Abstract
There is rapidly growing interest in learning how to engineer immune cells, such as T lymphocytes, because of the potential of these engineered cells to be used for therapeutic applications such as the recognition and killing of cancer cells. At the same time, our knowhow and capability to logically engineer cellular behavior is growing rapidly with the development of synthetic biology. Here we describe how synthetic biology approaches are being used to rationally alter the behavior of T cells to optimize them for therapeutic functions. We also describe future developments that will be important in order to construct safe and precise T cell therapeutics.
Introduction: synthetic biology meets immunology
Cells are capable of remarkably sophisticated behavior. In particular, immune cells exhibit a wide range of characteristics that are well suited for therapeutic applications. Research in cell biology and immunology has focused on dissecting the molecular mechanisms underlying these complex behaviors. However, there is now growing interest in understanding how to engineer immune cells to carry out controlled and redirected natural behavior and new, non-natural behaviors. This shift comes from the convergence of two exciting emerging areas of research. First is the establishment that engineered immune cells can be used as therapeutics to treat cancer or autoimmunity. Second is the development of synthetic biology – a field in which our understanding of molecular regulatory systems has been combined with our increasing ability to genetically modify and edit cellular systems. Thus this is a particularly exciting time: our ability to rationally engineer cells is exponentially growing, as are the potential therapeutic applications of engineered immune cells.
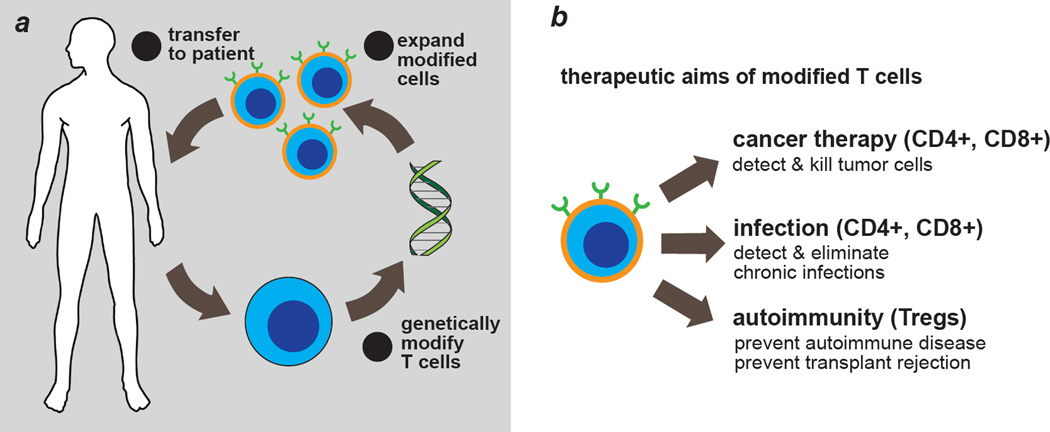
Synthetic biologists seek to understand the design principles of biological systems by dissecting, rebuilding and repurposing natural and synthetic components [1–6]. The biomedical relevance of engineered T cells demonstrated in recent clinical trials is one reason why T cells are emerging as an important model system for synthetic biologists. In adoptive immunotherapy, T cells are isolated from blood, processed ex vivo, and re-infused into patients [7,8,9] (Fig. 1A). Although best known for cancer therapy, the application of engineered T cells includes and is not limited to treating intracellular pathogens and auto-immunity [10,11] (Fig. 1B). Remarkably, engineered T cells can safely persist for years in vivo [12,13]. Progress towards allogeneic, universal donor T cells is underway, and so are methods of differentiating induced pluripotent stem cells into T cells [14,15]. Both technologies are envisioned to significantly increase the availability of therapeutic T cells.
Fig. 1. Engineering T cells for diverse clinical needs.
a, Overview of adoptive immunotherapy using genetically modified T cells. b, Current and future applications for engineered T cells.
