Abstract
Rationale
Notwithstanding the uncertainties regarding the outcomes of BMC therapy for heart repair, further insights are critically needed to improve this promising approach.
Objective
To delineate the true impact of BMC therapy for cardiac repair and gain insights for future trials through systematic review and meta-analysis of data from eligible randomized controlled trials (RCTs).
Methods and Results
Database searches through August 2014 identified forty-eight eligible RCTs (enrolling 2602 patients). Weighted mean differences for changes in left ventricular (LV) ejection fraction (EF), infarct size, LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV) were analyzed with random-effects meta-analysis. Compared with standard therapy, BMC transplantation improved LVEF (2.92%; 95% confidence interval [CI], 1.91 to 3.92; P<0.00001), reduced infarct size (−2.25%; 95% CI, −3.55 to −0.95; P=0.0007) and LVESV (−6.37 ml; 95% CI, −8.95 to −3.80; P<0.00001), and tended to reduce LVEDV (−2.26 ml; 95% CI, −4.59 to 0.07; P=0.06). Similar effects were noted when data were analyzed after excluding studies with discrepancies in outcomes reporting. The benefits also persisted when cardiac catheterization was performed in control patients as well. Although imaging modalities partly influenced the outcomes, LVEF improved in BMC-treated patients when assessed by MRI. Early (<48h) BMC injection after MI was more effective in reducing infarct size, while BMC injection between 3 and 10 days proved superior toward improving systolic function. A minimum of 50 million BMCs seemed to be necessary, with limited additional benefits seen with increasing cell numbers. BMC therapy was safe and improved clinical outcomes, including all-cause mortality, recurrent MI, ventricular arrhythmia, and cerebrovascular accident (CVA) during follow-up, albeit with differences between acute MI and chronic IHD subgroups.
Conclusions
Transplantation of adult BMCs improves LVEF, reduces infarct size and ameliorates remodeling in patients with IHD. These effects are upheld in analyses of studies employing MRI, and also after excluding studies with discrepant outcomes reporting. BMC transplantation may also reduce the incidence of death, recurrent MI, ventricular arrhythmia, and CVA during follow-up.
Keywords: Acute myocardial infarction, bone marrow mononuclear cells, meta-analysis, ischemic heart disease, stem cell
INTRODUCTION
Acute myocardial infarction (MI) and chronic ischemic heart disease (IHD) cause significant mortality, morbidity, and economic burden. A significant number of these patients develop heart failure due to progressive myocardial remodeling and left ventricular (LV) dysfunction. The existing pharmacological modalities have been able to slow the progression, but not reverse this deleterious process. This acute need for new therapies, coupled with early positive results, have led to quick embracing of adult bone marrow cell (BMC) therapy for heart repair by scientists and clinicians alike. Although results from individual trials have been discordant,1 emerging evidence from several large meta-analyses of pooled data suggest that therapy with bone marrow cells (BMCs) may exert multi-faceted salubrious effects in patients with acute MI as well as chronic IHD, enhancing LV function and remodeling and improving outcomes.2–4
Despite this wide appreciation of cell therapy as a viable yet experimental option, several trials have been unable to document benefits of BMC therapy,5,6 and the overall effects of cell therapy have remained controversial. The lack of benefit has been attributed to differences in trial design, and variability in imaging modalities used for assessment of efficacy endpoints. However, meta-analytic approaches have also failed to identify significant survival benefits of BMC therapy,7 thereby raising questions about the therapeutic efficacy of BMC injection. Moreover, a recent review of BMC therapy trials has raised concerns regarding the presence of significant inconsistencies in the reporting of several cohort studies and even a few RCTs.8
Notwithstanding the above inevitable vagaries associated with an emerging therapy with complex biological products, there remains an acute need to identify ways to improve the outcomes of cell therapy. Therefore, we sought to perform a careful systematic review and meta-analysis of all RCTs of heart repair with BMCs. In addition, separate analyses were performed after excluding RCTs with discrepancies in the reporting of outcome parameters and based on procedures in control groups that ensure appropriate blinding. We also performed several subgroup analyses to delineate the impact of cell number, route of injection, imaging modalities used for the assessment of end-points, cell preparation techniques, and timing of BMC injection after acute MI.
METHODS
Search strategy
We searched MEDLINE, the Web of Science, the Cochrane Central Register of Controlled Trials, and the reference lists of retrieved reports from December 1966 through August 31, 2014 for studies of BMC transplantation in patients with ischemic heart disease using the following terms: “stem cells,” “progenitor cells,” “bone marrow cells,” “coronary artery disease,” “myocardial infarction,” “chronic ischemic heart disease,” “acute myocardial infarction,” “ischemic cardiomyopathy,” “cardiomyopathy,” and “heart failure.” The complete search strategy is provided in Online Figure I.
Study selection
RCTs fulfilling the following criteria were included: i) enrolled patients with acute MI or chronic IHD; ii) patients received percutaneous coronary intervention (PCI) or thrombolysis or coronary artery bypass surgery (CABG); iii) patients in the intervention arm received BMC therapy via intracoronary (including bypass grafts) or intramyocardial (epicardial or endocardial) injection and patients in the control arm received standard therapy; iv) at least 1 month of follow-up; and v) sample size ≥10 patients. Search criteria were set to include only human studies conducted in adults aged ≥18 years. Studies published in languages other than English were excluded except those for which abstracts including the outcomes of interest were available in English. Because we used mean and standard deviation, studies that reported data using median and range could not be included. Studies using circulating progenitor cells after granulocyte colony-stimulating factor (G-CSF) mobilization were excluded to avoid confounding direct effects of G-CSF on the myocardium and BMCs. Studies that did not report pre-intervention and post-intervention outcomes of interest were excluded.9 Studies where the primary manuscript was not accessible were included, if the abstract contained data regarding the outcomes of interest. All studies with discrepancies in the reporting of primary outcomes of interest were excluded from a sub-analysis.5,10–15
Data extraction
Three investigators (M.A., A.S., Z.S.) independently screened all titles and abstracts to identify studies that met the inclusion criteria and extracted relevant data using a standardized form. The outcome measures included changes in LV ejection fraction (LVEF), infarct size, LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV). The clinical outcome measures included all-cause mortality, cardiac mortality, heart failure, stent thrombosis, in-stent restenosis, target vessel revascularization, cerebrovascular accident (CVA), transient ischemic attack (TIA), and ventricular arrhythmia. Data with the longest duration of follow-up were included for primary and secondary outcome measures. LV volumes were not included in the primary analysis if reported as an index. Cardiac magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT) data were preferred over echocardiographic data for primary analysis when available. In one study MRI data was excluded from the analysis as baseline MRI was performed 2 to 3 weeks after acute MI.5 When multiple imaging modalities were used in one study, data from each modality were extracted to be included in subgroup analyses. Clinical trials with multiple publications with sequential follow-up durations or different outcomes were considered as one study.16–18 For studies with 2 intervention arms19–23 that involved 2 different doses (low dose and high dose of BMCs) or different routes of administration (intracoronary and intramuscular), data were combined by the use of methods described in Cochrane handbook. In a study with two treatment arms based on different time-points of cell transplantation but single control group,24 the early time-point was included in the primary analysis to avoid duplication of controls during analysis. In a study with 2 different time-points of cell transplantation, the combined data as reported by the authors were used for the primary analysis.
Quality assessment
The quality of included RCTs was assessed by the use of criteria established by Juni et al.25
Data analysis
Statistical analysis was performed with Cochrane RevMan version 5, and results were expressed as weighted mean differences for continuous outcomes with 95% confidence intervals (CIs). Data were pooled using DerSimonian-Laird random-effects model, however, a fixed-effects model was also used to ensure the robustness of model chosen and the susceptibility to outliers. Heterogeneity was analyzed with the I2 statistic, with a significance level of α=0.05. For the I2 statistic, heterogeneity was defined as low (25%–50%), moderate (50%–75%), or high (>75%). For studies that reported mean±SD at baseline and follow-up but did not report the actual change (from baseline to follow-up) as mean±SD, the change in SD was calculated with a standardized formula.26 The Peto odds ratio (OR) was calculated for clinical outcomes.
Subgroup analysis
Planned subgroup analyses were conducted based on: i) type of IHD (acute MI vs. chronic IHD); ii) location of MI (anterior vs. multiple areas); iii) baseline LVEF of <41% vs. ≥41% (41% was the median LVEF at baseline) and <50% vs. ≥50% (LVEF <50% represents LV dysfunction); iv) type of density gradient media used for cell preparation (Lymphoprep vs. other Ficoll-Paque based methods); v) the use of heparin in the final cellular suspension; vi) route of injection in chronic IHD trials; vii) timing of BMC transplantation after acute MI and/or PCI (0 to 2 days, 3 to 10 days, and more than 10 days); viii) number of BMCs injected (<50 million, 50 to 100 million, >100 to 250 million, and >250 million); and ix) modes of imaging (echocardiography, MRI, left ventriculography [LVG] and SPECT) used for the assessment of primary outcomes. Additional analyses based on the duration of follow-up (0–3 months, 4–6 months, 7–12 months and >12 months) were performed to examine the persistence of effects.
Analysis of outcomes in trials with rigorous study design
A recent study examined the impact of rigor of BMC trials on outcomes in acute MI patients, and concluded that unblinding might lead to overestimation of effects.27 However, due to inherent difficulties associated with ascertaining proper blinding of investigators, treating physicians and patients based only on published reports, we elected to perform separate analyses based on: i) whether control patients underwent cardiac catheterization as a sham procedure; and ii) whether control patients underwent bone marrow aspiration (Online Table I).
Analysis of outcomes without discrepancies in reporting of trial results
With regard to BMC trials, a recent report has also identified numerous discrepancies in reporting of study design, methods, and results.8 However, discrepancies in reporting of design and methods8 may only have limited potential to influence the overall conclusions of these studies. Therefore, to delineate the impact of discrepancies on collective outcomes of cell therapy trials, we performed separate meta-analysis after excluding studies with discrepancies in reporting of primary outcomes, such as LVEF. Discrepancies in reporting results included incorrect calculation of effects (LVEF, infarct size, LVESV and LVEDV) or discrepant reporting of results in different published reports (Online Table II).
