Abstract
The platelet thrombus is the major pathologic entity in acute coronary syndromes, and antiplatelet agents are a mainstay of therapy. However, individual patient responsiveness to current antiplatelet drugs is variable, and all drugs carry a risk of bleeding. An understanding of the complex role of Prostaglandin E2 (PGE2) in regulating thrombosis offers opportunities for the development of novel individualized antiplatelet treatment. However, deciphering the platelet regulatory function of PGE2 has long been confounded by non-standardized experimental conditions, extrapolation of murine data to humans, and phenotypic differences in PGE2 response. This review synthesizes past and current knowledge about PGE2 effects on platelet biology, presents a rationale for standardization of experimental protocols, and provides insight into a molecular mechanism by which PGE2-activated pathways could be targeted for new personalized antiplatelet therapy to inhibit pathologic thrombosis without affecting hemostasis.
Keywords: Antiplatelet agent, platelet reactivity regulation, platelet aggregation, platelet inhibitor, prostaglandin E2, EP3 antagonist
Introduction
Platelets perform a critical function by maintaining hemostasis and initiating thrombus formation. However, platelet hyperreactivity has been implicated in the pathophysiology of cardiovascular diseases, including acute coronary syndromes, myocardial infarction, and ischemic stroke. Antiplatelet therapy, such as with aspirin and P2Y12 antagonists, is a cornerstone in the management of cardiovascular disease. Despite use of these agents, platelet hyperreactivity persists in a substantial fraction of patients, contributing to on-treatment major adverse cardiovascular events including cardiovascular mortality. The benefit of antiplatelet drugs is counterbalanced by the risk of major bleeding, because they inhibit both arterial thrombosis and hemostasis. There is considerable interest in developing novel antiplatelet agents that decrease platelet reactivity, but do not affect hemostasis.
There are many naturally occurring molecules that inhibit platelet function, including prostaglandin I2 (prostacyclin, PGI2), prostaglandin D2 (PGD2), nitric oxide, and adenosine. Prostacyclin has been the most widely studied of these agents. It is produced by vascular endothelium and acts as a potent vasodilator that inhibits platelet aggregation. PGD2 is produced by mast cells and activated platelets [1], is more stable than prostacyclin in blood plasma, is a vasodilator and inhibits platelet aggregation [2]. Prostaglandin E2 (PGE2) also modulates vascular tone and platelet reactivity. It is produced by the vascular endothelium, activated macrophages within atherosclerotic plaques, and platelets. Despite decades of research, the role of PGE2 on platelet reactivity remains not well understood. Findings have long been confounded by differing effects based on experimental conditions, differences in murine and human biology, the use of synthetic PGE2 receptor agonists, and unappreciated phenotypic differences in PGE2 response. This review synthesizes past investigations and current research into the important role of PGE2 in regulating thrombosis and provides insight into a potential new therapeutic approach that could inhibit pathologic thrombosis without affecting hemostasis.
The role of prostanoids in platelet function
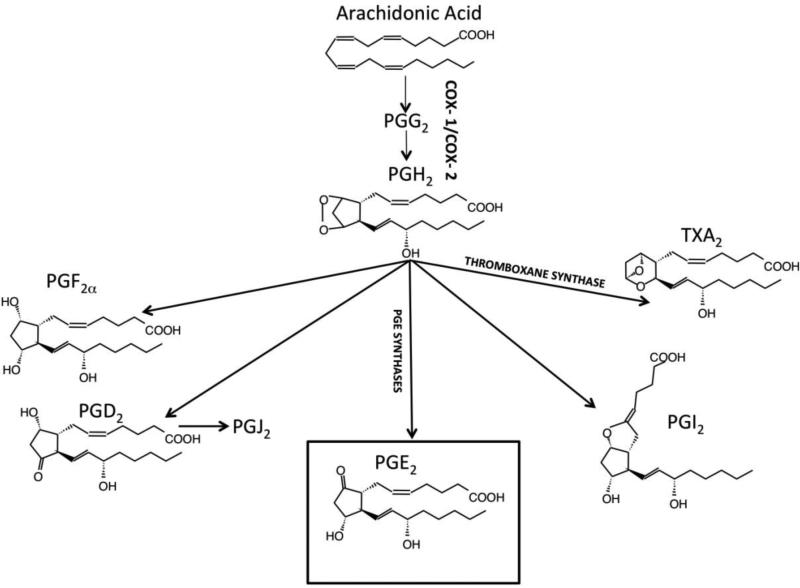
The role of PGE2 in regulating platelet function is best understood in the context of the larger family of related prostanoids that are produced in the vasculature from common polyunsaturated fatty acid precursors. Prostanoids are bioactive receptor ligands that trigger a variety of G-protein coupled receptor signaling pathways characteristic of individual cell types and activation states. Inflammation, endothelial function, and platelet reactivity interact in contributing to cardiovascular disease and form the mechanistic basis for targeting treatments and preventive strategies. In physiologic and pathophysiologic states, cellular activation releases arachidonic acid via phospholipase cleavage of membrane phospholipids (Figure 1). Arachidonic acid is the key polyunsaturated fatty acid metabolized to the precursor prostaglandin (PG) endoperoxides, PGG2 and PGH2, by cyclooxygenases COX-1 in platelets or COX-2 in the vasculature. PGH2 is further converted to bisenoic prostanoids via specific synthases and isomerases. The most relevant prostanoids for platelet function are thromboxane A2 (TxA2), PGD2, PGE2, and prostacyclin (PGI2). Originating from arachidonic acid and PG endoperoxides, prostanoids exhibit subtle differences in chemical structure that confer striking differences in biological responses attributable to characteristic specificity and activity profiles as ligands for the diverse family of prostanoid receptors. Prostanoids also exhibit a broad range of chemical stability and differences in susceptibility to and consequences of metabolism. The varieties and amounts of prostanoids produced vary depending on the specific cell type and activation status. Cell types that generate prostanoids in the vascular system include endothelial cells, vascular smooth muscle cells, platelets, leukocytes, mast cells, and macrophages. Prostanoids play key roles in vascular tone, inflammation, and thrombosis-hemostasis.
Fig. 1. Prostanoid Metabolism.
Arachidonic acid is released from cellular membrane phospholipids by phospholipase A2. Shown here is the metabolism by the cyclic pathway to prostaglandins and thromboxane. Leukotrienes are also derived from arachidonic acid by a separate linear pathway (not shown for clarity).
Thromboxane A2
TxA2 is the predominant prostanoid generated in activated platelets and has been studied extensively. Though chemically among the most unstable prostanoids, having a half-life of less than one minute, it acts locally via the TPα receptor as an autocoid to potently activate platelets, stimulate platelet shape change, release platelet granule contents, and produce irreversible platelet aggregation. Besides its effects on platelets, TxA2 mediates a number of physiologic and pathophysiologic responses, including vasoconstriction, inflammation, and angiogenesis [3]. TxA2 is among the most potent known vasoconstrictors of both venous and arterial smooth muscle. Evidence suggests a significant, but not fully characterized function in mediating inflammatory responses. In septic shock models, TxA2 may be protective by reducing systemic vasodilation [4], but at the same time has been shown to contribute to renal dysfunction [5]. TxA2 also has a role in angiogenesis and endothelial cell differentiation and proliferation. A TxA2 mimetic that acts on the TPβ receptor was shown to inhibit VEGF-mediated endothelial cell migration and angiogenesis [6]. However, the TxA2 agonist U46,619 stimulated IL-1beta-induced angiogenesis. Whereas the preferential agonist of the TP receptor is TxA2, the metabolic precursors of TxA2, PGG2 and PGH2, are also TP agonists and thus may produce similar responses.
Excess thromboxane, measured as inactive metabolites including TxB2, has been shown to contribute to thrombosis and myocardial ischemic events [7]. Deficiencies in thromboxane signaling have been associated with bleeding disorders [8, 9]. Irreversible inhibition of COX-mediated PG endoperoxide and TxA2 production, primarily in platelets, is the key action of aspirin, leading to its benefit in reducing the risk of acute coronary syndromes and stroke. TxA2 synthase inhibitors were developed as potential alternatives to aspirin that may have more favorable physiological properties. It was found that such drugs not only decrease TxA2 formation, but they also favor PGI2, PGE2, PGF2α, and PGD2 production [10-13]. A limitation of the clinical use of thromboxane synthase inhibitors is that while TxA2 production is reduced and platelet inhibitory PGI2 and PGD2 are increased, there is an opposing increase in proaggregatory cyclic endoperoxides [11, 12]. In particular, the parent PGH2, which binds the TP receptor, accumulates in this setting. Therefore, it is understandable why the net anti-platelet effect of the TxA2 synthase antagonist dazoxiben was minimal, unless combined with a TP antagonist [12]. Several TxA2 synthase inhibitors and TxA2 / PG endoperoxide (TP) receptor antagonists have been advanced to clinical development, but not one has been adopted into clinical use in the United States [14-16]. The TP receptor antagonist, terutroban, was compared to, and found to be no different from, aspirin in a large clinical trial (PERFORM) for prevention of stroke in patients with prior stroke or transient ischemic attacks [17].
