (Kresch et al. 1999) or 30 nm ghrelin (Peptides International, Louisville, KY, USA) (Nunes et al. 2008) and with one of the following treatments: 10−6 m RA (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.1% dimethylsulfoxide (DMSO), 0.1% DMSO alone, 1 μm bombesin plus 10−6 m RA, 30 nm ghrelin plus 10−6 m RA, 10−7 m bombesin antagonist (Phoenix Pharmaceuticals, Mannheim, Germany) (Hosli et al. 1993) or 10−6 m ghrelin antagonist (Bachem AG, Bubendorf, Switzerland) (Nunes et al. 2008). Thus, this set of experiments included the following 14 groups: control (n = 27), control plus ghrelin (n = 27), control plus bombesin (n = 27), control plus ghrelin antagonist (n = 27), control plus bombesin antagonist (n = 27), control plus DMSO (n = 27), control plus RA dissolved in DMSO (n = 27), control plus bombesin and RA (n = 27), control plus ghrelin and RA (n = 27), nitrofen (n = 27), nitrofen plus ghrelin (n = 27), nitrofen plus bombesin (n = 27), nitrofen plus bombesin antagonist (n = 27) and nitrofen plus ghrelin antagonist (n = 27). The culture medium was replaced every 48 h. Lungs were collected for Western blotting after 4 days in culture (96 h).
Morphometric analysis
Branching morphogenesis was monitored daily by photographing the explants using a stereomicroscope equipped with a camera (DP71, Olympus). At day 0 (D0: 0 h) and day 4 (D4: 96 h) of culture, branching, the epithelial and total perimeters, and the epithelial and total areas were measured using ImageJ image processing and analysis software (version 1.44; ImageJ, US National Institutes of Health, Bethesda, MD, USA).
For all experimental conditions, the results were expressed as the D4/D0 ratio.
Western blotting
Lung explants were processed for Western blot analysis using an RARα antibody at a 1:500 dilution, an RARβ antibody at a 1:200 dilution and an RARγ antibody (Santa Cruz Biotechnology Inc.) at a 1:500 dilution.
Protein lysates of pooled lung explants (n = 9) from each group were obtained via homogenisation of the fetal tissue with a pellet pestle motor (Kimble Kontes, Chicago, IL, USA) as previously described (Nogueira-Silva et al. 2013). Different pooled lung samples were used, and three independent experiments were performed. A quantity of 10 μg of protein was loaded onto 8% acrylamide minigels, and the procedure was performed as previously described (Piairo et al. 2011). The blots were incubated with RARα, RARβ or RARγ antibodies overnight at 4 °C, followed by an appropriate secondary anti-rabbit (1:5000 dilution) (Santa Cruz Biotechnology Inc.) or anti-mouse (1:2000 dilution) (Cell Signaling Technology, Danvers, MA, USA) horseradish peroxidase-conjugated antibody. The blots were subsequently developed with the Super Signal West Femto Substrate (Pierce Biotechnology, Waltham, MA, USA), and the chemiluminescent signal was captured using a Chemidoc XRS system (Bio-Rad, Philadelphia, PA, USA). As a loading control, the blots were probed with a β-tubulin antibody (1:150,000 dilution; Abcam). Quantitative analysis was performed using Quantity One analysis software (version 4.6.5 1D, Bio-Rad).
Statistical analysis
All quantitative data are presented as means ± SEM. Statistical analysis was performed via one-way ANOVA using SigmaStat 3.5 (Systat Software Inc., San Jose, CA, USA). The Bonferroni test was applied for post-test analysis. The threshold for statistical significance was set at P < 0.05.
Results
RAR expression pattern in normal and hypoplastic 17.5 dpc rat lungs
Immunohistochemistry analyses revealed that RARα, β and γ were expressed in the epithelial and mesenchymal cells of both control and hypoplastic lungs (Fig.1). Moreover, RARα and RARγ expression appeared to be higher in hypoplastic lungs than in control lungs (Fig.1).
Figure 1.
