Abstract
The purpose of this study was to evaluate elasticity and viscoelasticity in the anterior and deeper stromal regions of the cornea after cross linking with three different protocols using atomic force microscopy (AFM) through indentation. A total of 40 porcine corneas were used in this study and were divided into 4 groups (10 corneas per group): control (no treatment), Dresden (corneal epithelial debridement, riboflavin pretreatment for 30 minutes and a 3mw/cm2 for 30 minutes UVA irradiation), accelerated (corneal epithelial debridement, riboflavin pretreatment for 30 minutes and a 30mw/cm2 for 3 minutes UVA irradiation), and genipin (corneal epithelial debridement and submersion of anterior surface in a 1% genipin solution for 4 hours). Elasticity and viscoelasticity were quantified using AFM through indentation for all corneas, for the anterior stroma and at a depth of 200μm. For the control, Dresden, accelerated, and Genipin groups, respectively, the average Young’s modulus for the anterior stromal region was 0.60±0.58MPa, 1.58 ±1.04MPa, 0.86±0.46MPa, and 1.71±0.51MPa; the average for the 200μm stromal depth was 0.08±0.06MPa, 0.08±0.04MPa, 0.08±0.04MPa, and 0.06±0.01MPa. Corneas crosslinked with the Dresden protocol and genipin were significantly stiffer than controls (p<0.05) in the anterior region only. For the control, Dresden, Accelerated, and Genipin groups, respectively, the average calculated apparent viscosity for the anterior stroma was 88.2±43.7kPa-s, 8.3±7.1kPa-s, 8.1±2.3kPa-s, and 9.5±3.8kPa-s; the average for the 200μm stromal depth was 35.0±3.7kPa-s, 49.6±35.1kPa-s, 42.4±17.6kPa-s, and 41.8±37.6kPa-s. All crosslinking protocols resulted in a decrease in viscosity in the anterior region only (p<0.05). The effects of cross-linking seem to be limited to the anterior corneal stroma and do not extend to the deeper stromal region. Additionally, the Dresden and genipin protocols seem to produce a stiffer anterior corneal stroma when compared to the accelerated protocol.
Keywords: Cornea crosslinking, ultraviolet light, corneal elasticity, corneal viscoelasticity, Atomic Force Microscopy
INTRODUCTION
Cross linking of the cornea has been gaining popularity over the past 10 years as an approach to halt the progression of corneal ectatic disorders such as keratoconus and post-LASIK corneal ectasia (O’Brart, 2014). The original protocol described by Seiler et al., also known as the Dresden protocol, is based on a photo-chemical reaction between ultraviolet light-A (UV-A) and riboflavin (Wollensak et al., 2003a, Wollensak et al., 2003b). Theoretically, cross linking of the cornea inhibits the progression of corneal ectatic disorders by increasing corneal stiffness; this is accomplished by the induction of chemical covalent bonds at the surface of collagen fibrils and within the surrounding proteoglycans (Hayes et al, 2013). The above theory has been proven indirectly by clinical studies, which demonstrate stabilization of keratometric values and refraction (Vinciguerra et al., 2013, Kymionis et al., 2012) and by a few experimental studies that assessed the increase of corneal stiffness (Wollensak et al., 2003b, Kohlhaas et al., 2006, Lanchares et al., 2011, Spoerl et al., 1998, Wollensak & Iomdina, 2009b, Wollensak & Iomdina, 2009a). A land mark report by Wollensak et al demonstrates a significant increase in corneal stiffness and in Young’s modulus after crosslinking assessed using stress-strain measurements (Wollensak et al., 2003b).
After the initial Dresden protocol, a series of other protocols have also been proposed focusing on the retention of the corneal epithelium (Caporossi et al., 2013), the delivery of riboflavin to achieve corneal stromal saturation (Arboleda et al., 2014, Seiler et al., 2014), the increase of UV-A intensity (Tomita et al., 2014), the decrease of the treatment time (Tomita et al., 2014), and even the use of other crosslinking agents (Avila et al., 2012, Avila & Navia, 2010). The purpose of this experimental study is to quantify the elasticity and viscoelasticity of the anterior stroma and at a stromal depth of 200μm after three different crosslinking protocols (Dresden (Wollensak et al., 2003a), Accelerated (Tomita et al., 2014) and Genipin (Avila & Navia, 2010)) using atomic force microscopy (AFM) in porcine corneas. Through the principle of nanoindentation and its ability to implement low indentation depths, AFM can independently characterize the distinct layers of corneal samples and perform depth-dependent characterization studies with proper hydration (Dias et al., 2013, Dias & Ziebarth, 2013).
