Abstract
Macrophages coexpress both the interleukin (IL)-2Rγ chain (γc) and IL-13Rα1. These receptor chains can heterodimerize with IL-4Rα to form type I or type II IL-4 receptor complexes, respectively. We used macrophages derived from Il2rg and Il13ra1 knockout (KO) mice to evaluate the requirements for these receptor chains for induction of the alternative macrophage activation (AMA) pathway by IL-4 and IL-13. Absence of γc significantly decreased activation of STAT6 by IL-4 but not IL-13. However, although activation of STAT6 by IL-4 was markedly reduced in γc KO macrophages, it was not abolished, indicating that IL-4 can still signal through type II IL-4 receptors via the IL-13Rα1 chain. IL-13 failed to activate STAT6 in macrophages derived from Il13ra1 KO mice; however, these cells remained fully responsive to IL-4. The inability of IL-13 but not IL-4 to signal in Il13ra1−/- macrophages correlated with the inability of IL-13 but not IL-4 to induce expression of genes such as Arg1, Retnla and Ccl11 that are characteristically expressed by alternatively activated macrophages. In addition, IL-13 but not IL-4 failed to induce membrane fusion and giant cell formation by Il13ra1 KO macrophages. These findings demonstrate that the IL-13Rα1 chain is essential for induction of the AMA pathway by IL-13 but not IL-4.
Key Words: Alternative macrophage activation, Arginase, Interleukin-4, Interleukin-13, Interleukin-13 receptor-α1, STAT6
Introduction
T cell-derived cytokines such as interferon-γ (IFN-γ) and interleukin (IL)-4 induce distinct gene expression profiles and distinct activation states in macrophages by differentially activating the transcription factors STAT1 and STAT6, respectively. The classical macrophage activation pathway is activated by the Th1-type cytokine, IFN-γ [1, 2]. Signaling through IFN-γ receptors on macrophages induces activation of STAT1 and expression of STAT1-responsive genes such as Nos2 (inducible nitric oxide synthetase, iNOS), Cxcl9 (Mig) and Cxcl10 (IP-10) [3]. The alternative macrophage activation (AMA) pathway was originally defined to distinguish the phenotype of IL-4-treated macrophages from that of classically activated macrophages induced by treatment with IFN-γ [4]. Signaling through IL-4 receptors on macrophages induces activation of STAT6 and expression of STAT6-responsive genes such as Arg1 (arginase I), Retnla (Fizz1) and Ccl11 (eotaxin) [5, 6, 7]. Alternatively activated macrophages also exhibit elevated expression of certain cell surface markers, including mannose receptor C type 1 (CD206) and dectin-1 (Clec7a) [4, 8, 9]. In addition to IL-4, it was subsequently found that the AMA pathway can also be activated by IL-13 [10, 11].
IL-4 and IL-13 induce functional responses by binding to specific cell surface receptors and activating a signal transduction cascade that results in rapid activation of latent cytosolic STAT6 [12, 13]. Once activated, STAT6 translocates from the cytosol to the nucleus, and binds to STAT-binding elements (SBE) in the promoters of various STAT6-responsive genes. SBEs typically contain the core nucleotide sequence, TTCNNNGAA, where NNN can be any nucleotide. However, unlike most SBEs that contain an N3-type spacer between the palindromic TTC and GAA nucleotides, STAT6-binding elements frequently contain N4-type spacers [14, 15]. As a consequence, the promoter regions of IL-4/IL-13-responsive genes usually contain N4-type SBEs that have a high affinity for STAT6. In contrast, the promoter regions of IFN-γ-responsive genes usually contain N3-type SBEs that have a much higher affinity for STAT1 than STAT6. This difference explains, at least in part, why the transcriptional signature induced by IL-4 and IL-13 is so distinct from the gene signature induced by IFN-γ.
Activation of STAT6 is critical for induction of the AMA pathway by both IL-4 and IL-13 [16, 17, 18]. Although both IL-4 and IL-13 can induce AMA, IL-4 is generally more potent than IL-13 in terms of its ability to induce activation of STAT6 and gene expression in both murine macrophages and human monocytes [19, 20]. The molecular basis for the greater potency of IL-4 versus IL-13 in macrophages is not completely understood, but likely results, at least in part, from the overall higher affinity of type I IL-4 receptors for IL-4 versus the lower affinity of type II IL-4 receptors for IL-13. In this study, we used bone marrow-derived macrophages from mice with targeted deletions of the genes for the IL-4Rα chain (Il4ra), IL-2Rγ (Il2rg) or IL-13Rα1 (Il13ra1) to define the requirements for these receptor chains in the activation of STAT6 and induction of alternatively activated macrophages by IL-4 and IL-13.
Materials and Methods
Culture Medium and Reagents
The complete medium used for culturing macrophages consisted of RPMI-1640 medium (Life Technologies, Grand Island, N.Y., USA) supplemented with 10% FBS (HyClone, Logan, Utah, USA), 2 mML-glutamine and 50 µg/ml gentamycin. Recombinant murine IL-4, IL-13 and IFN-γ were obtained from R&D Systems Inc. (Minneapolis, Minn., USA). The rabbit polyclonal anti-phospho-(Tyr641)-STAT6 antibody (catalog No. 9361) was obtained from Cell Signaling Technology (Danvers, Mass., USA), and the rabbit polyclonal anti-STAT6 antibody (catalog No. sc-621) was obtained from Santa Cruz Biotechnology Inc. (Dallas, Tex., USA).
Cells
Bone marrow-derived macrophage cultures were generated as described previously [21] from bone marrow aspirates extracted from the femurs of wild-type BALB/c or C57BL/6 mice (Taconic Farms, Germantown, N.Y., USA) or from gene knockout (KO) mice, including Il4ra-/-[22], Il2rg-/- [23] or Il13ra1−/-[24] mice. The cells were cultured at 1 × 106 cells/ml in complete RPMI-1640 medium containing 50 ng/ml of recombinant murine M-CSF (R&D Systems Inc.) for 7–10 days at 37°C. After that time, the M-CSF was washed off the cultures and the cells were treated with recombinant murine IL-4, IL-13 and/or IFN-γ, as indicated in the text.
The methods used to generate primary mouse lung fibroblast cultures have been described previously [24]. Briefly, the lungs from wild-type or Il13ra1 KO mice were harvested and then perfused with 10 ml of HBSS containing 2 mg/ml of collagenase D (Roche Diagnostics, Indianapolis, Ind., USA), excised, minced and incubated with 2 mg/ml of collagenase in DMEM for 45 min at 37°C. The enzymatically digested tissue was subsequently forced through a 100-μm nylon sieve and the cells were plated on 100-mm petri dishes containing Iscove's media supplemented with 5% FBS, 2 mML-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin, and incubated for 2 weeks at 37°C. The resulting fibroblast monolayers were harvested and plated onto fresh 100-mm petri dishes, and allowed to expand for an additional 7 days prior to use.