Prostacyclin
Prostacyclin is the primary prostaglandin product of endothelial cells. Though chemically more stable than TxA2, prostacyclin non-enzymatically converts to an inactive product, 6-keto-PGF1α. Prostacyclin enhances microvascular blood flow by potent vasodilation. Through IP receptor mediated activation of platelet adenylate cyclase, prostacyclin impairs platelet adhesion and physiologically antagonizes platelet aggregation in response to all agonists as well as to vascular injury [18]. The counterbalancing actions of stimulatory thromboxane and inhibitory prostacyclin are thought, in part, to regulate systemic platelet activation, limit bleeding, and prevent thrombus overgrowth. In general, the potent hemodynamic effects of IP receptor agonists limit their clinical utility as antiplatelet agents. The stable prostacyclin mimetics epoprostenol, treprostinil, and iloprost, are used as vasodilators in patients with pulmonary arterial hypertension [19], and iloprost has been explored as a potential therapy in scleroderma and Raynaud's phenomenon [20]. Iloprost has also been studied as a potential benefit in critical limb ischemia [21-25], likely due to both antiplatelet and vasodilatory effects.
Prostaglandin D2
PGD2 is the primary prostaglandin produced by mast cells. PGD2 has predominantly been studied in association with inflammation, particularly its role in asthma and other allergic disorders [26, 27]. More recently, PGD2 has been linked to androgenic male pattern baldness. Prostaglandin D2 inhibits hair growth and is elevated in the bald scalp of men with androgenetic alopecia [28]. PGD2 signals in leukocytes and hair follicles through the DP2 receptor (CRTH2) to induce Th2, eosinophil, and basophil chemotaxis and inhibition of hair growth via G-alpha(i) [28, 29]. An important mechanism for niacin-induced flushing is vasodilation following release of PGD2. This prompted development of laropiprant, a DP1 receptor antagonist, for use in combination with niacin to optimize niacin-induced cholesterol reduction and prevent niacin-induced facial flushing. However, this combination was abandoned because it caused more harm than good in a large clinical trial (HPS2-THRIVE) [30]. PGD2 may also play roles in the central nervous system, including effects on sleep, body temperature regulation, and nociception.
PGD2 interacts with DP1 receptors on both platelets and vascular smooth muscle cells that couple to G-alpha(s) to stimulate adenylate cyclase, which inhibits platelet aggregation and causes vasodilation [31]. However, the clinical importance of PGD2 as an inhibitor of platelet function is not currently known, though laropiprant did not produce an overt prothrombotic effect.
Prostaglandin E2
PGE2 is produced in several cell types and contributes to homeostasis in the GI tract, kidney, immune system, and vasculature. PGE2 is produced from PGH2 by PGE synthase or spontaneous rearrangement of PGH2 in aqueous solution [32]. PGE2 is the major prostaglandin secreted by cultured vascular smooth muscle cells and macrophages. PGE2 has been found to be the predominant prostanoid from endothelial cells in human microvessels [33] and rabbit coronary microvessels [34], and it likely plays a significant role in angiogenesis [35] and vascular tone [36, 37]. PGE2 is also present in atherosclerotic plaque [38, 39]. Resident macrophages in atherosclerotic plaque constitutively express COX-1 and make small amounts of PGE2 at baseline. Induction of COX-2 with lipopolysaccharide was shown to preferentially increase production of PGE2. High nanomolar concentrations of PGE2 produced by activated macrophages are implicated in cardiovascular pathologies such as plaque rupture and abdominal aortic aneurysm formation [40]. COX-2 and PGE synthase co-localize in symptomatic carotid plaques, and their expression correlates with the extent of inflammatory infiltration and matrix metalloproteinase activity [41]. PGE2 derived from atherosclerotic plaque can exit the plaque and act directly on platelets [39]. Activated platelets themselves produce a large amount of PGE2 [42]. The significant presence of PGE2 in platelets, microvasculature, atherosclerotic plaque, and activated macrophages makes it natural to extrapolate that PGE2 contributes importantly to vascular physiology and the pathophysiology of thrombosis. The remainder of the review will focus on the evolution to the current understanding of the role of PGE2 in regulating platelet function.
Cyclic AMP role in platelet activation
Platelet homeostasis has long been ascribed to a balance of mechanisms that either raise or decrease cyclic adenosine 3'5'-monophosphate (cAMP) [43]. Prior to the discovery of the various prostaglandin receptors, Ashby et al. hypothesized that prostaglandins exert their effects via either stimulation or inhibition of cAMP production [44, 45]. While it is now recognized that cAMP is but one of many factors in the platelet regulatory pathway, a general appreciation of the role of cAMP is essential to understanding how prostaglandins, including PGE2, modulate platelet activity. cAMP was shown to mediate the stronger antiplatelet effect of TxA2 synthase inhibitors observed in pathologies associated with in vivo platelet activation [46]. An increase in cAMP is associated with platelet inhibition, and a decrease in cAMP promotes platelet aggregation induced by calcium mobilization[43] [47]. cAMP levels can be regulated by agents that either enhance production via adenylate cyclase or decrease its metabolism via cAMP phosphodiesterases. In general, Gs-coupled receptors stimulate adenylate cyclase, raising cAMP and inhibiting platelet function. For example, PGI2 inhibits platelet aggregation by increasing intracellular cAMP via activation of its Gs-coupled IP receptor. In contrast, Gi-coupled receptors, such as the ADP receptor, P2Y12, inhibit adenylate cyclase, decrease cAMP, and facilitate platelet aggregation. Importantly, Gi receptor stimulation alone does not directly induce platelet aggregation, but acts synergistically with other receptors that induce mobilization of intracellular calcium, such as the Gq-coupled thromboxane receptor, TP [48, 49]
PGE2 acts via multiple receptors
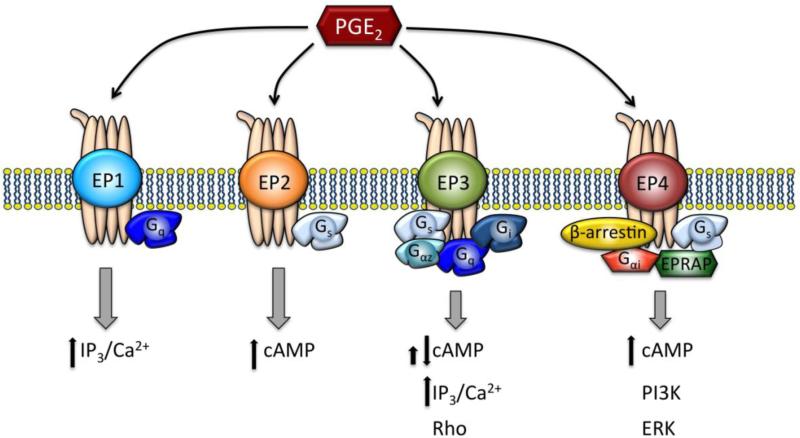
Initially it was thought that PGE2 may act via the PGI2 / IP receptor; however, it was later determined that PGE2 preferably activates its own specific receptors [50]. PGE2 has four receptor subtypes that were identified and subsequently cloned [51-53], termed EP1, EP2, EP3 and EP4. The EP receptors are present in numerous tissues, and the distribution of EP receptor subtypes varies among different tissues [54]. Eggerman et al. first showed that PGE2 has a receptor on human platelets that is distinct from prostacyclin's receptor [50]. Using RT-PCR and Southern blot, Paul et al. showed that the EP3 and EP4 receptors are much more prominent in human platelets than the EP2 receptor [55]. The EP1 receptor is sparse in most tissues compared to the other EP receptor subtypes [54], and it has not been detected in human platelets thus far. These G protein coupled receptors differ in structure and signal transduction coupling (Figure 2). In order to understand the role of PGE2 in regulating platelet activity, it is necessary to delve into the specific function of the different EP receptor subtypes.
Fig. 2. EP receptors and signaling pathways.
PGE2 binds to four receptor subtypes: EP1, EP2, EP3, and EP4. Each receptor has distinct signaling pathways depending on the cell type in which it is expressed. All major mediators and second messengers are shown for each receptor subtype. The details of these pathways continue to be refined.
Elucidating EP receptor function with specific agonists/antagonists
Much research on the role of PGE2 in platelet function has focused on the use of EP receptor subtype-selective agonists and antagonists. Frequently used selective EP receptor agonists include butaprost (EP2) [56], sulprostone (EP3) [57], and PGE1-OH (EP4) [57]. Iloprost is an EP1 agonist, but is poorly selective [57]. Newer synthetic EP receptor agonists include ONO-DI-004 (EP1) [58], ONO-AE1-259 (EP2) [58, 59], ONO-AE-248 (EP3) [60], and ONO-AE1-329 (EP4) [60]. Novel EP receptor antagonists include ONO-8713 (EP1) [58], ONO-AE3-240 (EP3) [61], DG-041 (EP3) [62], ONO-AE208 (EP4) [63], and MF-191 (EP4) [56].
EP1 Receptor Biochemistry and Function
The EP1 receptor acts predominantly via Gq, activating phospholipase C, protein kinase C, and releasing intracellular calcium [54]. However, it does not seem that EP1 is expressed in human platelets, as neither the selective EP1 agonist ONO-DI-004 nor the EP1 antagonist ONO-8713 have any effect on platelet aggregation induced by platelet activating factor (PAF) [58].
EP2 Receptor Biochemistry and Function
The EP2 receptor couples to Gs, leading to increased production of cAMP. Thus, EP2 stimulation leads to inhibition of platelet aggregation. The selective EP2 agonist ONO-AE1-259 inhibits platelet aggregation [58, 59]. This inhibitory effect is also seen with the EP2 agonist butaprost [56].