MATERIALS AND METHODS
Tissue acquisition
A total of 40 porcine globes obtained from an abattoir were used in this study. Upon receipt, the corneal epithelium was removed and the corneas ware excised, leaving a generous scleral rim (between 2 to 4 mm). The corneas were then placed in 20% Dextran overnight to restore the cornea to its physiological thickness range of 500 to 800μm (Borja et al., 2004). Pachymetry measurements were taken to ensure thickness restoration (DGH 55 Pachmate, DGH Technology Inc., Exton, PA, USA). The corneas were then divided into four groups (10 corneas per group): control, Dresden, accelerated crosslinking, and genipin:
-
-
Control group (Group 1): the corneas in this group were subjected to no treatments
-
-
Dresden group (Group 2): the de-epithelialized corneas were instilled with 0.1% riboflavin solution (10mg riboflavin-5-phosphate in 10mL Dextran 20% solution) at a rate of one drop every 5 minutes for 30 minutes. After corneal stromal saturation with riboflavin, the corneas were irradiated using UV-A light with a wavelength of 370 nm and with an intensity of 3mW/cm2. The irradiance was performed for 30 minutes, corresponding to a total surface dose of 5.4 J/cm2. During UVA irradiation, riboflavin solution was applied every 5 minutes to maintain corneal saturation with riboflavin.
-
-
Accelerated group (Group 3): the de-epithelialized corneas were instilled with 0.1% riboflavin solution (10mg riboflavin-5-phosphate in 10mL Dextran 20% solution) one drop every 5 minutes for 30 minutes. After corneal stromal saturation with riboflavin, the corneas were irradiated using UV-A light with a wavelength of 370 nm and with an intensity of 30mW/cm2. The irradiance was performed for 3 minutes, corresponding to a total surface dose of 5.4 J/cm2.
-
-
Genipin group (Group 4): genipin is a natural chemical crosslinker, derived from the Gardenia jazminoides plant. Based on the described protocol by Avila et al, the de-epithelialized corneas were placed anterior side down in 1% genipin solution (1g genipin/100mL balanced salt solution) for a duration of 4 hours (the corneas were not bathed in the solution, only the stromal side was exposed and not the endothelial side) (Avila et al., 2012, Avila & Navia, 2010).
After treatment, corneas within their respective experimental group were evenly divided for elasticity and viscoelasticity assessment of the anterior stroma and stromal region at a depth of 200μm. Since the porcine cornea lacks Bowman’s membrane, the superficial anterior stromal region was readily accessed (the corneal epithelium was removed for Dextran solution pretreatment). The depth of 200μm was accessed using a corneal microkeratome (Moria, LSK Evolution 2, Moria SA, Antony, France) with a 200μm head (CBSU 200 Head, Moria-SA, Antony, France). The corneal thickness was measured again to determine how much stroma was removed by the microkeratome. The corneal samples were then placed in a custom cornea holder with 15% Dextran solution to maintain corneal hydration prior to mechanical testing22.
Elasticity and Viscoelasticity assessment
Mechanical property measurements were performed using a custom-built AFM system with elastic and viscoelastic characterization capability (Dias et al., 2013, Dias & Ziebarth, 2013, Ziebarth et al., 2011, Ziebarth et al., 2007). AFM cantilevers (NSC12 series, Mikromasch, San Jose, CA) were modified with glass microspheres (59–74μm diameter, 15926-100, Polysciences Inc) and then calibrated using a reference force calibration cantilever (CLFC-NOBO, Bruker, Camarillo, CA) (spring constant of modified tip: 29.8N/m). The modified cantilever tips were used to indent the corneal samples with an approach speed of 15μm/s. For elasticity testing, a maximal indentation force of 1000mV (<20 nN) was applied by the cantilever on the cornea and then was immediately retracted at the same speed. For stress-relaxation testing, the same indentation force of 1000mV was applied on the cornea and remained at that indentation depth for a minimum stress hold time of 10 seconds. With the use of custom MATLAB programs, the indentation force-indentation depth curves were analyzed using the Hertz model for a spherical indenter (Hertz, 1881):
where F [N] is the applied force, E [N/m2 or Pa] is the Young’s modulus of elasticity of the sample of interest, R [m] is the radius of the indenter, ν [dimensionless] is the Poisson’s ratio, and D [m] is the indentation. In addition, the stress relaxation force response curves were analyzed using the Darling viscoelastic model (Darling et al., 2006):
where δ0 [m] is the penetration indentation depth that the stress relaxation occurs, ER [N/m2 or Pa] is the relaxed modulus, ν [dimensionless] is the Poisson’s ratio of the material, τ0 [s] is the relaxation time under constant load, τε [s] is the relaxation time under constant deformation, R [m] is the radius of the indenter, and μ [Pa-s] is apparent viscosity. These recordings were repeated at least 15 times per sample. All experiments were performed at room temperature. The accuracy of the curve fits was visually verified.