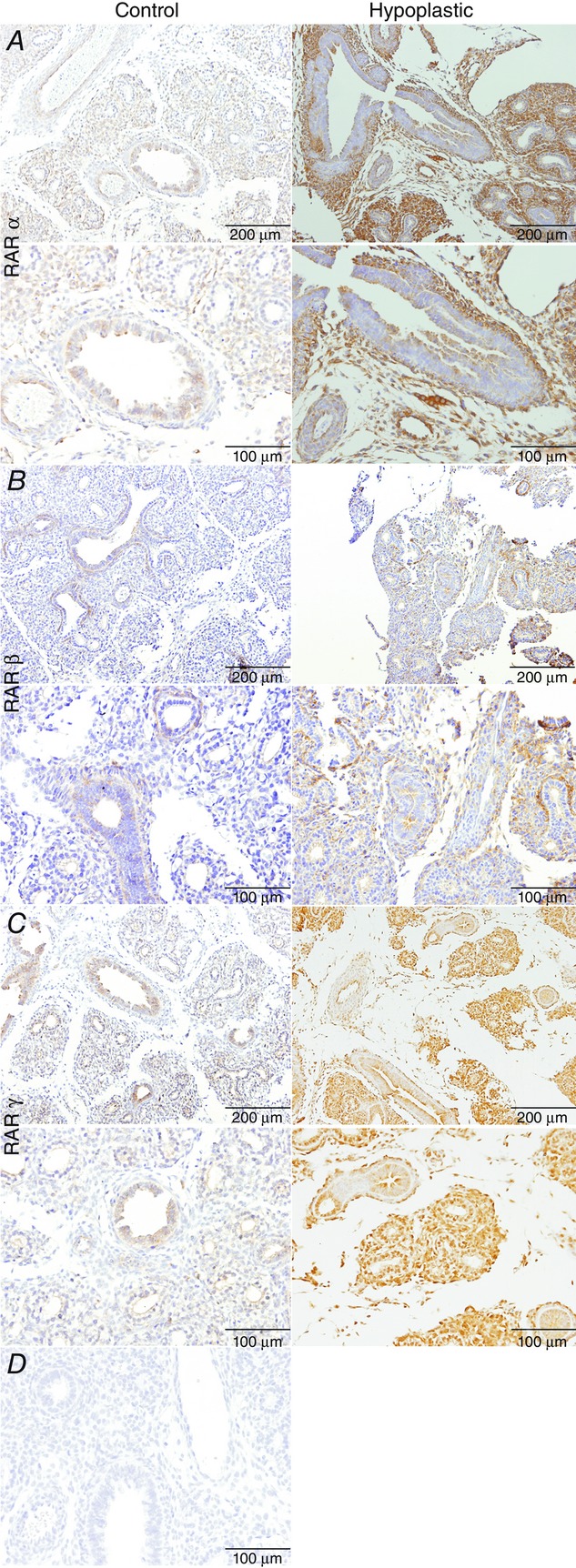
Retinoic acid receptor (RAR) expression patterns in 17.5 days post-conception rat lungs under normal and hypoplastic conditions
A, RARα. B, RARβ. C, RARγ. D, negative immunohistochemistry control: omission of the primary antibodies.
Bombesin, ghrelin, RA, bombesin plus RA and ghrelin plus RA supplementation analyses
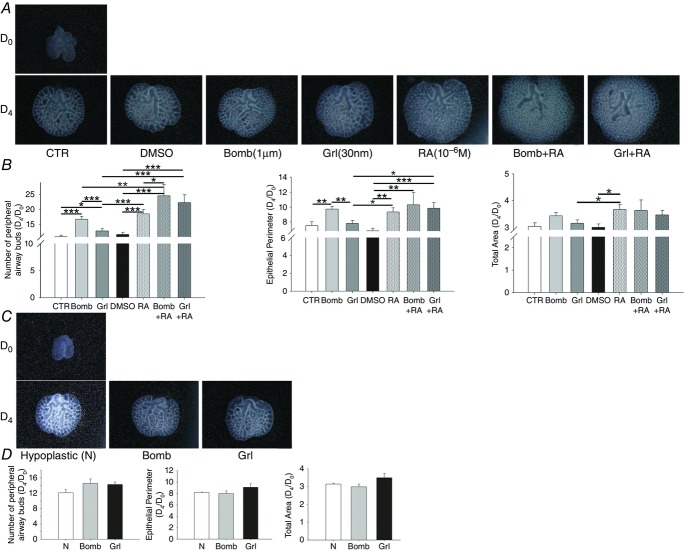
To evaluate the effects of neuroendocrine factors (bombesin and ghrelin), RA, and bombesin or ghrelin plus RA on lung growth, control and hypoplastic fetal lung explants were performed. Figure2A shows representative examples of control fetal lung explants treated with bombesin (1 μm), ghrelin (30 nm), RA (10−6 m), bombesin plus RA or ghrelin plus RA. In normal lungs, bombesin, ghrelin, RA and the combination of bombesin or ghrelin with RA appear to stimulate lung growth (Fig.2A). In fact, all of these compounds increased the total number of peripheral airway buds, and bombesin, RA, bombesin plus RA and ghrelin plus RA also increased the epithelial perimeter (Fig.2B). Moreover RA increases the total area of the lungs (Fig.2B).
Figure 2.
Branching morphogenesis of rat lung explant cultures treated with bombesin (Bomb), ghrelin (Grl), retinoic acid (RA), bombesin plus RA (Bomb + RA) or ghrelin plus RA (Grl + RA)
A, the upper panel is representative of normal lung explants (CTR) at day 0 (D0); the bottom panel represents untreated lung explants (CTR) and explants treated with DMSO, Bomb, Grl, RA, Bomb plus RA or Grl plus RA on day 4 (D4). B, morphometric analysis of the number of peripheral airway buds, epithelial perimeter and total area. C, the upper panel is representative of the hypoplastic lung explants (N) at D0; the bottom panel represents untreated hypoplastic lung explants (N) and hypoplastic explants treated with Bomb or Grl on D4. D, morphometric analysis of the number of peripheral airway buds, epithelial perimeter and total area. The results are expressed as the D4/D0 ratio. *P < 0.05; **P < 0.01; ***P < 0.001.