Electrophoretic Mobility Shift Assays
Nuclear protein extracts were prepared from cells after treatment with IL-4 or IL-13 as previously described [25]. A double-stranded oligonucleotide, based on a DNA sequence in the promoter of the human IL-1 receptor antagonist gene IL1RN, was used as a probe for STAT6 in the gel shift assays [26]. This oligonucleotide contains SBE1, which has a high affinity for STAT6 complexes. The GAS probe used to measure STAT1 activity was derived from a sequence in the promoter of the human IgG FcγR1 gene, FCGR1a[27]. These probes were end-labeled with [γ-32P]-ATP using T4 polynucleotide kinase, and binding reactions were performed as described previously [25]. A portion of each binding reaction mixture (8 μl per sample) was electrophoresed on nondenaturing, 6% polyacrylamide gels (Invitrogen, Carlsbad, Calif., USA) using 0.25× Tris-borate-ethylenediamine-N,N,N′,N′-tetraacetic acid buffer (22 mM Tris-HCl, pH 8.0, 22 mM borate and 0.5 mM ethylenediamine-N,N,N′,N′-tetraacetic acid). The gels were subsequently dried and visualized by autoradiography.
Western Blots
The levels of tyrosine-phosphorylated STAT6 (pY-STAT6) were measured by immunoblotting as described previously [28]. After treatment with recombinant IL-4, IL-13 or IFN-γ for 30 min at 37°C, the cells were washed three times with Dulbecco's PBS and whole cell protein lysates were prepared. Total STAT6 protein was immunoprecipitated with rabbit anti-STAT6 Ab (sc-621; Santa Cruz Biotechnology). Immunoprecipitated proteins were resolved by electrophoresis on 8% SDS-PAGE gels (Invitrogen) and then transferred to polyvinylidene difluoride membranes. The levels of pY-STAT6 and total STAT6 were visualized by enhanced chemiluminescence using a rabbit antibody specific for Tyr641-phosphorylated STAT6 (catalog No. 9361; Cell Signaling Technology) or rabbit anti-STAT6 Ab, respectively.
Quantitative Real-Time PCR
Changes in gene expression were measured by qRT-PCR analyses of individual ISGs. Total RNA was isolated from macrophages using RNAzol B (Tel-Test, Friendswood, Tex., USA) by the acid/guanidinium thiocyanate/phenol/chloroform extraction method, as described previously [25]. The RNA samples were DNAse treated to remove any residual genomic DNA using RT2 qPCR-grade RNA isolation kits from Qiagen Inc. (Valencia, Calif., USA). One microgram of purified RNA from each treatment group was used as a template for synthesis of first-strand cDNAs using ReactionReady First Strand cDNA synthesis kits from Qiagen. Specific primer assays for selected ISGs were obtained from Qiagen and analyzed on the Mx3000P system (Agilent Technologies, Santa Clara, Calif., USA). PCR amplification was performed by thermal cycling using SuperArray RT2 Real-Time SYBR Green PCR Master Mix with real-time detection by SYBR Green and 5-carboxy-X-rhodamine (ROX) dyes on the Mx3000P instrument according to the manufacturer's instructions.
Changes in gene expression levels were analyzed using MxPro software v.4.10 (Agilent), and the results are expressed as the mean fold increase relative to the control levels after normalization to the housekeeping gene GAPDH. Changes in the relative levels of gene expression in treated versus nontreated control cells were calculated using the 2−ΔΔCT method as described previously [29]. Graphing and statistical analysis of qPCR results were performed using Prism 5.0 (Graph Pad Software, San Diego, Calif., USA). The data were analyzed using a two-tailed Student t test. Values represent the mean ± SD of triplicate determinations. p values <0.05 were considered statistically significant. All experiments were repeated at least three times with similar results.
Cell Fusion Assays
The cell fusion assays were performed as described previously [9]. Briefly, bone marrow-derived macrophages were resuspended in OptiMEM-10 medium and plated on Permanox plastic slides at 1 × 105 cells/well. IL-4 or IL-13 (100 ng/ml) were added and the cultures were incubated for 48 h. The slides were stained using the Hemacolor staining kit (EMD Millipore), and photographs were taken using a Nikon Coolscope slide scanner. Four to eight independent images per well were acquired, and the number of giant and single cell nuclei were counted. Percent fusion values were determined based on the number of giant cell nuclei (>2 nuclei) divided by the number of total nuclei.
Results
The IL-4Rα Chain Is Required for Activation of STAT6 by both IL-4 and IL-13
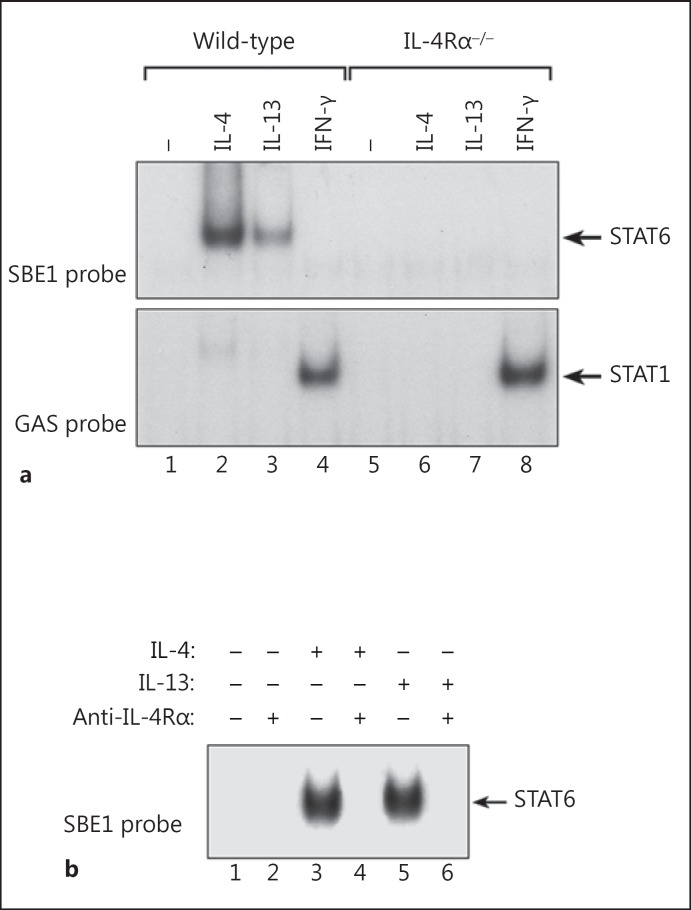
To determine if the IL-4Rα chain is required for signaling by both IL-4 and IL-13 in macrophages, we examined the ability of IL-4 and IL-13 to induce activation of STAT6 in macrophages derived from control (wild-type) and IL-4Rα gene KO (Il4ra−/-) mice [22]. Bone marrow-derived macrophages from wild-type or Il4ra−/- mice were treated with recombinant murine IL-4, IL-13 or IFN-γ (20 ng/ml) for 30 min at 37°C. Nuclear protein extracts were then prepared and analyzed by electrophoretic mobility shift assays (EMSA) for DNA-binding activity using an N4-type GAS probe (SBE1) that preferentially binds activated STAT6 [26]. As shown in figure 1a, both IL-4 and IL-13 induced activation of STAT6 in wild-type macrophages; however, neither IL-4 nor IL-13 induced STAT6 activity in IL-4Rα chain-deficient macrophages. IL-4 induced higher levels of STAT6 DNA-binding activity than IL-13 even though the cells were treated with equivalent concentrations of each cytokine. The inability of IL-4 and IL-13 to induce activation of STAT6 in IL-4Rα-null macrophages did not reflect a global inability of these cells to respond to cytokines because treatment with IFN-γ induced equivalent levels of STAT1 activation in wild-type and Il4ra−/- macrophages.