T lymphocytes and their signaling systems are an ideal test bed for synthetic engineering, thanks to decades of rigorous basic research that has generated extensive knowledge on T cell biology. The proliferative capacity of T cells also makes it relatively simple to obtain large numbers of cells for experimental and treatment purposes. Transient or stable expression of synthetic molecules in T cells can be achieved using multiple methods (Box 1)[16–20], and genome engineering via CRISPR or ZFN approaches carries immense potential for construction of complex circuits involving re-wiring, modifying, or disabling endogenous pathways. Finally, T cells provide a rich context for intercellular interactions that is amenable to engineering and can be used to explore key parameters in cell-cell communication and dynamic population behaviors [21,22].
Box 1. Methods to engineer T cells.
Thus the field of T cell engineering (synthetic immunology) is rapidly growing. This review will discuss selected examples T cell engineering and how this field might expand in the future to enhance precision control over therapeutic T cells.
Progress in rewiring T cells
Detection of disease signals through synthetic T cell receptors
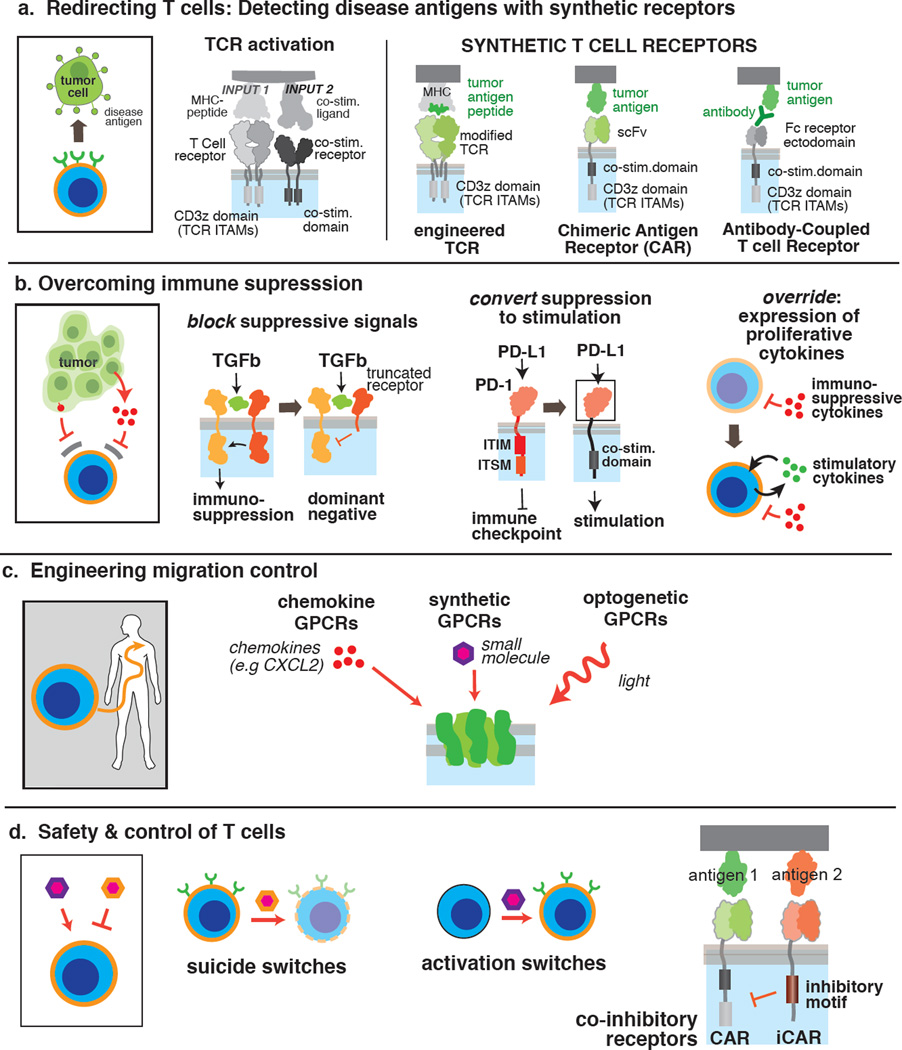
T cells normally use their T cell receptor (TCR) to detect antigens presented by the MHC. To harness T cells in treating disease, it is critical to be able to alter T cells such that they recognize specific, selected disease signals (e.g. a tumor antigen). A streamlined way to modulate a T cell’s specificity for input signals is to employ synthetic receptors, which are typically chimeras of motifs and domains of natural or synthetic origin. Synthetic TCRs, chimeric antigen receptors (CARs) and antibody-coupled T cell receptors redirect cells to recognize disease associated ligands or antigens on target cells [7,9,23,24] (Fig. 2A). The first generation of these synthetic receptors was developed nearly 20 years ago and generally only contained signaling modules from the TCR. The current generation of CARs and antibody-coupled T cell receptors typically combine intracellular signaling modules from both the TCR and co-stimulatory receptors. In this regard antigen-dependent activation of T cells equipped with such receptors is independent of MHC and costimulatory interactions. The single antigen input activates two intracellular responses -- both signal 1 (antigenic stimulation) and signal 2 (co-stimulation) of the TCR pathway to produce desired outcomes such as cytokine production, proliferation, and survival. With the advent of current generation CARs containing costimulatory motifs, CAR T cells have shown strong in vivo proliferation and durability required for therapeutic efficacy. A tremendously exciting example of current CAR therapy is the CD19 CAR T cell trials for treatment of B cell cancers, which highlight the potential of cell-based therapeutics to transform cancer therapy [7].