EP3 Receptor Biochemistry and Function
The EP3 receptor is more complex than the other EP receptors, such that six isoforms have been identified [53, 64-66]. These isoforms result from alternative mRNA splicing, which alters the cytoplasmic domain and signal transduction pathways. Although EP3 was originally described to couple to Gi leading to reduction of intracellular cAMP [67], subsequent evidence suggests that different isoforms can variably increase cAMP via Gs or IP3 via Gq [68, 69]. Recent evidence shows that EP3 also can couple to Gαz [70, 71].
Stimulation of EP3 via the EP3-specific agonist sulprostone does not directly lead to platelet aggregation, but potentiates aggregation in response to agonists such as ADP and U46,619 in human [38, 56, 62, 72] and murine [73] platelet rich plasma. Additionally, PGE2-mediated stimulation of EP3 decreased cAMP, consistent with its primary signaling in platelets being coupled to Gi [73]. The selective EP3 antagonist DG-041 abolishes the pro-aggregatory effects of both sulprostone [58] and low concentrations of PGE2 [56, 63, 74].
EP4 Receptor Biochemistry and Function
Similar to the EP2 receptor, the EP4 receptor was initially characterized as a Gs-coupled receptor, which stimulates adenylate cyclase and cAMP production [75]. These cAMP-dependent pathways can involve Protein Kinase A (PKA), Epac (i.e. exchange protein activated by cAMP), and AMP Kinase (AMPK) [75-77]. Subsequent studies by Fujino and Regan have shown that stimulation of EP4 evokes coupling to a pertussis toxin-sensitive Gαi/0 leading to PI3-kinase-dependent activities [78-80]. Takayama and Libby described a novel EP4 receptor-associated protein (EPRAP) that participates in anti-inflammatory signaling in humans [81].
The EP4 agonist AE1-329 inhibited platelet aggregation induced by platelet activating factor (PAF), ADP, and collagen [58, 59, 82]. AE1-329 also prevented platelets from binding fibrinogen through the GPIIb/IIIa receptor [82]. Similarly, we have shown that inhibition of EP4 with a selective antagonist MF-191 completely abrogated the effect of PGE2 on inhibiting U46,619-induced platelet aggregation [56]. While this inhibitory function is in keeping with its known coupling to Gs, other EP4-mediated signaling pathways could contribute to PGE2 signaling in platelets. For example, when activated by PGE2, EP4 can also recruit arrestins [83], leading to c-Src activation, a signaling pathway independent of G protein activation [84]. Interestingly, Arrestin 2 was also shown to regulate the PAR4-dependent signaling pathway in human platelets [85]. Thus, the complexity of the EP4 signaling pathways suggests that EP4 might function through both cAMP-dependent and -independent pathways.
Deciphering the physiologic role of PGE2 in platelets
Early Studies – Conflicting results and biphasic concentration-dependent response
Early studies into the role of PGE2 in regulating platelet function in humans have produced conflicting results. A consistent theme among studies is the finding that PGE2 alone, without an additional agonist, does not lead to platelet aggregation. Thus, determining the effects of PGE2 on platelet aggregation must be done with PGE2 in combination with other agonists. An early study by Kloeze and colleagues showed that PGE2 at concentrations of 5-10 μmol/L inhibited platelet aggregation induced by ADP [86]. Shio et al. further characterized two phases of aggregation, an initial phase, followed by a plateau, and then a secondary phase. They found that PGE2 (50-200 μg/L = 0.14-0.57 μmol/L) decreased the primary phase of ADP-induced aggregation in all individuals, while it enhanced the secondary phase of aggregation in some individuals [87]. Though the same primary phase inhibition with second phase potentiation was also reported by McDonald et al. [88], enhancement of the primary phase of aggregation was described by Andersen et al. [89].
Whereas early investigations into the effects of low concentrations of PGE2 on platelet aggregation were conflicting, studies using higher doses produced more consistent results. Heptinstall and Gray described a bimodal effect of human platelets to PGE2, whereby it potentiated platelet aggregation at lower concentrations (0.1-10 μmol/L) and inhibited aggregation at a much higher concentration (100 μmol/L) [90, 91]. Such a biphasic effect of PGE2 was demonstrated in multiple other studies [87, 92-94]. This biphasic response was hypothesized to be due to inhibition of cAMP production at low concentrations and stimulation of cAMP production at higher concentrations. Indeed, Robison et al. showed that cAMP levels were raised in human platelet rich plasma when PGE2 was delivered at a high concentration known to inhibit ADP-induced platelet aggregation [95]. The studies of Ashby demonstrated that low dose PGE2-mediated platelet aggregation was associated with decreased levels of cAMP [96]. Thus, well before the EP receptors were discovered, it was postulated that PGE2 may act through multiple receptors that have opposing effects. It was thought that low concentrations of PGE2 acted via a Gi-coupled receptor, and higher concentrations bound to a Gs-coupled receptor, such as the PGI2 receptor [90].
High doses of PGE2 bind the PGD2 Receptor
Both the PGI2 receptor (IP) and PGD2 receptor (DP1) mediate platelet inhibition, and it was hypothesized that high concentrations of PGE2 could interact with one of these receptors. PGE2 has a relatively low affinity for IP in humans, and selective inhibition of IP does not prevent the inhibitory effects of PGE2 [58]. PGE2 does act, however, on the DP1 receptor [2]. The affinity of PGE2 for DP1 has been shown to be at a Ki of 307 +/− 106 nmol/L [97]. This Ki predicts that PGE2 concentrations above 500 nmol/L will activate DP1.
Studies in mice – important data, but limited translation to humans
In part because of the high variability in the response of human platelets to PGE2, particularly at low doses, investigators extensively studied the effect of PGE2 on platelets using mice. In contrast to humans, low concentrations of PGE2 consistently have a pro-aggregatory effect in mice. Concentrations of PGE2 up to 100 μmol/L potentiate platelet aggregation induced by sub-efficacious concentrations of a thromboxane receptor agonist, U46,619, and by collagen (18-21).
Within many tissue types, including platelets, the presence of the EP receptors differs in mice compared to humans. In murine platelets, EP1 is essentially absent, and the expression levels of EP3 are over 200-fold higher than EP4 and over 1000-fold higher than EP2 [98]. Therefore, the relative contribution of EP2 and EP4 pathways to PGE2 effects on platelets is negligible in mice. For example, the dose of the EP2 specific agonist butaprost required for platelet inhibition is much higher in mice than humans [IC50 87 ± 3 μmol/L vs. 2.57 ± 0.82 μmol/L, respectively) [58] [59]. Likewise, the EP4 agonist AE1-329 had a much less robust inhibitory effect in mouse platelets compared to human platelets [58, 59, 82]. EP3 activation almost entirely drives the effect of PGE2 in mice, explaining why PGE2 has no detectable effect on platelets in EP3 −/− knockout mice [73].
Similar to humans, very high doses of PGE2 (greater than 1 μmol/L) in mice inhibit platelets [73]. However, in mice, this inhibitory effect is not mediated by PGE2 binding of DP1, as this receptor is not present in murine platelets [99]. Rather, high doses of PGE2 bind the murine prostacyclin receptor IP [73]. Thus, for numerous reasons the findings in mice cannot be extrapolated directly to humans.
As PGE2 mediates in vitro platelet aggregation in mice via EP3, investigators explored how modulating EP3 may contribute to in vivo thrombosis. Mice deficient in EP3 had decreased susceptibility to atherothrombosis triggered by intravenous arachidonic acid infusion [98] or mechanical plaque rupture [39]. Tilly et al showed that blocking EP3 in mice with the EP3 antagonist DG-041 inhibited atherothrombosis in vivo caused by ferric chloride or arachidonic acid exposure followed by mechanical plaque disruption [100]. Whereas mice that completely lack functional EP3 demonstrate increased in vivo bleeding times [98], mice treated with DG-041 have preserved hemostasis [100, 101].
Biased agonism – PGE2 and EP specific agonists may have different effects
Early studies in humans were done without the understanding that human platelets express three different PGE2 receptors. The discovery of the EP receptor subtypes launched a number of investigations into how the diverse effects of each receptor mediate the overall response to PGE2. Most of these studies were performed using EP receptor specific agonists, and the results were extrapolated to determine the effects of PGE2 itself. However, our data suggest that activation of EP2 in human platelets by its selective agonist butaprost or by PGE2 itself may have different effects: although butaprost inhibits platelet aggregation induced by U46,619, PGE2 does not affect platelet aggregation when both EP3 and EP4 are blocked with their respective antagonists (DG-041 and MF-191) [56]. These results are in keeping with the growing evidence suggesting that synthetic ligands of a target GPCR may activate distinct pathways, leading to coupling of the receptor to different intracellular effectors [102, 103] in a process termed “biased agonism” [104, 105]. Similar to EP2, differences based on “biased agonism” have recently been illustrated with subtype selective ligands of EP4 [106].
Current understanding - PGE2 response is determined by a relative effect of EP receptor activation
In 2010, we and others reported that the net effect of PGE2 in human platelets reflects a balance between the potentiating effects mediated by EP3 and the inhibitory effects mediated by EP4 [56, 58, 59, 107]. These data suggest that PGE2 may contribute to platelet homeostasis by modulating the overall response to other activators and inhibitors. The following section will discuss how inter-individual phenotypic differences in the effects of low dose PGE2 initially hindered the field, but eventually led to an improved understanding of the role of PGE2.