Fig. 1.
The IL-4Rα chain is required for induction of STAT6 activity by both IL-4 and IL-13. a Bone marrow-derived macrophages from control (wild-type) and IL-4Rα chain KO (Il4ra−/-) mice (BALB/cJ background) were treated with IL-4, IL-13 or IFN-γ (20 ng/ml) for 30 min at 37°C. Nuclear protein extracts were then prepared and analyzed by EMSA for DNA-binding activity using a radiolabeled oligonucleotide N4-type GAS probe (SBE1) that preferentially binds activated STAT6 (upper panel) and for STAT1 activity using an N3-type GAS probe (GRR) that has a high affinity for STAT1 (lower panel). b Wild-type macrophages were pretreated or not with the anti-IL-4Rα mAb, M1, for 30 min at 37°C. IL-4 or IL-13 (20 ng/ml) was then added, and the cultures were incubated for an additional 30 min. Nuclear protein extracts were then prepared and analyzed by EMSA for STAT6-binding activity using the SBE1 probe.
To confirm the essential role of the IL-4Rα chain for productive signaling by IL-4 and IL-13, we treated normal (wild-type) bone marrow-derived macrophages with IL-4 or IL-13 in the presence or absence of a blocking monoclonal antibody (M1) to the murine IL-4Rα chain [30]. As shown in figure 1b, the M1 mAb completely blocked activation of STAT6 by both IL-4 and IL-13. These results confirmed that the IL-4Rα chain is an essential component of the receptor complexes for both IL-4 and IL-13 in macrophages.
Deletion of the Common Gamma Chain Diminishes the Ability of IL-4 but Not IL-13 to Activate STAT6 in Macrophages
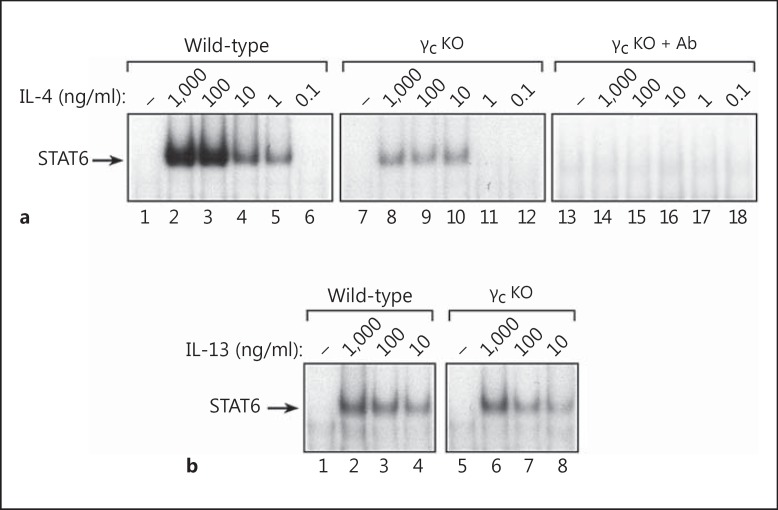
Most cell types express either the common gamma chain (γc) or the IL-13Rα1 chain, but not both. However, monocytes and macrophages are unique because they coexpress γc and IL-13Rα1 [19, 20, 31]. To determine if macrophages require γc to mediate responsiveness to IL-4 and/or IL-13, we prepared bone marrow-derived macrophages from control (wild-type) and γc-deficient (Il2rg-/-) mice [23, 32] and examined their responsiveness to IL-4 and IL-13. As shown in figure 2a, IL-4 induced strong activation of STAT6 in wild-type macrophages (lanes 1–6); however, the induction of STAT6 activity by IL-4 was much weaker but not abrogated in γc-deficient macrophages (lanes 7–12). Treatment of γc KO macrophages with IL-4 in the presence of the inhibitory anti-IL-4Rα mAb (M1) blocked the residual activation of STAT6 by IL-4 in these cells (lanes 13–18). Higher concentrations of IL-4 were required to activate STAT6 in γc−/- macrophages compared to wild-type macrophages. Furthermore, IL-4 appeared to function more like IL-13 in γc−/- macrophages because the IL-4-induced dose response curve in Il2rg−/- macrophages was very similar to the IL-13-induced dose response in wild-type macrophages. Unlike the response to IL-4, the absence of γc expression did not decrease the ability of IL-13 to activate STAT6 in macrophages (fig. 2b). These findings indicate that IL-4 responsiveness is markedly diminished but not abrogated in γc-deficient macrophages, whereas responsiveness to IL-13 is unaffected. The residual STAT6 activity induced by IL-4 in Il2rg−/- macrophages results from default signaling through type II IL-4 receptor complexes because these cells still express the IL-13Rα1 chain in the absence of γc.
Fig. 2.
Activation of STAT6 by IL-4 is diminished but not abrogated in γc-deficient macrophages. a Bone marrow-derived macrophages from control (wild-type) and γc KO (Il2rg-/-) mice (C57BL/6J background) were treated with log10 concentrations of IL-4 ranging from 1,000 to 0.1 ng/ml for 30 min at 37°C. An additional set of bone marrow-derived macrophages from γc KO mice were treated with IL-4 in the presence of the neutralizing anti-IL-4Rα1 mAb, M1 (lanes 13–18). Nuclear protein extracts were then prepared and the levels of activated STAT6 were measured by EMSA using a radiolabeled SBE1 probe. b Another set of macrophages derived from the same mice were incubated with IL-13 (1,000-10 ng/ml) for 30 min at 37°C (lower panel). Nuclear protein extracts were then prepared and analyzed for STAT6 activity by EMSA using the SBE1 probe.
