Abstract
Voltage-gated sodium (NaV) and calcium (CaV) channels are involved in electrical signaling, contraction, secretion, synaptic transmission, and other physiological processes activated in response to depolarization. Despite their physiological importance, the structures of these closely related proteins have remained elusive because of their size and complexity. Bacterial NaV channels have structures analogous to a single domain of eukaryotic NaV and CaV channels and are their likely evolutionary ancestor. In this article, we review recent work that has led to new understanding of NaV and CaV channels through high-resolution structural studies of their prokaryotic ancestors. New insights into their voltage-dependent activation and inactivation, ion conductance, and ion selectivity provide realistic structural models for the function of these complex membrane proteins at the atomic level.
Keywords: voltage-gated sodium channel, voltage-gated calcium channel, NaChBac, NavAb, voltage sensor, selectivity filter, slow inactivation
Voltage-gated NaV and CaV channels and their bacterial ancestors
Voltage-gated NaV channels (NaVs) initiate action potentials in excitable cells and are crucial for electrical signaling from bacteria to man [1]. Voltage-gated CaV channels (CaVs) are activated by depolarization during action potentials, and Ca2+ entry through them initiates synaptic transmission, muscle contraction, hormone secretion, and many other biochemical and physiological processes [2, 3]. These channels are thought to share similar voltage-dependent activation and inactivation processes, whose structural basis is fundamental for electrical signaling. Moreover, how these channels can rapidly and selectively conduct Na+ or Ca2+ ions in response to changes of the electrical membrane potential is a crucial question in biology.
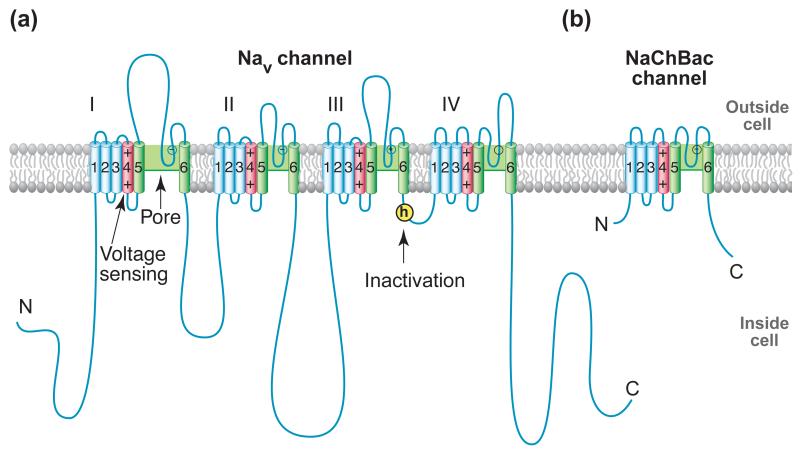
Mammalian NaV channels are complexes of a large α subunit of 260 kDa and smaller β subunits of 30-40 kDa [4]. cDNA encoding the pore-forming α subunits is sufficient for expression of functional NaV channels, whereas the β subunits enhance expression, modulate NaV channel gating, and serve as cell adhesion molecules (reviewed in [5, 6]). NaV channel α subunits are polypeptides of approximately 2000 amino acid residues organized into four homologous domains, each containing six transmembrane segments (Figure 1a) [5, 6]). Each homologous domain consists of two functional modules: a voltage-sensing module (VSM) composed of the S1-S4 segments, and a pore-forming module (PM) composed of the S5 and S6 segments and the P loop between them (Figure 1a).
Figure 1. NaV Channel Structure.
(a) Two-dimensional schematic map of NaV channel structure and function. The α subunit of NaV1.2 channels is illustrated as a transmembrane folding diagram in which cylinders represent transmembrane alpha helices and lines represent connecting amino acid sequences in proportion to their length. The roman numerals indicate the four homologous domains and the Arabic numerals are used to label the six transmembrane helices. The S4 helices are colored in red with “+” signs indicating gating charges. The S5-S5 helices are colored in green and the small white circles indicate key residues in the selectivity filter with “+” and “−“ signs indicating their charge states. The yellow circle with an “h” indicates inactivation gate. (b) Schematic map of the bacterial NaChBac channel, which contains the minimal functional elements of a single homologous domain in a mammalian NaV channel.
Mammalian CaV channels are also multi-subunit complexes [3]. They have a central, pore-forming α1 subunit that is analogous in structure and function to the NaV channel α subunit. The α1 subunit is associated with an intracellular β subunit, a membrane-associated α2δ disulfide-linked complex, and a transmembrane γ subunit, all of which are involved in channel regulation but not in voltage-dependent gating or ion conductance. Analysis of the pore-forming sequences of the 143 voltage-gated ion channels and their relatives in the human genome predicted a common core domain composition with many key conserved structural elements. These conserved features led to the proposal that NaV and CaV channels derive from a common ancestor and have a similar structural basis for their function [7]. Despite their high biological significance, the sheer sizes and complex transmembrane architectures of these channel proteins have posed a major challenge for structural and mechanistic analyses of their functions.