Phenotypic differences in PGE2 effect on human platelets
Despite the conclusion by many investigators that low dose PGE2 promotes aggregation, a number of studies have noted variable responses among human subjects [72, 87, 91, 108, 109]. In the study by Shio et al., certain subjects seemed to lack the second phase of potentiation, such that the overall response to PGE2 was only inhibition [87]. Gresele et al. showed that PGE2 promoted platelet aggregation to arachidonic acid in patients deemed “nonresponders” to thromboxane synthase inhibitors, but inhibited aggregation in those termed “responders” [109]. At that time, there was significant interest in the development of thromboxane synthase inhibitors, and several other investigators described the phenomenon of responders and non-responders to these drugs [110-113]. Matthews et al. showed that PGE2 (0.01-5 μmol/L) inhibited aggregation in response to ADP in 2 out of 8 blood donors and potentiated aggregation in the other 6 donors [72]. This inter-individual variability in humans remained unexplained, but was suggested to be due to the differential “response of their adenylate cyclase to PGE2” [109] or variation in the ratio of PGD2 to TxA2 + PGE2 produced [112].
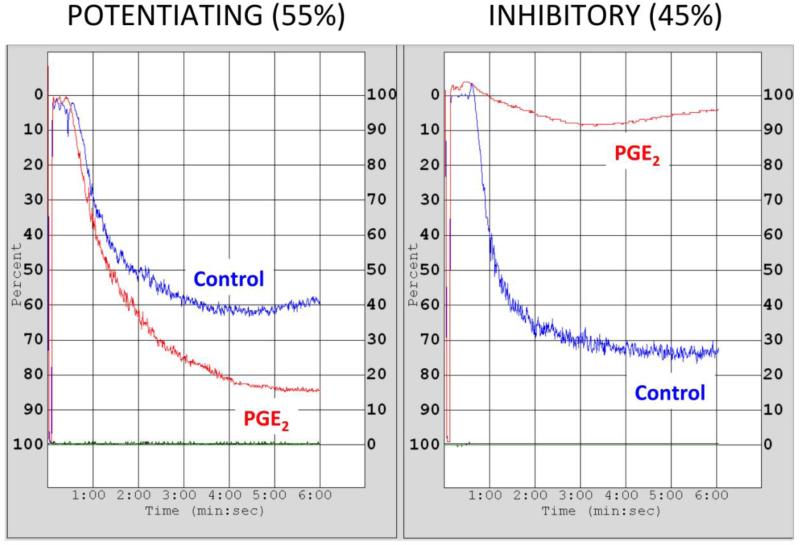
Our group first described in 2010 that the effects of PGE2 at low concentration (100 nmol/L) on human platelets segregates into different phenotypic groups [56]. Since then, we have refined our characterization of these groups and clarified the terminology. The phenotypic groups are: inhibitory (PGE2 inhibits platelet aggregation), and potentiating (PGE2 permits or potentiates aggregation) (Figure 3). Testing 104 healthy volunteers revealed that 45% have the inhibitory phenotype and 55% have the potentiating phenotype. This variable PGE2 effect was lost when platelets were activated with higher concentrations of agonist that elicit full activation [56]. Importantly, the volunteers have been tested on multiple occasions over several years without variation in the phenotypic group assignment, demonstrating reproducibility and suggesting independence from environmental factors. We have also analyzed patients with coronary artery disease who are receiving dual antiplatelet therapy (aspirin and P2Y12 inhibitors) and have shown that this treatment does not affect phenotypic determination.
Fig. 3. Representative traces of the two phenotypic groups analyzed by light transmission aggregometry.
Healthy volunteers or patients undergoing PCI were consented following a protocol approved by the Vanderbilt Institutional Review Board. Platelet rich plasma (PRP) was obtained from citrated blood, and platelet count was adjusted to 250,000 cells per μl with autologous platelet poor plasma (PPP). PRP was preincubated with PGE2 100 nmol/L (PGE2) or vehicle (control) for 30 s, followed by a sub-maximal concentration of U46,619. Aggregation was recorded for 6 min as described by Smith et al [56]. The frequency of each phenotypic group is indicated (n=104).
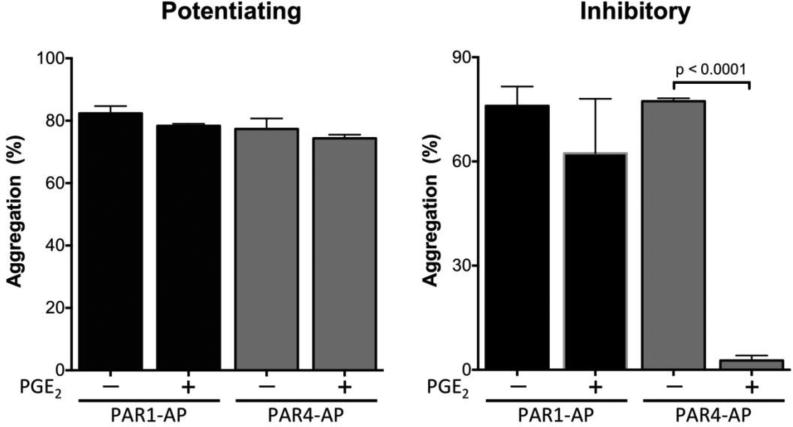
PGE2 has the same inhibitory or potentiating effect on platelet aggregation whether in the presence of agonist for the thromboxane receptor [56] or the thrombin receptor protease activated receptor type 4 (PAR4) (Figure 4). In contrast, PGE2 has no effect on platelets activated with a protease activated receptor type 1 (PAR1) specific agonist peptide (Figure 4). Of note, MacIntyre and Gordon showed that PGE2 had no effect on platelet aggregation induced by collagen in citrated platelet rich plasma [114], results that we reproduced (data not shown). These data outline the complexity of the regulation of platelet aggregation by PGE2 and reinforce the need to better understand the mechanism responsible for the two phenoptypes.
Fig. 4. Effect of PGE2 on platelet aggregation induced by agonist peptides specific for the two thrombin receptors expressed on human platelets.
Healthy volunteers were consented following a protocol approved by the Vanderbilt Institutional Review Board. PRP was obtained from citrated blood, and platelet count was adjusted to 250,000 cells per μl with autologous platelet poor plasma (PPP). 250 μl PRP was preincubated with vehicle (−) or with 100 nM PGE2 (+) for 30 s, followed by sub-maximal concentrations of agonist peptides specific for PAR1 (PAR1-AP) or PAR4 (PAR4-AP). Aggregation was recorded for 6 min as described previously [56]. Values represent mean ± S.E.M (n = 3). Statistical significance was evaluated by paired t-test.
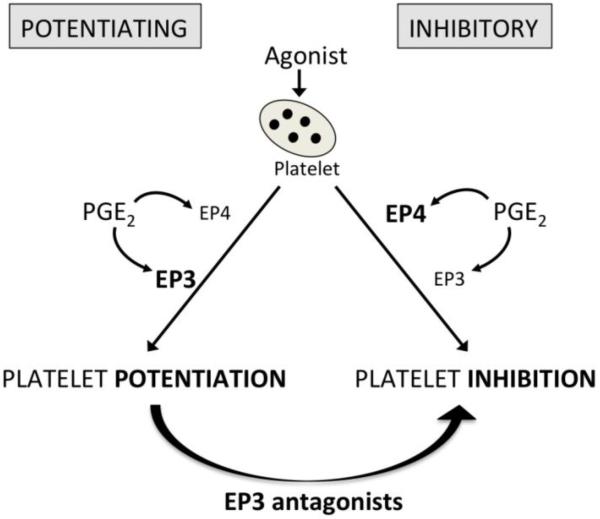
We observed that all individuals express EP2, EP3, and EP4, and that these receptors respond similarly to their specific agonists butaprost, sulprostone, and PGE1-OH, respectively [56]. However, the net result of activation of these three receptors by their endogenous agonist, PGE2, leads to the different phenotypes, suggesting that there are differences in specific signaling pathways. Importantly, our data showed that an EP3 antagonist, DG-041, converts subjects from the potentiating to the inhibitory phenotype [56] (Figure 5). Further work is necessary to determine the signaling mechanisms by which PGE2 affects platelet aggregation in each phenotypic group.
Fig. 5. Effect of PGE2 on platelet activation in humans.
The net effect of PGE2 reflects the differential activation of the two receptors EP3 and EP4. EP3 antagonists shift phenotype from potentiating to inhibitory.
Several reasons may explain why the two different PGE2 response phenotypes were not recognized and characterized earlier: 1) The presence of the different EP receptor subtypes in human platelets was not initially appreciated; 2) Much of the data was obtained using murine models, in which the PGE2 response results entirely from EP3 activation; 3) Many experiments have been conducted with higher concentrations of agonists that cause full platelet activation, a condition in which PGE2 has no effect; and 4) Most recent experiments with human platelets have been performed using EP-specific agonists, rather than PGE2, possibly leading to “biased agonist” effects.