The IL-13Rα1 Chain Is Required for Activation of STAT6 by IL-13 but Not IL-4 in Macrophages
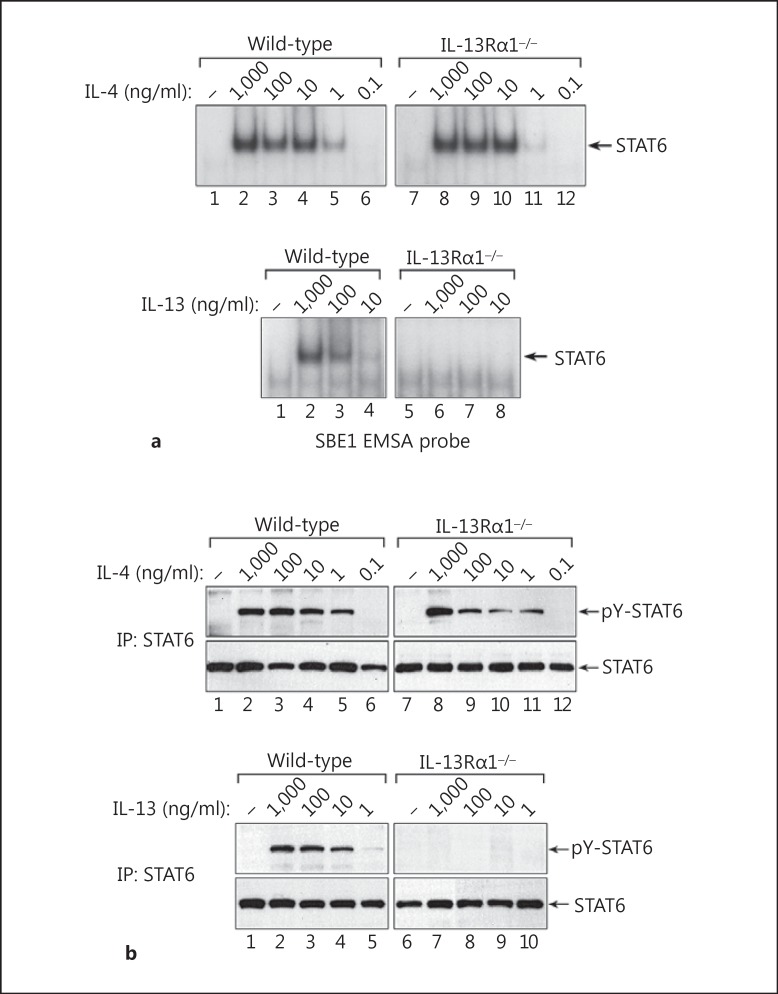
Both IL-4 and IL-13 can signal through type II IL-4 receptor complexes in nonhematologic cell types such as fibroblasts and endothelial cells [33, 34]; however, it is not clear if the IL-13Rα1 chain is required for signaling by both IL-4 and IL-13 in macrophages. To define the importance of the IL-13Rα1 chain in IL-4/IL-13 signaling by macrophages, we prepared bone marrow-derived macrophages from control wild-type and Il13ra1−/- mice [24, 35], and measured their responsiveness to IL-4 and IL-13. As shown in figure 3a, IL-4 induced equivalent levels of STAT6 activity in wild-type and IL-13Rα1-deficient macrophages. However, although IL-13 induced activation of STAT6 in wild-type macrophages, the ability of IL-13 to activate STAT6 was abrogated in Il13ra1−/- macrophages. Similar results were obtained when we measured the levels of tyrosine-phosphorylated STAT6 by Western blotting (fig. 3b). These findings demonstrate that the IL-13Rα1 chain is essential for IL-13 signaling but not for IL-4 signaling in macrophages.
Fig. 3.
The IL-13Rα1 chain is required for activation of STAT6 by IL-13 but not IL-4 in macrophages. a Macrophages derived from wild-type or Il13ra1−/- mice (C57BL/6J background) were treated with IL-4 (1,000-0.1 ng/ml) or IL-13 (1,000-10 ng/ml) for 30 min at 37°C. Nuclear protein extracts were then prepared, and STAT6 activity was measured by EMSA using a radiolabeled SBE1 probe. b A companion set of whole cell lysates was also prepared from wild-type and Il13ra1−/- macrophages, and the levels of tyrosine-phosphorylated STAT6 were measured by Western blotting with rabbit anti-phospho-STAT6 (Tyr641) antibody. IP = Immunoprecipitation.
To determine if IL-4 and IL-13 induce similar responses in peritoneal macrophages, we obtained peritoneal macrophages from wild-type and Il13ra1 KO mice and examined their responsiveness to IL-4 and IL-13. Similar to the findings with bone marrow-derived macrophages, both IL-4 and IL-13 induced activation of STAT6 in wild-type peritoneal macrophages, whereas only IL-4 induced activation of STAT6 in peritoneal macrophages derived from Il13ra1 KO mice (online suppl. fig. 1; see www.karger.com/doi/10.1159/000376579 for all online suppl. material).
The IL-13Rα1 Chain Is Required for Induction of the AMA Pathway by IL-13 but Not IL-4
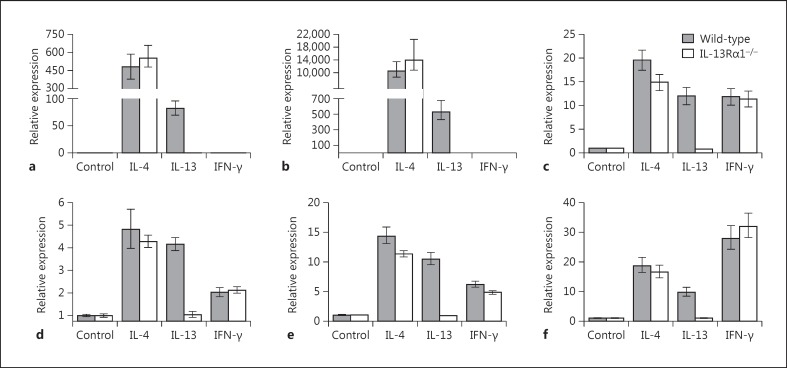
Both IL-4 and IL-13 can induce the AMA pathway [4, 5, 6, 7]. The phenotype of alternatively activated macrophages is defined in part by expression of a characteristic set of STAT6-responsive genes that includes Arg1 (arginase-1), Retnla (Fizz1), Ccl11 (eotaxin) and Clec7a (dectin-1, β- glucan receptor), among others. To determine if the IL-13Rα1 chain is required for induction of IL-4/IL-13-responsive genes, we treated control wild-type macrophages and IL-13Rα1-null macrophages with IL-4, IL-13 or IFN-γ for 6 h, and then harvested total RNA from these cells to measure the expression levels of several genes that are characteristically expressed by alternatively activated macrophages. As shown in figure 4, both IL-4 and IL-13 induced expression of Arg1 and Retnla in wild-type macrophages; however, only IL-4 induced expression of Arg1 and Retnla in IL-13Rα1-null macrophages. IFN-γ did not induce expression of Arg1 or Retnla in either wild-type or Il13ra1−/- macrophages. The inability of IL-13 to upregulate gene expression in IL-13Rα1-deficient macrophages extended to several other genes, including Ccl11, Clec7a and the transcriptional regulatory genes Nfil3 (E4BP4) and Socs1. Like the Arg1 and Retnla genes, expression of these genes was upregulated by both IL-4 and IL-13 in wild-type macrophages, but only IL-4 upregulated expression of these genes in Il13ra1−/- macrophages.
Fig. 4.