Fig. 2. Diverse synthetic modules have been developed to reprogram therapeutic T cells.
a, Synthetic receptor designs for targeting disease antigens. ITAM: immunoreceptor tyrosine-based activation motif, scFv: single chain variable fragment. b, Methods for engineering T cells resistant to immunosuppressive microenvironments. ITIM: immunoreceptor tyrosine-based inhibitory motif, ITSM: immunoreceptor tyrosine-based switch motif. c, Approaches to engineer migration/trafficking control. d, Engineering safety switches and gated activation in gene modified T cells.
Overcoming immune suppression
The immunosuppressive conditions in tumor microenvironments remain a major challenge in cancer immunotherapy. High levels of inhibitory signals in tumor microenvironments in the form of cytokines (e.g. TGF-β and IL-4) and cell surface co-inhibitory ligands (PD-L1) can globally suppress effector T cell functions (Fig. 2B). Additional synthetic receptors have been developed to overcome such immunosuppressive mechanisms. For example, a dominant negative allele of the TGF-β receptor can abrogate TGF-β mediated inhibition, rescue the survival defects of T cells, and enhance their anti-tumor activities in distinct mouse models [25,26]. In another example, a chimeric receptor consisting of the IL-4Rα extracellular domain and IL-7Rα transmembrane + intracellular domains induces Th1 polarization in the presence of IL-4, which normally suppresses Th1 effector functions. T cells expressing this chimeric receptor showed enhanced immunity against IL-4-producing tumors, likely due to the ability to continuously utilize tumor-derived IL-4 to proliferate and sustain Th1 activities [27].
The concept of converting inhibitory signals into activating signals has also been explored to mitigate PD-L1 mediated immunosuppression. A chimeric receptor consisting of the PD-1 extracellular domain and CD28 intracellular segment converts co-inhibitory signaling of the PD-1 pathway to co-stimulatory signaling of the CD28 pathway [28]. Primary human CD8+ T cells expressing this chimeric receptor produced more effector cytokines when stimulated with PD-L1+ target cells, and retained granzyme B expression more effectively after prolonged exposure to the PD-L1 ligand in vitro, suggesting sustained anti-tumor functions.