Statistical analysis
Statistical analysis of data was performed by a custom made data base in Excel (Microsoft Office). A Student’s t-test was used to analyze the elasticity and viscoelasticity of the control versus treated corneas. A p value less than 0.05 was considered to be statistically significant.
RESULTS
Thickness
The average central corneal thickness for all the eyes at the start of the experiments was 662.8±30.7μm (range: 600–705 μm). For the porcine corneas subjected to mechanical testing at a depth of 200μm, the average amount of stroma removed using the 200μm microkeratome head was 222.2±41.3μm (range: 141–284 μm). The percentage change of corneal thickness (change in thickness relative to initial thickness) before and after treatment (Figure 1) demonstrated a statistical significance (p<0.05), for groups 2, 3 and 4; for groups 2 and 3 a decrease in thickness was revealed, while for group 4 an increase.
Figure 1.
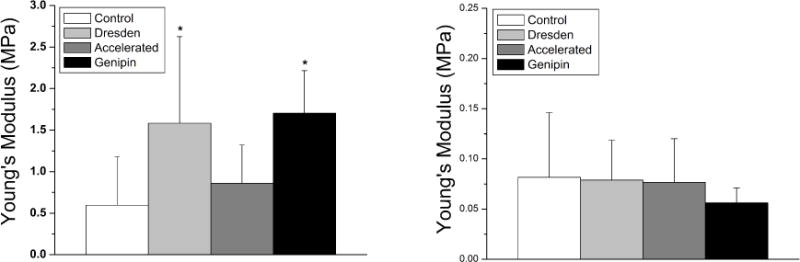
Young’s modulus of elasticity for the anterior (left) and 200μm stromal depth (right). The Dresden and genipin protocols significantly increased the stiffness of the cornea in the anterior region only (indicated by an asterisk on the graph). Note that the vertical scale for the 200μm stromal depth is smaller than the anterior for clarity.
Elasticity
The average Young’s modulus for each experimental group at the anterior stromal region was: 0.60±0.58MPa, 1.58 ±1.04MPa, 0.86±0.46MPa, and 1.71±0.51MPa for groups 1, 2, 3 and 4, respectively (Figure 1, left). For the 200μm depth stromal depth, the average Young’s modulus was: 0.08±0.06MPa, 0.08±0.04MPa, 0.08±0.04MPa, and 0.06±0.01MPa for groups 1, 2, 3 and 4, respectively (Figure 1, right). Concerning the anterior stromal region, the Dresden and genipin crosslinking treatments proved statistically stiffer when compared to the control (p<0.05), while the accelerated protocol was comparable (p=0.23); at the 200μm stromal depth, all groups deemed comparable to the control (p=0.47 for Dresden, p=0.45 for accelerated, and p=0.25 for genipin).
Viscoelasticity
The average calculated apparent viscosity for each experimental group at the anterior stromal region was: 88.2±43.7kPa-s, 8.3±7.1kPa-s, 8.1±2.3kPa-s, and 9.5±3.8kPa-s for groups 1, 2, 3 and 4, respectively (Figure 2, left). A statistically significant decrease in apparent viscosity was demonstrated in all groups (p<0.05) when compared to the control group. For the 200μm stromal depth, the average calculated apparent viscosity was 35.0±3.7kPa-s, 49.6±35.1kPa-s, 42.4±17.6kPa-s, and 41.8±37.6kPa-s for groups 1, 2, 3 and 4, respectively (Figure 2, right); at the 200μm stromal depth, all groups were comparable to the control (p=0.27 for Dresden, p=0.33 for accelerated, and p=0.37 for genipin).
Figure 2.