The IL-13Rα1 chain is required for induction of STAT6-responsive genes by IL-13 but not IL-4 in macrophages. Bone marrow-derived macrophages from wild-type or Il13ra1−/- mice were incubated with medium alone (control), IL-4 (10 ng/ml), IL-13 (100 ng/ml) or IFN-γ (10 ng/ml) for 6 h at 37°C. At the end of this incubation period, RNA extracts were prepared and analyzed by quantitative RT-PCR to measure expression levels of several IL-4-responsive genes, including Arg1 (arginase-1; a), Retnla (Fizz1, Relm-α; b), Ccl11 (eotaxin; c), Clec7a (dectin-1, β-glucan receptor; d), Nfil3 (E4BP4; e) and Socs1 (SOCS-1; f). The gene expression levels were determined relative to the housekeeping gene Gapdh and represent the mean of triplicate determinations.
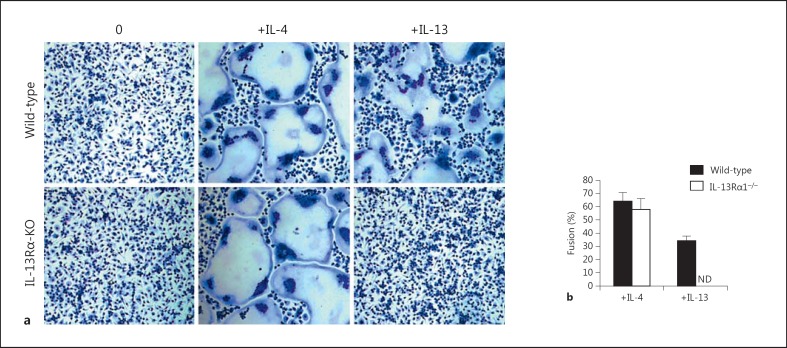
Induction of the AMA pathway by IL-4 or IL-13 is also characterized in part by membrane fusion and the formation of multinucleated giant cells [9, 36, 37]. To determine if the IL-13Rα1 chain is required for membrane fusion and the formation of multinucleated giant cells by IL-4 and/or IL-13, we incubated bone marrow-derived macrophages from control wild-type and Il13ra1−/- mice with IL-4 or IL-13 (100 ng/ml) for 48 h, and then measured the percentage of fused cells. As shown in figure 5a, both IL-4 and IL-13 induced membrane fusion and giant cell formation by the control wild-type macrophages. However, only IL-4 induced giant cell formation by IL-13Rα1-null macrophages. The percentage of fused cells was quantified microscopically, and the results shown in figure 5b represent the mean ± SD of quadruplicate determinations for each treatment group. The cell fusion results in figure 5b correlated well with the observed differences in giant cell formation shown in figure 5a. Together, these findings demonstrate that deletion of the IL-13Rα1 chain abrogates the ability of IL-13 but not IL-4 to induce macrophage fusion and giant cell formation.
Fig. 5.
Absence of the IL-13Rα1 chain abrogates the ability of IL-13 but not IL-4 to induce macrophage fusion and giant cell formation. Bone marrow-derived macrophages from wild-type and Il13ra1 KO mice were cultured in the presence or absence of IL-4 or IL-13 (100 ng/ml) for 48 h. The cells were then stained with Hemacolor solution to facilitate the detection of giant cells (a), and the percentage of fused cells was measured microscopically (b). The results shown represent the mean ± SD of quadruplicate determinations for each treatment group. ND = Not detectable.
The IL-13Rα1 Chain Is Required for IL-13 to Inhibit IFN-γ-Induced Signaling and Gene Expression in Macrophages
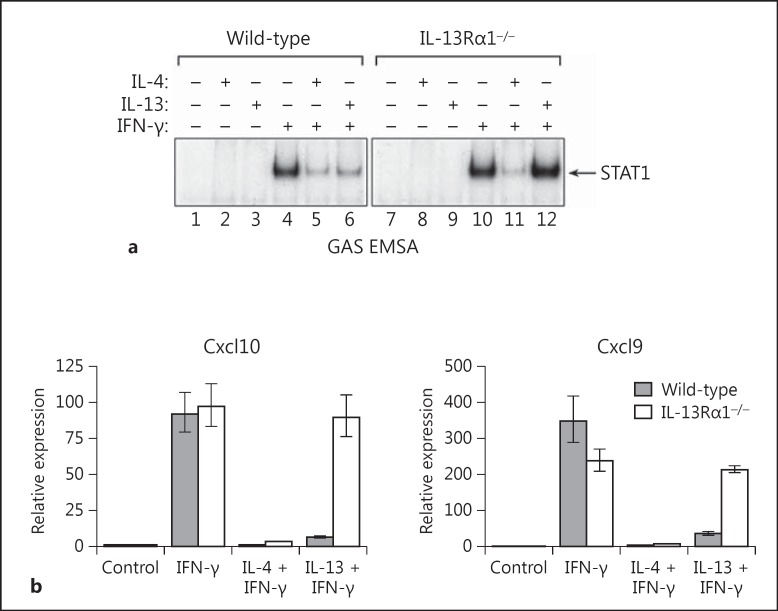
Previous studies have shown that IL-4 and IL-13 can inhibit classical macrophage activation by IFN-γ [38, 39]. To determine if the IL-13Rα1 chain is required for the ability of IL-4 and/or IL-13 to inhibit signal transduction and gene expression induced by IFN-γ, we pretreated cultures of wild-type or IL-13Rα1-null macrophages overnight (18 h) with IL-4 or IL-13, and then added IFN-γ (10 ng/ml) to the cultures. After incubation for an additional 30 min, nuclear protein extracts were prepared, and the levels of STAT1 activity were assayed by EMSA using the N3 GAS probe (GRR). As shown in figure 6a, both IL-4 and IL-13 markedly inhibited activation of STAT1 in wild-type macrophages; however, only IL-4 inhibited activation of STAT1 in Il13ra1−/- macrophages.
Fig. 6.
The IL-13Rα1 chain is required for IL-13 but not IL-4 to inhibit induction of signaling and gene expression by IFN-γ in macrophages. a Bone marrow-derived macrophages from control (wild-type) or IL-13Rα1 KO mice were incubated overnight (18 h) with IL-4 (10 ng/ml) or IL-13 (100 ng/ml), and then IFN-γ (10 ng/ml) was added as indicated. After incubation for an additional 30 min, nuclear protein extracts were prepared, and the levels of STAT1 activity were measured by EMSA using a radiolabeled GAS (GRR) probe. b A matched set of macrophages derived from wild-type and Il13ra1−/- mice were pretreated overnight with IL-4 (10 ng/ml) or IL-13 (100 ng/ml), and then IFN-γ (10 ng/ml) was added as indicated. The cells were incubated for an additional 4 h, and then total RNA extracts were prepared. The levels of Cxcl9 (Mig) and Cxcl10 (IP-10) gene expression were measured by qRT-PCR.