The unexpected discovery of the bacterial NaV channel NaChBac was a landmark in ion channel research [8]. The sequence of NaChBac is analogous to one domain of the eukaryotic NaV or CaV channel, and it functions as a homotetramer (Figure 1b) [8]. Orthologs of NaChBac are found in gram-positive and gram-negative eubacteria and in archaea, suggesting a truly ancient origin [8]. Although its amino acid sequence in the pore region is closer to CaV channels, NaChBac selectively conducts Na+. These properties further confirmed its identity as an ancestor of both mammalian NaV or CaV channels. The bacterial NaV channels are ‘minimalist’ in structure, as they have a VSM and PM but no large intracellular and extracellular linkers. This minimalist structure makes them ideally suited for structural studies of the conserved core functions of NaV and CaV channels. This article reviews the mechanistic insights into the conserved core functions of NaV and CaV channels gained from structural analyses of the NaChBac family of bacterial ion channels.
The first glimpse of NaV channel structure
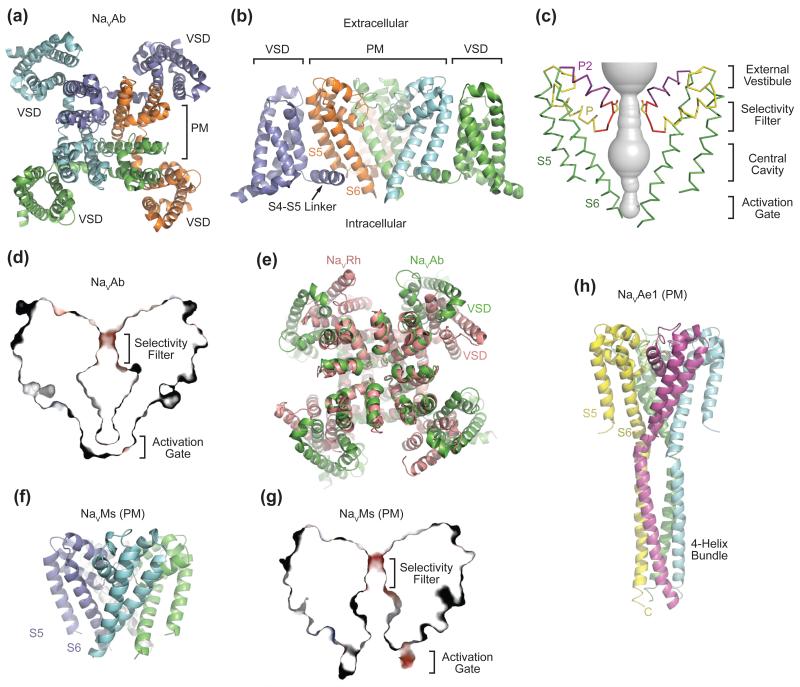
One decade after identification of NaChBac, the X-ray crystal structure of its ortholog from Arcobacter butzleri (NaVAb) was determined at 2.7 Å resolution [9], opening the way to understanding NaV channel structure and function at the atomic level. The NaVAb structure revealed a homotetrameric architecture, in which four subunits pack in a symmetric manner, giving rise to a functional channel with a central ion-conducting pore (Figure 2a). The four S5 and S6 helices and the P loops form the pore, which is surrounded by the form VSM composed of the S1-S4 helices. Interestingly, although the VSM of each subunit is coupled to the pore via an α-helical S4-S5 linker, its closest noncovalent contacts are with the S5 helix of its neighboring subunit (Figure 2a, 2b). The S4-S5 linker helix of each subunit also directly intersects the S5 and S6 helices of the adjacent subunit. Such a tightly interlocked subunit arrangement suggests that the four VSMs cooperatively control concerted opening of the pore.
Figure 2. Overall Structures of Prokaryotic NaV Channels.