RESULTS
Search results
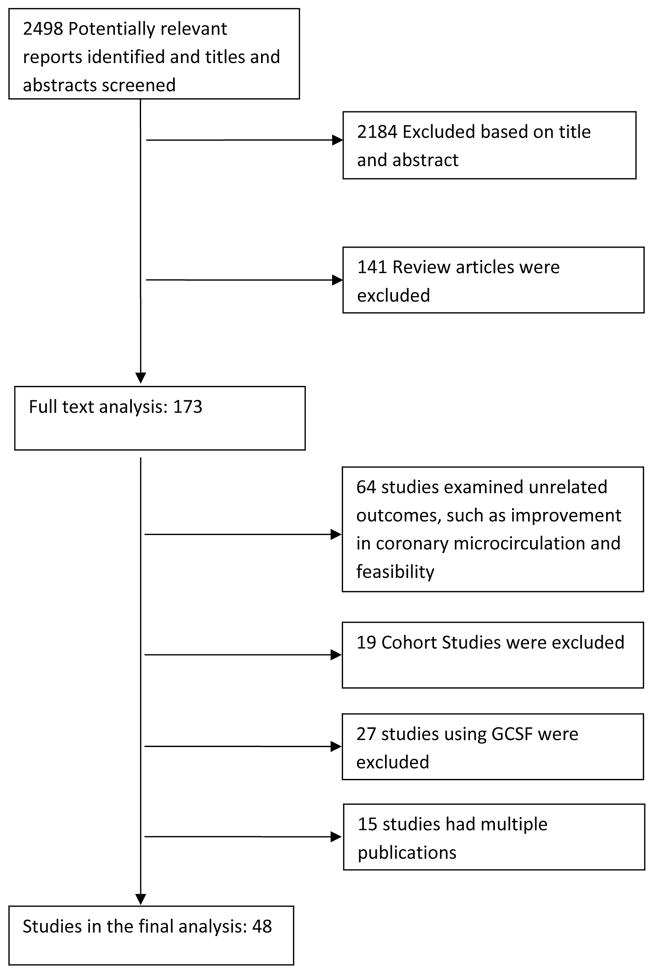
The initial search retrieved 2498 reports, of which 2184 were excluded on the basis of the title and abstract. After the exclusion of 141 review articles, full-text analysis was performed on 173 reports, of which 64 were excluded because of unrelated outcomes; 27 were excluded due to the use of G-CSF; 19 were cohort studies; and 15 articles represented additional publications from previously reported trials, leaving forty-eight RCTs5,6,11–14,16–20,22–24,28–63 (enrolling a total of 2602 patients) for inclusion in the final analysis (Figure 1).
Figure 1. Flow diagram of included RCTs.
The selection of eligible studies of bone-marrow cell transplantation in patients with acute myocardial infarction and chronic ischemic heart disease. G-CSF, granulocyte colony stimulating factor; RCT, randomized controlled trial.
Study characteristics
Table 1 summarizes the characteristics of included studies. The median follow-up duration was 6 months (range, 3–60 months), and the median sample size was 43 patients (range, 10–204 patients). The timing of BMC transplantation in patients with acute MI varied among the included studies (median, 7 days after MI; range, 1 to 18 days), and the median number of BMCs injected was 125×106 (range, 2×106–60×109). The median EF of patients at baseline was 41% (range, 21%–58%).
TABLE 1.
Characteristics of RCTs included in the Meta-Analysis
| Source | Sample size |
Mean follow-up duration (months) |
Cell type | Preparation | No. of cells transplanted |
Route of injection |
Type of IHD |
Location of MI |
Time from PCI and/or MI to cell transplant |
Imaging modalities |
|---|---|---|---|---|---|---|---|---|---|---|
| Ang et al, 200819 | 25 | 6 | BMMNC | Lymphoprep, autologous serum, injected same day | 85±56×106 (IM) 115 ± 73 × 106 (IC) | IM or IC w/CABG | CIHD | NR | >6 wk | MRI |
| Assmus et al, 200628 | 46 | 3 | BMMNC | Ficoll, X-VIVO 10 medium, injected same day | 205±110×106 | IC | CIHD | Multiple | 2470±2196 d | LVG |
| Bartunek et al, 201329 | 36 | 6 | MSC | BMC cultured to purify MSC, treatment with cardiopoietic factors for 5 days in culture, injected 4–6 weeks after marrow aspiration | 733 × 106 (605–1168) | Endomyo | CIHD | NR | > 2 mo | Echo |
| Cao et al, 200930 | 86 | 48 | BMMNC | Lymphoprep, heparinized saline | 5±1.2×107 | IC | AMI | Anterior | 7 d | Echo (EF, Vol), SPECT (IS) |
| Chen et al, 200431 | 69 | 6 | MSC | BMC cultured to purify MSC, heparinized saline, injected 10 days after marrow aspiration | 48–60×109 | IC | AMI | Multiple | 18.4±0.5 d | LVG (EF), PET (IS) |
| Colombo et al, 201114 | 10 | 12 | CD133+ BMMNC | Magnetic bead isolation, normal saline with 10% HAS, injection BMC cultured to purify | 5.9 (4.9 to 13.5)×106 | IC | AMI | Anterior | 10 to 14 d | Echo (EF, Vol) |
| Gao et al, 201332 | 43 | 24 | MSC | MSC, heparinized saline, injected 14 days after marrow aspiration | 3.08 ± 0.52 × 106 | IC | AMI | Multiple | 14 d | Echo |
| Ge et al, 200633 | 20 | 6 | BMMNC | Lymphoprep, heparinized saline, injected 2–3 hours after marrow aspiration | 40×106 | IC | AMI | Multiple | 1 d | Echo (EF), SPECT (IS) |
| Grajek et al, 201034 | 45 | 12 | BMMNC | Ficoll, X-VIVO 15+2 % heat-inactivated autologous plasma, injected next day | 2.34±1.2×109 | IC | AMI | Anterior | 5–6 d | Echo |
| Hendrikx et al, 200635 | 20 | 4 | BMMNC | Lymphoprep, heparinized saline, injected next day | 60.25±31.35×106 | IM | CIHD | Multiple | 217±162 d | MRI |
| Hirsch et al, 201136 | 134 | 4 | BMMNC | Lymphoprep, heparinized saline+4% HSA, injected same day | 296±164 × 106 | IC | AMI | Multiple | 6 (4–7) d | Echo, MRI |
| Huang et al (Abstract), 200637 | 40 | 6 | BMMNC | NA | NA | IC | AMI | Inferior | NA | MRI |
| Huikuri et al, 200811 | 80 | 6 | BMMNC | Ficoll-Hypaque, heparinized, saline+autologous serum, injected within 3 h | 402±196×106 | IC | AMI | Multiple | 2–6 d | Echo (EF), LVG (Vol) |
| Janssens et al, 200638 | 67 | 4 | BMMNC | Ficoll, saline+ autologous serum, injected within 24 h | 172±72×106 | IC | AMI | Multiple | 1–2 d | MRI |
| Jazi et al, 201239 | 32 | 6 | BMMNC | Ficoll-Paque, incubated overnight in growth factors supplemented serum, injected next day | 24.6 ± 8.4 × 108 | IC | AMI | Anterior | < 30 d | Echo |
| Lipiec et al, 200940 | 36 | 6 | BMMNC | Ficoll-Paque Plus, saline, injected within 2–3 h | 0.33±0.17×106 CD133+ 3.36±0.1.87×106 CD34+ |
IC | AMI | Anterior | 3–10 d | SPECT |
| Lu et al, 201341 | 50 | 12 | BMMNC | Ficoll, saline, injected same day | 13.38±8.14×107 | IC | CIHD | Multiple (triple vessel disease) | n/a | MRI |
| Lunde and coworkers, 2006,2008,2 0095,42,70 | 100 | 36 | BMMNC | Lymphoprep, storage in 20 % heparin-plasma, injected next day | 87±47.7×106 | IC | AMI | Anterior | 6±1.3 d | SPECT (EF, EDV, IS), Echo (ESV) |
| Maureira et al, 201243 | 14 | 6 | BMMNC | Ficoll, injected same day | 1 × 107 | Transmyo | CIHD | Multiple | n/a | MRI |
| Meluzin et al. 2006,200820, 21 | 66 | 12 | BMMNC | Histopaque, incubated overnight in serum-free medium before injection | High dose: 1×108 Low dose: 1×107 |
IC | AMI | Multiple | 7±0.3 d | SPECT |
| Meyer and coworkers, 2004,2006,2 00910,44,71 | 60 | 18 | BMMNC | Gelatin polysuccinate density gradient, heparinized saline, injected within 6–8 h | 24.6±9.4×108 | IC | AMI | Multiple | 4.8±1.3 d | MRI |
| Nogueira et al, 200945 | 20 | 6 | BMMNC | Ficoll-Paque Plus, saline+5% HSA, injected within 9 h | 1.0×108 | IC | AMI | Multiple | 5.5±1.2 d | Echo |
| Penicka et al, 200746 | 27 | 4 | BMMNC | NR | 26.4×108 | IC | AMI | Anterior | 4–11 d | Echo (EF, Vol), PECT (IS) |
| Perin et al, 201147 | 30 | 6 | BMMNC | Ficoll, saline+5% HSA, injected same day ALDHbr cells isolated | 484.1 ± 313.0 106 | Endomyo | CIHD | Multiple | n/a | SPECT |
| Perin et al, 201248 | 20 | 6 | BMMNC | by a commercial cell sorter, saline+5 % HSA, injected same day | 2.37±1.31 × 106 | Endomyo | CIHD | Multiple | n/a | Echo |
| Perin et al, 201249 | 82 | 6 | BMMNC | Ficoll-based separation using automated processor (Sepax), saline+5% HSA, injected within 8–9 hours | 99.03±5.58 × 106 | Endomyo | CIHD | Multiple | n/a | Echo |
| Piepoli et al, 2010, 201316,72 | 38 | 24 | BMMNC | Ficoll-Hypaque, saline, 5% HSA, injected same day | 24.88 X 107 | IC | AMI | Anterior | 4–7 d | Echo |
| Plewka et al, 2009,201117, 73 | 56 | 24 | BMMNC | Ficoll-Paque, saline, injected within 2 hours. | 14.4 ± 4.9 × 107 | IC | AMI | Anterior | 7 ± 2 hours | Echo |
| Pokushalov et al, 201050 | 109 | 12 | BMMNC | Ficoll-Paque Plus, heparinized saline, injected same day | 41±16×106 | IM | CIHD | Multiple | 9±8 y | Echo |
| Quyyumi et al, 201151 | 31 | 6 | CD34+ | Magnetic bead isolation (Dynabeads) on Isolox 300i, final solution (10 ml) contained PBS (6ml) & 40% autologous serum (4ml)+1% HSA, injected within 24–48 h | 5–15×106 | IC | AMI | NR | 8.3 d (median) | MRI |
| van Ramshorst et al, 200974 | 49 | 3 | BMMNC | Ficoll, PBS+0.5% HSA, injected same day | 100×106 | IM | CIHD | NA | >6 mo | MRI |