Tumor-targeting T cells can also be engineered to conditionally secrete cytokines that promote anti-tumor functions and T cell survival in tumor microenvironments. In such a controlled manner, toxicities that would otherwise result from systemic administration of the cytokine might be minimized. One example is antigen-dependent production of IL-12 through an NFAT-responsive promoter that is activated upon tumor antigen recognition by a CAR or TCR, although a recent clinical study reveals the necessity for greater stringency to avoid severe toxicities [29,30]. Increased stringency of IL-12 production could be achieved through administering a synthetic small molecule to specifically regulate the onset, duration, and magnitude of IL-12 transcription [31]. As summarized in earlier work, IL-12 exerts multiple advantageous immunomodulatory effects in tumor microenvironments through engaging both the innate and the adaptive immune components as well as altering the extracellular matrix [32]. One major effect of IL-12 is promoting neo-antigen recognition by the immune system so that tumor cells that have evaded T cell recognition by loss of MHC or target antigen expression can be eliminated. Controlled production of other cytokines (e.g. IL-2, IL-15) to promote T cell proliferation and survival can be achieved with ribozyme-based switches responsive to small molecules [33]. As co-stimulatory signals and cytokines act in concert to regulate T cell functions, the potential synergy among the synthetic devices discussed in this section would be exciting to explore in adoptive immunotherapy.
Re-directed migration of T cells
Migration of T cells can be controlled using natural or synthetic G-protein coupled receptors (GPCRs) recognizing soluble ligands of interest to increase the T cells’ therapeutic potential (Fig. 2C). For example, preclinical tumor models showed enhanced T cell migration to tumor sites and improved tumor regression when CAR T cells expressed a natural GPCR for the tumor-derived chemokine CCL2 or CXCR2 [34,35]. GPCRs engineered to recognize orthogonal ligands, known as receptors activated solely by a synthetic ligand (RASSLs), have been developed using directed molecular evolution [36]. Migration of human primary T cells expressing one such RASSL was exogenously regulated using the small molecule clozapine-N-oxide, an inert metabolite of the FDA-approved drug clozapine. Both in vitro and in vivo, RASSL-expressing T cells migrated up concentration gradients towards the sources of ligand [37]. Similarly, Xu et. al. designed a light-responsive rhodopsin-CXCR4 chimeric GPCR for controlled phototaxis of engineered T cells in vitro and in vivo. In an OVA tumor model, light stimulation induced significant intratumoral infiltration of antigen-reactive cytotoxic T cells, along with enhanced T cell proliferation and tumor regression [38]. In summary, synthetic motility control systems using orthogonal small molecules or light could promote infiltration and retention of engineered T cells in tissues that lack T cell surveillance, such as certain solid tumors and immune privileged sites.
It is worth noting the exciting development of synthetic molecules specifically designed to trigger immune cell functions, such as engineered cytokines and their mimics [39] as well as bi-specific antibodies [40]. While most of these molecules are designed to interact with natural immunoreceptors, further engineering could be applied to yield highly specific, orthogonal pairs of synthetic ligands and receptors, which in principle would afford more precise exogenous control over engineered immune cells [41].
Safety devices
A critical design aspect moving forward will be incorporation of robust safety mechanisms to prevent unrestrained activity of engineered cells. There have been serious adverse effects associated with many therapeutic T cell clinical trials, including severe cytokine release syndrome, and in some cases death due to cross reaction with healthy tissues [7,9,23]. A number of approaches have been described to make engineered T cells safer. Arguably the simplest strategy is the “kill switch” or “suicide gene” that results in death of engineered cells upon addition of an artificial stimulus. Principal amongst these approaches is ectopic expression of the herpes simplex virus (HSV) thymidine kinase that causes DNA replication defects in engineered cells after addition of the FDA-approved antiviral ganciclovir, and is currently under investigation in a Phase I CAR T cell trial. An alternative is ectopic expression of an engineered protein designed to mediate inducible apoptosis. The leading example is an inducible Caspase 9 system that has also been tested in a Phase I trial. This FKBP-Caspase9 (iCasp9) fusion homo-dimerizes and stimulates the cell intrinsic apoptotic pathway upon small molecule addition [42]. Finally, cells may be engineered to express surface receptors or epitope tags such that addition of antibodies leads to depletion of therapeutic cells [43].
Each of these strategies has its associated challenges. HSV thymidine kinase has documented immunogenicity [44], which could allow rejection of therapeutic cells in immunocompetent patients; a similar concern holds for synthetic epitope tags employed for antibody depletion of target cells. Alternatively, expression of native molecules (such as EGFR) for antibody depletion strategies could have unintended consequences in the cells of interest, and may require significant engineering to render them biologically inert.
