Abstract
SREC-I is a class F scavenger receptor with key role in the immune response, particularly in antigen presenting cell (APC) such as macrophages and dendritic cells (DC). This receptor is able to mediate engulfment of dead cells as well as endocytosis of heat shock protein (HSP)-antigen complexes. SREC-I could thus potentially mediate the tolerizing influence of apoptotic cells or the immunostimulatory effects of HSP-peptide complexes, depending on context. This receptor was able to mediate presentation of external antigens, bound to HSPs through both the Class II pathway as well as cross presentation via MHC class I complexes. In addition to its recently established role in adaptive immunity, emerging studies are indicating a broad role in innate immunity and regulation of cell signaling through Toll Like Receptors (TLR). SREC-I may thus play a key role in APC function by coordinating immune responses to internal and external antigens in APC.
Introduction
Scavenger receptors are a family of receptors that have in common the ability to bind to covalently modified proteins, most notably oxidized low density lipoprotein (oxidized LDL). The scavenging of oxidized LDL by endothelial cells plays a significant role in sparing organisms from pathologies such as atherosclerosis1. Interestingly the scavenger receptor family is grouped along functional lines and most such proteins have little sequence similarity2, 10. One mystery associated with this protein family is that, although there is minimal homology in primary structures among scavenger receptor families, they can associate with a similar and equally diverse group of ligands1,3SREC-I (Scavenger Receptor expressed by Endothelial cells), a member of the class F scavenger receptor family, 85.7 kD protein was first cloned from HUVEC (Human Umbilical Vein Endothelial Cells) cells and termed as scavenger receptor expressed by endothelial cells2,26 The primary structure of this scavenger receptor had minimal similarity with those of most other scavenger receptors previously characterized, although SREC-II which is a member of this same family was shown to have some common features in terms of the extracellular domains12, 26. The extracellular domain of SREC is also similar to that of FEEL-122. The extended extracellular domain (ED) of the class F receptors is comprised of epidermal growth factor like cysteine rich motifs (EGF repeats) while the unusually long intracellular domain contains a serine-proline rich region25. SREC-1, particularly in the ED, has significant homology with the C. elegans protein CED-1, a polypeptide involved in the uptake of apoptotic bodies27. Additional cell corpse engulfing proteins such as MEGF10, MEGF11 and MEGF12 are also CED-1paralogs and, like SREC-I contain multiple EGF repeats within the ED 20, 27. Like other scavenger receptors the class F family are defined by their ability to bind, internalize and metabolize modified LDL species, such as acetylated (Ac) LDL, oxidized (Ox) LDL, a process involved in the pathogenesis of atherosclerosis15, 25. In addition to their roles in binding and internalizing these modified lipids, SREC-I was also shown to participate in other cellular functions such as cell-cell adhesion, antigen cross presentation, engulfment of apoptotic cells and innate immunity. The cell adhesion properties may involve SREC-I interaction with SREC-II counter-receptors on the partner cell26. SREC-I can also cooperate with pattern recognition receptor function in innate immunity (see below). This receptor has an additional role in mediating morphological changes when overexpressed in a fibroblasts suggesting its participation in morphogenesis of cells (A. Murshid, unpublished studies). The intracellular domain of SREC-1, which is extensive compared with that of other scavenger receptors, is largely uncharacterized. However, it has been shown that this cytosolic domain is capable of interacting with protein phosphatase 1α (PP1 α) in L cells and thus mediating morphological changes13.
SREC-I and Antigen Cross Presentation
SREC-I has been shown to be a key receptor for heat shock proteins8, 29. The physiological significance of extracellular HSPs is not entirely clear, although they are known to play key roles in the immune response7. HSPs can be immunostimulatory when associated with tumor antigens, transporting the chaperoned antigens into AP. In contrast, in different contexts, HSPs can play immunoregulatory roles and suppress T cell mediated immunity in inflammatory diseases5,24. We have attempted to discover physiologically relevant HSP receptors. We carried out forced expression of candidate receptors in a cell line, CHO that is null for HSP binding then assayed binding of fluorescently labeled Hsp70 or Hsp90 to those receptors expressing cell. Hsp70 was found to bind Class E receptor Lox-1, Class F receptor SREC-I and Class H receptor Feel-1/stabilin-129. In addition, Hsp70 was found to bind to some NK receptors found on the surface of natural killer cells 29. As most of our studies have centered on SREC-I we will discuss this receptor in more detail in this review. SREC-I , in common with another scavenger receptor, LOX-I can bind with high avidity to HSPs, including Hsp70, Hsp90, Grp94, Hsp110 and Grp170 with or without associated antigens and appears to be an important common receptor for these proteins3,19,21. In addition, among all the scavenger receptors that have been characterized so far, we found that SREC-I and LOX-1 each appeared to mediate the majority of the cross presentation of the Ova SIINFEKL epitope chaperoned by Hsp90 or Hsp70 in BMDC21.