(a) Structure of the NaVAb bacterial NaV channel determined at 2.7Å resolution and viewed from the extracellular side [9]. The four subunits of the homotetrameric channel are shown in different colors. The central pore is surrounded by four VSMs. (b) Side view of NaVAb with the same coloring scheme as shown in (a). S5 and S6 helices of one subunit (slate) and the VSM of another subunit (cyan) are omitted for clarity. (c) Architecture of the NaVAb pore with pore volume shown in grey and the high-strength-field (HSF) site residue Glu177 shown in sticks. P and P2 indicate the P and P2 helices. (d) Cross-section of the NaVAb pore showing the closed activation gate. The selectivity filter is shown with electrostatic surface potential colored from −50 to 50 kT (red to blue). (e) Superposition of NaVRh and NaVAb with their PMs superimposed [9, 11]. The VSM of the two channels packs against the PM at different angles. (f) Structure of the NaVMs pore module [13]. (g) Cross-section of the NaVMs pore showing the open conformation of its activation gate [18]. The selectivity filter is shown with electrostatic surface potential colored from −50 to 50 kT (red to blue). (h) Structure of the NaVAe1 pore with well-resolved C-terminal domain forming a four-helix bundle [15].
The NaVAb structure unveiled the detailed anatomy of a NaV pore. The NaVAb pore consists of an external vestibule, a narrow ion selectivity filter, a spacious water-filled central cavity, and an activation gate in closed conformation at the inner end of the S6 helices, completely sealing off the ion-conduction pathway (Figure 2c, 2d). Because NaVAb activates at very negative membrane potentials, it is not surprising that the channel was crystallized with an activated VSMs at the 0-mV potential in a protein crystal. The combination of four activated VSMs and a closed pore strongly suggests that the NaVAb Ile217Cys mutant used for high-resolution structure determination was crystallographically trapped in a pre-open state, which is a required intermediate for a homotetrameric channel that opens in a single concerted transition (see Glossary). The NaVAb crystal structure ended the long wait for a high-resolution view of a voltage-gated NaV channel and heralded further analyses of the structural underpinnings of both NaV and CaV channel functions.
Prokaryotic NaV channel structure in greater detail
Following the NaVAb structure, an explosion of structural studies of bacterial NaV channels provided additional insights into the structural basis of channel functions. Wild-type NaVAb (NaVAb-WT) succumbed to crystallographic analysis, which fortuitously captured the channel in two slow-inactivated states (see below and [10]). Structure determination of NaVRh from Richettsiales sp. HIMB114 offered an independent view of a NaV channel, also captured in a slow-inactivated state [11]. The activated VSMs of NaVRh wrap around the central pore at a different angle and the S4 segment has moved further outward than in NaVAb, implying a considerable degree of flexibility in VSM-pore interactions (Figure 2e). Recently, electron crystallography was used to determine the overall architecture of a third NaChBac family member from Caldalkalibacillus thermarum (NaVCt). Despite relatively low 9Å resolution, two distinguishable conformations of NaVCt were readily recognized [12], marked by different degrees of openness of the S6 intracellular activation gate (see Glossary) and, surprisingly, by a partially blurred S4-S5 linker helix.
The modular architecture of bacterial NaV channels allows their S5-S6 segments to assemble into a functional pore in the absence of VSMs. Such a strategy has been adopted to obtain crystal structures of two pore-only bacterial channels from Magnetococcus sp. (NaVMs [13]) and Alkalilimnicola ehrlichei (NaVAe1 [14, 15]). The NaVMs pore shares nearly an identical selectivity filter with NaVAb, but adopts an open conformation at the intracellular S6 activation gate (Figure 2f, 2g). Superposition analysis of the S6 helix in NaVMs and NaVAb indicates that gate opening is mediated by a slight rotation about a single residue in the middle of the S6 helix in close proximity to a strictly conserved Asn residue facing directly toward the S4-S5 linker. By capturing the partially or fully open pore, the NaVMs pore structures gave additional insight into structural mechanisms of pore opening and closing [16]. Based on these structures, VSM activation may open the activation gate by splaying the S6 helix at a central hinge residue, as in the NaVMs structure. However, the absence of the interacting S4-S5 linker may alter conformational movement of the S6 segment in the pore-only NaVMs structure relative to the complete channels with VSMs. Future studies are needed to validate this mechanism.
In contrast to NaVMs, the pore-only structure of NavAe1 shows a closed activation gate [15], as in NaVAb. A unique observation from this structure is the well-resolved C-terminal tail extending from the S6 helix in a four-helix bundle that would project into the cytosol in a bacterial cell (Figure 2h) [15]. This C-terminal sequence is found in many bacterial NaV channels. Mutational and structural analyses of this sequence have established its functional role in assembly, gating, and inactivation [12, 15, 18, 19].
Led by NaVAb, the bacterial NaChBac family NaV channel structures not only revealed the overall architecture of a NaV channel in different functional states, but also offered detailed insights into the structural mechanisms underlying their essential functions. These include voltage sensing, channel inactivation, and ion selectivity and conductance, which are highlighted next.