Clinical Implications
Risk for cardiovascular events
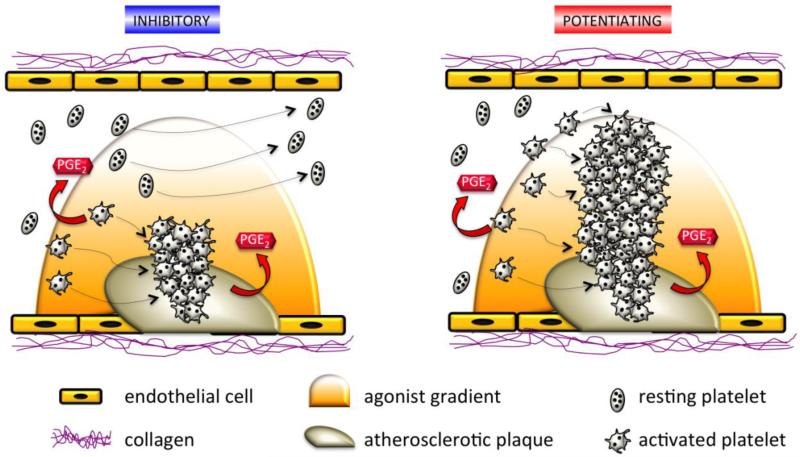
It is understood that vascular injury stimulates platelet adhesion in part via activation of platelet glycoproteins, followed by release of chemical mediators, such as thrombin, ADP, TxA2, and norepinephrine that recruit more platelets to the site of the wound. However, a gradient of agonist forms around the thrombus, and platelets circulating in the low concentration part of the gradient may not be recruited to the wound, but would be partially activated, making them more “sticky.” These “sticky” platelets express adhesion molecules at their surface and may contribute to pathologic thrombus formation [115]. PGE2 may be an underappreciated mediator, generated from the macrophages in atherosclerotic plaques and activated platelets, that plays a role in regulation of platelet reactivity. We have shown that low concentrations of PGE2, such as those generated locally by activated platelets, can exert either inhibitory or potentiating effects. In individuals with the inhibitory phenotype, the local PGE2 could slow the growth of the associated thrombus by increasing the threshold for activation of circulating platelets in the proximity of the atheroma (Figure 5). This inhibition would be mediated by the effects of PGE2 on EP4. In contrast, in individuals with the potentiating phenotype, platelet aggregation may be enhanced via the potentiating EP3 receptors without effective inhibition through EP4 receptors. Thus, we would postulate that in the setting of atherosclerotic plaque rupture, individuals with the potentiating phenotype might be at higher risk of thrombotic cardiovascular events including myocardial infarction or stroke. We further would postulate that phenotyping individuals for their platelet responses to PGE2 could serve as a risk prediction tool that might also guide treatment selectively for those at higher risk.
Potential for novel therapeutic options
Increasing platelets threshold to agonist stimulation could constitute an appealing new therapeutic approach in the management of cardiovascular thrombotic events [115], as it might inhibit thrombosis without affecting hemostasis. Proposed targets for intervention include the EP3 and EP4 receptors via EP3 antagonists and EP4 agonists.
Based on encouraging preclinical studies in mice [100, 101], the in vivo effects of EP3 antagonism have been investigated in human subjects. Tilly et al. treated 10 healthy volunteers with DG-041 for 7 days and found that the drug reduced platelet aggregation to collagen, while not affecting cutaneous bleeding time [100]. In a separate study by Fox et al., healthy volunteers were given DG-041 alone or in various combinations with clopidogrel and aspirin [116]. The study showed that DG-041 neither increased bleeding time alone nor further increased bleeding time to clopidogrel. At the same time, the effect of DG-041 further decreased platelet aggregation. In 2006, deCODE Genetics (Reykjavik, Iceland) began a Phase IIa randomized, placebo-controlled clinical trial of DG-041 in 144 patients with peripheral arterial disease, examining the safety and tolerability of the drug and its effect on various markers of disease severity. While the results have not been published, the company internally reported no serious adverse events and favorable effects on inflammatory biomarkers, such as c-reactive protein and monocyte chemotactic protein 1, as well as ankle-brachial index measurements [117].
While these studies are very promising, Schober et al. cautioned that the concentration of PGE2 in human atherosclerotic plaques is low, which may render the effects of PGE2 in vessels minimal. In their study PGE2 from plaque homogenates had no effect on platelet aggregation [38]. Furthermore, the EP3 antagonists AE5-599 and AE3-240 did not prevent platelet aggregation, dense granule release, or GP IIb/IIIa exposure induced by plaque homogenates. These results are important as they indicate that more studies are needed to understand fully the effect of PGE2 on atherothrombosis in humans. However, our data predict that plaque homogenates should not be used as platelet agonist: 1) plaque homogenate contains collagen and thrombin and other agonists that are not regulated by PGE2. Moreover, the concentration of these agonists in the assay must have elicited full platelet activation, a condition in which PGE2 has no effect. 2) The platelet donor must be phenotyped for PGE2 response prior to doing the experiment to make sure that the data can be interpreted correctly: a donor with the potentiating phenotype would fail to show platelet inhibition. 3) PGE2 half-life in biological milieu such as blood or plasma is in the minute range, and the cellular machinery generating it in plaques is disturbed during homogenization, suggesting that there might have been no native PGE2 left in the homogenate. In studying the complex downstream effects of PGE2, it is essential to have a precise and sophisticated experimental design, which takes into account the concentration-sensitive effects of the molecule and the interplay with the variable characteristics of the donor platelets.
In addition to the clinical use of an EP3 receptor antagonist, a selective EP4 receptor agonist has been proposed to be of clinical utility. Apart from its effects on platelet aggregation, PGE2 via EP4 may have beneficial effects on reducing inflammation and myocardial injury in the setting of ischemia [118, 119]. In vitro antithrombotic effects of the EP4 agonist AE1-329 have been seen using platelet aggregation assays [58, 59, 82] and flow chamber experiments that studied thrombus formation in human blood passed over a collagen column [82]. Indeed, platelet inhibition to AE1-329 was similar to the effects of aspirin and was synergistic in combination [82]. Whether the EP4 agonist increases bleeding time is not known, and in vivo studies in mice cannot be relied upon to predict effects in humans for the reasons described above. Additionally, as the EP4 receptor plays a regulatory role in numerous tissues, the non-platelet effects of systemic administration would need to be closely monitored. For example, in an animal model of type 1 diabetes, an EP4 agonist exerted negative pro-inflammatory effects on the kidney leading to fibrosis and albuminuria [120]. EP4 agonists also may promote tumor progression and metastasis in various malignancies [121-123]. Nevertheless, an EP4 agonist was explored as a potential therapy for ulcerative colitis in a phase II clinical trial, and the safety profile was favorable [124].
Platelets and Tumor Growth
It is becoming increasingly recognized that there is a significant interaction between tumor cells and platelets. Platelet activation in cancer patients increases the risk of thrombosis, a major complication of cancer [125]. More recent data has shown that platelets may contribute to metastasis by inducing epithelial-mesenchymal transition, vascular remodeling, endothelial adhdesion, and even immune evasion [126-128]. Platelets recently were shown possibly to function as a chemo-attractant to cancer cells [129].
Platelets can also induce COX-2 overexpression in cancer cells themselves, demonstrated in a colon cancer cell line [130]. Subsequently, in both platelets and tumor cells, PGE2 is thought to contribute to a variety of signaling pathways that may ultimately lead to tumor growth and metastasis. Inhibition of COX via aspirin or NSAIDs has already been explored as agents to inhibit this cascade [131]. Specific antagonists that act on the EP receptors have been proposed to be novel anti-tumor agents in colorectal and breast cancer [132-134].
Conclusions
The critical need to elucidate platelet biology has driven over 40 years of research into the function of PGE2. PGE2 can have either stimulatory or inhibitory roles in human platelets depending on experimental conditions, and individuals segregate into different phenotypic groups exhibiting either PGE2-mediated potentiation or inhibition of platelet aggregation. The discovery of the EP receptors launched a series of investigations into their effects in platelets. Using specific agonists, it was shown that the EP3 receptor promotes platelet aggregation and that the EP4 and EP2 receptors inhibit platelet aggregation. Investigators including our group and Heptinstall et al. have shown that the effect of PGE2 on human platelets is determined by the net activation of EP4 and EP3 [56, 58]. Since mice functionally only express EP3 in platelets, murine studies cannot be expected to predict outcomes in humans. In addition, biased agonism must be appreciated as a potential confounder when designing experiments with synthetic agonists to study the effect of PGE2. Taken together, these considerations demand that we standardize the experimental conditions when studying the effect of PGE2 on platelet function (Table 1).
Table 1.
Proposed Standardization of PGE2 Platelet Assays
| Key Points |
|---|
| • Studies must be performed on human platelets, because the relative expression of EP receptor subtypes in mice is different. |
| • PGE2 must be utilized rather than EP-specific agonists due to the potential for biased agonism; the contribution of each receptor sub-type can be studied by blocking the other sub-types with highly selective antagonists. |
| • The concentration of PGE2 must not exceed 100-200 nmol/L to ensure that it does not stimulate DP1 receptors. |
| • The concentration of agonist used to stimulate platelet aggregation must be sub-maximal and determined for each individual by constructing a dose-response relationship with and without PGE2. |
| • Recognition of the presence of two human phenotypic groups should be reflected in the data analysis and its interpretation. |
We have made the seminal observation that humans segregate into two distinct platelet response phenotypes: 1) inhibitory phenotype, whereby a low concentration of PGE2 increases the activation threshold for agonists; 2) potentiating phenotype, whereby a low concentration of PGE2 decreases the activation threshold for agonists. The presence of these phenotypes may explain much of the inter-individual variability in PGE2-mediated responses that has been described over the years, including the variable responses to TxA2 synthase inhibitors. When performing and analyzing human platelet function studies, especially those involving prostanoids, platelets with different phenotypes may be best studied with slightly different protocols and analyzed separately to avoid confounding results.