The most severe limitation of “suicide gene” strategies in settings such as CAR T cell therapy, where the CAR is constitutively “on” in the presence of antigen, is the requirement for 100% efficacy of the switch to avoid toxicity issues. Even small numbers of cells that inactivate the kill switch or evade deletion could expand and cause significant toxicity. Indeed, in a phase I trial of iCasp9 expressing cells, treatment with the small molecule dimerizer led to only ~95% depletion of gene modified cells [42].
An alternative approach would be to design cells that are “off” in the basal state and exhibit controlled gating of activation (“activation switches”, Fig. 2D). Here cells could be rendered therapeutically inert until addition of an activating signal such as a small molecule, or detection of specific environmental antigens such as a defined tissue localization (Wu et al, unpublished results).
Alternatively, improved safety could be engineered with negative regulatory systems that mitigate activity or enhance specificity. For example, Fedorov et al. have reported the construction of inhibitory CARs (iCARs) that contain domains from the co-inhibitory receptors CTLA-4 or PD-1 [45]. In T cells expressing a conventional active CAR targeting Antigen A and an iCAR targeting Antigen B, ligation of the iCAR “overrides” the active CAR and allows discrimination between cells expressing Antigen A alone versus both Antigens A and B. Extending this approach to more complex synthetic circuits could allow construction of T cells with exquisitely specific behaviors and feedback control that allow defined periods of activation, multi-antigen gating, etc. Analogously, other groups have begun constructing CARs with tandem extracellular recognition domains [46]. These and other combinatorial recognition CARs might exhibit more specific antigen recognition, or could be used to reduce the chance of tumor escape via target antigen loss or mutation.
Future needs: the promise of synthetic T cell circuits
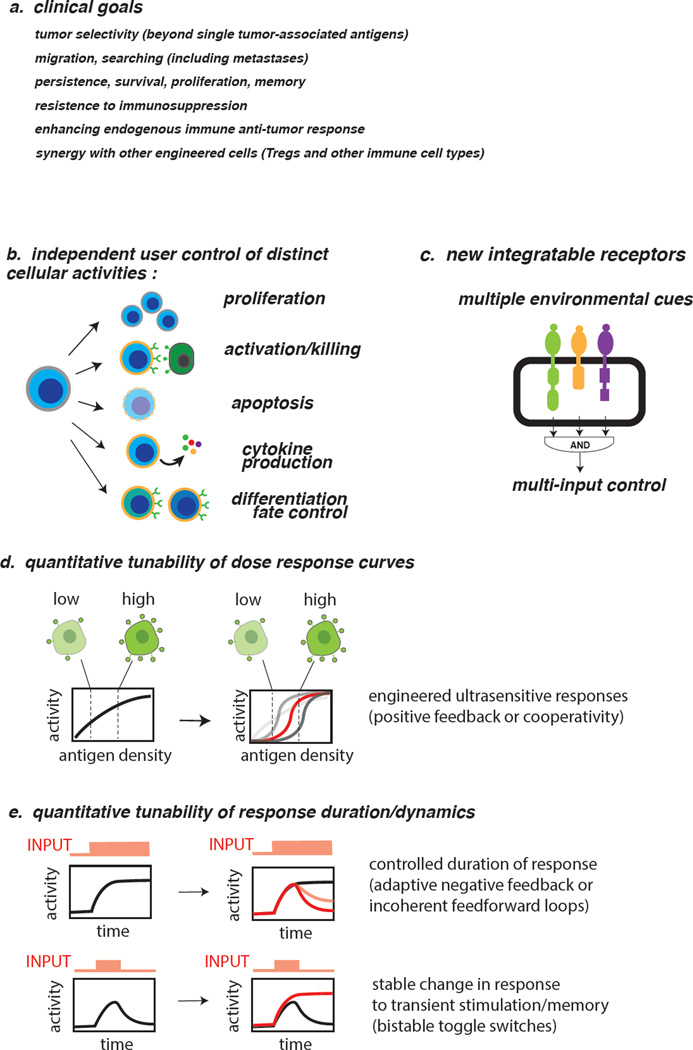
Numerous clinically desirable behaviors would be useful in next generation therapeutic T cells (Fig. 3A). Desired properties include, but are not limited to, increased tumor recognition specificity, directed migration/trafficking, enhanced persistence/survival/differentiation, resistance to immunosuppressive microenvironments, and recruitment of endogenous anti-tumor response. To date current approaches to engineering T cells have been relatively simplistic, involving single transgenes or relatively static approaches that address a single challenge detailed above. We propose that going forward it will be vital to develop a diverse toolbox of dynamically regulated engineered behaviors and functionalities that can be rapidly integrated to generate customized cell therapies for diverse applications.