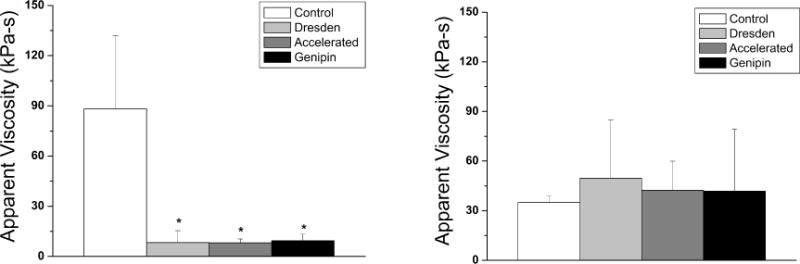
Viscosity for the anterior (left) and 200μm stromal depth (right). All crosslinking protocols resulted in a significant decrease in viscosity in the anterior region only (indicated by an asterisk on the graph).
DISCUSSION
The cross-linkage theory of aging was first proposed in 1942 by Bjorksten et al, and he applied this theory to several aging diseases, such as sclerosis and the loss of elasticity in the skin (Bjorksten, 1968). Cross-linking occurs between protein molecules, with the most prominent example in animals being collagen tissue. Collagen is the most abundant protein in vertebrates, found in the skin, tendons, ligaments, bone, and cartilage. The theory maintains that in young humans there are few cross-links between the collagen proteins while aging increases the number of cross-links, causing, for example, the skin to shrink and become less soft and pliable. The same applies for the corneal stroma as it is mainly composed from collagen; the cornea gets stiffer with age and that is why in most cases keratoconus does not progress after the age of 35 to 40 years.
The original corneal crosslinking treatment (Dresden protocol), which is based on a photochemical reaction between ultraviolet light-A (UV-A) and riboflavin (Wollensak et al., 2003a), basically aims to ‘age’ the corneal stroma and produce a stiffer cornea (Wollensak et al., 2003a, Wollensak, 2006). The introduction of cross-linking in routine clinical practice has changed the management of corneal ectatic disorders; furthermore, it provides a ‘true’ treatment, by inhibiting their progression. Prior to crosslinking, all interventions (glasses, contact lenses and intra-corneal ring segment implantation (Kymionis et al., 2007)) were used to improve visual function of patients, while they did not treat the underlying pathophysiology of the corneal tissue. Other protocols (accelerated cross linking (Tomita et al., 2014)) and different cross-linking agents have been also described to produce a stiffer cornea. A promising alternate to the well-established UVA and riboflavin cross-linking, is the use of genipin (Avila & Navia, 2010, Avila et al., 2012). Genipin is a natural chemical cross-linker, derived from the Gardenia jazminoides plant and its installation on the bear corneal stroma resulted in increase of the stromal mechanical strength as described by Avila et al (Avila & Navia, 2010, Avila et al., 2012).
Our study revealed that the Dresden and genipin crosslinking treatments were most effective in increasing corneal stiffness within the anterior stromal region. However, the accelerated crosslinking protocol did not produce any effect on the corneal elasticity as compared to the control. Concerning the 200μm stromal depth, none of the treatments produced significant changes in corneal elasticity. This signifies that the effects of all treatment protocols were limited only to the anterior stroma; this can be attributed to several factors including the limited diffusion of the riboflavin and genipin solutions into the deeper stromal regions as well as the limited exposure of these deeper stromal regions to UV-A irradiation due to the Beer-Lambert Law, which describes the exponential decrease of light attenuation with increasing distances. The control group demonstrated elasticity that was statistically comparable between the anterior and 200μm stromal depth. Such result was also observed in the study of Kohlhaas et al (Kohlhaas et al., 2006). This observation is believed to be correlated to the stromal organization of the porcine corneal model, which is highly organized and consistent through its stromal depth, compared to the varied stromal organization of the human cornea.
Viscosity classically represents the resistance of a material to the occurrence of fluid flow within its microstructure. Using this definition, it would be reasonable to assume that the viscous properties of the cornea correspond to the nature of the proteoglycan-keratocyte content within the stroma. A study by Zhang et al demonstrated that riboflavin-UVA cross-linking yielded not only crosslinking between the collagen fibers but also crosslinking between the proteoglycan content (Zhang et al., 2011). However, the viscosity measurement could also be indicative of modifications to the intrinsic viscoelasticity of the proteoglycans and collagen chains that comprise the cornea. This would mean that crosslinking modifies the collagen-proteoglycan interface or the collagen-collagen fibrillar interaction, thereby preventing slippage of lamellae past each other. Within the anterior stromal region, the crosslinking treatments produced notable decreases in corneal viscosity compared to the control corneas. Such decrease observed corresponds to modification to the stroma, but future studies should be conducted to establish a correlation between the viscosity parameter, proteoglycan-keratocyte matrix, collagen-proteoglycan interface, and collagen-collagen fibrillary interaction. With regards to the 200μm stromal depth, none of the crosslinking treatments produced statistically significant differences in corneal viscosity, compared to the control group. These results reveal that the deeper stromal regions remain unaffected by the crosslinking treatments.