| Roncalli et al, 201153 | 101 | 3 | BMMNC | Ficoll, 4% HSA, injected same day. | 98.3±8.7 × 106 | IC | AMI | Anterior | 9.3±1.7 d | MRI Echo |
| Ruan et al, 200554 | 20 | 6 | BMC | NR | NR | IC | AMI | Anterior | 1 d | Echo |
| Schachinger et al, 2006,200655, 56 | 204 | 4 | BMMNC | Ficoll, storage in X- vivo-10 medium+20% serum, injected the same or next day | 236±174×106 | IC | AMI | Multiple | 4.3±1.3 d | LVG |
| Silva et al, 200922 | 30 | 6 | BMMNC | Ficoll-Paque Plus, saline, injected within 8.5 h | 1×108 | IC | AMI | Multiple | 5.5±1.3 d | RNV |
| Srimahachot a et al, 201115 | 23 | 6 | BMMNC | Isoprep, saline+2% autologous serum, injected same day | 420±221×106 | IC | AMI | Multiple | 57±122 d | MRI |
| Suarez de Lezo et al, 200757 | 20 | 3 | BMMNC | Ficoll-based separation using semi- automated processor (COBE 2991), heparinized saline, injected same day | 9±3×108 | IC | AMI | Anterior | 7±2 d | LVG |
| Surder et al, 201324 | 133 | 4 | BMMNC | Ficoll, 20% autologous serum, injected same day | 159.7 ± 125.8 × 106 | IC | AMI | Anterior (90%) | 6 (2) d | MRI |
| Traverse et al, 2012,20146,58 | 95 | 12 | BMMNC | Ficoll-based separation using automated processor (Sepax), saline+5% HSA, injected within 8–9 hours | 150 × 106 | IC | AMI | Anterior (>90%) | 3–7 d | MRI |
| Traverse et al, 201159 | 87 | 6 | BMMNC | Ficoll-based separation using automated processor (Sepax), saline+5% HSA, injected within 8–9 hours | 1.47±17×108 | IC | AMI | Multiple | 14–21 d (17.4) | MRI |
| Traverse et al, 201013 | 40 | 6 | BMMNC | Ficoll-based separation using semi- automated processor (COBE 2991), saline+5% HSA, injected within 8 hours | 1×108 1.67±0.34×107 | IC | AMI | Anterior | 3–10 d | MRI |
| Tse et al, 200760 | 28 | 6 | BMMNC | Ficoll, PBS+10% autologous serum, injected same day | (low), 4.20±2.80×107 (High) | IM | CIHD | NA | NA | MRI |
| Turan et al, 201262 | 62 | 12 | BMMNC | Automated point of care cell processor (Harvest BMAC system), injected same day | 9.6±3.2×107 | IC | AMI | Multiple | 7 d | LVG |
| Turan et al, 201161 | 56 | 12 | BMMNC | Automated point of care cell processor (Harvest BMAC system), injected same day | 99 ± 25 × 106 | IC | CIHD | NA | n/a | LVG |
| Wohrle et al, 2010,201318, 75 | 40 | 36 | BMMNC | Ficoll, saline+ 2% HSA, Injected within 6 hours | 381±130×106 | IC | AMI | Multiple | 6.1 (5.5–7.3) d | MRI |
| Yao et al, 2008 | 47 | 6 | BMMNC | Ficoll-Hypaque, heparin-treated plasma, injected same day | 180×106 1.9±1.2×108 (single | IC | CIHD | Multiple | 13±8 mo | MRI |
| Yao et al, 2009 | 39 | 12 | BMMNC | Ficoll-Hypaque, heparin-treated plasma, injected same day | transfusion), 2.0±1.4×108 (Repeat transfusion) | IC | AMI | Anterior | 3 to 7 d repeat at 3 mo | MRI |
| Zhao et al, 2008 | 36 | 6 | BMMNC | Ficoll, heparinized saline, injected same day | 6.59 ± 5.12 × 108 | IM w/CABG | CIHD | Multiple | NA | Echo |
Abbreviations: AMI, acute myocardial infarction; BMC, bone marrow cells; BMMNC, bone marrow mononuclear cells; CABG, coronary artery bypass graft; CIHD, chronic ischemic heart disease; Echo, echocardiography; EF, ejection fraction; EPC, endothelial progenitor cell; HSA, human serum albumin; IC, intracoronary; IM, intramuscular; IS, infarct size; LVG, left ventriculography; MI, myocardial infarction; MRI, magnetic resonance imaging; MSC, mesenchymal stem cells; NA, not available; NR, not reported; PCI, percutaneous coronary intervention; PET, positron emission tomography; RCT, randomized controlled trial; RNV, radionuclide ventriculography; SPECT, single-photon emission computed tomography; ALDHbr, Aldehyde dehydrogenas–bright
Study quality
The quality metrics of included studies are shown in Online Table III. At least 22 studies failed to blind participants and/or caregivers; 7 studies did not provide adequate information on blinding of participants and caregivers; and blinding of outcome assessors was unclear in at least 4 studies. The attrition rate and adequacy of follow-up are provided in Online Table III. The follow-up was complete in most studies with shorter follow-up duration. In studies with longer follow-up, the percent of patients lost to follow-up was acceptable. The inter-reviewer agreement on these quality domains was >90%.
Cardiac parameters
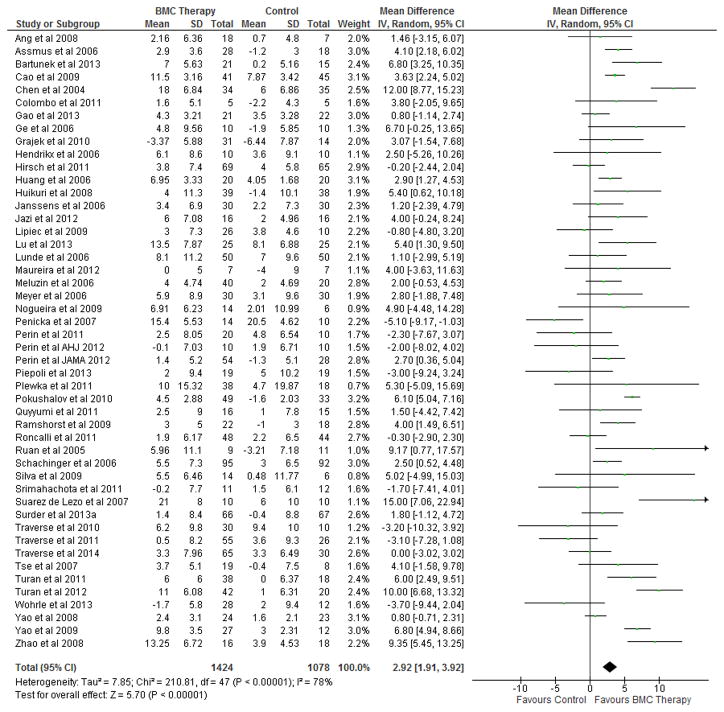
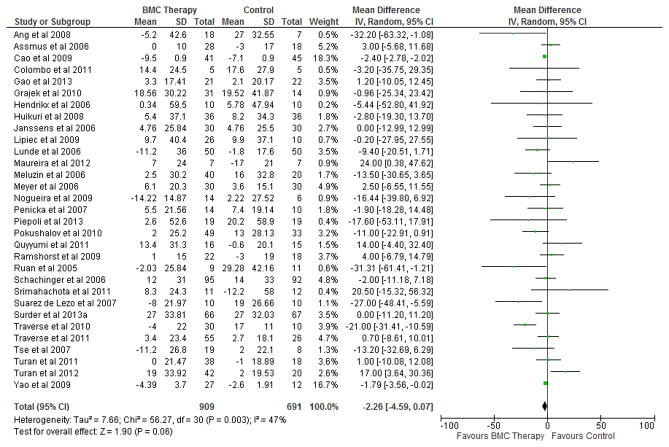
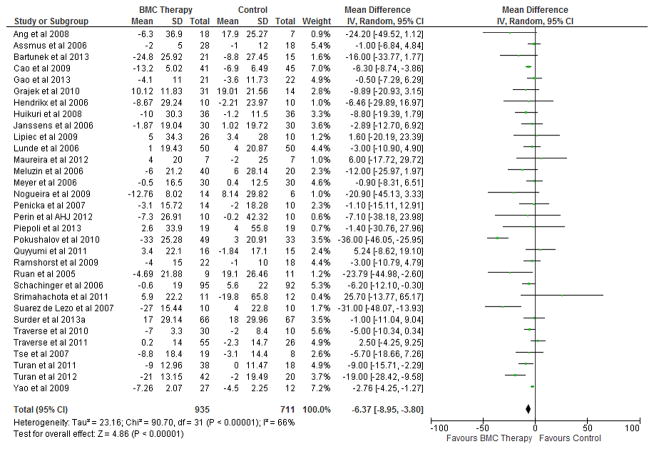
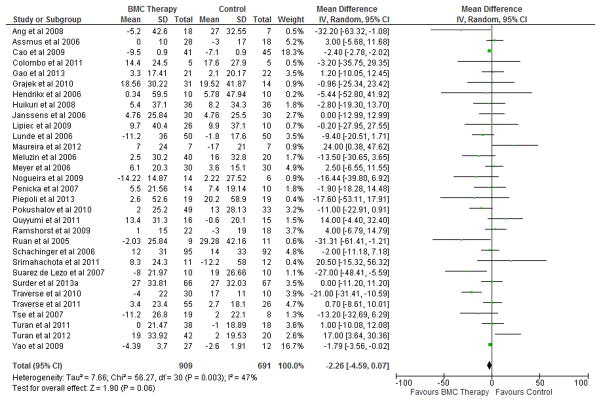
BMC transplantation resulted in improvement in LVEF (2.92%; 95% CI, 1.91 to 3.92; P<0.00001; Figure 2), reduction in infarct size (−2.25%; 95% CI, −3.55 to −0.95; P=0.0007; Figure 3) and LVESV (−6.37 ml; 95% CI, −8.95 to −3.80; P<0.00001; Figure 4) compared with standard therapy. There was a trend toward reduction in LVEDV (2.26 ml; 95% CI, − 4.59 to 0.07; P=0.06; Figure 5).
Figure 2. Impact of BMC transplantation on LV ejection fraction.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in left ventricular ejection fraction (LVEF) in patients treated with bone marrow cells (BMCs) compared with controls in included RCTs. Transplantation of BMCs resulted in a 2.92% (95% CI, 1.91–3.92; P<0.00001) increase in mean LVEF. The overall effect was statistically significant in favor of BMC transplantation. IV, inverse variance.
Figure 3. Impact of BMC transplantation on infarct size.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in infarct scar size in patients treated with bone marrow cells (BMCs) compared with controls in included RCTs. Transplantation of BMCs resulted in a 2.25% (95% CI, −3.55 to −0.95; P<0.0007) decrease in mean infarct scar size. The overall effect was statistically significant in favor of BMC transplantation. IV, inverse variance.
Figure 4. Impact of BMC transplantation on LVESV.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in left ventricular end-systolic volume (LVESV) in patients treated with bone marrow cells (BMCs) compared with controls in included RCTs. Transplantation of BMCs resulted in 6.37 ml (95% CI, − 8.95 to − 3.80; P<0.00001) decrease in LVESV. The overall effect was statistically significant in favor of BMC transplantation. IV, inverse variance.
Figure 5. Impact of BMC transplantation on LVEDV.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in left ventricular end-diastolic volume (LVEDV) in patients treated with bone marrow cells (BMCs) compared with controls in included RCTs. BMC transplantation resulted in a 2.26 ml (95% CI, −4.59 to 0.07; P=0.06) decrease in mean LVEDV. The overall effect was not significant statistically. IV, inverse variance.
Persistence of benefits during long-term follow-up
The sub-analysis performed on the basis of duration of follow-up showed that improvement in LVEF, infarct size, and LVESV persisted beyond 12 months (Table 2). An improvement in LVEDV was noted in the first 12 months; however, this did not persist beyond one year.
TABLE 2.