Earlier it was demonstrated that HSP-antigen complexes could be bound to SREC-I and internalized in antigen presenting cells such as dendritic cells (DC) and macrophages (as well as a large variety of tissue culture cell lines21). An Hsp90-antigen-SREC-I internalization pathway was characterized in these cell types which was similar to a previously described mechanism involving tubule like vesicles formation upon uptake of ligand-receptor complex and known as CLIC (Clathrin independent carriers) or GEEC (GPI anchored protein-enriched endocytic compartments)9,10. This pathway is distinct from endocytic mechanisms involving Clathrin and is heavily utilized by GPI-anchored proteins32. Although the significance of entry of HSP-SREC-I complexes through the CLIC/GEEC pathway is not entirely clear, this mechanism does appear to permit regulation of antigen cross presentation by signal transducing molecules as discussed below. Hsp90-polypeptide-SREC-I complexes were able to mediate cross presentation of external chaperoned antigens, mediating processing in both endosomal and proteasomal compartments.
It is not clear to what degree the antigen presentation pathways involved with HSP-chaperoned antigens are similar to those used by other forms of antigens. For free, unchaperoned antigens, dedicated receptors have been shown to direct antigens to either the MHC class II pathway or to cross-presentation via the MHC class I complexes6,11. We have demonstrated that antigens bound to Hsp90 could be internalized via SREC-I and later processed. Internalized antigens could be loaded onto either MHC class I (cross presentation) or MHC class II molecules (Class II presentation). It is not known whether the scavenger receptor mediates triage between the two MHC pathways or whether the choice of pathways is stochastic. Antigen presentation then led to specific activation of both CD8+ and CD4+ T cells. In these parallel MHCI and MHCII antigen presentation pathways, SREC-I engagement by Hsp90-bound antigens increased Cdc42 GTPase activity, regulating actin assembly and polymerization and other signaling pathways such as Src kinase signaling41.
Receptor mediated internalization of Hsp90 bound antigens rather than non-specific internalization of free antigens has two potential advantages. Such HSP chaperoned antigens can be protected from proteolysis during trafficking through the cell compartments and thus reduced amount of antigen would be required to initiate both CD4+ and CD8+ T cell priming 23. It is however clear that we understand chaperone mediated antigen cross priming only in outline so far and that considerable further investigations are required in order to understand the basic mechanisms involved. SREC-I thus plays a key role in receptor-mediated uptake of chaperone-bound antigen presentation, protecting and transporting its charges to the key intracellular sites.
Role of SREC-I in apoptotic cells engulfment
The elimination of defective and unwanted cells by apoptosis is an essential process for maintenance of tissue homeostasis as well as contributing to tumor regression in cytotoxic therapies. A rapid and immunologically clean removal of these apoptotic cells is crucial for evading inflammation, immune tolerance and homeostasis34. Phagocytic cells recognize and engulf these dying cells through several surface receptors expressed by these cells or by the interaction of bridging soluble proteins that recognize “find-me” and “eat-me” signals presented in apoptotic cells, as lipid lysophosphatidylcholine (LPC) and Phosphatidylserine (PS)35.
The first suggestion that SREC-I could participate in the recognition and engulfment of apoptotic cells was when the transmembrane protein CED-1 from C. elegans was identified as an ortholog of human SREC-I. CED-1 was reported to be responsible for the recognition and internalization of apoptotic cells by C. elegans. This receptor has a sequence similarity and shares a similar overall structure with SREC-I36. Using GFP under control of ced-1 promoter, it was demonstrated that CED-1 is expressed at high levels in cells that can act as endocytic cells along the surface of cell corpses but not in the dying cell. Mutations in the ced-1 gene that cause loss of protein function resulted in a phenotype characterized by cell corpse retention, indicating that CED-1 is required for identification and engulfment of apoptotic cells in C. elegans36.