Finally, persistent on-treatment platelet hyper-reactivity remains a challenge in the management of cardiovascular diseases, as it is correlated with adverse clinical outcomes. There is great interest in developing novel antiplatelet agents that allow true personalized or tailored antiplatelet therapy. Thus far, studies using point-of-care platelet function testing have failed to show benefit to this approach. DG-041, an EP3 antagonist, showed promise in its ability to inhibit platelet function while not increasing bleeding. The use of an EP3 receptor antagonist in conjunction with knowledge of the individual's PGE2 response phenotype offers a simple and elegant approach to personalize anti-platelet therapy. An EP4 agonist also may inhibit platelet function, but the effects on bleeding and on normal function in other tissues are not known. Further research into the biological properties of the EP receptors and their signaling pathways that underlie the inhibitory and potentiating phenotypes may create additional options for safe and effective treatment of cardiovascular diseases.
Highlights.
Human platelet's response to PGE2 segregates in two phenotypic groups
PGE2 effects on mouse platelets cannot be translated to human platelets
Studies must use PGE2 rather than receptor-specific agonist because of biased agonism
Published data in platelets are inconsistent from lack of standardized protocols
PGE2 receptors could be new targets for antiplatelet drugs that preserve hemostasis
Fig. 6. Schematic representation of a blood vessel demonstrating the hypothetical effects of PGE2 on thrombus growth in inhibitory and potentiating phenotypes.
At the site of atherosclerotic plaque rupture, a gradient of agonist is released, and platelets are recruited to form an initial plug. PGE2 is produced by macrophages in the plaque as well as by activated platelets. The inhibitory phenotype is accompanied by a increased threshold for platelet aggregation, preventing platelet hyperreactivity. In contrast, the potentiating phenotype is associated with a decreased threshold for platelet aggregation, promoting formation of an occlusive thrombus.
Acknowledgement
The authors would like to thank Dr. E. V. Gurevich in the Department of Pharmacology at Vanderbilt for the anti-arrestin antibody and for his help in the analysis of arrestins in platelets. This study was funded from by grants from the NIH (HL081009, 5T32HL007411) and from the AHA (14GRNT20460090).
Abbreviations
- AMPK
AMP Kinase
- cAMP
cyclic adenosine 3'5'-monophosphate
- COX
Cyclooxygenase
- PAF
Platelet activating factor
- PG
Prostaglandin
- DP
Prostaglandin D2 receptor
- EP
Prostaglandin E2 receptor
- PKA
Protein Kinase A
- Tx
Thromboxane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- 1.Watanabe T, et al. Characterization of the biosynthetic pathway of prostaglandin D2 in human platelet-rich plasma. J Biol Chem. 1982;257(24):14847–53. [PubMed] [Google Scholar]
- 2.Whittle BJ, et al. Specificity between the anti-aggregatory actions of prostacyclin, prostaglandin E1 and D2 on platelets. Adv Exp Med Biol. 1985;192:109–25. doi: 10.1007/978-1-4615-9442-0_9. [DOI] [PubMed] [Google Scholar]
- 3.Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118(1):18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Yamada T, et al. Thromboxane A2 regulates vascular tone via its inhibitory effect on the expression of inducible nitric oxide synthase. Circulation. 2003;108(19):2381–6. doi: 10.1161/01.CIR.0000093194.21109.EC. [DOI] [PubMed] [Google Scholar]
- 5.Boffa JJ, et al. Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J Am Soc Nephrol. 2004;15(9):2358–65. doi: 10.1097/01.ASN.0000136300.72480.86. [DOI] [PubMed] [Google Scholar]
- 6.Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95(4):372–9. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- 7.Oates JA, et al. Clinical implications of prostaglandin and thromboxane A2 formation (1). N Engl J Med. 1988;319(11):689–98. doi: 10.1056/NEJM198809153191106. [DOI] [PubMed] [Google Scholar]
- 8.Hirata T, et al. Arg60 to Leu mutation of the human thromboxane A2 receptor in a dominantly inherited bleeding disorder. J Clin Invest. 1994;94(4):1662–7. doi: 10.1172/JCI117510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuse I, Higuchi W, Aizawa Y. Pathogenesis of a bleeding disorder characterized by platelet unresponsiveness to thromboxane A2. Semin Thromb Hemost. 2000;26(1):43–5. doi: 10.1055/s-2000-9802. [DOI] [PubMed] [Google Scholar]
- 10.Defreyn G, Deckmyn H, Vermylen J. A thromboxane synthetase inhibitor reorients endoperoxide metabolism in whole blood towards prostacyclin and prostaglandin E2. Thromb Res. 1982;26(6):389–400. doi: 10.1016/0049-3848(82)90311-5. [DOI] [PubMed] [Google Scholar]
- 11.Defreyn G, et al. Familial bleeding tendency with partial platelet thromboxane synthetase deficiency: reorientation of cyclic endoperoxide metabolism. Br J Haematol. 1981;49(1):29–41. doi: 10.1111/j.1365-2141.1981.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 12.Gresele P, et al. Role of proaggregatory and antiaggregatory prostaglandins in hemostasis. Studies with combined thromboxane synthase inhibition and thromboxane receptor antagonism. J Clin Invest. 1987;80(5):1435–45. doi: 10.1172/JCI113223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermylen J, et al. Thromboxane synthetase inhibition as antithrombotic strategy. Lancet. 1981;1(8229):1073–5. doi: 10.1016/s0140-6736(81)92241-8. [DOI] [PubMed] [Google Scholar]
- 14.Patrono C. Biosynthesis and pharmacological modulation of thromboxane in humans. Circulation. 1990;81(1 Suppl):I12–5. discussion I22-3. [PubMed] [Google Scholar]
- 15.Rosenfeld L, Grover GJ, Stier CT., Jr. Ifetroban sodium: an effective TxA2/PGH2 receptor antagonist. Cardiovasc Drug Rev. 2001;19(2):97–115. doi: 10.1111/j.1527-3466.2001.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 16.Kawano KI, et al. Thromboxane A(2) synthase inhibitor enhanced antithrombotic efficacy of GPIIb-IIIa receptor antagonist without increasing bleeding. Eur J Pharmacol. 2001;417(3):217–22. doi: 10.1016/s0014-2999(01)00904-9. [DOI] [PubMed] [Google Scholar]
- 17.Bousser MG, et al. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet. 2011;377(9782):2013–22. doi: 10.1016/S0140-6736(11)60600-4. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296(5567):539–41. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin VV, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 20.Garcia de la Pena Lefebvre P, et al. Efficacy of Raynaud's phenomenon and digital ulcer pharmacological treatment in systemic sclerosis patients: a systematic literature review. Rheumatol Int. 2015 doi: 10.1007/s00296-015-3241-1. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch AT, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 22.Two randomised and placebo-controlled studies of an oral prostacyclin analogue (Iloprost) in severe leg ischaemia. The Oral Iloprost in severe Leg Ischaemia Study Group. Eur J Vasc Endovasc Surg. 2000;20(4):358–62. doi: 10.1053/ejvs.2000.1175. [DOI] [PubMed] [Google Scholar]
- 23.Balzer K, et al. Reduction of ischaemic rest pain in advanced peripheral arterial occlusive disease. A double blind placebo controlled trial with iloprost. Int Angiol. 1991;10(4):229–32. [PubMed] [Google Scholar]
- 24.Diehm C, et al. [Iloprost, a stable prostacyclin derivative, in stage 4 arterial occlusive disease. A placebo-controlled multicenter study]. Dtsch Med Wochenschr. 1989;114(20):783–8. doi: 10.1055/s-2008-1066673. [DOI] [PubMed] [Google Scholar]
- 25.Meini S, et al. Short-term and long-term effects of one-week treatment with intravenous iloprost in critical limb ischemia patients (Leriche-Fontaine stage III and IV). Int Angiol. 2005;24(1):64–9. [PubMed] [Google Scholar]
- 26.Hardy CC, et al. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N Engl J Med. 1984;311(4):209–13. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- 27.Murray JJ, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315(13):800–4. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- 28.Garza LA, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4(126):126ra34. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirai H, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193(2):255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Group HTC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–12. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 31.Giles H, Leff P. The biology and pharmacology of PGD2. Prostaglandins. 1988;35(2):277–300. doi: 10.1016/0090-6980(88)90093-7. [DOI] [PubMed] [Google Scholar]
- 32.Salomon RG, et al. Solvent-induced fragmentation of prostaglandin endoperoxides. New aldehyde products from PGH2 and a novel intramolecular 1,2-hydride shift during endoperoxide fragmentation in aqueous solution. J Am Chem Soc. 1984;106(20):11. [Google Scholar]
- 33.Charo IF, et al. Prostaglandin I2 is not a major metabolite of arachidonic acid in cultured endothelial cells from human foreskin microvessels. Journal of Clinical Investigation. 1984;74(3):914–9. doi: 10.1172/JCI111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerritsen ME, Cheli CD. Arachidonic acid and prostaglandin endoperoxide metabolism in isolated rabbit and coronary microvessels and isolated and cultivated coronary microvessel endothelial cells. Journal of Clinical Investigation. 1983;72(5):1658–71. doi: 10.1172/JCI111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwano T, et al. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18(2):300–10. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- 36.Hristovska AM, et al. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007;50(3):525–30. doi: 10.1161/HYPERTENSIONAHA.107.088948. [DOI] [PubMed] [Google Scholar]