Unadjusted difference in mean change in outcome parameters in BMC-treated patients compared with controls based on the duration of follow-up.
| Follow-up duration | BMC Therapy (n) | Control (n) | Difference in mean (95% CI) | P value |
|---|---|---|---|---|
|
| ||||
| LVEF | ||||
| 0 – 3 months | 499 | 385 | 3.53 (2.05, 5.00) | <0.00001 |
| 4 – 6 months | 1196 | 958 | 2.92 (1.88, 3.96) | <0.00001 |
| 7 – 12 months | 591 | 446 | 4.43 (0.48, 3.89) | <0.00001 |
| > 12 months | 330 | 298 | 2.19 (0.48, 3.89) | 0.01 |
|
| ||||
| Infarct size | ||||
| 0 – 3 months | 123 | 88 | −6.02 (−11.37, −0.67) | <0.03 |
| 4 – 6 months | 443 | 373 | −2.25 (−3.77, −0.72) | <0.004 |
| 7 – 12 months | 167 | 93 | −4.39 (−7.20, −1.57) | 0.002 |
| > 12 months | 99 | 87 | −2.25 (−3.14, −1.36) | <0.00001 |
|
| ||||
| LVESV | ||||
| 0 – 3 months | 322 | 244 | −7.26 (−11.11 to −3.41) | 0.0002 |
| 4 – 6 months | 907 | 751 | −5.50 (−7.78, −3.22) | <0.00001 |
| 7 – 12 months | 424 | 353 | −10.58 (−14.90, −6.27) | <0.00001 |
| > 12 months | 285 | 271 | −4.76 (−7.46, −2.06) | 0.0006 |
|
| ||||
| LVEDV | ||||
| 0 – 3 months | 322 | 244 | −2.34 (−7.03, 2.34) | 0.33 |
| 4 – 6 months | 877 | 725 | −2.57 (−4.98, −0.16) | <0.04 |
| 7 – 12 months | 432 | 358 | −5.84 (−11.03, −0.64) | <0.00001 |
| > 12 months | 262 | 266 | −2.08 (−4.98, 0.82) | 0.16 |
Abbreviations: BMC, bone marrow cell; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; CI, confidence interval; n, number of patients in each group
Subgroup analysis
Subgroup analysis showed that improvements in LV function, infarct size, and LVESV with BMC transplantation were significant regardless of the type of IHD (acute MI vs. chronic IHD), except that there was a greater reduction in infarct size in patients with chronic IHD (Table 3). The benefits of BMC therapy were similar regardless of the location of MI, except that BMC transplantation resulted in significant reduction in LVEDV (− 9.57 ml, 95% CI,−6.50 to −2.63; P=0.002) in patients with anterior wall MI. The impact of baseline LVEF was analyzed separately on the basis of median LVEF (41%) and the presence of LV systolic dysfunction (LVEF <50%). Results from both analyses showed that BMC recipients experienced similar improvement in EF regardless of the baseline EF; however improvements in LVESV were more pronounced in patients with lower baseline EF (Table 3). As for the route of injection, all patients with acute MI received intracoronary injection of BMCs. Therefore, the impact of intracoronary and intramyocardial routes of injection was analyzed only in patients with chronic IHD. In these patients, the outcomes were not significantly different between the 2 routes of BMC administration, except that intramyocardial injection was associated with greater reduction in LVESV (Table 3). Analysis based on the methods of cell preparation (Ficoll vs. Lymphoprep) did not identify any significant interaction (Table 3). When studies were compared based on the presence or absence of heparin in the final cell suspension, there was greater improvement in LVEF and infarct size in heparin group. With regard to cell preparation, in 31 studies, BMCs were injected on the same day as BM harvest; in 5 studies, cells were injected by the next day; and the time-frame was unclear in 8 studies. BMCs were culture expanded in 4 studies leading to a delay in cell injection for a variable period of time, ranging from 3 days to 6 weeks. Because information on storage condition, especially temperature during storage, was not available in the vast majority, subgroup analysis was not performed.
TABLE 3.
Subgroup analysis to identify any impact of the type of ischemic heart disease, location of myocardial infarction, left ventricular ejection fraction at baseline, route of bone marrow cell transplantation, and methods of cell preparation on outcome variables.
| Outcome | Difference in mean (95% CI) | BMC therapy (n) | Control (n) | Difference in mean (95% CI) | BMC therapy (n) | Control (n) | P value for Interaction |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Acute MI | Chronic IHD | ||||||
| LVEF | 2.49 (1.19, 3.79) | 1065 | 832 | 3.84 (2.30, 5.38) | 361 | 248 | 0.19 |
| Infarct scar size | −2.13 (−3.49, −0.78) | 668 | 545 | −5.90 (−8.03, −3.78) | 56 | 25 | 0.003 |
| LVESV | −4.87 (−7.29, −2.45) | 713 | 567 | −10.37 (−18.56, −2.18) | 222 | 144 | 0.21 |
| LVEDV | −2.55 (−5.00, −0.10) | 718 | 572 | −1.84 (−9.13, 5.46) | 201 | 129 | 0.86 |
|
| |||||||
| Anterior Wall MI | MI in Other Territories | ||||||
| LVEF | 2.04 (0.18, 3.89) | 497 | 373 | 3.13 (1.79, 4.47) | 833 | 644 | 0.35 |
| Infarct scar size | −1.90 (−3.72, −0.07) | 311 | 258 | −3.04 (−5.70, −0.38) | 369 | 280 | 0.49 |
| LVESV | −5.57 (−9.34, −1.81) | 294 | 225 | −7.34 (−12.52, −2.16) | 516 | 390 | 0.59 |
| LVEDV | −9.57 (−16.50, −2.63) | 272 | 218 | −1.48 (−3.04, 0.08) | 518 | 397 | 0.03 |
|
| |||||||
| Baseline LVEF <41 % | Baseline LVEF ≥41% | ||||||
| LVEF | 2.85 (1.34, 4.35) | 602 | 454 | 2.97 (1.63, 4.31) | 824 | 626 | 0.90 |
| Infarct scar size | −2.16 (−3.97, −0.34) | 265 | 217 | −2.38 (−4.76, 0.00) | 449 | 343 | 0.88 |
| LVESV | −9.42 (−14.53, −4.32) | 369 | 287 | −4.70 (−7.40, −1.99) | 566 | 424 | 0.11 |
| LVEDV | −3.79 (−7.60, 0.02) | 348 | 272 | −1.60 (−6.12, 2.91) | 571 | 429 | 0.47 |
|
| |||||||
| Baseline LVEF <50 % | Baseline LVEF ≥50 % | ||||||
| LVEF | 3.03 (1.94, 4.11) | 1240 | 910 | 2.77 (0.86, 4.69) | 206 | 170 | 0.82 |
| Infarct scar size | −2.84 (−4.24, −1.45) | 832 | 702 | −0.72 (−3.63, 2.19) | 84 | 67 | 0.20 |
| LVESV | −7.24 (−10.31, −4.17) | 770 | 565 | −3.43 (−7.69, 0.84) | 165 | 146 | 0.15 |
| LVEDV | −4.38 (−7.88, −0.87) | 777 | 578 | 1.66 (−4.02, 7.34) | 165 | 146 | 0.08 |
|
| |||||||
| IC - CIHD | IM - CIHD | ||||||
| LVEF | 2.37 (0.38, 4.37) | 199 | 151 | 4.12 (2.33, 5.92) | 238 | 164 | 0.20 |
| Infarct scar size | −3.02 (−7.67, 1.64) | 70 | 48 | −5.90 (−16.36, 4.56) | 80 | 55 | 0.62 |
| LVESV | −4.86 (−11.15, 1.44) | 74 | 43 | −11.95 (−23.04, −0.86) | 202 | 136 | 0.28 |
| LVEDV | 1.61 (−5.12, 8.34) | 74 | 43 | −5.37 (−17.71, 6.98) | 127 | 93 | 0.33 |
|
| |||||||
| Other Ficoll-based methods | Lymphoprep | ||||||
| LVEF | 2.93 (1.66, 4.20) | 1167 | 894 | 2.31 (−0.08, 4.69) | 148 | 137 | 0.65 |
| Infarct scar size | −1.47 (−3.37, 0.42) | 389 | 300 | −1.67 (−4.56, 1.22) | 136 | 126 | 0.91 |
| LVESV | −6.45 (−9.91, −2.99) | 641 | 504 | −6.46 (−8.88, −4.05) | 69 | 62 | 1.00 |
| LVEDV | −4.21 (−7.97, −0.45) | 641 | 504 | −9.54 (−27.93, 8.85) | 69 | 62 | 0.58 |
|
| |||||||
| No Heparin | Heparinized saline | ||||||
| LVEF | 1.75 (0.54, 2.96) | 680 | 431 | 5.15 (3.17, 7.12) | 347 | 321 | 0.004 |
| Infarct scar size | −0.49 (−2.74, 1.75) | 275 | 165 | −3.89 (−5.96, −1.82) | 202 | 190 | 0.03 |
| LVESV | −3.74 (−6.30, −1.18) | 429 | 295 | −7.62 (−12.20, −3.04) | 260 | 233 | 0.15 |
| LVEDV | −4.40 (−9.94, 1.14) | 413 | 285 | −1.87 (−3.95, 0.22) | 260 | 233 | 0.40 |
Abbreviations: CIHD, chronic ischemic heart disease; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; IC, intracoronary; IM, intramuscular; MI, myocardial infarction; BM, Bone marrow
Impact of BMC number on cardiac parameters
The median BMC number used in the included trials was 125 million. To find the optimal cell dose, an analysis based on different numbers of injected cells revealed that there was no significant improvement in cardiac parameters in trials that used fewer than 50 million cells (Table 4). Transplantation of 50 to 100 million cells induced significant improvement in LVEF and LVESV. With further increase beyond 100 million and 250 million cells, there were incremental benefits, the significance of which remains unclear. There was no improvement in infarct size seen in each subgroup, primarily due to limited number of studies in each group.
TABLE 4.
Unadjusted difference in mean change in outcome parameters in bone marrow cell–treated patients compared with controls based on the number of injected cells.
| Cell Count (Millions) | BMC Therapy (n) | Control (n) | Difference in mean (95% CI) | P value |
|---|---|---|---|---|
|
| ||||
| LVEF | ||||
| < 50 | 153 | 110 | 2.29 (−0.48, 5.06) | 0.11 |
| 50 to 100 | 431 | 292 | 3.32 (1.79, 4.85) | <0.0001 |
| > 100 to 250 | 472 | 360 | 2.14 (0.36, 3.92) | 0.02 |
| > 250 | 339 | 285 | 3.41 (0.25, 6.57) | 0.03 |
|
| ||||
| Infarct size | ||||
| < 50 | 16 | 15 | 1.50 (−2.55, 5.55) | 0.16 |
| 50 to 100 | 243 | 193 | −2.87 (−4.65, −1.09) | 0.02 |
| > 100 to 250 | 267 | 187 | −1.82 (−4.50, 0.86) | 0.18 |
| > 250 | 186 | 164 | −2.83 (−8.33, 2.68) | 0.31 |
|
| ||||
| LVESV | ||||
| < 50 | 148 | 105 | −6.01 (−19.63, 7.61) | 0.39 |
| 50 to 100 | 305 | 204 | −7.45 (−10.46, −4.44) | <0.00001 |
| > 100 to 250 | 320 | 264 | −2.60 (−3.95, −1.26) | 0.0002 |
| > 250 | 153 | 127 | −8.24 (−16.36, −0.13) | 0.05 |
|
| ||||
| LVEDV | ||||
| < 50 | 143 | 100 | 0.50 (−9.05, 10.04) | 0.92 |
| 50 to 100 | 305 | 204 | −5.23 (−11.85, 1.39) | 0.12 |
| > 100 to 250 | 320 | 264 | −1.52 (−3.15, 0.12) | 0.07 |
| > 250 | 132 | 112 | −2.52 (−11.50, 6.46) | 0.58 |
Abbreviations: LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; CI, confidence interval; n, number of patients in each group
Impact of timing of BMC transplantation after acute MI
The median time interval for BMC transplantation in patients with acute MI was 7 days (range, 1 to 18 days). BMCs were injected within 48 hours after acute MI/PCI in 4 studies, within 3 to 10 days in 22 studies, and after 10 days in 6 studies. BMC injection on days 3 to 10 resulted in greatest improvement in EF, LVESV and LVEDV; however, there was no significant reduction in infarct size in this subgroup. The greatest reduction in infarct size was seen when BMCs were transplanted within the first 48 hours after MI (Table 5).