Fig. 3. Looking forward: design principles for next generation therapeutic T cells.
a, Desirable overall clinical properties for next generation therapeutic T cells. b, Cellular activities to be independently controlled by user for enhanced therapeutic cell safety and precision. c, Multi-input systems required for complex Boolean logic and sophisticated decision making. d, Enhanced ligand/antigen density discrimination to distinguish normal vs disease target cells via tuning dose responses in engineered T cells. e, Engineered control of T cell response duration and state-switching.
A critical challenge in cell based therapies is either insulating engineered circuits from the endogenous response, or understanding native behavior sufficiently to integrate the desired functionality. This is particularly relevant for T cell engineering because of developmental plasticity and the immense impact that environmental factors play in determining T cell fate, function, and localization [47,48]. Ultimately, we must develop tools for independent control of multiple T cell functions, including but not limited to survival, proliferation, trafficking, targeted cytolysis or cytokine production, differentiation, and maintenance of cell fate (Fig. 3B). Designing artificial circuits for these capacities will require careful and systematic dissection of native systems, particularly given the interconnectedness of many of the parameters described above [47,48].
Another significant need is development of multi-input control that allows for complex Boolean logic gating, analogous to the way cells integrate diverse stimuli to encode downstream outputs (Fig. 3C). Such combinatorial sensing could aid in discriminating healthy tissue from cancer, or limit production of secondary outputs (such as cytokines) to specific tissues to avoid side-effects of systemic production.
Along these lines, it will be critical to move beyond constitutive transgenes and/or reliance upon endogenous pathways to regulate duration and intensity of programmed behaviors. Incorporation of classical synthetic biology motifs such as positive feedback for amplification, negative feedback for limiting duration, or bistable switches to promote mixed population states (Fig. 3D, E) should allow for rational control of therapeutic behaviors, both temporally and spatially [1–6].
A primary technical limitation to application of complex circuits lies in the ability to deliver large genetic payloads (3–5+ transgenes and promoters) reproducibly at high efficiency. Transposon-based approaches may prove superior to viral vectors in this regard due to larger payload delivery; alternatively, CRISPR-based homologous recombination could overcome this hurdle. Barring advancement of this technology, it may also be possible to deploy CRISPR to selectively rewire endogenous signaling pathways by tweaking promoter strength/responsiveness, introducing destabilizing mutations, or creating dominant negative alleles in engineered cells.
In summary, T cells are one of the most exciting platforms for cellular engineering and development of mammalian synthetic biology tools. Advancing clinical efficacy of next-generation cell therapies will necessitate the development of a diverse toolkit that allows independent user control of multiple cellular behaviors and functionalities. Building upon foundational knowledge of the native behavior of T cells, this may ultimately allow rational design of customized therapeutic cells for a diverse range of unmet clinical needs.
Highlights.