Voltage-dependent activation
Voltage-dependent activation of NaV channels depends on voltage-driven transmembrane movement of gating charges (see Glossary) in the S4 segment [1]. This charge movement was first detected as a small capacitive gating current in voltage clamp studies [20, 21]. The S4 transmembrane segments contain 4-8 repeated motifs of a positively charged amino acid residue (usually arginine) flanked by two hydrophobic residues. They were proposed to carry the gating charges in the sliding helix model of voltage sensing [5]. This conceptual model was placed on a solid structural foundation by molecular modeling using the Rosetta Membrane algorithm together with structural information on the KV1.2 channel [22] and the NaVAb channel [17]. Extensive structure-function studies now provide strong support for this model [23], and a consensus mechanism for voltage sensor function based on the sliding helix model has emerged [24].
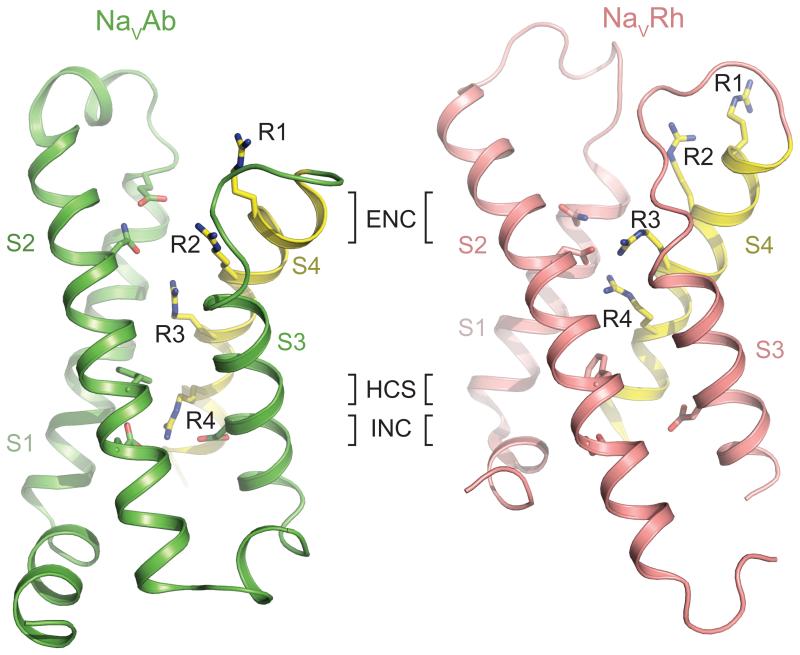
The high-resolution structures of the VSMs of KV1.2 and NaVAb channels revealed the functional conformation of their voltage sensors [9, 25-27]. The S4 transmembrane segment arrays the gating charges across the membrane. Three gating charges (R1-R3 in NaVAb) are exposed to the external solution and interact with negatively charged and hydrophilic amino acid side chains in the Extracellular Negative Cluster (ENC) in the S1-S3 segments (Figure 3) [9]. The fourth conserved gating charge (R4 in NaVAb) interacts with negatively charged amino acid side chains in the Intracellular Negative Cluster (INC) [9]. Between the third and fourth gating charges, the highly conserved Hydrophobic Constriction Site (HCS) seals the voltage sensor and prevents transmembrane movement of water and ions [9]. In the context of the sliding helix model, the S4 segment is thought to move through the HCS in response to changes in voltage, transfer its gating charges through the transmembrane electric field, and exchange ion-pair partners between the INC and ENC [23] [17]. Comparison of the structures of NaVAb and NaVRh provides a snapshot of this transmembrane movement, as the S4 segment in NaVRh has moved farther outward, such that its fourth gating charge is located above the HCS (Figure 3)[11]. A similar sliding-helix motion is observed during activation of a voltage-sensitive phosphatase [28]. Most X-ray crystallographic structures of voltage sensors reveal part or all of the S4 segment in a 310 rather than α-helical conformation [9-11]. These results raise the possibility that S4 may undergo a transition from α-helical to 310 helical conformation during activation, which would allow outward movement of gating charges and exchange of ion pair partners with less rotation of the helix core [17, 23]. This outward movement of the gating charges in the VSM is coupled to pore opening and inactivation as described in the following sections.
Figure 3. A Comparison of the Voltage-sensor Domains of NaVAb and NaVRh.
The VSMs of NaVAb (left, green) and NaVRh (right, salmon) are shown in ribbon diagram [9, 11]. The sliding S4 helix is highlighted in yellow with the side chains of the four gating charge arginine residues (R1-R4) shown in sticks. Key residues forming the extracellular negative cluster (ENC), intracellular negative cluster (INC), and hydrophobic constriction site (HCS) are shown in sticks. The R4 residue of NaVAb and NaVRh interacts with the INC and ENC residues, respectively.