An important limitation of this study is that porcine corneas were used as the experimental model. These corneas are approximately 50% thicker in comparison to humans and the porcine corneal stroma is highly organized and consistent through all its depth, compared to the varied stromal organization of the human cornea. These factors may influence the effect of the cross-linking treatment and an extrapolation of these study findings to humans is difficult. However, the results of this study show that there is no significant stiffening or change in viscosity of the cornea at a depth of 200μm, which is still clinically relevant to the human cornea, since this depth corresponds to the mid-stromal region of the human.
With the spherical indenter geometry used, the Hertz model was used to calculate Young’s modulus of elasticity from the force and indentation information (Hertz, 1881). The Darling model that was used to calculate viscosity also uses the Hertz model for spherical indenters as a starting point in the derivation. However, it is important to note that the Hertz model assumes that the sample is isotropic, homogeneous, linearly elastic, and infinitely thick, none of which accurately describe the cornea. Although the use of the Hertz model will not provide absolute values for elasticity and viscosity, it will still give important relative numbers to compare differences between groups. Because of this, the use of the Hertz model, and associated variations for cantilever tip geometry, has become standard among groups using Atomic Force Microscopy to characterize tissue mechanics, including the cornea (Vinckier and Semenza, 1998; Ikai et al, 2003; Last et al, 2012; Lombardo et al, 2012). In the current study, all experimental conditions remained constant between groups, so any differences in Young’s modulus and viscosity measured are indicative of modifications due to the treatment.
CONCLUSIONS
In conclusion cross-linking treatments seem to produce a stiffer cornea with a lower viscosity. Treatments appear to be depth-dependent, with the majority taking place in the anterior stromal region and less in the deeper stromal regions.
Research Highlights.
Cornea stiffness was quantified after crosslinking with three different protocols
The traditional Dresden protocol was more effective than an accelerated protocol
The chemical crosslinker genipin significantly increased corneal stiffness
Crosslinking effects are limited to the anterior corneal stroma
Acknowledgments
Grant support: UNCF/MERCK Science Research Dissertation Fellowship (JD); NIH National Research Service Award Individual Predoctoral Fellowship (1F31EY021714-01, JD), Hellenic Society of Intraocular Implants and Refractive Surgery scholarship for fellowship training (VFD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial interest in the material described within this manuscript.
References
- Arboleda A, Kowalczuk L, Savoldelli M, Klein C, Ladraa S, Naud MC, Aguilar MC, Parel JM, Behar-Cohen F. Evaluating in vivo delivery of riboflavin with coulomb-controlled iontophoresis for corneal collagen cross-linking: a pilot study. Invest Ophthalmol Vis Sci. 2014;55:2731–2738. doi: 10.1167/iovs.14-13931. [DOI] [PubMed] [Google Scholar]
- Avila MY, Gerena VA, Navia JL. Corneal crosslinking with genipin, comparison with UV-riboflavin in ex-vivo model. Molecular vision. 2012;18:1068–1073. [PMC free article] [PubMed] [Google Scholar]
- Avila MY, Navia JL. Effect of genipin collagen crosslinking on porcine corneas. Journal of cataract and refractive surgery. 2010;36:659–664. doi: 10.1016/j.jcrs.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Bjorksten J. The crosslinkage theory of aging. Journal of the American Geriatrics Society. 1968;16:408–427. doi: 10.1111/j.1532-5415.1968.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Borja D, Manns F, Lamar P, Rosen A, Fernandez V, Parel JM. Preparation and hydration control of corneal tissue strips for experimental use. Cornea. 2004;23:61–66. doi: 10.1097/00003226-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D, Caporossi T. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39:1157–1163. doi: 10.1016/j.jcrs.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2006;14:571–579. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Dias J, Diakonis VF, Kankariya VP, Yoo SH, Ziebarth NM. Anterior and posterior corneal stroma elasticity after corneal collagen crosslinking treatment. Exp Eye Res. 2013;116:58–62. doi: 10.1016/j.exer.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias JM, Ziebarth NM. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp Eye Res. 2013;115:41–46. doi: 10.1016/j.exer.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Kamma-Lorger CS, Boote C, Young RD, Quantock AJ, Rost A, Khatib Y, Harris J, Yagi N, Terrill N, Meek KM. The Effect of Riboflavin/UVA Collagen Cross-linking Therapy on the Structure and Hydrodynamic Behaviour of the Ungulate and Rabbit Corneal Stroma. PLoS ONE. 8:e52860. doi: 10.1371/journal.pone.0052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz H. The contact of elastic solids. Journal für die Reine und Angewandte Mathematik. 1881;92:156. [Google Scholar]