More recently it was demonstrated that DC, macrophages and endothelial cells expressing SREC-I could bind phosphatidyl serine moieties exposed on the apoptotic cell surface37. Additionally, the same group demonstrated that CD8α+ DCs expressing higher levels of SREC-I were more capable of engulfing dying cells or apoptotic cells than those of SREC-I−/− mice. Forced expression of SREC-I in these SREC-I−/− DCs reversed the phenotypes and enhanced uptake of dying cells. These findings indicated a role of SREC-I in apoptotic cell engulfment and removing dying cells. Additionally these knock-out mice had a spontaneous lupus-like disease, with the presence of autoantibodies, indicating that impairment in the SRECI-mediated clearance of apoptotic cells contributes to development of this autoimmune disorder37.
Signaling through SREC-I
In addition to internalizing HSP-bound peptides, ligand-bound SRECI appears to play a significant role in cell signaling. These signaling properties appear to be related to the appearance of SREC-I in lipid rafts after binding ligands such as Hsp9021. Lipid rafts are cholesterol and sphingolipid-rich membrane microdomains, floating in the bulk membrane, that can concentrate molecules involved in cell signaling18. Although lacking the glycerphophoinositide anchor domain motifs found in many raft-associated membrane proteins, SREC-I contains other motifs that would permit it to associate with lipid rafts22. The S-acylation of cysteine residues close to the transmembrane domain, with highly saturated palmitate residues, that can dissolve in the environment of the lipid raft has been associated with the ability of cells without GPI anchor domains to enter lipid rafts16, 18. SREC-I has five cysteine residues immediately adjacent to the transmembrane domain, making this a likely mechanism for the entry of SREC-I into lipid rafts. Although SREC-I activities, such as ability of ligand-binding and localization in the cell, has been shown to be regulated by glycosylation of specific sites of this receptor, it is not clear how ligand binding localizes SREC-I to lipid microdomains of plasma membrane. The N-glycan of Asparagine N382 of SREC-I modulates the affinity to its ligand, whereas N393 is responsible for its cellular localization 42.
We have demonstrated that Hsp90-SRECI complexes, but not unliganded SREC-I, could associate with the small GTPase Cdc42 and non-receptor tyrosine kinase Src, molecules tightly associated with lipid rafts21. Cdc42 and Src activity appeared to be important in regulating antigen cross presentation of Hsp90-associated antigens in DC.
Lipid micro domains such as rafts also concentrate intermediates in the TLR4 signaling pathway in response to innate immune stimuli30. We have found that SREC-I causes TLR4 to translocate to lipid microdomain in the presence of LPS (A. Murshid & SK Calderwood, in preparation). Our preliminary studies also showed significant co-localization of SREC-I ligand Hsp90 along with SREC-I and TLR4 in similar lipid raft domains (A. Murshid & SK Calderwood, in preparation). HSP-triggered signaling through SRECI may thus be involved both in amplifying antigen cross presentation and in stimulating innate immunity. It may be significant that the other major HSP-binding scavenger receptor associated with antigen cross presentation, LOX-1, although bearing no sequence similarity compared with SRECI appeared to associate with TLRs on ligand binding and mediate immune responses in a similar way to SREC-I14.
SREC-I, a potent receptor for inflammatory ligands
SREC-I can initiate immunological responses upon interacting with and binding to ligands such as peptide-bearing HSPs. This ligand-receptor interaction had distinct outcomes. In HSP-Ag uptake through SREC-I, binding could activate Src signaling which appeared to initiate internalization of the HSP-peptide-SREC-I complex to endocytic vesicles21.