Fast and slow inactivation
Mammalian NaV channels are inactivated by both fast and slow processes, which involve distinct mechanisms and recover on very different time scales. NaV channels in nerve and muscle open in response to depolarization and then inactivate within 1-2 ms [29]. This classical fast inactivation process is required for repetitive firing of action potentials in neural circuits and for controlling excitability in nerve and muscle cells, and it recovers within a few milliseconds upon repolarization. The short intracellular loop connecting homologous domains III and IV of the eukaryotic NaV channel α subunit mediates fast inactivation by folding into the intracellular mouth of the pore and blocking it (Figure 1a; [5]). Bacterial NaV channels lack such a fast inactivation gate, but they have a slow inactivation process.
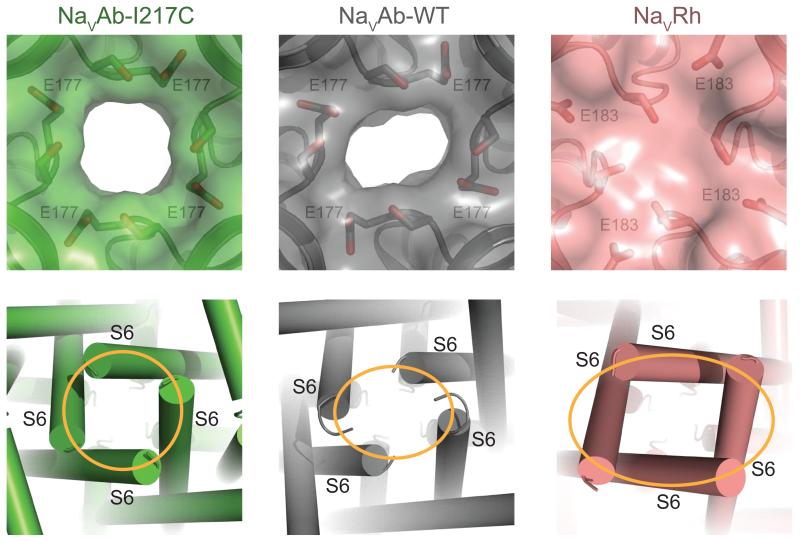
Slow inactivation increases during trains of action potentials over hundreds of milliseconds and reverses even more slowly [30, 31]. Structure-function studies of mammalian NaV channels suggested that the ion selectivity filter and the pore-lining S6 segments move during slow inactivation [30], which is corroborated by structure-function studies of the slow inactivation process of bacterial NaV channels [32-34]. The NaVAb-WT and NaVRh structures revealed a distorted selectivity filter and a dramatic breakdown of the four-fold symmetry around the central axis (Figure 4) [10, 11], suggesting they represent the slow-inactivated state, which is the most stable functional state at the membrane potential of 0 mV in a protein crystal. In contrast to the original NaVAb-I217C structure [9], these potentially slow-inactivated channels have partially collapsed pore domains, such that two S6 segments and P loops have moved toward the central axis of the pore and two have moved away [10, 11]. It is likely that this structural change underlies slow inactivation of NaV channels in both prokaryotes and eukaryotes. Moreover, because voltage-dependent inactivation of CaV channels occurs on the same time scale as slow inactivation of NaV channels, it is plausible that it involves a similar structural mechanism, as revealed in the ancestral NaV channels from which both eukaryotic NaV and CaV channels evolved. These structural studies revealed much about the mechanisms of opening and inactivation of the pores of NaV and CaV channels. The following section considers how the open pores can rapidly and selectively conduct Na+ or Ca2+.
Figure 4. Structural Basis of Slow Inactivation.
Specific structural features of NaVAb-WT and NaVRh, including a distorted selectivity filter and the breakdown of the four-fold symmetry of the pore, as expected for the slow inactivation state. Top panel: Close-up views of the extracellular entrance of NaVAb-I217C (green, symmetric), NaVAb-WT (grey, asymmetric), and NaVRh (salmon, collapsed) with semi-transparent surface representation of the three channels [9-11]. The high-strength-field site glutamate residue in each structure along with its nearby serine residue is shown in sticks. Bottom panel: Close-up view of the intracellular closed activation gate of NaVAb-I217C (symmetric, orange circle), NaVAb-WT (asymmetric, orange oval), and NaVRh (asymmetric, orange oval). .