TABLE 5.
Unadjusted difference in mean change in outcome parameters in bone marrow cell-treated patients compared with controls based on timing of cell injection after acute MI.
| Time of injection | BMC Therapy (n) | Control (n) | Difference in mean (95% CI) | P value |
|---|---|---|---|---|
|
|
||||
| LVEF | ||||
| 0 – 2 days | 98 | 81 | 1.85 (−0.67, 4.37) | 0.15 |
| 3 – 10 days | 814 | 625 | 2.09 (0.54, 3.64) | 0.008 |
| > 10 days | 142 | 116 | 2.74 (−1.88, 7.37) | 0.25 |
|
| ||||
| Infarct size | ||||
| 0 – 2 days | 40 | 40 | −4.41 (−8.22, −0.60) | 0.02 |
| 3 – 10 days | 494 | 400 | −1.00 (−2.52, 0.53) | 0.20 |
| > 10 days | 100 | 72 | −7.13 (−16.06, 1.81) | 0.12 |
|
| ||||
| LVESV | ||||
| 0 – 2 days | 39 | 41 | −11.14 (−31.17, 8.88) | 0.28 |
| 3 – 10 days | 587 | 466 | −5.76 (−8.37, −3.14) | 0.0001 |
| > 10 days | 87 | 60 | 1.37 (−3.39, 6.12) | 0.57 |
|
| ||||
| LVEDV | ||||
| 0 – 2 days | 39 | 41 | −12.59 (−42.68, 17.50) | 0.41 |
| 3 – 10 days | 587 | 466 | −2.95 (−5.65, −0.25) | 0.03 |
| > 10 days | 92 | 65 | 0.81 (−5.53, 7.16) | 0.45 |
Abbreviations: LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; CI, confidence interval; n, number of patients in each group
Imaging modalities and outcomes
Analysis based on imaging modalities revealed important interactions with regard to LVEF and LVESV. Improvement in LVEF in BMC-treated patients was significant when MRI, echocardiography or LVG were used, whereas the increase was insignificant by SPECT (Table 6). Infarct scar size reduction was significant by SPECT but not by MRI. BMC therapy reduced LVESV when assessed by echocardiography, MRI, and LVG, but not by SPECT, while reduction in LVEDV was significant by echocardiography and SPECT only (Table 6).
TABLE 6.
Unadjusted differences in mean change in outcome parameters in bone marrow cell-treated patients compared with controls based on the mode of imaging.
| Follow-up duration | BMC Therapy (n) | Control (n) | Difference in mean (95% CI) | P value for Z | P value for subgroup differences |
|---|---|---|---|---|---|
|
| |||||
| LVEF | |||||
| Echo | 642 | 533 | 2.69 (1.27, 4.12) | 0.0002 | 0.05 |
| SPECT | 150 | 96 | 0.93 (−0.83, 2.68) | 0.30 | |
| MRI | 642 | 533 | 1.60 (0.30, 2.90) | 0.02 | |
| LVG | 372 | 305 | 5.26 (2.47, 8.05) | 0.0002 | |
|
| |||||
| Infarct size | |||||
| SPECT | 155 | 135 | −2.41 (−2.78, −2.03) | <0.00001 | 0.19 |
| MRI | 416 | 306 | −1.18 (−2.97, 0.61) | 0.20 | |
|
| |||||
| LVESV | |||||
| Echo | 249 | 195 | −11.76 (−19.09, −4.43) | 0.002 | 0.02 |
| SPECT | 116 | 80 | −4.57 (−11.13, 1.99) | 0.17 | |
| MRI | 341 | 252 | −2.59 (−3.90, −1.27) | 0.0001 | |
| LVG | 211 | 176 | −11.12 (−19.27, −2.97) | 0.008 | |
|
| |||||
| LVEDV | |||||
| Echo | 283 | 235 | −2.41 (−2.79, −2.03) | 0.00001 | 0.42 |
| SPECT | 116 | 80 | −9.56 (−18.39, −0.72) | 0.03 | |
| MRI | 391 | 302 | −1.48 (−6.21, 3.25) | 0.54 | |
| LVG | 211 | 176 | −0.31 (−10.47, 9.86) | 0.95 | |
Abbreviations: Echo, echocardiography; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVG, left ventriculography; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography; CI, confidence interval; n, number of patients in each group
Impact of the rigor of study design and blinding on outcomes
The analysis of studies wherein control patients also underwent cardiac catheterization as a sham procedure mimicking cell injection showed that BMC transplantation improved LVEF, reduced infarct size, LVESV and LVEDV (Table 7). The analysis of studies wherein control patients underwent bone marrow aspiration revealed significant reduction in LVESV with BMC therapy. There was also a trend toward improvement in LVEF with BMC therapy, however, it did not reach statistical significance (P=0.05) (Table 7).
Table 7.
Subgroup analysis to examine any potential impact of procedures in control patients on outcome variables.
| Outcome | Difference in mean (95% CI) | BMC Therapy (n) | Control (n) | P value |
|---|---|---|---|---|
|
| ||||
| Cardiac catheterization in control group | ||||
| 2.86 (1.40, 4.32) | ||||
| LVEF | −3.41 (−5.38, −1.44) | 694 | 513 | 0.0001 |
| Infarct scar size | −3.17 (−4.47, – 1.86) | 314 | 222 | 0.0007 |
| LVESV | −2.76 (−4.97, – 0.55) | 374 | 277 | <0.00001 |
| LVEDV | 405 | 312 | 0.01 | |
|
| ||||
| No cardiac catheterization in control group | ||||
| LVEF | 2.97 (1.54, 4.40) | 732 | 567 | <0.0001 |
| Infarct scar size | −1.16 (−3.07, 0.74) | 398 | 337 | 0.23 |
| LVESV | −7.93 (−12.35, −3.51) | 561 | 434 | 0.0004 |
| LVEDV | −1.43 (−6.57, 3.71) | 504 | 379 | 0.59 |
|
| ||||
| BM aspiration in control group | ||||
| LVEF | 1.58 (0.00, 3.17) | 443 | 299 | 0.05 |
| Infarct scar size | 0.05 (−2.72, 2.81) | 178 | 97 | 0.97 |
| LVESV | −3.51 (−6.40, −0.62) | 261 | 194 | 0.02 |
| LVEDV | −4.68 (−12.64, 3.27) | 251 | 184 | 0.25 |
|
| ||||
| No BM aspiration in control group | ||||
| LVEF | 3.40 (2.21, 4.60) | 981 | 779 | <0.00001 |
| Infarct scar size | −2.59 (−4.01, −1.18) | 534 | 462 | 0.0003 |
| LVESV | −7.67 (−10.96, −4.39) | 674 | 517 | <0.00001 |
| LVEDV | −1.73 (−4.13, 0.68) | 658 | 507 | 0.16 |
Abbreviations: LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; BM, Bone marrow; CI, confidence interval; n, number of patients in each group
Impact of BMC therapy on survival and other clinical outcomes
During follow-up, BMC therapy was associated with significantly lower incidence of adverse outcomes, including all-cause mortality (OR, 0.55; 95% CI, 0.34 to 0.89; I2=37%; P=0.01), recurrent MI (OR, 0.50; 95%CI, 0.27 to 0.92; I2=21%; P=0.03), ventricular tachycardia/fibrillation (OR, 0.45; 95% CI, 0.22 to 0.93; I2=10%; P=0.03) and CVA/TIA (OR, 0.25; 95% CI, 0.08 to 0.81; I2=0%; P=0.02) (Table 8). There was also a trend toward reduced incidence of heart failure, stent thrombosis, and cardiac death in BMC-treated patients, while the incidence of in-stent restenosis and target vessel revascularization was similar in BMC-treated patients compared with controls. In addition, we also estimated OR for patients with acute MI and CIHD separately (Table 8). Although the ORs were uniformly directionally concordant between these two subgroups of IHD patients, differences were noted in statistical significance of observations. These differences may perhaps be explained on the basis of variable numbers of patients in different analyses.
Table 8.
Clinical outcomes in bone marrow cell-treated patients compared with patients receiving standard therapy.
| IHD patients | AMI patients | CIHD patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | BMC (n) | Control (n) | Peto OR (95% CI) | P value | BMC (n) | Control (n) | Peto OR (95% CI) | P value | BMC (n) | Control (n) | Peto OR (95% CI) | P value |
| All-cause mortality | 1397 | 980 | 0.55 (0.34, 0.89) | 0.01 | 1053 | 741 | 0.77 (0.41, 1.44) | 0.40 | 344 | 239 | 0.35 (0.16, 0.73) | 0.005 |
| Cardiac deaths | 970 | 666 | 0.52 (0.24, 1.13) | 0.10 | 712 | 496 | 0.58 (0.25, 1.38) | 0.22 | 258 | 170 | 0.36 (0.07, 1.86) | 0.22 |
| Recurrent MI | 1159 | 799 | 0.50 (0.27, 0.92) | 0.03 | 912 | 634 | 0.52 (0.27, 1.01) | 0.05 | 247 | 165 | 0.34 (0.05, 2.19) | 0.26 |
| Heart failure | 1027 | 761 | 0.62 (0.37, 1.05) | 0.08 | 841 | 626 | 0.77 (0.42, 1.42) | 0.40 | 186 | 135 | 0.36 (0.11, 1.14) | 0.08 |
| Stent thrombosis | 599 | 458 | 0.48 (0.21, 1.09) | 0.08 | 527 | 407 | 0.51 (0.22, 1.18) | 0.12 | 72 | 51 | 0.13 (0.00, 6.54) | 0.31 |
| In-stent restenosis | 432 | 332 | 0.92 (0.55, 1.54) | 0.75 | 383 | 284 | 0.91 (0.53, 1.57) | 0.74 | 49 | 48 | 0.97 (0.13, 7.10) | 0.97 |
| TVR | 866 | 606 | 0.84 (0.59, 1.21) | 0.36 | 778 | 546 | 0.83 (0.57, 1.21) | 0.33 | 88 | 60 | 1.00 (0.32, 3.11) | 1.00 |
| CVA | 640 | 462 | 0.25 (0.08, 0.81) | 0.02 | 491 | 355 | 0.47 (0.11, 1.95) | 0.30 | 149 | 107 | 0.07 (0.01, 0.55) | 0.01 |
| VT / VF | 481 | 419 | 0.45 (0.22, 0.93) | 0.03 | 264 | 209 | 0.38 (0.17, 0.85) | 0.02 | 217 | 210 | 0.95 (0.19, 4.79) | 0.95 |
Abbreviations: BMC, bone marrow cell; CVA, cerebrovascular accident; MI, myocardial infarction; OR, odds ratio; TVR, target vessel revascularization; VF, ventricular fibrillation; VT, ventricular tachycardia; CI, confidence interval; n, number of patients in each group; AMI, acute myocardial infarction,; CIHD: chronic ischemic heart disease
Exclusion of trials with discrepancies in result reporting
Meta-analysis after excluding 7 trials with discrepancies in outcomes reporting produced very similar results. BMC transplantation led to improvement in LVEF (2.90%; 95% CI, 1.83 to 3.97; P<0.00001; Online Figure II), reduction in infarct size (− 2.21%; 95% CI, − 3.61 to − 0.82; P<0.00001; Online Figure III) and LVESV (−7.14 ml; 95% CI, −10.14 to −4.15; P<0.00001; Online Figure IV) compared with standard therapy. There was a trend toward reduction in LVEDV (−1.52 ml; 95% CI, −3.78 to 0.74; P=0.19; Online Figure V). Thus, after excluding 7 studies, which were noted to have discrepant reporting of outcomes in various publications,8 the primary outcomes of this meta-analysis remained essentially unchanged.