-
·
Synthetic biology uses modular molecular components to control cellular behavior
-
·
T cells are emerging as a viable platform for cell therapy in cancer and autoimmunity
-
·
Convergence of cell therapy and synthetic biology has demonstrated ways to rewire input sensing and activation of T cells
-
·
Rewiring approaches generate insights into T cell decision making and lead to more precision controlled therapeutic cells
ACKNOWLEDGEMENTS
The authors sincerely thank Dr. Russell Gordley and Dr. Amir Mitchell for thoughtful discussions on synthetic gene circuits. We thank other members of the Lim lab for helpful discussions. We apologize to authors whose relevant work was not cited in this review due to space constraints. The authors are supported by the National Institutes of Health (P50 GM081879, PN2 EY016546, R01 GM055040, R01 CA196277), the Jane Coffin Childs Memorial Fund for Medical Research, and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lim WA. Designing customized cell signalling circuits. Nat Rev Mol Cell Biol. 2010;11(6):393–403. doi: 10.1038/nrm2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim WA, Lee CM, Tang C. Design principles of regulatory networks: searching for the molecular algorithms of the cell. Mol Cell. 2013;49(2):202–212. doi: 10.1016/j.molcel.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nandagopal N, Elowitz MB. Synthetic biology: integrated gene circuits. Science. 2011;333(6047):1244–1248. doi: 10.1126/science.1207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purnick PE, Weiss R. The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol. 2009;10(6):410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 5.Way JC, Collins JJ, Keasling JD, Silver PA. Integrating biological redesign: where synthetic biology came from and where it needs to go. Cell. 2014;157(1):151–161. doi: 10.1016/j.cell.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Bacchus W, Aubel D, Fussenegger M. Biomedically relevant circuit-design strategies in mammalian synthetic biology. Mol Syst Biol. 2013;9:691. doi: 10.1038/msb.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123(17):2625–2635. doi: 10.1182/blood-2013-11-492231. ** A summary of recent clinical results from blood cancer patients treated with CAR T cells, and an outlook for future CAR T cell therapeutic development.
- 8.Sadelain M. T-cell engineering for cancer immunotherapy. Cancer J. 2009;15(6):451–455. doi: 10.1097/PPO.0b013e3181c51f37. [DOI] [PubMed] [Google Scholar]
- 9. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. ** An up-to-date overview of the development of adoptive cell therapy for cancer using tumor infiltrating lymphocytes and T cells engineered with synthetic TCRs or CARs.
- 10.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: the next pillar of medicine. Sci Transl Med. 2013;5(179):179ps177. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bluestone JA, Bour-Jordan H. Current and future immunomodulation strategies to restore tolerance in autoimmune diseases. Cold Spring Harb Perspect Biol. 2012;4(11) doi: 10.1101/cshperspect.a007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132):132ra153. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biasco L, Scala S, Basso Ricci L, Dionisio F, Baricordi C, Calabria A, Giannelli S, Cieri N, Barzaghi F, Pajno R, Al-Mousa H, et al. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med. 2015;7(273):273ra213. doi: 10.1126/scitranslmed.3010314. ** This study presents new evidence for long term persistence and safety of genetically engineered T cells. It also describes a high-throughput method to track individual T cell clones using retrovirus integration sites.
- 14.Torikai H, Reik A, Soldner F, Warren EH, Yuen C, Zhou Y, Crossland DL, Huls H, Littman N, Zhang Z, Tykodi SS, et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122(8):1341–1349. doi: 10.1182/blood-2013-03-478255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31(10):928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh H, Huls H, Kebriaei P, Cooper LJ. A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19. Immunol Rev. 2014;257(1):181–190. doi: 10.1111/imr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife. 2014;3 doi: 10.7554/eLife.04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart Y, Reich-Zeliger S, Antebi YE, Zaretsky I, Mayo AE, Alon U, Friedman N. Paradoxical signaling by a secreted molecule leads to homeostasis of cell levels. Cell. 2014;158(5):1022–1032. doi: 10.1016/j.cell.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Youk H, Lim WA. Sending mixed messages for cell population control. Cell. 2014;158(5):973–975. doi: 10.1016/j.cell.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. ** A summary of the molecular structure of CARs and the components that influence CAR signaling. Additional factors that support CAR T cell functions and insights gained in recent clinical studies into the safety and future development of CARs are discussed.