In contrast, bombesin and ghrelin did not interfere with hypoplastic lung growth, as shown in Fig.2C and confirmed through morphometric analysis (Fig.2D).
RAR protein expression after bombesin, ghrelin, bombesin plus RA, and ghrelin plus RA supplementation
To investigate the possible mechanisms underlying the effects of bombesin, ghrelin and neuroendocrine factors plus RA interactions, the protein levels of RARα, β and γ were assessed. RARα and RARγ expression levels were higher in untreated hypoplastic lungs than in control lungs (Fig.3A and C). Bombesin and ghrelin supplementation induced RARα and RARγ expression in normal lungs (Fig.3A and C). Bombesin plus RA and ghrelin plus RA also induced RARα expression (Fig.3A). In contrast, ghrelin-treated hypoplastic lungs exhibited decreased RARα and RARγ expression (Fig.3A and C). The expression of RARβ was similar in all of the studied groups (Fig.3B).
Figure 3.
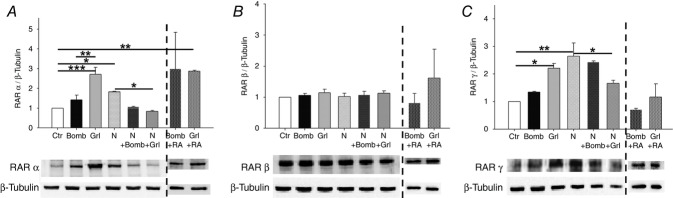
Protein expression levels of retinoic acid receptors (RARs) in normal (Ctr) and hypoplastic (N) lung explants treated with bombesin (Bomb), ghrelin (Grl) as well as bombesin plus RA (Bomb + RA) or ghrelin plus RA (Grl + RA) in normal lung explants
A, RARα expression. B, RARβ expression. C, RARγ expression. Examples of representative blots are shown. Protein levels were normalised to β-tubulin, which was used as a loading control. The data are presented as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Effect of RA on RAR expression in normal fetal lungs
To assess the effect of RA on RARα, β and γ, the levels of these three proteins were evaluated. In control lungs, RA led to decreased RARα and RARγ expression but increased RARβ expression (Fig.4).
Figure 4.
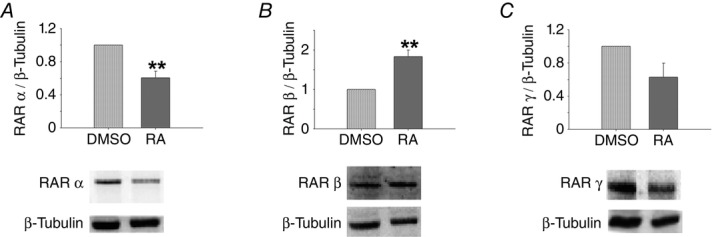
Protein expression levels of retinoic acid receptors (RARs) in normal lung explants (DMSO) treated with retinoic acid (RA)
A, RARα. B, RARβ. C, RARγ. Representative blots are shown. Protein levels were normalised to β-tubulin, which was used as a loading control. The data are presented as means ± SEM. **P < 0.01 vs. DMSO.
Effects of bombesin and ghrelin antagonists on normal and hypoplastic lung growth and RAR expression
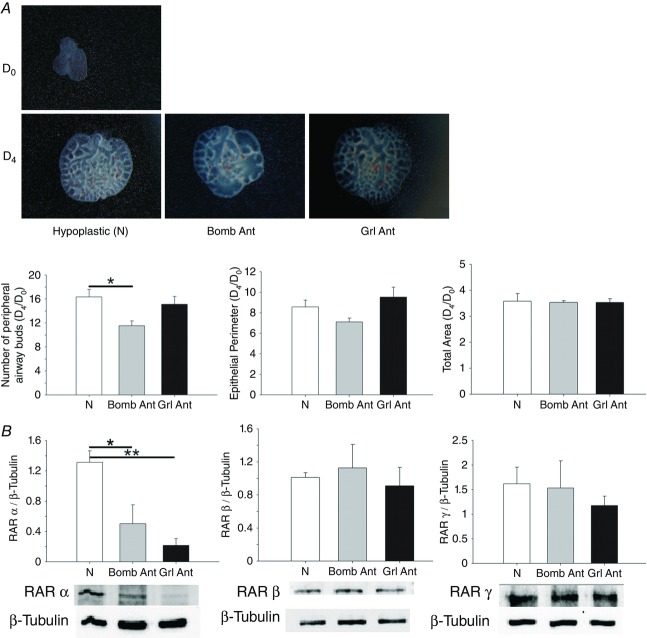
To confirm the roles of bombesin and ghrelin in normal and hypoplastic lung development, antagonists of these peptides were added to lung explants. The bombesin and ghrelin antagonists significantly inhibited lung growth, as indicated by decreases in the total number of peripheral airway buds and the epithelial perimeter (Figs5 and 7A). However, the total area remained unchanged after treatment with the antagonists (data not shown). Regarding RAR protein expression, explants treated with the bombesin and ghrelin antagonists exhibited a statistically significant decrease in RARα expression (Figs 6A and 7B). In contrast, RARβ expression in treated explants was similar to that in untreated explants, and a slight decrease in RARγ expression was observed in explants treated with these antagonists (Figs 6B and C, and 7B and C).