Next, we used a matched set of bone marrow-derived macrophages derived from wild-type and Il13ra1−/- mice to determine if the inability of IL-13 to inhibit IFN-γ signaling in IL-13Rα1-null macrophages correlated with an inability to inhibit expression of STAT1-inducible genes. Macrophages derived from wild-type and Il13ra1 KO mice were pretreated overnight with IL-4 or IL-13, and then IFN-γ (10 ng/ml) was added as indicated. The cultures were incubated for an additional 4 h, and then total RNA extracts were prepared and analyzed by qPCR to measure the levels of Cxcl9 (Mig) and Cxcl10 (IP-10) gene expression. As shown in figure 6b, pretreatment with IL-4 or IL-13 markedly suppressed the levels of Cxcl9 (Mig) and Cxcl10 (IP-10) gene expression induced by IFN-γ in wild-type macrophages. In contrast, although IL-4 markedly inhibited Cxcl9 and Cxcl10 gene expression in IL-13Rα1-null macrophages, IL-13 did not. In separate but related experiments, we also found that IL-4 but not IL-13 markedly inhibited LPS-induced production of IL-12 by Il13ra1 KO macrophages, whereas both IL-4 and IL-13 inhibited IL-12 production by wild-type macrophages (online suppl. fig. 2).
The IL-13Rα1 Chain Is Essential for Signaling by both IL-4 and IL-13 in Fibroblasts
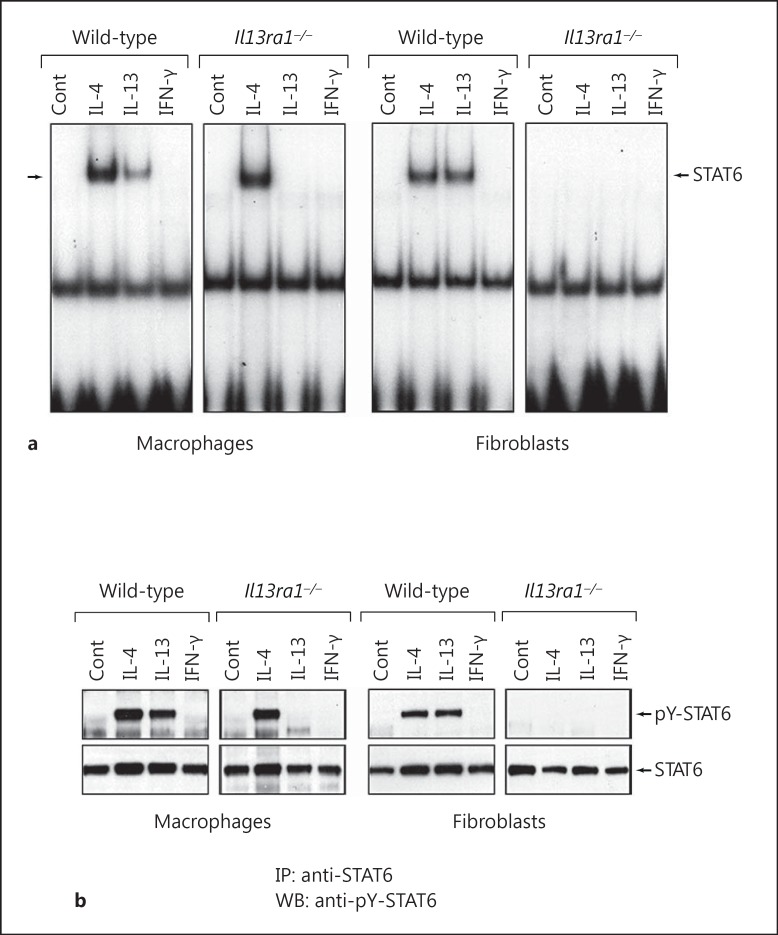
As shown in figure 3, the ability of IL-4 to activate STAT6 was essentially unchanged in Il13ra1−/- macrophages because, despite the absence of IL-13Rα1, these cells still express γc, and IL-4 can signal effectively via type I IL-4 receptor complexes. In contrast to monocytes and macrophages which coexpress γc and IL-13Rα1, nonhematologic cell types, such as fibroblasts and endothelial cells, express IL-13Rα1 but not γc [33, 34]. We compared the ability of IL-4 and IL-13 to activate STAT6 in macrophages and fibroblasts derived from wild-type mice and Il13ra1−/- mice. The fibroblasts used in these experiments were generated from the lungs of wild-type and Il13ra1−/- mice as described previously [24]. As shown in figure 7a, both IL-4 and IL-13 induced activation of STAT6 in wild-type macrophages, but only IL-4 was able to activate STAT6 in Il13ra1−/- macrophages. In contrast, although both IL-4 and IL-13 induced activation of STAT6 in wild-type fibroblasts, neither IL-4 nor IL-13 induced activation of STAT6 in Il13ra1−/- fibroblasts. Similar results were obtained when we measured the levels of tyrosine-phosphorylated STAT6 by immunoblotting with anti-pY-STAT6 Ab (fig. 7b). Both IL-4 and IL-13 induced phosphorylation of STAT6 in wild-type macrophages, but only IL-4 induced phosphorylation of STAT6 in Il13ra1 KO macrophages. However, unlike in macrophages, neither IL-4 nor IL-13 induced phosphorylation of STAT6 in fibroblasts. These findings demonstrate that the IL-13Rα1 chain is required for signaling by IL-4 in fibroblasts but not in macrophages. However, in the case of IL-13, the IL-13Rα1 chain is essential for signaling by both macrophages and fibroblasts.
Fig. 7.
The IL-13Rα1 chain is required for activation of STAT6 by IL-4 in fibroblasts but not in macrophages. a Macrophages derived from wild-type or Il13ra1−/- mice were treated with IL-4, IL-13 or IFN-γ (20 ng/ml) for 30 min at 37°C. Nuclear protein extracts were then prepared and assayed for STAT6 activity by EMSA using a radiolabeled SBE1 probe. Lung fibroblast cultures were also prepared from wild-type and Il13ra1−/- mice, and treated with IL-4, IL-13 or IFN-γ in a similar manner. b A companion set of whole cell lysates was prepared from wild-type and Il13ra1−/- macrophages and fibroblasts, and the levels of tyrosine-phosphorylated STAT6 were measured by Western blotting with rabbit anti-phospho-Tyr641 STAT6 antibody. IP = Immunoprecipitation; WB = Western blot.
Discussion
Our findings demonstrate that expression of the IL-13Rα1 chain is necessary for induction of the AMA pathway by IL-13 but not IL-4. Macrophages express two distinct receptor complexes by which IL-4 can signal to induce the AMA pathway. The first is mediated by signaling through type I IL-4 receptor complexes that consist of the IL-4Rα chain and γc. The second is activated by signaling through type II IL-4 receptor complexes which are heterodimers composed of the IL-4Rα and IL-13Rα1 chains. In γc-deficient macrophages, IL-4 can still signal via type II IL-4 receptor complexes because these cells maintain expression of IL-13Rα1. In other words, the binary complex formed by the initial binding of IL-4 to the IL-4Rα chain can still recruit the IL-13Rα1 chain if γc is not present on the cell membrane. In contrast to IL-4, IL-13 can only signal via type II IL-4 receptor complexes to induce activation of STAT6 and downstream STAT6-responsive gene expression. Interestingly, we observed that the magnitude of STAT6 activation and gene expression induced by IL-4 in wild-type macrophages is generally greater than that induced by IL-13. These results are consistent with related findings by others [19, 20]. However, in γc-deficient macrophages, the magnitude of STAT6 activity induced by IL-13 was comparable to that induced by IL-4. This is likely due to the fact that, in the absence of γc, both IL-4 and IL-13 can only signal via type II IL-4 receptor complexes.