- 24. Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res. 2014;74(1):93–103. doi: 10.1158/0008-5472.CAN-13-1365. * This study describes the design and the preclinical characterization of a chimeric receptor featuring the extracellular domain of a high affinity Fc receptor and the intracellular domain of a second generation CAR. Tumor cells with surface antigens bound by the variable regions of antibodies are subject to recognition by engineered T cells through the interaction between the Fc portion of the antibodies and the FcR domain on T cells. The design bridges antibody therapeutics and cell based therapies. It potentiates multiplexed tumor antigen recognition by T cells expressing the same receptor, as well as a means to regulate therapeutic T cell activities through controlling the dosage of administered antibodies.
- 25.Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31(5):500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Yu Z, Muranski P, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. Inhibition of TGF-beta signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther. 2013;20(5):575–580. doi: 10.1038/gt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leen AM, Sukumaran S, Watanabe N, Mohammed S, Keirnan J, Yanagisawa R, Anurathapan U, Rendon D, Heslop HE, Rooney CM, Brenner MK, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol Ther. 2014;22(6):1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol. 2012;51(3–4):263–272. doi: 10.1016/j.molimm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, Nahvi AV, Ngo LT, Sherry RM, Phan GQ, Hughes MS, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21(10):2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komita H, Zhao X, Katakam AK, Kumar P, Kawabe M, Okada H, Braughler JM, Storkus WJ. Conditional interleukin-12 gene therapy promotes safe and effective antitumor immunity. Cancer Gene Ther. 2009;16(12):883–891. doi: 10.1038/cgt.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chmielewski M, Abken H. CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunol Immunother. 2012;61(8):1269–1277. doi: 10.1007/s00262-012-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YY, Jensen MC, Smolke CD. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc Natl Acad Sci U S A. 2010;107(19):8531–8536. doi: 10.1073/pnas.1001721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, Whittington M, Yang Y, Overwijk WW, Lizee G, Hwu P. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010;16(22):5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, Powell DJ, Jr, Riley JL, June CH, Albelda SM. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17(14):4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conklin BR, Hsiao EC, Claeysen S, Dumuis A, Srinivasan S, Forsayeth JR, Guettier JM, Chang WC, Pei Y, McCarthy KD, Nissenson RA, et al. Engineering GPCR signaling pathways with RASSLs. Nat Methods. 2008;5(8):673–678. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park JS, Rhau B, Hermann A, McNally KA, Zhou C, Gong D, Weiner OD, Conklin BR, Onuffer J, Lim WA. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc Natl Acad Sci U S A. 2014;111(16):5896–5901. doi: 10.1073/pnas.1402087111. * See 38.
- 38. Xu Y, Hyun YM, Lim K, Lee H, Cummings RJ, Gerber SA, Bae S, Cho TY, Lord EM, Kim M. Optogenetic control of chemokine receptor signal and T-cell migration. Proc Natl Acad Sci U S A. 2014;111(17):6371–6376. doi: 10.1073/pnas.1319296111. * These articles present two modes of orthogonal control over the migration of engineered T cells using synthetic GPCRs, a promising approach to enhance T cell infiltration into tumor microenvironments and immune privileged sites that are normally devoid of T cells.
- 39.Spangler JB, Moraga I, Mendoza JL, Garcia KC. Insights into cytokine-receptor interactions from cytokine engineering. Annu Rev Immunol. 2015;33:139–167. doi: 10.1146/annurev-immunol-032713-120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakur A, Lum LG. Cancer therapy with bispecific antibodies: Clinical experience. Curr Opin Mol Ther. 2010;12(3):340–349. [PMC free article] [PubMed] [Google Scholar]
- 41.Urbanska K, Lynn RC, Stashwick C, Thakur A, Lum LG, Powell DJ., Jr Targeted cancer immunotherapy via combination of designer bispecific antibody and novel gene-engineered T cells. J Transl Med. 2014;12(1):347. doi: 10.1186/s12967-014-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118(5):1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, Bonini C, Bordignon C. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109(11):4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 45. Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. * A proof-of-principle study demonstrating the potential of combinatorial antigen recognition using more than one synthetic receptors. Increasing the number of recognized antigens promotes the targeting specificity of engineered T cells and thereby could improve the treatment efficacy through reduced off-target toxicities.
- 46.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schonfeld K, Koch J, Dotti G, Heslop HE, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15(12):1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]