Figure 5.
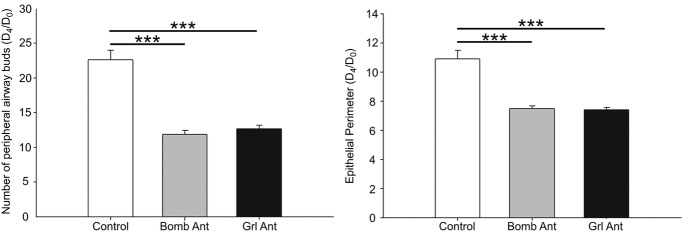
Branching morphogenesis in rat lung explant cultures (Control) treated with a bombesin or ghrelin antagonist (Bomb Ant or Grl Ant)
The number of peripheral airway buds and the epithelial perimeter. The results are expressed as the D4/D0 ratio. ***P < 0.001.
Figure 7.
Branching morphogenesis and RAR expression levels of rat hypoplastic lung explant (N) cultures treated with a bombesin or ghrelin antagonist (Bomb Ant or Grl Ant)
A, the number of peripheral airway buds, epithelial perimeter and total area. Results are expressed as the D4/D0 ratio. *P < 0.05. B, RARα, RARβ and RARγ protein expression levels. Representative blots are shown. Protein levels were normalised to β-tubulin, which was used as a loading control. The data are presented as means ± SEM. *P < 0.05; **P < 0.01.
Figure 6.
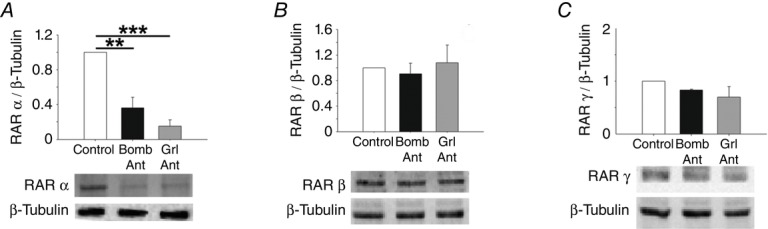
Protein expression levels of retinoic acid receptors (RARs) in normal lung explants (Control) treated with a bombesin or ghrelin antagonist (Bomb Ant or Grl Ant)
A, RARα. B, RARβ. C, RARγ. Representative blots are shown. Protein levels were normalised to β-tubulin, which was used as a loading control. The data are presented as means ± SEM. **P < 0.01; ***P < 0.001.
Discussion
Congenital diaphragmatic hernia (CDH) is associated with pulmonary hypoplasia, and it has been shown that RA signalling is involved in the establishment of this disease. Furthermore, CDH has been linked to an increased levels of neuroendocrine factors. This report describes a putative link between neuroendocrine factors and the RA pathway in normal and hypoplastic lung development for the first time.
Retinoids such as vitamin A and its derivatives are crucial for growth, development and tissue differentiation (Blomhoff & Blomhoff, 2006). RA acts by binding to nuclear receptors that exist in three different isoforms: α, β and γ (Mactier & Weaver, 2005). RA receptors are expressed from the earliest stages of embryonic lung development into postnatal life. RARα is ubiquitously expressed at consistent levels over time during lung development, whereas RARβ is excluded from the distal epithelium during branching but is still present in the epithelial cells of proximal and mid-sized airways (Wongtrakool et al. 2003; Roth-Kleiner & Post, 2005). In this study, we show that in the canalicular stage, RARs are expressed in epithelial and mesenchymal cells in both normal and hypoplastic lungs; moreover, the levels of RARα and RARγ appear to be increased in hypoplastic lungs, in contrast to what Rajatapiti et al. (2006) have shown previously in qualitative analysis (Fig.1).
Classically, RA is described as one of the major stimulators of lung development. In CDH, several growth factors, including RA, have been observed to promote lung branching when exogenously administered (Baptista et al. 2005; Keller et al. 2010). Furthermore, the RA signalling pathway has been shown to be involved in CDH pathogenesis and pulmonary hypoplasia. For instance, the incidence rate of CDH is higher in litters born to dams fed vitamin A-deficient diets (Andersen et al. 1941). RARα/RARβ double knockout mice display diaphragmatic hernia (Chen et al. 2003); however, when mutations affect only one isoform of one receptor, no fetal anomalies are detected, suggesting functional redundancy between various receptors and isoforms (Azais-Braesco & Pascal, 2000). Nitrofen suppresses the RA response element (Major et al. 1998), and in vitro assays have shown that nitrofen inhibits Retinaldehyde dehydrogenase 2 (RALDH2) (Mendelsohn et al. 1994). In humans, infants with CDH exhibit lower plasma retinol levels than healthy infants (Mey et al. 2003; Beurskens et al. 2007). Additionally, it has been demonstrated that antenatal administration of vitamin A reduces the incidence of CDH and restores lung maturation in the nitrofen rat model and that RA improves lung branching in CDH lungs in fetal lung explant cultures (Thébaud et al. 1999, 2001; Babiuk et al. 2004).