Structural basis for Na+ conductance and selectivity
The four available crystal structures of NaChBac family members share a highly similar selectivity filter, which forms the narrowest constriction in the extracellular half of their pore. The prototypical NaVAb selectivity filter is made of a short loop flanked by two short helices, the P helix and P2 helix, which are connected to the S5 and S6 helix, respectively (Figure 2c) [9]. Classic studies of eukaryotic NaV channels depicted a selectivity filter with an oxygen-lined pore about 3 Å by 5 Å in cross section [1, 35-37]. An ionized carboxylic group was proposed to serve as a high-field-strength (HFS) site, which can displace tightly bound waters of hydration and interact with Na+ in a partially hydrated form. According to Eisenman’s theory [1], such an anionic site favors small cations and confers Na+ selectivity. This hallmark HFS site can be readily identified at the extracellular opening of the NaVAb selectivity filter, where four Glu residues from the four subunits create a constriction with dimensions of 4.6 Å × 4.6 Å (Figure 4, 5a). The size of the orifice is just sufficient for Na+ to go through together with at least two water molecules in the same plane, and possibly two additional ones “behind” and “in front” of the ion. Further into the conduction pathway, the NaVAb selectivity filter features two additional central and inner sites, which are formed by two quartets of backbone carbonyls (Figure 5b). The symmetric geometry and nearby electron density of these two sites indicate that they could coordinate four planar water molecules around the pore axis to fully hydrate Na+.
Figure 5. Selectivity Filter of NaVAb.
(a) Close-up view of the extracellular entrance of NaVAb-I217C with the high-strength-field site, Glu177, highlighted in yellow [9]. (b) Side view of the NaVAb-I217C selectivity filter showing Glu177 (yellow) and the backbone carbonyls of Leu176 and Thr175, all of which are involved in selecting and permeating partially hydrated sodium ions. (c) Superposition of NaVAb and a K+-channel (PDB code 1K4C) selectivity filter. The structural alignment, which is based on the common P-helices of the two channels, highlights the significant differences in the width of the two selectivity filters. (d) Different conformational states of the four high-strength-field site Glu177 residues captured in equilibrium molecular dynamics simulation of NaVAb in a hydrated lipid bilayer with Na+ moving in and out of the pore [40]. The side chains of Glu177 point either out toward the mouth of the selectivity filter or into the lumen (0-4 indicate the number of Glu side chains dunked; Na+ ions not shown). (e) Axial distribution of Na+ atoms in the selectivity filter and central cavity of NaVAb. Three distinguishable states are highlighted in which Na+ is directly bound to Glu177 (green), to both Glu177 and the backbone carbonyl of Leu176 (yellow), or to neither (brown). (d) and (e) are adapted from reference [40].
Other bacterial NaV channel structures also revealed an aqueous selectivity filter with a wide lumen, an HFS site formed by Glu side chains, and two central and inner sites formed by backbone carbonyls [11, 15, 38]. Although the selectivity filter of NaVAb was empty in the crystal, an island of electron density was found in the selectivity filters of both NaVRh and NaVMs [11, 38]. In the case of NaVRh, crystallographic analysis assigned the density as a blocking Ca2+ ion in a mostly hydrated form [38]. A similarly hydrated ion has also been observed at an outer site in the extracellular vestibule of the NaVAe1 and NaVAb selectivity filters [9, 11]. This site could represent the entry point for permeant ions ready to be partially dehydrated.
While higher resolution structures of bacterial NaV channels will be needed to better delineate the binding mode of hydrated Na+, the overall features of their selectivity filters are in full agreement with the classical four-barrier, three-site model proposed for the asymmetric four-domain eukaryotic NaV channels [37]. The size and chemical nature of the prokaryotic and eukaryotic NaV channel selectivity filters are in stark contrast to K+ channels, which select K+ by completely removing its hydration shell through interaction with a much narrower array of backbone carbonyl oxygen atoms in the absence of negatively charged coordinating ligands (Figure 5c)[39]. Evidently, two fundamentally different modes of ion conductance and selectivity had evolved in bacterial Na+ and K+ channels, long before four-domain NaV channels arose through evolution in eukaryotes.
Molecular dynamics of Na+ permeation
The structure of bacterial NaV channels provided crystallographic snapshots of the pore structure, but the chemical details of their ion permeation mechanism can be better understood through molecular dynamics simulations. A single open NaV channel conducts ~107 Na+ per second, with greater than 10-fold selectivity over K+ and nearly 50-fold selectivity over Ca2+ [1]. This high rate of conductance precludes high-affinity binding because the residence time of each ion in the pore would be too long. The similarity in size of these ions also precludes ion-sieving as the primary mechanism of selectivity. How are high rates of permeation and high ion selectivity achieved without high-affinity binding and molecular sieving?