Publication bias
There was no significant publication bias for the primary outcomes (LVEF, LVESV, LVEDV and infarct size) as assessed by Funnel plots and Egger’s test (Online Figure VI).
DISCUSSION
Salient findings
The current meta-analysis of data from 48 RCTs (2602 patients) demonstrates the safety and efficacy of adult BMC transplantation for heart repair in patients with acute MI and chronic IHD. Although the improvements in LVEF, infarct scar size and LVESV in BMC-treated patients were numerically small, the differences were significant. Very similar results were generated from analysis after excluding studies with discrepancies in outcomes reporting. Perhaps more importantly, these benefits seemed to translate into improved clinical outcomes (all-cause mortality, recurrent MI, ventricular arrhythmia, and CVA/TIA) during follow-up, also reflecting the long-lasting nature of BMC effects. Importantly, our results document improvement in LVEF in studies that employed MRI for cardiac functional assessment. Moreover, these results identify BMC number less than 50 million to be ineffective for cardiac repair, and identify the 3–10-day period after MI/PCI as the preferred interval for cell therapy. Together, these findings provide a strong rationale for large-scale clinical trials, and offer significant insights that may prove useful for future trial design.
BMC therapy improves LV function and reduces infarct size
Because of the variability in study design, patient characteristics, cell types, and other study-related variables, the outcomes of individual BMC therapy trials have been discordant.1 In addition, due to differences in search strategies, study inclusion criteria, and analysis specifics, the interpretation of outcomes from various meta-analyses of pooled data have also been different.2,4,7,64 Therefore, and although a beneficial impact of BMC therapy has been noted in several previous meta-analyses, the debate over the efficacy of BMC therapy continues. The results of this meta-analysis, which included data from only RCTs that collectively enrolled a total of 2602 patients, indicate that injection of BMCs in patients with both acute MI and chronic IHD improves LVEF. Importantly, the improvement in EF was also noted when data specifically from studies using MRI for outcome assessment were analyzed. Although the 3% increase in EF seems rather small, it is important to note that the potential benefits from other therapeutic options for these patients are quite similar.65 Furthermore, BMC therapy also improved remodeling as evidenced by reduction in infarct scar size and LVESV. In contrast to our previous meta-analysis,4 which also included cohort studies and showed a significant reduction in LVEDV, the current results did not show a statistically significant reduction in LVEDV, although there was a trend toward improvement.
Additional analyses based on the duration of follow-up showed that improvements in LVEF, infarct size, and LVESV were persistent for more than 12 months. Consistent with our previous analysis,4 the current data from RCTs alone indicate that benefits of BMC transplantation on LV function and remodeling are not transient. Although the molecular mechanisms giving rise to these benefits remain unclear, the potential impact of these changes in terms of improvement in patient symptoms and quality of life may provide strong rationale for larger clinical trials of BMC therapy.
Patient characteristics
In order to identify patient variables that may influence outcomes of BMC therapy, we analyzed data on the basis of predefined subgroups. Analysis on the basis of the type of IHD showed greater reduction in infarct size with BMC therapy in patients with chronic IHD compared with patients with acute MI (Table 3). There was similar improvement in EF and LVESV in both groups. These findings indicate that BMC transplantation can effectively ameliorate LV remodeling and improve cardiac function in both acute and chronic settings. Additional analysis revealed that cardiac parameters were similarly improved with BMC transplantation regardless of the location of MI, although the reduction in LVEDV was significantly greater in patients with anterior wall MI compared with MI involving other territories, suggesting potentially greater benefits in patients with anterior wall MI. This is important as patients with anterior wall MI are at greater risk of developing heart failure during long-term follow-up.66
Analysis based on the median LVEF (41%) showed greater reduction in LVEDV and infarct size in patients with LVEF <41% at baseline; however, improvements in EF and LVESV were similar (Table 3). These differences in outcomes were similar when subgroup data were analyzed using a baseline LVEF of 50%, below which LV dysfunction is considered present, except that LVESV was not significantly reduced when baseline EF was ≥50% (Table 3). Overall, these results suggest that LV remodeling outcomes with BMC therapy may be superior in patients with a lower LVEF at baseline.
Safety of BMC therapy and improvement in clinical outcomes
From a clinical standpoint, the overall patient-important outcomes, including survival, are critically important toward evaluating the merits of any new therapy. In this regard, several previous meta-analyses have documented the safety of BMC therapy, including our previous analysis which also included cohort studies.2–4 Results from our current analysis, based on RCTs only, not only demonstrate the safety of BMC transplantation in patients with IHD, but also suggest that BMC therapy may reduce all-cause mortality, recurrent MI, ventricular arrhythmia, and CVA/TIA. These data also show a trend toward reduced incidence of cardiac deaths, heart failure, and stent thrombosis. However, these highly encouraging findings should be tempered with the knowledge that hypothesis-generating inferences from meta-analysis ought to be validated in prospective large RCTs of BMC therapy, such as BAMI.
Timing of BMC injection after acute MI
The survival of injected cells in the first few days after acute MI may be jeopardized by the local inflammation that renders the myocardium a hostile environment for injected cells. In the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial, the authors stratified data according to the timing of BMC injection after acute MI and showed that transplantation of BMC was more beneficial when performed at least 5 days after an acute MI.55 The issue of optimal timing for BMC injection was specifically studied in subsequent trials (TIME, LateTIME and SWISS-AMI).6,24,58,59 The TIME trial evaluated outcomes of cell delivery at day 3 or day 7 after reperfusion, while LateTIME evaluated cell delivery 2 to 3 weeks post-reperfusion. The SWISS-AMI trial compared the effects of delivery on days 5 to 7 versus 3 to 4 weeks post-PCI. Overall, the primary results for TIME, LateTIME, and SWISS-AMI did not show any discernible benefit of cell therapy irrespective of the timing of cell injection.6,24,58,59 In the current meta-analysis, transplantation of BMCs between 3 to 10 days after MI/PCI produced greater improvement in cardiac parameters (LVEF, LVESV and LVEDV) (Table 5). The reduction in infarct size was greater when cells were injected within the first 48 hours. However, these results need to be interpreted cautiously as the majority of trials (22 out of 32 trials) in patients with acute MI injected cells in the 3–10-day window after MI/PCI.
BMC number
Myocardial retention of BMCs after transplantation is variable and may depend on the total number of transplanted cells. Since BMC numbers have varied widely in individual trials, we analyzed the impact of BMC number (<50×106; 50–100×106; 100–250×106; and >250×106 BMCs) on different cardiac parameters. There was no improvement in cardiac parameters in trials that used fewer than 50 million cells. However, there was consistent improvement in EF and LVESV in trials using 50 to 100 million cells highlighting that a minimum of 50 million BMCs are needed for the benefits to manifest. Incremental increases in EF and LVESV were noted with further increase in BMC number beyond 100 million (Table 4). However, these observations need to be validated in prospective RCTs directly comparing different BMC numbers. One such study that compared the effects of variable cell doses showed significant improvement in cardiac function with high dose (100 million vs. 10 million) and the beneficial effects persisted beyond 12 months favoring the higher dose.20,21
Cell processing techniques
Differences in outcomes with similar cells have suggested that cell processing methods may impact outcomes significantly.67,68 The results of subgroup analysis based on the specific methods of density-gradient centrifugation for cell isolation showed significant improvement in EF and LVEDV in trials using Ficoll-based methods compared with Lymphoprep. However, there was similar improvement in LVESV with both techniques (Table 3). Additionally, greater improvements in LVEF and infarct size were noted in studies using heparin in the final cell suspension compared with studies that did not. Since BMCs were stored for various lengths of time in disparate storage conditions, direct comparisons will be necessary to identify the optimal BMC processing methods for future trials.
Imaging modalities and outcomes
One of the proposed reasons for discrepant results among various BMC trials is the use of dissimilar imaging modalities for the assessment of end-points. In this regard, cardiac MRI is often considered to be the gold standard for the assessment of cardiac structure and function. Importantly, no benefit of BMC therapy has been noted in analysis of pooled MRI data from a relatively small number of studies in a previous meta-analysis.64 Our analysis of MRI data from 20 eligible studies showed that BMC therapy improved LVEF and reduced LVESV without any significant change in infarct size and LVEDV (Table 6). Overall, the observed improvements in LVEF and LVESV were more pronounced in studies that used echocardiography, MRI, and LVG compared with those using SPECT. However, the reduction in infarct size was noted only with SPECT. These results indicate that the benefits of BMC therapy are perhaps not merely stemming from the use of imprecise techniques, and can be documented even when assessed with rigorous methodologies.
Rigorous study design
The analysis of studies where control group underwent cardiac catheterization as sham procedure was consistent with the overall result of the meta-analysis. On the other hand, the studies where bone marrow aspiration was performed as a sham procedure did not show significant improvement in ejection fraction; although there was a significant reduction in LVESV. However, these results should be interpreted with caution as the number of studies performing bone marrow aspiration were limited (n=7).
Studies with discrepancies
Various apparent discrepancies in several BMC trials have been enumerated in a recent article that identified over 600 discrepancies in 49 BMC trials and conducted an analysis based on the number of discrepancies and positive outcomes.8 The authors concluded that the number of discrepancies correlated with the positive outcomes reported in these studies. Although this methodology has been questioned in a recent paper,69 we elected to perform a separate analysis using this information.8 Since discrepancies in the description of methods and baseline characteristics may potentially result from innocuous editing inaccuracies, we considered discrepancies in outcome reporting alone serious enough to merit exclusion. However, meta-analysis performed after excluding 7 RCTs that had discrepancies in result reporting (Online Table I) produced results that were very similar to the overall findings of the current meta-analysis. These observations suggest that the beneficial effects of BMC therapy in heart repair may not represent mere artifacts produced by studies with discrepant data.