Focusing on neuroendocrine cells, studies conducted during the last decade have revealed a complex functional role for pulmonary neuroendocrine cells (PNECs). In the early stages of lung development, PNECs act as modulators of fetal lung growth and differentiation, whereas at the time of birth, they act mainly as airway O2 sensors involved in neonatal adaptation (Andersen et al. 1941). The major neuroendocrine peptides in the lung are bombesin and ghrelin. Recent data from our group showed that ghrelin is overexpressed in human hypoplastic lungs and the lungs of CDH model rats compared with normal lungs (Santos et al. 2006). Bombesin expression has been shown to be higher in CDH lungs than in normal lungs (Santos et al. 2006; Cutz et al. 2007). These increases in some growth factors observed in CDH lungs might represent a compensatory response of hypoplastic lungs attempting to recover normal levels of lung growth (Santos et al. 2006).
Given that PNECs behave as lung sensors and that RA is a classical lung growth factor, and the levels of both neuroendocrine peptides and RA are affected in hypoplastic lungs, we hypothesised a putative link between these two important lung regulators.
In the present study, it was demonstrated that bombesin, ghrelin and particularly RA, or bombesin or ghrelin combined with RA, promote normal lung branching and increase the lung epithelial perimeter (Fig.2A and B). Moreover, exogenous supplementation of bombesin or ghrelin in normal lungs increased RARα and RARγ expression but did not affect RARβ expression (Fig.3). In contrast, inhibition of bombesin or ghrelin decreased lung branching as well as RARα and RARγ expression (Figs 5 and 6, respectively). Thus, these results demonstrate, for the first time, that there is a link between neuroendocrine factors and the RA signalling pathway. Moreover, RARα and RARγ appear to be the major mediators between these two apparently different pathways. Indeed, these results suggest that the action of neuroendocrine factors during lung growth is at least mediated by an increase in RARα and RARγ expression, indicating that RA pulmonary sensitisation is mediated by neuroendocrine factors through RARα and RARγ.
Furthermore, the results showed that RA supplementation in normal lungs decreased RARα and RARγ expression and increased RARβ expression (Fig.4). A possible explanation for this observation is that neuroendocrine cells sense the increase in lung growth mediated by RA and consequently decrease the production of neuroendocrine factors, which in turn leads to decreased RARα and RARγ expression. However, the possibility of a direct down-regulation effect of RA on RAR expression cannot be excluded. RARβ expression was stimulated by RA supplementation and remained unchanged by bombesin and ghrelin modulation. These results exclude the involvement of RARβ in the RA/PNEC interaction with respect to lung growth mechanisms.
Regarding pulmonary hypoplasia, according to the literature, RARα and RARγ are overexpressed in hypoplastic lungs compared with normal lungs. In vitro bombesin and ghrelin supplementation in hypoplastic lung explant cultures did not affect the examined lung morphometric parameters (Fig.2B and D), probably because these receptors are already overexpressed. The expression of RARα and RARγ slightly decreases in hypoplastic lungs after ghrelin supplementation (Fig.3). We do not have a plausible explanation for this result. However, this finding reinforces the idea that there is an association between neuroendocrine factors and RA signalling. Moreover, inhibition of bombesin or ghrelin significantly decreased RARα expression as well as lung branching (Fig.7), reinforcing our hypothesis.
It has largely been demonstrated that RA significantly increases lung branching in hypoplastic lungs after RA supplementation in vitro. Moreover, hypoplastic lungs overexpress bombesin and ghrelin (Cutz et al. 2007). In this study, we observed an increase in RARα and RARγ expression in hypoplastic lungs that might be explained by the compensatory overexpression of bombesin and ghrelin in these lungs (Fig.3). This is in agreement with the effect of neuroendocrine factor supplementation in normal lungs. In Fig.8, a putative mechanism for the link between neuroendocrine cells and the RA pathway is presented.
Figure 8.