Long, unbiased molecular dynamics simulations were carried out for NaVAb at 0 mV, approximately the peak of Na+ conductance during an action potential. More than 200 Na+ ions passed inward through the ion selectivity filter at 6 × 106 per second and reached equilibrium across it [40]. As inward-moving Na+ approaches the HFS site, molecular simulations revealed dynamic interactions between the carboxylates and Na+, such that 1-3 waters of hydration in the inner shell were displaced by interaction with 1-3 carboxylates [40]. Upon binding Na+, the carboxylates bend (or “dunk”) deeper into the pore by rotating around a single torsion angle in the Glu side chains (Figure 5d). As they move inward past the HFS site, Na+ ions regain a complete inner shell of water during interaction with the central and inner coordination sites formed by backbone carbonyls of Leu and Thr residues [40]. The primary sites of interaction during conductance were the carboxylates of the HFS site alone and combined interaction with the HFS site and the backbone carbonyls of Leu in the central site (Figure 5e). These molecular dynamics results reveal active catalysis of Na+ conductance by correlated dunking movements of the Glu side chains. This novel feature of the HFS site in the selectivity filter, and the interactions with backbone carbonyls, are both essential steps in the ion permeation process.
Molecular dynamics simulations of NaVMs and NaVRh for shorter times at large negative and positive membrane potentials provide complementary views of the permeation process. Movement of multiple Na+ ions was required for effective conductance, and two to three sites of interaction within the selectivity filter involving the HFS site and the Leu backbone carbonyls were observed [38, 41], consistent with the unbiased simulations [40]. Glu dunking was observed in some studies, but large negative membrane potentials might have reduced the extent of dunking observed [38] due to electrostatic repulsion of carboxylates by the negative internal membrane potential. Overall, molecular dynamics simulations performed in different ways give a generally consistent view of Na+ conductance in bacterial NaV channels.
From NaVAb to CaVAb: structural insight into Ca2+ selectivity
CaV channels have evolved a selectivity mechanism that allows them to discriminate Ca2+ over the similar-sized Na+, even though the latter is far more abundant in the extracellular solution. How can CaV channels achieve this high ion selectivity while maintaining high-throughput conductance? At the sequence level, CaV channels are closely related to NaV channels, whose ion selectivity can be altered to favor Ca2+ with simple mutations in the selectivity filter [42-44]. Inspired by early work converting NaChBac to a Ca2+-selective form with such mutations [45], crystallographic analysis of a similarly engineered NaVAb mutant, known as CaVAb, has provided valuable structural insights into Ca2+ selectivity and permeation in CaV channels [46].
CaVAb was created by substituting three amino acid residues in the ion selectivity filter of NaVAb (including the HFS site Glu) with Asp (Figure 6a; TLESWSM to TLDDWSD). These substitutions confer Ca2+ selectivity with a permeability ratio of PCa:PNa~400:1, equivalent to mammalian CaV channels. The crystal structure of CaVAb revealed little conformational change in the backbone geometry of the selectivity filter, indicating that ion selectivity is exclusively dictated by the amino acid side chains (Figure 6a). Previous experimental and theoretical studies of mammalian CaV channels have postulated that CaV channels select Ca2+ over Na+ on the basis of affinity and that the high flux of Ca2+ is mediated by a “knock-off” mechanism (see Glossary), in which a second Ca2+ entering the pore “knocks off” a previous resident Ca2+ by charge repulsion. However, this concept was inconsistent with the single high-affinity Ca2+-binding site suggested by careful analyses of mutations and cation block [43]. With Ca2+ included in the crystallization medium, the CaVAb structure clearly revealed three ion-binding sites lining up along the axis of the selectivity filter (Figure 6b) [46]. Leading from the entry to the exit of the ion conduction pathway, these three sites are coordinated by a quartet of Asp residues (Site 1), a box of four Asp side chains and four backbone carbonyls of Leu residues (Site 2), and a quartet of backbone carbonyls of Thr residues alone (Site 3). The distances between all three ion-binding sites and their surrounding oxygen atoms suggest that Ca2+ is bound and conducted in hydrated form, in agreement with the 6 Å diameter estimated for the selectivity filter in mammalian CaV channels [47]. Favorable crystal structures revealed waters of hydration surrounding Ca2+ ions [46]. By titrating Ca2+ concentration, the central site of CaVAb was identified as the high affinity site, whereas the third site close to the central cavity showed lowest affinity [46]. Consistent with studies of mammalian CaV channels, divalent cations such as Cd2+ and Mn2+ block CaVAb by occupying only the central high-affinity site [46].
Figure 6. Ca2+ Selectivity and Permeation by CaVAb.
(a) Side view of the superimposed selectivity filters of CaVAb and NaVAb [9, 46]. The side chains of the three residues of NaVAb mutated to generate CaVAb are colored in yellow in the CaVAb structure. The backbone structure of the selectivity filters of the two channels are nearly identical. (b) Side view of the CaVAb selectivity filter with three Ca2+ (green spheres) binding sites and their coordinating oxygen atoms. The distances between Ca2+ and the oxygen atoms indicated with dash lines range from 4.0 Å to 5.0 Å. (c) A proposed mechanism of Ca2+ permeation by CaVAb based on a combination of the “knock-out” and “stepwise permeation” models. The selectivity filter oscillates between two proposed ionic occupancy states where Ca2+ ions either bind to position 2, the high-affinity binding site, or position 1 and 3, which bind the ion at lower affinities.