SREC-I has been shown to recognize modified self-ligands, such as acetylated LDL but also non-self molecules present in invading pathogens 25,27. This feature indicated SREC-I as an important receptor for recognition of danger signals and the maintenance of tissue homeostasis as well as the control of infection. SREC-I was reported to trigger inflammatory signaling through the crosstalk with co-receptors, as TLR family members. The outer membrane protein A (OmpA) from Klebsiella pneumoniae was shown to be a ligand for SREC-I and LOX-1. In DCs and macrophages, exposure to OmpA induced the production of pro-inflammatory cytokines and chemokines, as IL-6 and IL-8 in a TLR2-dependent manner, suggesting a cooperative pathway between SREC-I / LOX-1 and TLR238. SREC-I also bound to the fungal pathogens Cryptococcus neoformans and Candida albicans, through the recognition of β-glucan residues exposed on the cell surface of these organisms. This scavenger receptor in cooperation with TLR2 triggered the production of IL-1β, CXCL2 and CXCL1 upon exposure to C. neoformans39. SREC-I expressed by DCs was also demonstrated to bind to non-structural protein 3 (NS3) of the hepatitis C virus, leading to IL-6 production by these cells, in crosstalk with TLR2. Endocytosis of NS3 was required to NS3-induced IL-6 production, underlying the importance of SREC-I as a scavenger receptor in the control of infections40.
Recently, TLR3 and TLR4 were also shown to cooperate with SREC-I in ligand mediated signaling and cytokine production41. SREC-I was demonstrated to enhance poly:IC-mediated TLR3 activation and downstream signaling (A. Murshid and SK Calderwood in preparation). TLR3 and SREC-I were shown to colocalize after poly:IC treatment and the formation of TLR3-SREC-I complexes increased IL-8 production by THP-1 macroophage/monocyte cells. Also, it was demonstrated that poly:IC-induced SREC-I-TLR3 interaction led to amplified NF-κB pathway activity and an increase in activated, phosphorylated forms of the MAP kinases p38 and c-jun kinase (JNK). MAPK activation was required for IL-8 and IL-6 production by THP-1 cells expressing both SREC-I and TLR3, upon poly:IC stimulation (A. Murshid and SK Calderwood in preparation). We also demonstrated that pathways downstream of LPS-TLR4 such as MAPK and NfκB were activated in cells expressing SREC-I (A. Murshid and SK Calderwood, in preparation).
Concluding Remarks
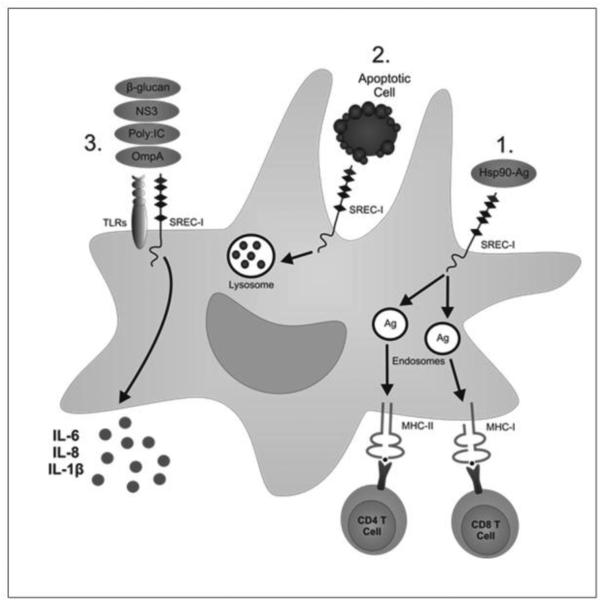
Developing studies indicate a broad role for SREC-I in many areas of cell physiology with important functions in vascular endothelium, fibroblasts and immune cells. In immune cells, this receptor appears to play roles in both innate and adaptive immunity (Fig. 1). Its scavenger function also permits SREC-I to function in engulfment of dead cells as well as internalization of extracellular HSPs. It may thus be involved in immune tolerance when apoptotic cells are engulfed or by contrast in T cell stimulation when HSP-peptide complexes are internalized and chaperone antigens are presented by MHC class I and II complexes. SREC-I is thus an important receptor in APC such as macrophages and DC (Fig. 1). SREC-I may also be a key component of innate immunity and may recognize molecules involved in sterile inflammation such as HSPs as well as PAMPS such as LPS and TLR3 ligands. The receptor may thus coordinate immune responses to internal and external antigens in DC.
Figure 1. Different roles of SREC-I in immunity and dead cell removal.
1. Antigen Presentation: Hsp-Ag interacts with SREC-I on antigen presenting cells and thus becomes internalized by these cells. Cells then process the antigens and processed antigens can be loaded to either MHC-I or MHC-II molecules to activate adaptive immunity. 2. Apoptotic cell engulfment. SREC-I binds to apoptotic cells through phosphatidylserine moiety exposed on apoptotic cells and can thus engulf them. Apoptotic bodies are then internalized and processed in the lysosome. 3. Pathogens are recognized by both TLRs and SREC-I. This is accompanied by internalization of pathogens, activation of signaling and transcription and release of cytokines.