- 37.Qian YM, et al. Potent contractile actions of prostanoid EP3-receptor agonists on human isolated pulmonary artery. Br J Pharmacol. 1994;113(2):369–74. doi: 10.1111/j.1476-5381.1994.tb16997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schober LJ, et al. The role of PGE(2) in human atherosclerotic plaque on platelet EP(3) and EP(4) receptor activation and platelet function in whole blood. J Thromb Thrombolysis. 2011;32(2):158–66. doi: 10.1007/s11239-011-0577-6. [DOI] [PubMed] [Google Scholar]
- 39.Gross S, et al. Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. Journal of Experimental Medicine. 2007;204(2):311–20. doi: 10.1084/jem.20061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes DR, et al. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. Journal of Vascular Surgery. 1997;25(5):810–5. doi: 10.1016/s0741-5214(97)70210-6. [DOI] [PubMed] [Google Scholar]
- 41.Cipollone F, et al. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation. 2001;104(8):921–7. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- 42.Smith JB, Willis AL. Formation and release of prostaglandins by platelets in response to thrombin. Br J Pharmacol. 1970;40(3):545P–546P. [PMC free article] [PubMed] [Google Scholar]
- 43.Haslam RJ, Dickinson NT, Jang EK. Cyclic nucleotides and phosphodiesterases in platelets. Thromb Haemost. 1999;82(2):412–23. [PubMed] [Google Scholar]
- 44.Ashby B. Model of prostaglandin-regulated cyclic AMP metabolism in intact platelets: examination of time-dependent effects on adenylate cyclase and phosphodiesterase activities. Mol Pharmacol. 1989;36(6):866–73. [PubMed] [Google Scholar]
- 45.Ashby B. Novel mechanism of heterologous desensitization of adenylate cyclase: prostaglandins bind with different affinities to both stimulatory and inhibitory receptors on platelets. Mol Pharmacol. 1990;38(1):46–53. [PubMed] [Google Scholar]
- 46.Vezza R, Nenci GG, Gresele P. Thromboxane synthase inhibitors suppress more effectively the aggregation of thromboxane receptor-desensitized than that of normal platelets: role of adenylylcyclase up-regulation. J Pharmacol Exp Ther. 1995;275(3):1497–505. [PubMed] [Google Scholar]
- 47.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99(12):1293–304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 48.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95(14):8070–4. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniel JL, et al. Role of intracellular signaling events in ADP-induced platelet aggregation. Thromb Haemost. 1999;82(4):1322–6. [PubMed] [Google Scholar]
- 50.Eggerman TL, Andersen NH, Robertson RP. Separate receptors for prostacyclin and prostaglandin E2 on human gel-filtered platelets. J Pharmacol Exp Ther. 1986;236(3):568–73. [PubMed] [Google Scholar]
- 51.An S, et al. Cloning and expression of the EP2 subtype of human receptors for prostaglandin E2. Biochem Biophys Res Commun. 1993;197(1):263–70. doi: 10.1006/bbrc.1993.2470. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, et al. Cloning and expression of the EP3-subtype of human receptors for prostaglandin E2. Biochem Biophys Res Commun. 1994;198(3):999–1006. doi: 10.1006/bbrc.1994.1142. [DOI] [PubMed] [Google Scholar]
- 53.Regan JW, et al. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol Pharmacol. 1994;46(2):213–20. [PubMed] [Google Scholar]
- 54.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46(2):205–29. [PubMed] [Google Scholar]
- 55.Paul BZ, Ashby B, Sheth SB. Distribution of prostaglandin IP and EP receptor subtypes and isoforms in platelets and human umbilical artery smooth muscle cells. Br J Haematol. 1998;102(5):1204–11. doi: 10.1046/j.1365-2141.1998.00910.x. [DOI] [PubMed] [Google Scholar]
- 56.Smith JP, et al. PGE2 decreases reactivity of human platelets by activating EP2 and EP4. Thromb Res. 2010;126(1):e23–9. doi: 10.1016/j.thromres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Iyu D, et al. The role of prostanoid receptors in mediating the effects of PGE(2) on human platelet function. Platelets. 2010;21(5):329–42. doi: 10.3109/09537101003718065. [DOI] [PubMed] [Google Scholar]
- 59.Kuriyama S, et al. Selective activation of the prostaglandin E2 receptor subtype EP2 or EP4 leads to inhibition of platelet aggregation. Thromb Haemost. 2010;104(4):796–803. doi: 10.1160/TH10-01-0043. [DOI] [PubMed] [Google Scholar]
- 60.Suzawa T, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141(4):1554–9. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 61.Amano H, et al. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197(2):221–32. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heptinstall S, et al. DG-041 inhibits the EP3 prostanoid receptor--a new target for inhibition of platelet function in atherothrombotic disease. Platelets. 2008;19(8):605–13. doi: 10.1080/09537100802351073. [DOI] [PubMed] [Google Scholar]
- 63.Glenn JR, et al. PGE(2) reverses G(s)-mediated inhibition of platelet aggregation by interaction with EP3 receptors, but adds to non-G(s)-mediated inhibition of platelet aggregation by interaction with EP4 receptors. Platelets. 2012;23(5):344–51. doi: 10.3109/09537104.2011.625575. [DOI] [PubMed] [Google Scholar]
- 64.An S, et al. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry. 1994;33(48):14496–502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- 65.Kotani M, et al. Molecular cloning and expression of multiple isoforms of human prostaglandin E receptor EP3 subtype generated by alternative messenger RNA splicing: multiple second messenger systems and tissue-specific distributions. Mol Pharmacol. 1995;48(5):869–79. [PubMed] [Google Scholar]
- 66.Schmid A, et al. Splice variants of the human EP3 receptor for prostaglandin E2. Eur J Biochem. 1995;228(1):23–30. doi: 10.1111/j.1432-1033.1995.tb20223.x. [DOI] [PubMed] [Google Scholar]
- 67.Sugimoto Y, et al. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. Journal of Biological Chemistry. 1992;267(10):6463–6. [PubMed] [Google Scholar]
- 68.Irie A, et al. Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. European Journal of Biochemistry. 1993;217(1):313–8. doi: 10.1111/j.1432-1033.1993.tb18248.x. [DOI] [PubMed] [Google Scholar]
- 69.Namba T, et al. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993;365(6442):166–70. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, et al. Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J Biol Chem. 2002;277(48):46035–42. doi: 10.1074/jbc.M208519200. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, et al. Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc Natl Acad Sci U S A. 2000;97(18):9984–9. doi: 10.1073/pnas.180194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matthews JS, Jones RL. Potentiation of aggregation and inhibition of adenylate cyclase in human platelets by prostaglandin E analogues. Br J Pharmacol. 1993;108(2):363–9. doi: 10.1111/j.1476-5381.1993.tb12810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fabre JE, et al. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. Journal of Clinical Investigation. 2001;107(5):603–10. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iyu D, et al. P2Y(1)(2) and EP3 antagonists promote the inhibitory effects of natural modulators of platelet aggregation that act via cAMP. Platelets. 2011;22(7):504–15. doi: 10.3109/09537104.2011.576284. [DOI] [PubMed] [Google Scholar]
- 75.Coleman RA, et al. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994;47(2):151–68. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 76.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4(9):733–8. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 77.Faour WH, Gomi K, Kennedy CR. PGE(2) induces COX-2 expression in podocytes via the EP(4) receptor through a PKA-independent mechanism. Cell Signal. 2008;20(11):2156–64. doi: 10.1016/j.cellsig.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Journal of Biological Chemistry. 2002;277(4):2614–9. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 79.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. Journal of Biological Chemistry. 2003;278(14):12151–6. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 80.Fujino H, Regan JW. EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol Pharmacol. 2006;69(1):5–10. doi: 10.1124/mol.105.017749. [DOI] [PubMed] [Google Scholar]
- 81.Takayama K, et al. A novel prostaglandin E receptor 4-associated protein participates in antiinflammatory signaling. Circulation Research. 2006;98(4):499–504. doi: 10.1161/01.RES.0000204451.88147.96. [DOI] [PubMed] [Google Scholar]
- 82.Philipose S, et al. The prostaglandin E2 receptor EP4 is expressed by human platelets and potently inhibits platelet aggregation and thrombus formation. Arterioscler Thromb Vasc Biol. 2010;30(12):2416–23. doi: 10.1161/ATVBAHA.110.216374. [DOI] [PubMed] [Google Scholar]
- 83.Desai S, Ashby B. Agonist-induced internalization and mitogen-activated protein kinase activation of the human prostaglandin EP4 receptor. FEBS Lett. 2001;501(2-3):156–60. doi: 10.1016/s0014-5793(01)02640-0. [DOI] [PubMed] [Google Scholar]