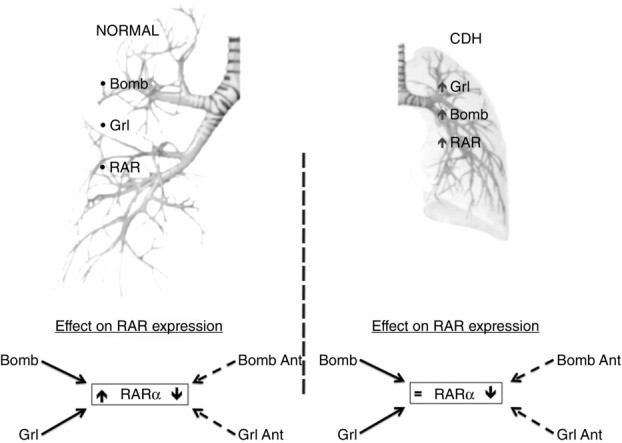
Schematic representation of the putative link between neuroendocrine cells and the retinoic acid signalling pathway
In the upper panel, on the left side of the scheme we have represented the normal fetal lung expressing neuroendocrine products: bombesin (Bomb) and ghrelin (Grl) as well as retinoic acid receptors (RAR). On the right side of the scheme we have represented the CDH fetal lungs, which express increased (↑) levels of neuroendocrine products (Bomb and Grl) as well as RAR. On the bottom panel, we represent the RAR explants expression after modulation (agonists and antagonists) of neuroendocrine products. Focusing on isoform α, in control explants, RAR(α) expression is significantly increased (↑) and decreased (↓) with addition of agonists (full arrows, Bomb and Grl) and antagonists (dashed arrows, Bomb Ant and Grl Ant) of neuroendocrine products, respectively. Regarding CDH explants, addition of neuroendocrine products does not induce and additionally increment RAR(α) expression when compared with untreated CDH explants (=), but addition of neuroendocrine antagonists (Bomb Ant and Grl Ant) induces a significant decrease (↓) of RAR(α) expression. As a result of these findings, we propose a mechanism of interaction between bombesin and ghrelin with the retinoic acid signalling pathway through the modulation of RAR expression that seems to be working in the hypoplastic lungs.
In summary, based on these results, we can conclude that neuroendocrine factors act as regulators of lung growth, sensitising the lungs to the action of RA through RARα and RARγ up-regulation.
Acknowledgments
We would like to thank Luís Martins and Ana Lima for their help with animal euthanasia and processing tissues for paraffin blocks. We also wish to thank Emanuel Carvalho-Dias for comments on this manuscript.
Glossary
- CDH
congenital diaphragmatic hernia
- D0
day 0 of culture
- D4
day 4 of culture
- dpc
days post-conception
- PNECs
pulmonary neuroendocrine cells
- RA
retinoic acid
- RAR
retinoic acid receptor
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
P.P.-T. performed the experiments and prepared the manuscript. P.P.-T. and J.C-.P. conceived and designed the study. P.P.-T., R.S.M., C.N.-S. and J.C.-P. analysed and interpreted the results. P.P.-T., R.S.M., C.N.-S. and J.C.-P. drafted the manuscript for important intellectual content. Each author contributed to and approved the final manuscript. All the experiments were done in the Life and Health Sciences Research Institute (ICVS) in the University of Minho (Braga, Portugal).
Funding
Grants: P.P.-T. was supported by the Fundação para a Ciência e a Tecnologia (ref. SFRH/BD/73660/2010). R.S.M. was supported by the ON.2 SR&TD Integrated Program (N-01-01-01-24-01-07) (ref. UMINHO/BPD/31/2013). The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Andersen DH. Incidence of congenital diaphragmatic hernia in the young of rats bred on a diet deficient in vitamin A. Am J Dis Child. 1941;62:888–889. [Google Scholar]
- Antipatis C, Ashworth CJ, Grant G, Lea RG, Hay SM. Rees WD. Effects of maternal vitamin A status on fetal heart and lung: changes in expression of key developmental genes. Am J Physiol Lung Cell Mol Physiol. 1998;275:1184–1191. doi: 10.1152/ajplung.1998.275.6.L1184. [DOI] [PubMed] [Google Scholar]
- Asabe K, Tsuji K, Handa N, Kajiwara M. Suita S. Immunohistochemical distribution of bombesin-positive pulmonary neuroendocrine cells in a congenital diaphragmatic hernia. Surg Today. 1999;29:407–412. doi: 10.1007/BF02483031. [DOI] [PubMed] [Google Scholar]
- Azais-Braesco V. Pascal G. Vitamin A in pregnancy: requirements and safety limits. Am J Clin Nutr. 2000;71:1325S–1333S. doi: 10.1093/ajcn/71.5.1325s. [DOI] [PubMed] [Google Scholar]
- Babiuk RP, Thebaud B. Greer JJ. Reductions in the incidence of nitrofen-induced diaphragmatic hernia by vitamin A and retinoic acid. Am J Physiol Lung Cell Mol Physiol. 2004;286:L970–L973. doi: 10.1152/ajplung.00403.2003. [DOI] [PubMed] [Google Scholar]
- Baptista MJ, Melo-Rocha G, Pedrosa C, Gonzaga S, Teles A, Estevão-Costa J, Areias JC, Flake AW, Leite-Moreira AF. Correia-Pinto J. Antenatal vitamin A administration attenuates lung hypoplasia by interfering with early instead of late determinants of lung underdevelopment in congenital diaphragmatic hernia. J Pediatr Surg. 2005;40:658–665. doi: 10.1016/j.jpedsurg.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Beurskens N, Klaassens M, Rottier R, de Klein A. Tibboel D. Linking animal models to human congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol. 2007;79:565–572. doi: 10.1002/bdra.20370. [DOI] [PubMed] [Google Scholar]