Meta-analyses of BMC therapy
Since the publication of the first comprehensive meta-analysis on adult BMC therapy for heart repair, numerous meta-analyses have examined highly diverse combinations of pooled datasets from an increasing number of BMC therapy trials. Although the results of these meta-analyses have largely shown an overall beneficial impact of BMC injection, some differences in conclusions have also been noted. Although discussion of specific discrepancies is not possible due to space, it is critical to emphasize that findings in each meta-analysis are overwhelmingly dependent on specific criteria for study selection and outcomes examined. The results of meta-analyses therefore may vary, often greatly, depending on which studies/patients are included.
Limitations
Although we included data only from RCTs, given the differences in study variables, the degree of heterogeneity among BMC trials continues to be an inherent limitation of such analysis. Another limitation is the difference in sample size in various predefined subgroups, which may lead to nonsignificant associations (Table 8). However, the observations across most of these subgroups (Tables 3–7) suggest that the associations are likely valid. A few additional sub-analyses (types of cells, impact of storage conditions, and such) were not possible due to either limited number of studies in the subgroup or insufficient information in published reports. Moreover, the methods of ascertainment of adverse events for individual trials were not available in published documents.
Conclusions
BMC therapy in patients with IHD improves LV function, remodeling, and clinical outcomes. These observations remain valid even when assessed by the most rigorous methods, and after exclusion of trials with discrepancies in results reporting.
Supplementary Material
Novelty and Significance.
What Is Known?
Individual clinical trials of cardiac repair with bone marrow cells have produced dissimilar data, and the overall efficacy of BMC therapy continues to remain controversial.
Several recent meta-analyses of pooled data from several clinical trials have resulted in discordant conclusions.
What New Information Does This Article Contribute?
This meta-analysis includes the largest (n=48) number of randomized controlled trials of bone marrow cell (BMC) therapy in patients with ischemic heart disease, and because of large patient numbers, is able to offer robust inferences and insights regarding factors that influence cell therapy outcomes.
This meta-analysis also included the largest (n=20) number of BMC trials in which MRI was used to assess cardiac parameters, and shows an improvement in left ventricular ejection fraction with BMC therapy.
The benefits of BMC therapy persist after the exclusion of studies with discrepancy in outcomes reporting.
The safety and efficacy of bone marrow cell (BMC) therapy for cardiac repair continue to be evaluated in clinical trials. The results from early studies of BMC therapy as well as meta-analyses of diverse subsets of clinical trials have been discordant. We performed a systematic review and meta-analysis of pooled data from 48 randomized controlled trials (RCTs) of BMC therapy that enrolled 2,602 patients with ischemic heart disease (IHD). Our results show that BMC injection in patients with IHD is associated with modest yet significant improvements in left ventricular (LV) structure and function. This analysis also suggests significant improvement in LV ejection fraction when MRI was used to assess cardiac function. Importantly, the benefits of BMC therapy were also noted in meta-analysis performed after exclusion of studies with discrepancies in outcomes reporting; and when cardiac catheterization was performed in control patients. BMC-treated patients experienced substantive reduction in all-cause mortality, recurrent MI, ventricular arrhythmia, and cerebrovascular accident, indicating significantly favorable clinical outcomes despite numerically small improvements in cardiac parameters. These results provide a robust basis for the conduct of large RCTs using patient-important clinical outcomes as primary endpoints.
Acknowledgments
SOURCES OF FUNDING
This work were supported in part by National Institutes of Health grant R01 HL-117730.
Nonstandard Abbreviations and Acronyms
- BMC
Bone Marrow Cells
- CIHD
Chronic Ischemic Heart Disease
- MI
myocardial Infarction
- RCT
Randomized Controlled Trials
Footnotes
DISCLOSURES
None.
References
- 1.Rosenzweig A. Cardiac cell therapy--mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 4.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 6.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. Jama. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, Howard JP, Cole GD, Francis DP group Dw. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 11.Huikuri HV, Kervinen K, Niemela M, Ylitalo K, Saily M, Koistinen P, Savolainen ER, Ukkonen H, Pietila M, Airaksinen JK, Knuuti J, Makikallio TH. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29:2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Q, Sun Y, Xia L, Chen A, Wang Z. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann Thorac Surg. 2008;86:1833–1840. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 13.Traverse JH, McKenna DH, Harvey K, Jorgenso BC, Olson RE, Bostrom N, Kadidlo D, Lesser JR, Jagadeesan V, Garberich R, Henry TD. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. Am Heart J. 2010;160:428–434. doi: 10.1016/j.ahj.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo A, Castellani M, Piccaluga E, Pusineri E, Palatresi S, Longari V, Canzi C, Sacchi E, Rossi E, Rech R, Gerundini P, Viecca M, Deliliers GL, Rebulla P, Soligo D, Giordano R. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: results from a randomized controlled trial. J Cardiovasc Med (Hagerstown) 2011;12:239–248. doi: 10.2459/JCM.0b013e328343d708. [DOI] [PubMed] [Google Scholar]
- 15.Srimahachota S, Boonyaratavej S, Rerkpattanapipat P, Wangsupachart S, Tumkosit M, Bunworasate U, Nakorn TN, Intragumtornchai T, Kupatawintu P, Pongam S, Saengsiri AO, Pothisri M, Sukseri Y, Bunprasert T, Suithichaiyakul T. Intra-coronary bone marrow mononuclear cell transplantation in patients with ST-elevation myocardial infarction: a randomized controlled study. J Med Assoc Thai. 2011;94:657–663. [PubMed] [Google Scholar]
- 16.Piepoli MF, Vallisa D, Arbasi C, Cavanna L, Cerri L, Mori M, Passerini F, Tommasi L, Rossi A, Capucci A. Two year follow-up results of the CARDIAC (CARDIomyoplasty by Autologous intraCoronary bone marrow in acute myocardial infarction) randomised controlled trial. Int J Cardiol. 2013;168:e132. doi: 10.1016/j.ijcard.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Plewka M, Krzeminska-Pakula M, Peruga JZ, Lipiec P, Kurpesa M, Wierzbowska-Drabik K, Korycka-Wolowiec A, Kasprzak JD. The effects of intracoronary delivery of mononuclear bone marrow cells in patients with myocardial infarction: a two year follow-up results. Kardiol Pol. 2011;69:1234–1240. [PubMed] [Google Scholar]
- 18.Wohrle J, von Scheidt F, Schauwecker P, Wiesneth M, Markovic S, Schrezenmeier H, Hombach V, Rottbauer W, Bernhardt P. Impact of cell number and microvascular obstruction in patients with bone-marrow derived cell therapy: final results from the randomized, double-blind, placebo controlled intracoronary Stem Cell therapy in patients with Acute Myocardial Infarction (SCAMI) trial. Clin Res Cardiol. 2013;102:765–770. doi: 10.1007/s00392-013-0595-9. [DOI] [PubMed] [Google Scholar]
- 19.Ang KL, Chin D, Leyva F, Foley P, Kubal C, Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT, Galinanes M. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–670. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 20.Meluzin J, Janousek S, Mayer J, Groch L, Hornacek I, Hlinomaz O, Kala P, Panovsky R, Prasek J, Kaminek M, Stanicek J, Klabusay M, Koristek Z, Navratil M, Dusek L, Vinklarkova J. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128:185–192. doi: 10.1016/j.ijcard.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 21.Meluzin J, Mayer J, Groch L, Janousek S, Hornacek I, Hlinomaz O, Kala P, Panovsky R, Prasek J, Kaminek M, Stanicek J, Klabusay M, Koristek Z, Navratil M, Dusek L, Vinklarkova J. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152:975.e979–915. doi: 10.1016/j.ahj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Silva SA, Sousa AL, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, Soares AJ, Issa AF, Felipe LR, Branco RV, Addad JA, Moreira RC, Tuche FA, Mesquita CT, Drumond CC, Junior AO, Rochitte CE, Luz JH, Rabischoffisky A, Nogueira FB, Vieira RB, Junior HS, Borojevic R, Dohmann HF. Autologous bone-marrow mononuclear cell transplantation after acute myocardial infarction: comparison of two delivery techniques. Cell Transplant. 2009;18:343–352. doi: 10.3727/096368909788534951. [DOI] [PubMed] [Google Scholar]
- 23.Yao K, Huang R, Sun A, Qian J, Liu X, Ge L, Zhang Y, Zhang S, Niu Y, Wang Q, Zou Y, Ge J. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur J Heart Fail. 2009;11:691–698. doi: 10.1093/eurjhf/hfp062. [DOI] [PubMed] [Google Scholar]
- 24.Surder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, Turchetto L, Radrizzani M, Astori G, Schwitter J, Erne P, Zuber M, Auf der Maur C, Jamshidi P, Gaemperli O, Windecker S, Moschovitis A, Wahl A, Buhler I, Wyss C, Kozerke S, Landmesser U, Luscher TF, Corti R. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968–1979. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 25.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hristov M, Heussen N, Schober A, Weber C. Intracoronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: a meta-analysis. J Cell Mol Med. 2006;10:727–733. doi: 10.1111/j.1582-4934.2006.tb00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong H, Yim HW, Cho Y, Park HJ, Jeong S, Kim HB, Hong W, Kim H. The effect of rigorous study design in the research of autologous bone marrow-derived mononuclear cell transfer in patients with acute myocardial infarction. Stem Cell Res Ther. 2013;4:82. doi: 10.1186/scrt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 29.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 30.Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, Feng X, Zhang J, Duan Y, Wang J, Liu D, Wang H. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J. 2009;30:1986–1994. doi: 10.1093/eurheartj/ehp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, Wang ZG, Wang YF, Zhu ZM, Li TC, Liu HL, Tong ZC, Yang Y, Nan X, Guo F, Shen JL, Shen YH, Zhang JJ, Fei YX, Xu HT, Wang LH, Tian HT, Liu da Q, Yang Y. A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol. 2013;168:3191–3199. doi: 10.1016/j.ijcard.2013.04.112. [DOI] [PubMed] [Google Scholar]
- 33.Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y, Fan B, Liu X, Zhang S, Sun A, Zou Y. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT-STAMI) Heart. 2006;92:1764–1767. doi: 10.1136/hrt.2005.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grajek S, Popiel M, Gil L, Breborowicz P, Lesiak M, Czepczynski R, Sawinski K, Straburzynska-Migaj E, Araszkiewicz A, Czyz A, Kozlowska-Skrzypczak M, Komarnicki M. Influence of bone marrow stem cells on left ventricle perfusion and ejection fraction in patients with acute myocardial infarction of anterior wall: randomized clinical trial: Impact of bone marrow stem cell intracoronary infusion on improvement of microcirculation. Eur Heart J. 2010;31:691–702. doi: 10.1093/eurheartj/ehp536. [DOI] [PubMed] [Google Scholar]