- 84.Buchanan FG, et al. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci U S A. 2006;103(5):1492–7. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li D, et al. Arrestin-2 differentially regulates PAR4 and ADP receptor signaling in platelets. J Biol Chem. 2011;286(5):3805–14. doi: 10.1074/jbc.M110.118018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kloeze J. Influence of prostaglandins on platelet adhesiveness and platelet aggregation. In: Prostaglandins SS, Bergstrom B, editors. Nobel Symposium 11. Interscience Publishing Co.; New York: 1967. pp. 241–252. [Google Scholar]
- 87.Shio H, Ramwell P. Effect of prostaglandin E 2 and aspirin on the secondary aggregation of human platelets. Nat New Biol. 1972;236(63):45–6. doi: 10.1038/newbio236045a0. [DOI] [PubMed] [Google Scholar]
- 88.McDonald JW, Stuart RK. Interaction of prostaglandins E1 and E2 in regulation of cyclic-AMP and aggregation in human platelets: evidence for a common prostaglandin receptor. J Lab Clin Med. 1974;84(1):111–21. [PubMed] [Google Scholar]
- 89.Andersen NH, et al. On the multiplicity of platelet prostaglandin receptors. I. Evaluation of competitive antagonism by aggregometry. Prostaglandins. 1980;19(5):711–35. doi: 10.1016/0090-6980(80)90170-7. [DOI] [PubMed] [Google Scholar]
- 90.Gray SJ, Heptinstall S. Interactions between prostaglandin E2 and inhibitors of platelet aggregation which act through cyclic AMP. Eur J Pharmacol. 1991;194(1):63–70. doi: 10.1016/0014-2999(91)90124-9. [DOI] [PubMed] [Google Scholar]
- 91.Gray SJ, Heptinstall S. The effects of PGE2 and CL 115,347, an antihypertensive PGE2 analogue, on human blood platelet behaviour and vascular contractility. Eur J Pharmacol. 1985;114(2):129–37. doi: 10.1016/0014-2999(85)90620-x. [DOI] [PubMed] [Google Scholar]
- 92.Vezza R, et al. Prostaglandin E2 potentiates platelet aggregation by priming protein kinase C. Blood. 1993;82(9):2704–13. [PubMed] [Google Scholar]
- 93.Weiss HJ, et al. Prostaglandin E2 potentiation of platelet aggregation induced by LASS endoperoxide: absent in storage pool disease, normal after aspirin ingestion. Br J Haematol. 1976;32(2):257–72. doi: 10.1111/j.1365-2141.1976.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 94.Thierauch KH, Prior G. Modulation of platelet activation by prostaglandin E2 mimics. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:383–6. [PubMed] [Google Scholar]
- 95.Robison GAA,A, Hartmann RC. Divergent effects of epinephrine and prostaglandin E1 on the level of cyclic AMP in human blood platelets. Pharmacol Res Commun. 1969;1:325–332. [Google Scholar]
- 96.Ashby B. Cyclic AMP turnover in response to prostaglandins in intact platelets: evidence for separate stimulatory and inhibitory prostaglandin receptors. Second Messengers Phosphoproteins. 1988;12(1):45–57. [PubMed] [Google Scholar]
- 97.Abramovitz M, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483(2):285–93. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 98.Ma H, et al. Increased bleeding tendency and decreased susceptibility to thromboembolism in mice lacking the prostaglandin E receptor subtype EP(3). Circulation. 2001;104(10):1176–80. doi: 10.1161/hc3601.094003. [DOI] [PubMed] [Google Scholar]
- 99.Song WL, et al. Niacin and biosynthesis of PGD(2)by platelet COX-1 in mice and humans. J Clin Invest. 2012;122(4):1459–68. doi: 10.1172/JCI59262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tilly P, et al. Blocking the EP3 receptor for PGE2 with DG-041 decreases thrombosis without impairing haemostatic competence. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvt276. [DOI] [PubMed] [Google Scholar]
- 101.Singh J, et al. Antagonists of the EP3 receptor for prostaglandin E2 are novel antiplatelet agents that do not prolong bleeding. ACS Chem Biol. 2009;4(2):115–26. doi: 10.1021/cb8002094. [DOI] [PubMed] [Google Scholar]
- 102.Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28(8):423–30. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 103.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 104.Kenakin T. Allosteric theory: taking therapeutic advantage of the malleable nature of GPCRs. Curr Neuropharmacol. 2007;5(3):149–56. doi: 10.2174/157015907781695973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–86. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leduc M, et al. Functional selectivity of natural and synthetic prostaglandin EP4 receptor ligands. J Pharmacol Exp Ther. 2009;331(1):297–307. doi: 10.1124/jpet.109.156398. [DOI] [PubMed] [Google Scholar]
- 107.Iyu D, et al. PGE1 and PGE2 modify platelet function through different prostanoid receptors. Prostaglandins Other Lipid Mediat. 2011;94(1-2):9–16. doi: 10.1016/j.prostaglandins.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 108.Salzman EW, Kensler PC, Levine L. Cyclic 3′,5′-adenosine monophosphate in human blood platelets. IV. Regulatory role of cyclic amp in platelet function. Ann N Y Acad Sci. 1972;201:61–71. doi: 10.1111/j.1749-6632.1972.tb16287.x. [DOI] [PubMed] [Google Scholar]
- 109.Gresele P, et al. Adenylate cyclase activation determines the effect of thromboxane synthase inhibitors on platelet aggregation in vitro. Comparison of platelets from responders and nonresponders. J Pharmacol Exp Ther. 1988;246(1):301–7. [PubMed] [Google Scholar]
- 110.Heptinstall S, et al. Effects of a selective inhibitor of thromboxane synthetase on human blood platelet behaviour. Thromb Res. 1980;20(2):219–30. doi: 10.1016/0049-3848(80)90387-4. [DOI] [PubMed] [Google Scholar]
- 111.Bartele V, et al. Inhibition of thromboxane synthetase does not necessarily prevent platelet aggregation. Lancet. 1981;1(8228):1057–8. doi: 10.1016/s0140-6736(81)92224-8. [DOI] [PubMed] [Google Scholar]
- 112.Gresele P, et al. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. 1984;33(13):2083–8. doi: 10.1016/0006-2952(84)90577-x. [DOI] [PubMed] [Google Scholar]
- 113.FitzGerald GA, Reilly IA, Pedersen AK. The biochemical pharmacology of thromboxane synthase inhibition in man. Circulation. 1985;72(6):1194–201. doi: 10.1161/01.cir.72.6.1194. [DOI] [PubMed] [Google Scholar]
- 114.MacIntyre DE, Gordon JL. Calcium-dependent stimulation of platelet aggregation by PGE. Nature. 1975;258(5533):337–9. doi: 10.1038/258337a0. [DOI] [PubMed] [Google Scholar]
- 115.Gresele P, Falcinelli E, Momi S. Potentiation and priming of platelet activation: a potential target for antiplatelet therapy. Trends Pharmacol Sci. 2008;29(7):352–60. doi: 10.1016/j.tips.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 116.Fox SC, et al. Effects on platelet function of an EP3 receptor antagonist used alone and in combination with a P2Y12 antagonist both in-vitro and ex-vivo in human volunteers. Platelets. 2013;24(5):392–400. doi: 10.3109/09537104.2012.704648. [DOI] [PubMed] [Google Scholar]
- 117.Positive Clinical Results for DG041 Lead Product Development Highlights at deCODE R &D Event. 6/25/07; Available from: http://www.decode.com/positive-clinical-results-for-dg041-lead-product-development-highlights-at-decode-r-d-event/
- 118.Xiao CY, et al. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation. 2004;109(20):2462–8. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- 119.Hishikari K, et al. Pharmacological activation of the prostaglandin E2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2009;81(1):123–32. doi: 10.1093/cvr/cvn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mohamed R, Jayakumar C, Ramesh G. Chronic administration of EP4-selective agonist exacerbates albuminuria and fibrosis of the kidney in streptozotocin-induced diabetic mice through IL-6. Lab Invest. 2013;93(8):933–45. doi: 10.1038/labinvest.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pan MR, et al. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J Biol Chem. 2008;283(17):11155–63. doi: 10.1074/jbc.M710038200. [DOI] [PubMed] [Google Scholar]
- 122.Pozzi A, et al. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279(28):29797–804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 123.Yang L, et al. Host and direct antitumor effects and profound reduction in tumor metastasis with selective EP4 receptor antagonism. Cancer Res. 2006;66(19):9665–72. doi: 10.1158/0008-5472.CAN-06-1271. [DOI] [PubMed] [Google Scholar]
- 124.Nakase H, et al. Effect of EP4 agonist (ONO-4819CD) for patients with mild to moderate ulcerative colitis refractory to 5-aminosalicylates: a randomized phase II, placebo-controlled trial. Inflamm Bowel Dis. 2010;16(5):731–3. doi: 10.1002/ibd.21080. [DOI] [PubMed] [Google Scholar]
- 125.Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009;27(29):4821–6. doi: 10.1200/JCO.2009.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma D, et al. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229(8):1005–15. doi: 10.1002/jcp.24539. [DOI] [PubMed] [Google Scholar]
- 129.Orellana R, et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer. 2015;15(1):290. doi: 10.1186/s12885-015-1304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dovizio M, et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84(1):25–40. doi: 10.1124/mol.113.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 132.Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3(8):1031–9. [PubMed] [Google Scholar]
- 133.Ma X, et al. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66(6):2923–7. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 134.Kawamori T, et al. Chemopreventive effects of ONO-8711, a selective prostaglandin E receptor EP(1) antagonist, on breast cancer development. Carcinogenesis. 2001;22(12):2001–4. doi: 10.1093/carcin/22.12.2001. [DOI] [PubMed] [Google Scholar]