- Ikai A, Afrin R, Sekiguchi H, Okajima T, Alam MT, Nishida S. Nano-mechanical methods in biochemistry using atomic force microscopy. Curr Protein Pept Sci. 2003;4:181–193. doi: 10.2174/1389203033487171. [DOI] [PubMed] [Google Scholar]
- Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. Journal of cataract and refractive surgery. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Grentzelos MA, Kounis GA, Diakonis VF, Limnopoulou AN, Panagopoulou SI. Combined transepithelial phototherapeutic keratectomy and corneal collagen cross-linking for progressive keratoconus. Ophthalmology. 2012;119:1777–1784. doi: 10.1016/j.ophtha.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Siganos CS, Tsiklis NS, Anastasakis A, Yoo SH, Pallikaris AI, Astyrakakis N, Pallikaris IG. Long-term follow-up of Intacs in keratoconus. American Journal of Ophthalmology. 2007;143:236–244. doi: 10.1016/j.ajo.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Lanchares E, del Buey MA, Cristobal JA, Lavilla L, Calvo B. Biomechanical property analysis after corneal collagen cross-linking in relation to ultraviolet A irradiation time. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249:1223–1227. doi: 10.1007/s00417-011-1674-0. [DOI] [PubMed] [Google Scholar]
- Last JA, Thomas SM, Crasdale CR, Russell P, Murphy CJ. Compliance profile of the human cornea as measured by atomic force microscopy. Micron. 2012;43:1293–1298. doi: 10.1016/j.micron.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M, Lombardo G, Carbone G, De Santo MP, Barberi R, Serrao S. Biomechanics of the Anterior Human Corneal Tissue Investigated with Atomic Force Microscopy. Investigative Ophthalmology & Visual Science. 2012;53:1050–1057. doi: 10.1167/iovs.11-8720. [DOI] [PubMed] [Google Scholar]
- O’Brart DP. Corneal collagen cross-linking: a review. Journal of optometry. 2014;7:113–124. doi: 10.1016/j.optom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler TG, Fischinger I, Senfft T, Schmidinger G, Seiler T. Intrastromal application of riboflavin for corneal crosslinking. Invest Ophthalmol Vis Sci. 2014;55:4261–4265. doi: 10.1167/iovs.14-14021. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Experimental eye research. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- Tomita M, Mita M, Huseynova T. Accelerated versus conventional corneal collagen crosslinking. J Cataract Refract Surg. 2014;40:1013–1020. doi: 10.1016/j.jcrs.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Vinciguerra R, Romano MR, Camesasca FI, Azzolini C, Trazza S, Morenghi E, Vinciguerra P. Corneal Cross-Linking as a Treatment for Keratoconus: Four-Year Morphologic and Clinical Outcomes with Respect to Patient Age. Ophthalmology. 2013 doi: 10.1016/j.ophtha.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Vinckier A, Semenza G. Measuring elasticity of biological materials by atomic force microscopy. FEBS Letters. 1998;430:12–16. doi: 10.1016/s0014-5793(98)00592-4. [DOI] [PubMed] [Google Scholar]
- Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Current opinion in ophthalmology. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. Journal of cataract and refractive surgery. 2009a;35:540–546. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmologica. 2009b;87:48–51. doi: 10.1111/j.1755-3768.2008.01190.x. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. American Journal of Ophthalmology. 2003a;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. Journal of cataract and refractive surgery. 2003b;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Conrad AH, Conrad GW. Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking. The Journal of biological chemistry. 2011;286:13011–13022. doi: 10.1074/jbc.M110.169813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebarth NM, Arrieta E, Feuer WJ, Moy VT, Manns F, Parel JM. Primate lens capsule elasticity assessed using Atomic Force Microscopy. Experimental eye research. 2011;92:490–494. doi: 10.1016/j.exer.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebarth NM, Wojcikiewicz EP, Manns F, Moy VT, Parel JM. Atomic force microscopy measurements of lens elasticity in monkey eyes. Molecular vision. 2007;13:504–510. [PMC free article] [PubMed] [Google Scholar]