IL-4 receptor structural studies have shown that IL-4 and IL-13 catalyze differential assembly of IL-4 receptor complexes [40, 41]. IL-4 binds initially to the IL-4Rα chain to generate a binary complex which then recruits either γc or the IL-13Rα1 chain to form ternary type I or type II IL-4 receptor complexes, respectively. In contrast, the primary ligand-binding chain for IL-13 is IL-13Rα1, not IL-4Rα. Consequently, IL-13 binds initially to the IL-13Rα1 chain to generate IL-13/IL-13Rα1 binary complexes which then recruit the IL-4Rα chain to complete assembly of ternary type II IL-4 receptor complexes. Therefore, IL-4 and IL-13 induce rapid assembly of type II IL-4 receptor complexes by catalyzing physical association of the same receptor chains (i.e. IL-4Rα and IL-13Rα1), but the order of assembly is reversed. Although the receptor assembly sequences are distinct for IL-4 and IL-13, the intracellular signal transduction pathway and repertoire of genes induced by these two cytokines are largely the same.
We found that IL-13 failed to upregulate expression of several STAT6-inducible genes in IL-13Rα1-deficient macrophages. These genes included Arg1 (arginase-1), Retnla (Fizz1), Ccl11 (eotaxin-1), Clec7a (dectin-1), Nfil3 (E4BP4) and Socs1. Although the ability of IL-4 and IL-13 to induce expression of the Arg1, Retnla, Ccl11 and Clec7a genes in macrophages is well established, the ability of IL-4 and IL-13 to induce expression of Nfil3 and Socs1 has received less attention. We showed previously that IL-4 and IL-13 induce Socs1 gene expression in both mouse and human macrophages in a STAT6-dependent manner [28]. We also showed that Socs1 serves an autoregulatory role in macrophages by feedback inhibiting the expression of STAT6-responsive genes such as Arg1 and Retnla. Socs1 also strongly inhibits classical macrophage activation by IFN-γ [42, 43] and this may explain, at least in part, how IL-4 and IL-13 suppress the induction of STAT1-responsive genes in macrophages [38, 39].
In addition to the inability of IL-13 to induce expression of STAT6-responsive genes in IL-13Rα1-deficient macrophages, IL-13 also failed to induce membrane fusion and giant cell formation in these cells. The ability of IL-4 and IL-13 to induce membrane fusion and giant cell formation by mononuclear phagocytes was first reported more than 2 decades ago [36, 37]. Although the molecular basis of giant cell formation is not yet fully defined, several studies have shown that this process is STAT6 dependent and requires homotypic adhesion [44, 45]. The ability of IL-4 and IL-13 to induce membrane fusion and multinucleated giant cell formation is consistent with the association of giant cell formation with disease states such as asthma and certain helminth infections that are characterized by chronic activation of Th2 and type 2 innate lymphoid cells (ILC2) [46].
The differential responsiveness of macrophages to IL-4 versus IL-13 cannot be attributed to differences in the binding affinities of IL-4 and IL-13 for γc versus the IL-13Rα1 chain. LaPorte et al. [40] showed that the Kd values of IL-4/IL-4Rα binary complexes for γc versus the IL-13Rα1 chain are low but quite similar. In contrast, IL-13 binds initially to the IL-13Rα1 chain to form IL-13Rα1/IL-13 binary complexes which then heterodimerize with the IL-4Rα chain to generate type II IL-4 receptor complexes. The overall binding affinity of fully assembled IL-13Rα1/IL-13/IL-4Rα ternary complexes is much greater (Kd ≈ 30 pM) than the affinities of the individual receptor chains.
Macrophages coexpress γc and IL-13Rα1, and can respond to both IL-4 and IL-13. However, we found that IL-4 preferentially uses γc, not IL-13Rα1, to mediate signal transduction in macrophages, whereas IL-13 uses the IL-13Rα1 chain, not γc, to mediate signaling. If γc expression is diminished or absent in macrophages, IL-4 can default signal through type II IL-4 receptor complexes because the cells still express the IL-13Rα1 chain. Our studies demonstrate that the IL-13Rα1 chain is dispensable in the case of the ability of IL-4 to induce the AMA pathway. However, the IL-13Rα1 chain is essential for IL-13 to activate the AMA pathway. IL-4 can activate the AMA pathway by signaling through either type I or type II IL-4 receptor complexes, whereas IL-13 can only signal via type II IL-4 receptor complexes.
Unlike macrophages which coexpress γc and IL-13Rα1, nonhematologic cell types such as fibroblasts and endothelial cells do not express γc [33, 34]. Therefore, most nonhematologic cell types express only type II IL-4 receptor complexes, and the magnitude of IL-4- and IL-13-induced responses is comparable in these cells. We compared the ability of IL-4 and IL-13 to activate STAT6 in macrophages versus pulmonary fibroblasts derived from wild-type and Il13ra1 KO mice. We found that the IL-13Rα1 chain is essential for activation of STAT6 by IL-13, but is not required for IL-4 signaling in macrophages. In contrast, IL-13Rα1 was essential for signaling by both IL-4 and IL-13 in fibroblasts. This is because, unlike macrophages which coexpress γc and IL-13Rα1, fibroblasts express IL-13Rα1 but do not express γc.