Highlights.
* SREC-I contributes to immunosurveillance by scavenging damaged proteins, HSPs and cell corpses.
** SREC-I couples uptake and processing of antigens through both Class II and MHC class I pathways.
*** SREC-1 triggers inflammatory signaling pathways via entry into lipid raft membrane microdomains.
Acknowledgements
This work was supported by US National Institutes of Health research grants RO-1CA047407, RO1CA119045 and RO-1CA094397. A.M. is a recipient of JCRT and TJB is a recipient of CAPES fellowship. The authors alone are responsible for the content and writing of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi H, Tsujimoto M. Structure and function of a novel scavenger receptor expressed in human endothelial cells Tanpakushitsu kakusan koso Protein, nucleic acid, enzyme. 1999;44:1282–1286. [PubMed] [Google Scholar]
- 3.Greaves DR, Gough PJ, Gordon S. Recent progress in defining the role of scavenger receptors in lipid transport, atherosclerosis and host defence. Curr. Opin. Lipidol. 1998;9:425–432. doi: 10.1097/00041433-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J Biol Chem. 2004;279:51250–51257. doi: 10.1074/jbc.M406202200. [DOI] [PubMed] [Google Scholar]
- 3.Borges TJ, Lopes RL, Pinho NG, Machado FD, Souza AP, Bonorino C. Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBPbeta and C/EBPdelta. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2013;29:455–463. doi: 10.3109/02656736.2013.798037. [DOI] [PubMed] [Google Scholar]
- 4.Burgdorf S, Schuette V, Semmling V, Hochheiser K, Lukacs-Kornek V, Knolle PA, et al. Steady-state cross-presentation of OVA is mannose receptor-dependent but inhibitable by collagen fragments. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:E48–49. doi: 10.1073/pnas.1000598107. author reply E50-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderwood SK, Mambula SS, Gray PJ, Jr., Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS letters. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Calderwood SK, Theriault J, Gray PJ, Gong J. Cell surface receptors for molecular chaperones. Methods. 2007;43:199–206. doi: 10.1016/j.ymeth.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 8.Gupta GD, Swetha MG, Kumari S, Lakshminarayan R, Dey G, Mayor S. Analysis of endocytic pathways in Drosophila cells reveals a conserved role for GBF1 in internalization via GEECs. PLoS One. 2009;4:e6768. doi: 10.1371/journal.pone.0006768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba K, Inaba M. Antigen recognition and presentation by dendritic cells. International journal of hematology. 2005;81:181–187. doi: 10.1532/IJH97.04200. [DOI] [PubMed] [Google Scholar]
- 10.Ishii J, Adachi H, Aoki J, Koizumi H, Tomita S, Suzuki T, et al. SREC-II, a new member of the scavenger receptor type F family, trans-interacts with SREC-I through its extracellular domain. The Journal of biological chemistry. 2002;277:39696–39702. doi: 10.1074/jbc.M206140200. [DOI] [PubMed] [Google Scholar]
- 11.Ishii J, Adachi H, Shibata N, Arai H, Tsujimoto M. Scavenger receptor expressed by endothelial cells (SREC)-I interacts with protein phosphatase 1alpha in L cells to induce neurite-like outgrowth. Biochemical and biophysical research communications. 2007;360:269–274. doi: 10.1016/j.bbrc.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 12.Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Current opinion in lipidology. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 15.Levental I, Lingwood D, Grzybek M, Coskun U, Simons K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22050–22054. doi: 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingwood D, Kaiser HJ, Levental I, Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans. 2009;37:955–960. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- 17.Manjili MH, Henderson R, Wang XY, Chen X, Li Y, Repasky E, et al. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62:1737–1742. [PubMed] [Google Scholar]
- 18.McPhee CK, Baehrecke EH. The engulfment receptor Draper is required for autophagy during cell death. Autophagy. 2010;6:1192–1193. doi: 10.4161/auto.6.8.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murshid A, Gong J, Calderwood SK. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. Journal of immunology. 2010;2010185:2903–2917. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murshid A, Gong J, Calderwood SK. Molecular chaperone receptors: binding and trafficking of molecular chaperones by class F and class G scavenger receptors. Cellular trafficking of molecular chaperones in health and disease Ed B Henderson & A G Pockley. 2011 [Google Scholar]