- Beurskens LWJE, Tibboel D. Steegers-Theunissen RPM. Role of nutrition, lifestyle factors, and genes in the pathogenesis of congenital diaphragmatic hernia: human and animal studies. Nutr Rev. 2009;67:719–730. doi: 10.1111/j.1753-4887.2009.00247.x. [DOI] [PubMed] [Google Scholar]
- Blomhoff R. Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- Chen MH, MacGowan A, Ward S, Bavik C. Greer JJ. The activation of the retinoic acid response element is inhibited in an animal model of congenital diaphragmatic hernia. Biol Neonate. 2003;83:157–161. doi: 10.1159/000068932. [DOI] [PubMed] [Google Scholar]
- Cutz E1, Yeger H. Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease – recent advances. Pediatr Dev Pathol. 2007;10:419–435. doi: 10.2350/07-04-0267.1. [DOI] [PubMed] [Google Scholar]
- Doi T, Sugimoto K, Ruttenstock E, Dingemann J. Puri P. Prenatal retinoic acid upregulates pulmonary gene expression of PI3K and AKT in nitrofen-induced pulmonary hypoplasia. Pediatr Surg Int. 2010;26:1011–1015. doi: 10.1007/s00383-010-2654-x. [DOI] [PubMed] [Google Scholar]
- Goshe JR, Islam D. Boulanger SC. Congenital diaphragmatic hernia: searching for answers. Am J Surg. 2005;190:324–332. doi: 10.1016/j.amjsurg.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Hosli L, Hosli E, Winter T. Kaser H. Electrophysiological evidence for the presence of receptors for cholecystokinin and bombesin on cultured astrocytes of rat central nervous system. Neurosci Lett. 1993;163:145–147. doi: 10.1016/0304-3940(93)90367-t. [DOI] [PubMed] [Google Scholar]
- Keller RL, Tacy TA, Hendricks-Munoz K, Xu J, Moon-Grady AJ, Neuhaus J, Moore P, Nobuhara KK, Hawgood S. Fineman JR. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med. 2010;182:555–561. doi: 10.1164/rccm.200907-1126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresch M, Christian C, Zhu L, Obe M, Sanders MM. Hussain N. Bombesin inhibits apoptosis in developing fetal rat lung. Lung. 1999;177:241–251. doi: 10.1007/pl00007644. [DOI] [PubMed] [Google Scholar]
- Leeuwen L. Fitzgerald DA. Congenital diaphragmatic hernia. J Paediatr Child Health. 2014;50:667–673. doi: 10.1111/jpc.12508. [DOI] [PubMed] [Google Scholar]
- Mactier H. Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed. 2005;90:103–108. doi: 10.1136/adc.2004.057547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major D, Cadenas M, Fournier L, Leclerc S, Lefebvre M. Cloutier R. Retinol status of newborn infants with congenital diaphragmatic hernia. Pediatr Surg Int. 1998;13:547–549. doi: 10.1007/s003830050399. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Décimo D, Lufkin T, LeMeur M, Chambon P. Mark M. Function of the retinoic acid receptors (RARs) during development. (II) Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Mey J, Babiuk RP, Clugston R, Zhang W. Greer JJ. Retinal dehydrogenase-2 is inhibited by compounds that induce congenital diaphragmatic hernias in rodents. Am J Pathol. 2003;162:673–679. doi: 10.1016/s0002-9440(10)63861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Silva C, Carvalho-Dias E, Piairo P, Nunes S, Baptista MJ, Moura RS. Correia-Pinto J. Local fetal lung renin-angiotensin system as a target to treat congenital diaphragmatic hernia. Mol Med. 2011;18:231–243. doi: 10.2119/molmed.2011.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Silva C, Piairo P, Carvalho-Dias E, Peixoto FO, Moura RS. Correia-Pinto J. Leukemia inhibitory factor in rat fetal lung development: expression and functional studies. PLoS One. 2012;7:e30517. doi: 10.1371/journal.pone.0030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Siva C, Piairo P, Carvalho-Dias E, Veiga C, Moura RS. Correia-Pinto J. The role of glycoprotein 130 family of cytokines in fetal rat lung development. PLoS One. 2013;8:e67607. doi: 10.1371/journal.pone.0067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Nogueira-Silva C, Dias E, Moura RS. Correia-Pinto J. Ghrelin and obestatin: Different role in fetal lung development? Peptides. 2008;29:2150–2158. doi: 10.1016/j.peptides.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Pereira-Terra P, Deprest JA, Kholdebarin R, Khoshgoo N, DeKoninck P, Boerema-De Munck AA, Wang J, Zhu F, Rottier RJ, Iwasiow BM, Correia-Pinto J, Tibboel D, Post M. Keijzer R. Unique tracheal fluid microRNA signature predicts response to FETO in patients with congenital diaphragmatic hernia. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001054. (in press; DOI: 10.1097/SLA.0000000000001054 ) [DOI] [PubMed] [Google Scholar]