- 35.Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I101–107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch A, Nijveldt R, van der Vleuten PA, Tijssen JG, van der Giessen WJ, Tio RA, Waltenberger J, ten Berg JM, Doevendans PA, Aengevaeren WR, Zwaginga JJ, Biemond BJ, van Rossum AC, Piek JJ, Zijlstra F. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J. 2011;32:1736–1747. doi: 10.1093/eurheartj/ehq449. [DOI] [PubMed] [Google Scholar]
- 37.Huang RC, Yao K, Zou YZ, Ge L, Qian JY, Yang J, Yang S, Niu YH, Li YL, Zhang YQ, Zhang F, Xu SK, Zhang SH, Sun AJ, Ge JB. Long term follow-up on emergent intracoronary autologous bone marrow mononuclear cell transplantation for acute inferior-wall myocardial infarction. Zhonghua Yi Xue Za Zhi. 2006;86:1107–1110. [PubMed] [Google Scholar]
- 38.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 39.Jazi SM, Esfahani MH, Fesharaki M, Moulavi F, Gharipour M. Initial clinical outcomes of intracoronary infusion of autologous progenitor cells in patients with acute myocardial infarction. ARYA Atheroscler. 2012;7:162–167. [PMC free article] [PubMed] [Google Scholar]
- 40.Lipiec P, Krzeminska-Pakula M, Plewka M, Kusmierek J, Plachcinska A, Szuminski R, Robak T, Korycka A, Kasprzak JD. Impact of intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction on left ventricular perfusion and function: a 6-month follow-up gated 99mTc-MIBI single-photon emission computed tomography study. Eur J Nucl Med Mol Imaging. 2009;36:587–593. doi: 10.1007/s00259-008-0988-6. [DOI] [PubMed] [Google Scholar]
- 41.Lu M, Liu S, Zheng Z, Yin G, Song L, Chen H, Chen X, Chen Q, Jiang S, Tian L, He Z, Hu S, Zhao S. A pilot trial of autologous bone marrow mononuclear cell transplantation through grafting artery: a sub-study focused on segmental left ventricular function recovery and scar reduction. Int J Cardiol. 2013;168:2221–2227. doi: 10.1016/j.ijcard.2013.01.217. [DOI] [PubMed] [Google Scholar]
- 42.Lunde K, Solheim S, Forfang K, Arnesen H, Brinch L, Bjornerheim R, Ragnarsson A, Egeland T, Endresen K, Ilebekk A, Mangschau A, Aakhus S. Anterior myocardial infarction with acute percutaneous coronary intervention and intracoronary injection of autologous mononuclear bone marrow cells: safety, clinical outcome, and serial changes in left ventricular function during 12-months' follow-up. J Am Coll Cardiol. 2008;51:674–676. doi: 10.1016/j.jacc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Maureira P, Tran N, Djaballah W, Angioi M, Bensoussan D, Didot N, Fay R, Sadoul N, Villemot JP, Marie PY. Residual viability is a predictor of the perfusion enhancement obtained with the cell therapy of chronic myocardial infarction: a pilot multimodal imaging study. Clin Nucl Med. 2012;37:738–742. doi: 10.1097/RLU.0b013e318251e38a. [DOI] [PubMed] [Google Scholar]
- 44.Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira FB, Silva SA, Haddad AF, Peixoto CM, Carvalho RM, Tuche FA, Soares VE, Sousa AL, Rabischoffsky A, Mesquita CT, Borojevic R, Dohmann HF. Systolic function of patients with myocardial infarction undergoing autologous bone marrow transplantation. Arq Bras Cardiol. 2009;93:374–379. 367–372. doi: 10.1590/s0066-782x2009001000010. [DOI] [PubMed] [Google Scholar]
- 46.Penicka M, Horak J, Kobylka P, Pytlik R, Kozak T, Belohlavek O, Lang O, Skalicka H, Simek S, Palecek T, Linhart A, Aschermann M, Widimsky P. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction: a prematurely terminated randomized study. J Am Coll Cardiol. 2007;49:2373–2374. doi: 10.1016/j.jacc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, Herlihy JP, Fernandes MR, Cheong BY, Flamm SD, Traverse JH, Zheng Y, Smith D, Shaw S, Westbrook L, Olson R, Patel D, Gahremanpour A, Canales J, Vaughn WK, Willerson JT. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF) Am Heart J. 2011;161:1078–1087. e1073. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Perin EC, Silva GV, Zheng Y, Gahremanpour A, Canales J, Patel D, Fernandes MR, Keller LH, Quan X, Coulter SA, Moore WH, Herlihy JP, Willerson JT. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am Heart J. 2012;163:415–421. 421.e411. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. Jama. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pokushalov E, Romanov A, Chernyavsky A, Larionov P, Terekhov I, Artyomenko S, Poveshenko O, Kliver E, Shirokova N, Karaskov A, Dib N. Efficiency of intramyocardial injections of autologous bone marrow mononuclear cells in patients with ischemic heart failure: a randomized study. J Cardiovasc Transl Res. 2010;3:160–168. doi: 10.1007/s12265-009-9123-8. [DOI] [PubMed] [Google Scholar]
- 51.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 52.van Ramshorst J, Antoni ML, Beeres SL, Roes SD, Delgado V, Rodrigo SF, de Roos A, Holman ER, Fibbe WE, Lamb HJ, Zwaginga JJ, Boersma E, van der Wall EE, Schalij MJ, Atsma DE, Bax JJ. Intramyocardial bone marrow-derived mononuclear cell injection for chronic myocardial ischemia: the effect on diastolic function. Circ Cardiovasc Imaging. 2011;4:122–129. doi: 10.1161/CIRCIMAGING.110.957548. [DOI] [PubMed] [Google Scholar]
- 53.Roncalli J, Mouquet F, Piot C, Trochu JN, Le Corvoisier P, Neuder Y, Le Tourneau T, Agostini D, Gaxotte V, Sportouch C, Galinier M, Crochet D, Teiger E, Richard MJ, Polge AS, Beregi JP, Manrique A, Carrie D, Susen S, Klein B, Parini A, Lamirault G, Croisille P, Rouard H, Bourin P, Nguyen JM, Delasalle B, Vanzetto G, Van Belle E, Lemarchand P. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur Heart J. 2011;32:1748–1757. doi: 10.1093/eurheartj/ehq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruan W, Pan CZ, Huang GQ, Li YL, Ge JB, Shu XH. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chin Med J (Engl) 2005;118:1175–1181. [PubMed] [Google Scholar]
- 55.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 56.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM, Investigators R-A. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 57.Suarez de Lezo J, Herrera C, Pan M, Romero M, Pavlovic D, Segura J, Sanchez J, Ojeda S, Torres A. Regenerative therapy in patients with a revascularized acute anterior myocardial infarction and depressed ventricular function. Rev Esp Cardiol. 2007;60:357–365. [PubMed] [Google Scholar]
- 58.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Ellis SG. One-year follow-up of intracoronary stem cell delivery on left ventricular function following ST-elevation myocardial infarction. Jama. 2014;311:301–302. doi: 10.1001/jama.2013.282674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy R. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tse HF, Thambar S, Kwong YL, Rowlings P, Bellamy G, McCrohon J, Thomas P, Bastian B, Chan JK, Lo G, Ho CL, Chan WS, Kwong RY, Parker A, Hauser TH, Chan J, Fong DY, Lau CP. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT-CAD trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 61.Turan RG, Bozdag TI, Ortak J, Kische S, Akin I, Schneider H, Turan CH, Rehders TC, Rauchhaus M, Kleinfeldt T, Belu C, Brehm M, Yokus S, Steiner S, Sahin K, Nienaber CA, Ince H. Improved functional activity of bone marrow derived circulating progenitor cells after intra coronary freshly isolated bone marrow cells transplantation in patients with ischemic heart disease. Stem Cell Rev. 2011;7:646–656. doi: 10.1007/s12015-010-9220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turan RG, Bozdag TI, Turan CH, Ortak J, Akin I, Kische S, Schneider H, Rauchhaus M, Rehders TC, Kleinfeldt T, Belu C, Amen S, Hermann T, Yokus S, Brehm M, Steiner S, Chatterjee T, Sahin K, Nienaber CA, Ince H. Enhanced mobilization of the bone marrow-derived circulating progenitor cells by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction. J Cell Mol Med. 2012;16:852–864. doi: 10.1111/j.1582-4934.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao K, Huang R, Qian J, Cui J, Ge L, Li Y, Zhang F, Shi H, Huang D, Zhang S, Sun A, Zou Y, Ge J. Administration of intracoronary bone marrow mononuclear cells on chronic myocardial infarction improves diastolic function. Heart. 2008;94:1147–1153. doi: 10.1136/hrt.2007.137919. [DOI] [PubMed] [Google Scholar]
- 64.de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ Cardiovasc Interv. 2014;7:156–167. doi: 10.1161/CIRCINTERVENTIONS.113.001009. [DOI] [PubMed] [Google Scholar]
- 65.Reffelmann T, Konemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–308. doi: 10.1016/j.jacc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 66.O'Connor CM, Hathaway WR, Bates ER, Leimberger JD, Sigmon KN, Kereiakes DJ, George BS, Samaha JK, Abbottsmith CW, Candela RJ, Topol EJ, Califf RM. Clinical characteristics and long-term outcome of patients in whom congestive heart failure develops after thrombolytic therapy for acute myocardial infarction: development of a predictive model. Am Heart J. 1997;133:663–673. doi: 10.1016/s0002-8703(97)70168-6. [DOI] [PubMed] [Google Scholar]
- 67.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 68.Dawn B, Bolli R. Bone marrow for cardiac repair: the importance of characterizing the phenotype and function of injected cells. Eur Heart J. 2007;28:651–652. doi: 10.1093/eurheartj/ehm009. [DOI] [PubMed] [Google Scholar]
- 69.Moye L. DAMASCENE and Meta-Ecological Research: A Bridge Too Far. Circ Res. 2014;115:484–487. doi: 10.1161/CIRCRESAHA.114.304767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, Forfang K, Aakhus S. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95:1983–1989. doi: 10.1136/hrt.2009.178913. [DOI] [PubMed] [Google Scholar]
- 71.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 72.Piepoli MF, Vallisa D, Arbasi M, Cavanna L, Cerri L, Mori M, Passerini F, Tommasi L, Rossi A, Capucci A. Bone marrow cell transplantation improves cardiac, autonomic, and functional indexes in acute anterior myocardial infarction patients (Cardiac Study) Eur J Heart Fail. 2010;12:172–180. doi: 10.1093/eurjhf/hfp183. [DOI] [PubMed] [Google Scholar]
- 73.Plewka M, Krzeminska-Pakula M, Lipiec P, Peruga JZ, Jezewski T, Kidawa M, Wierzbowska-Drabik K, Korycka A, Robak T, Kasprzak JD. Effect of intracoronary injection of mononuclear bone marrow stem cells on left ventricular function in patients with acute myocardial infarction. Am J Cardiol. 2009;104:1336–1342. doi: 10.1016/j.amjcard.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 74.van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, de Roos A, Fibbe WE, Zwaginga JJ, Boersma E, Schalij MJ, Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 75.Wohrle J, Merkle N, Mailander V, Nusser T, Schauwecker P, von Scheidt F, Schwarz K, Bommer M, Wiesneth M, Schrezenmeier H, Hombach V. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol. 2010;105:804–812. doi: 10.1016/j.amjcard.2009.10.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.