- 21.Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Frontiers in immunology. 2012;3:63. doi: 10.3389/fimmu.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oura J, Tamura Y, Kamiguchi K, Kutomi G, Sahara H, Torigoe T, et al. Extracellular heat shock protein 90 plays a role in translocating chaperoned antigen from endosome to proteasome for generating antigenic peptide to be cross-presented by dendritic cells. International immunology. 2011;23:223–237. doi: 10.1093/intimm/dxq475. [DOI] [PubMed] [Google Scholar]
- 23.Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Zanin-Zhorov Alexandra, Cahalon Liora, Tal Guy, Margalit Raanan, Lider Ofer, Cohen Irun R. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116(7):2022–2032. doi: 10.1172/JCI28423. doi:10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Suzuki E, Nakayama M. The mammalian Ced-1 ortholog MEGF10/KIAA1780 displays a novel adhesion pattern. Experimental cell research. 2007;313:2451–2464. doi: 10.1016/j.yexcr.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Tamura Y, Osuga J, Adachi H, Tozawa R, Takanezawa Y, Ohashi K, et al. Scavenger receptor expressed by endothelial cells I (SREC-I) mediates the uptake of acetylated low density lipoproteins by macrophages stimulated with lipopolysaccharide. The Journal of biological chemistry. 2004;279:30938–30944. doi: 10.1074/jbc.M313088200. [DOI] [PubMed] [Google Scholar]
- 27.Theriault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. Journal of immunology. 2006;177:8604–8611. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
- 28.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 31.Adachi H, Tsujimoto M, Arai H, Inoue K. Expression cloning of a novel scavenger receptor from human endothelial cells. J. Biol. Chem. 1997;272:30220–31217. doi: 10.1074/jbc.272.50.31217. [DOI] [PubMed] [Google Scholar]
- 32.Mayor S, Sabharanjak S, Maxfield FR. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 199817:4626–38. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murshid A, Gong J, Calderwood SK. Hsp90-peptide complexes stimulate antigen presentation through the class II pathway after binding scavenger receptor SREC-I. Immunobiology. 2014 doi: 10.1016/j.imbio.2014.08.001. pii: S0171-2985(14)00135-1. doi: 10.1016/j.imbio.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–70. doi: 10.1083/jcb.201004096. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–74. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez-Ortiz ZG, Pendergraft WF, 3rd, Prasad A, Byrne MH, Iram T, Blanchette CJ, Luster AD, Hacohen N, El Khoury J, Means TK. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol. 2013;14:917–26. doi: 10.1038/ni.2670. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, Maina V, Magistrelli G, Haeuw JF, Hoeffel G, Thieblemont N, Corvaia N, Garlanda C, Delneste Y, Mantovani A. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–60. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206:637–53. doi: 10.1084/jem.20082109. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beauvillain C, Meloni F, Sirard JC, Blanchard S, Jarry U, Scotet M, Magistrelli G, Delneste Y, Barnaba V, Jeannin P. The scavenger receptors SRA-1 and SREC-I cooperate with TLR2 in the recognition of the hepatitis C virus non-structural protein 3 by dendritic cells. J Hepatol. 201052:644–51. doi: 10.1016/j.jhep.2009.11.031. doi: 10.1016/j.jhep.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 41.Murshid A, Gong J, Calderwood SK. Hsp90-peptide complexes stimulate antigen presentation through the class II pathway after binding scavenger receptor SREC-I. Immunobiology. 2014 doi: 10.1016/j.imbio.2014.08.001. pii: S0171-2985(14)00135-1. doi: 10.1016/j.imbio.2014.08.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sano M, Korekane H, Ohtsubo K, Yamaguchi Y, Kato M, Shibukawa Y, et al. N-glycans of SREC-I (scavenger receptor expressed by endothelial cells): essential role for ligand binding, trafficking and stability. Glycobiology. 201222(5):714–24. doi: 10.1093/glycob/cws010. doi: 10.1093/glycob/cws010. Epub 2012 Jan 25. [DOI] [PubMed] [Google Scholar]