It is generally agreed that the production of IL-4 by activated Th2 and ILC2 cells is tightly regulated and largely restricted to the immediate site(s) of immune reactivity. In contrast, IL-13 is often quantitatively more abundant than IL-4 in diseases such as asthma and parasitic infections that are characterized by the presence of activated ILC2 and/or Th2 cells. IL-13 is a primary regulator of airway hyperreactivity and mucus production in allergic lung inflammation, and it has been shown to play a more dominant role than IL-4 as an effector cytokine of Th2-mediated pathogenesis in several animal models [24, 35]. Alternatively activated macrophages are key participants in Th2-mediated inflammatory processes, and development of therapeutic agents that block the AMA pathway may provide an effective means to inhibit these processes.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
References
- 1.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray HW, Spitalny GL, Nathan CF. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-γ. J Immunol. 1985;134:1619–1622. [PubMed] [Google Scholar]
- 3.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein M, Keshav S, Harris N, Gordon S. Interleukin-4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raes G, De Baetselier P, Noël W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of Fizz1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 6.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 7.van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY, Gordon S, Brown GD. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171:4569–4573. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- 9.Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multi-stage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- 10.Doherty TM, Kastelein R, Menon S, Andrade S, Coffman RL. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151:7151–7160. [PubMed] [Google Scholar]
- 11.Doyle AG, Herbein G, Montaner LJ, Minty AJ, Caput D, Ferrara P, Gordon S. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-γ. Eur J Immunol. 1994;24:1441–1445. doi: 10.1002/eji.1830240630. [DOI] [PubMed] [Google Scholar]
- 12.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 13.Quelle FW, Shimoda K, Thierfelder W, Fischer C, Kim A, Ruben SM, Cleveland JL, Pierce JH, Keegan AD, Nelms K, Paul WE, Ihle JN. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995;15:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindler U, Wu P, Rothe M, Brasseur M, McKnight SL. Components of a STAT recognition code: evidence for two layers of molecular selectivity. Immunity. 1995;2:689–697. doi: 10.1016/1074-7613(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 15.Seidel HM, Milocco LH, Lamb P, Darnell JE, Jr, Stein RB, Rosen J. Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc Natl Acad Sci USA. 1995;92:3041–3050. doi: 10.1073/pnas.92.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signaling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J Immunol. 1996;157:3220–3222. [PubMed] [Google Scholar]
- 19.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, Paul WE. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Rα, IL-13Rα1, and γc regulates relative cytokine sensitivity. J Exp Med. 2008;205:2595–2608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, Keegan AD. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheikh F, Dickensheets H, Gamero AM, Vogel SN, Donnelly RP. An essential role for IFN-β in the induction of IFN-stimulated gene expression by LPS in macrophages. J Leukoc Biol. 2014;96:591–600. doi: 10.1189/jlb.2A0414-191R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, Paul WE, Katz SI, Love PE, Leonard WJ. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 24.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Jr, Donnelly RP, Wynn TA. Unique functions of the type II interleukin-4 receptor identified in mice lacking the interleukin-13 receptor α1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickensheets HL, Venkataraman C, Schindler U, Donnelly RP. Interferons inhibit activation of STAT6 by interleukin-4 in human monocytes by inducing SOCS1 gene expression. Proc Natl Acad Sci USA. 1999;96:10800–10805. doi: 10.1073/pnas.96.19.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohmori Y, Smith MF, Jr, Hamilton TA. IL-4-induced expression of the IL-1 receptor antagonist gene is mediated by STAT6. J Immunol. 1996;157:2058–2065. [PubMed] [Google Scholar]
- 27.Pearse RN, Feinman R, Ravetch JV. Characterization of the promoter of the human gene encoding the high-affinity IgG receptor: transcriptional induction by γ-interferon is mediated through common DNA response elements. Proc Natl Acad Sci USA. 1991;88:11305–11309. doi: 10.1073/pnas.88.24.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickensheets H, Vazquez N, Sheikh F, Gingras S, Murray PJ, Ryan JJ, Donnelly RP. Suppressor of cytokine signaling (SOCS)-1 is an IL-4-inducible gene in macrophages and feedback inhibits IL-4 signaling. Genes Immun. 2007;8:21–27. doi: 10.1038/sj.gene.6364352. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Beckmann MP, Schooley KA, Gallis B, Vanden Bos T, Friend D, Alpert AR, Raunio R, Prickett KS, Baker PE, Park LS. Monoclonal antibodies block murine IL-4 receptor function. J Immunol. 1990;144:4212–4217. [PubMed] [Google Scholar]
- 31.Hart PH, Bonder CS, Balogh J, Dickensheets HL, Vazquez N, Davies KV, Finlay-Jones JJ, Donnelly RP. Diminished responses to IL-13 by human monocytes differentiated in vitro: role of the IL-13R-α 1 chain and STAT6. Eur J Immunol. 1999;29:2087–2097. doi: 10.1002/(SICI)1521-4141(199907)29:07<2087::AID-IMMU2087>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 32.Andersson A, Grunewald SM, Duschl A, Fischer A, DiSanto JP. Mouse macrophage development in the absence of the common γ chain: defining receptor complexes responsible for IL-4 and IL-13 signaling. Eur J Immunol. 1997;27:1762–1768. doi: 10.1002/eji.1830270725. [DOI] [PubMed] [Google Scholar]
- 33.Schnyder B, Lugli S, Feng N, Etter H, Lutz RA, Ryffel B, Sugamura K, Wunderli-Allenspach H, Moser R. Interleukin-4 (IL-4) and IL-13 bind to a shared heterodimeric complex on endothelial cells mediating vascular cell adhesion molecule-1 induction in the absence of the common gamma chain. Blood. 1996;87:4286–4295. [PubMed] [Google Scholar]
- 34.Kotowicz K, Callard RE, Friedrich K, Matthews DJ, Klein N. Biological activity of IL-4 and IL-13 on human endothelial cells: functional evidence that both cytokines act through the same receptor. Int Immunol. 1996;8:1915–1925. doi: 10.1093/intimm/8.12.1915. [DOI] [PubMed] [Google Scholar]
- 35.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci USA. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McInnes A, Rennick DM. Interleukin-4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988;167:598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158:3385–3390. [PubMed] [Google Scholar]
- 38.Ohmori Y, Hamilton TA. IL-4-induced STAT6 suppresses IFN-γ-stimulated STAT1-dependent transcription in mouse macrophages. J Immunol. 1997;159:5474–5482. [PubMed] [Google Scholar]
- 39.Bogdan C, Thüring H, Dlaska M, Röllinghoff M, Weiss G. Mechanism of suppression of macrophage nitric oxide release by IL-13: influence of the macrophage population. J Immunol. 1997;159:4506–4513. [PubMed] [Google Scholar]
- 40.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, Keegan AD, Garcia KC. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito T, Suzuki S, Kanaji S, Shiraishi H, Ohta S, Arima K, Tanaka G, Tamada T, Honjo E, Garcia KC, Kuroki R, Izuhara K. Distinct structural requirements for interleukin-4 (IL-4) and IL-13 binding to the shared IL-13 receptor facilitate cellular tuning of cytokine responsiveness. J Biol Chem. 2009;284:24289–24296. doi: 10.1074/jbc.M109.007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 43.Brysha M, Zhang JG, Bertolino P, Corbin JE, Alexander WS, Nicola NA, Hilton DJ, Starr R. Suppressor of cytokine signaling-1 attenuates the duration of interferon γ signal transduction in vitro and in vivo. J Biol Chem. 2001;276:22086–22089. doi: 10.1074/jbc.M102737200. [DOI] [PubMed] [Google Scholar]
- 44.Moreno JL, Mikhailenko I, Tondravi MM, Keegan AD. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J Leukoc Biol. 2007;82:1542–1553. doi: 10.1189/jlb.0107058. [DOI] [PubMed] [Google Scholar]
- 45.Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Chang YJ, DeKruyff RH, Umetsu DT. The role of type 2 innate lymphoid cells in asthma. J Leukoc Biol. 2013;94:933–940. doi: 10.1189/jlb.0313127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data