- Pereira-Terra P, Kholdebarin R, Higgins M, Iwasiow BM, Correia-Pinto J. Keijzer R. Lower NPAS3 expression during the later stages of abnormal lung development in rat congenital diaphragmatic hernia. Pediatr Surg Int. 2015;31:659–663. doi: 10.1007/s00383-015-3703-2. [DOI] [PubMed] [Google Scholar]
- Piairo P, Moura RS, Nogueira-Silva C. Correia-Pinto J. The apelinergic system in the developing lung: expression and signalling. Peptides. 2011;32:2474–2483. doi: 10.1016/j.peptides.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Pober BR. Overview of epidemiology, genetics, birth defects, and chromosome abnormalities associated with CDH. Am J Med Genet C Semin Med Genet. 2007;145C:158–171. doi: 10.1002/ajmg.c.30126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajatapiti P, Keijzer R, Blommaart PE, Lamers WH, DeKrijger RR, Visser TJ, Tibboel D. Rottier R. Spatial and temporal expression of glucocorticoid, retinoid, and thyroid hormone receptors is not altered in lungs of congenital diaphragmatic hernia. Pediatr Res. 2006;60:693–698. doi: 10.1203/01.pdr.0000246245.05530.02. [DOI] [PubMed] [Google Scholar]
- Roth-Kleiner M. Post M. Similarities and dissimilarities of branching and septation during lung development. Pediatr Pulmonol. 2005;40:113–134. doi: 10.1002/ppul.20252. [DOI] [PubMed] [Google Scholar]
- Ruttenstock EM, Doi T, Dingemann J. Puri P. Prenatal administration of retinoic acid upregulates connective tissue growth factor in the nitrofen CDH model. Pediatr Surg Int. 2011;27:573–577. doi: 10.1007/s00383-010-2833-9. [DOI] [PubMed] [Google Scholar]
- Santos M, Bastos P, Gonzaga S, Roriz JM, Baptista MJ, Nogueira-Silva C, Melo-Rocha G, Henriques-Coelho T, Roncon-Albuquerque R. Leite-Moreira AF, De Krijger RR, Tibboel D, Rottier R. Correia-Pinto J. Ghrelin expression in human and rat fetal lungs and the effect of ghrelin administration in nitrofen-induced congenital diaphragmatic hernia. Pediatr Res. 2006;59:531–537. doi: 10.1203/01.pdr.0000202748.66359.a9. Jr, [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Takayasu H, Nakazawa N, Montedonico S. Puri P. Prenatal treatment with retinoic acid accelerates type 1 alveolar cell proliferation of the hypoplastic lung in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:367–372. doi: 10.1016/j.jpedsurg.2007.10.050. [DOI] [PubMed] [Google Scholar]
- Thébaud B, Barlier-Mur AM, Chailley-Heu B, Henrion-Caude A, Tibboel D, Dinh-Xuan AT. Bourbon JR. Restoring effects of vitamin A on surfactant synthesis in nitrofen-induced congenital diaphragmatic hernia in rats. Am J Respir Crit Care Med. 2001;164:1083–1089. doi: 10.1164/ajrccm.164.6.2010115. [DOI] [PubMed] [Google Scholar]
- Thébaud B, Tibboel D, Rambaud C, Mercier JC, Bourbon JR, Dinh-Xuan AT. Archer SL. Vitamin A decreases the incidence and severity of nitrofen-induced congenital diaphragmatic hernia in rats. Am J Physiol Lung Cell Mol Physiol. 1999;277:423–429. doi: 10.1152/ajplung.1999.277.2.L423. [DOI] [PubMed] [Google Scholar]
- van den Hout L, Sluiter I, Gischler S, De Klein A, Rottier R, Ijsselstijn H, Reiss I. Tibboel D. Can we improve outcome of congenital diaphragmatic hernia? Pediatr Surg Int. 2009;25:733–743. doi: 10.1007/s00383-009-2425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenhout RB, Tibboel D, Post M. Keijzer R. Congenital diaphragmatic hernia: comparison of animal models and relevance to the human situation. Neonatology. 2009;96:137–149. doi: 10.1159/000209850. [DOI] [PubMed] [Google Scholar]
- Volante M, Fulcheri E, Allìa E, Cerrato M, Pucci A. Papotti M. Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem. 2002;50:1013–1021. doi: 10.1177/002215540205000803. [DOI] [PubMed] [Google Scholar]
- Wongtrakool C, Malpel S, Gorenstein J, Sedita J, Ramirez MI, Underhill TM. Cardoso WV. Down-regulation of retinoic acid receptor αsignalling is required for sacculation and type I cell formation in the developing lung. J Biol Chem. 2003;278:46911–46918. doi: 10.1074/jbc.M307977200. [DOI] [PMC free article] [PubMed] [Google Scholar]