The spatial relationships among the three Ca2+-binding sites in the multi-ion pore of CaVAb and their relative affinities for Ca2+ point to an ion conductance mode that combines the “knock-off” mechanism [48-50] and a stepwise-permeation model (see Glossary) [51, 52] in which conducted Ca2+ moves in steps from an external low affinity binding site to high affinity sites in the selectivity filter and back to low affinity sites in the central cavity. In the CaVAb selectivity filter, the three Ca2+-binding sites are separated by a distance of ~4.5 Å, which would trigger electrical repulsion between Ca2+ ions. The combination of the central high-affinity site with outer and inner sites of lower affinity provides potential energy steps to facilitate the entry and exit of Ca2+ ions transiting the selectivity filter. In the presence of extracellular Ca2+ in high concentration, these two mechanisms could allow the selectivity filter to oscillate between a single-ion bound state with the high-affinity central site occupied and a two-ion bound state with central site empty, thereby achieving a high unidirectional Ca2+ flux (Figure 6c). Although CaVAb is engineered from NaVAb and does not contain a selectivity filter identical to that of the mammalian CaVs, it has very similar ion selectivity to a voltage-gated CaV channel. The basic principles governing Ca2+ conductance by CaVAb are most likely shared by the mammalian channels.
Concluding remarks
Structural studies of the bacterial NaV channels have given an unexpected wealth of new insights into ion channel function at molecular, mechanistic, and even atomic levels. The structures of NaVAb and CaVAb and the related molecular dynamics simulations revealed the mechanism of highly selective conductance of hydrated cations in the voltage-gated ion channel family. Ion selectivity is generated by interaction with the inner shell of waters of hydration rather than by direct interactions with the dehydrated ion itself, and molecular sieving based on ion size plays little role in the selectivity process. Glu side chains accompany conducted Na+ ions in a dunking motion. The structures of the VSMs of NaVAb and other bacterial NaV channels in the activated conformation and the structural models of the VSM of NaChBac in resting and activated states have given much-needed new definition to the mechanism of voltage sensor function. The nature of the conformational changes during slow inactivation have been revealed by the structures of wild-type NaVAb and NaVRh, which likely are good models for slow inactivation of eukaryotic NaV channels and voltage-dependent inactivation of eukaryotic CaV channels. With many questions to be addressed by future studies (see Outstanding Question Box), the new knowledge of these fundamental processes that underlie electrical signaling will have important implications for the many diseases caused by altered ion channel function [53] and for development of new generations of drugs targeted to specific ion channel subtypes [54].
Outstanding Questions Box.
1. What is the structural basis of the resting state of a NaV (or CaV) channel?
So far, the available bacterial NaV channel structures (NaVAb and NaVRh) have only captured the VSMs in their activated states, in which the S4 helices have already undergone substantial outward movement. The structure of the resting state of a bacterial NaV channel, in which the VSM is expected to have a distinct conformation with the gating charges on the intracellular side of the HCS, remains to be determined.
2. How does VSM activation lead to pore opening?
In voltage-gated ion channels, the VSM is believed to control the activation gate of the pore module via the S4-S5 linker. The structural mechanism underlying this allosteric regulation, however, remains elusive.
3. Can we use the prokaryotic channels to investigate drug binding?
One of the interesting properties of the prokaryotic NaV channels is their pharmacological sensitivity to eukaryotic NaV and CaV channel blockers [8]. A recent structural study of NaVMs has provided evidence for drug binding at the central cavity of its pore module[55]. Future structural studies of other NaChBac family members might reveal more detailed drug-channel interactions (see ([56]) for review).
4. How do the four-domain asymmetric eukaryotic Nav and Cav channels work?
While the bacterial NaV channel structures help to elucidate some fundamental principles of NaV and CaV channel functions, the eukaryotic NaV and CaV channels have a far more complex architecture and regulatory mechanisms. Structural determination of the eukaryotic NaV and CaV channels remains a “Holy Grail” of ion channel research.
Highlights.
Prokaryotic ancestors provide insights into voltage-gated Na+ and Ca2+ channels.
Voltage-gated Na+ channels permeate sodium ions in a partially hydrated form.
Slow inactivation of Na+ channels likely involves partial collapse of the pore.
New data support the sliding helix model for voltage-dependent gating.
Structural basis of Ca2+ selectivity and conductance by Ca2+-selective mutants.
Acknowledgements
W.A.C. and N.Z. are supported by NIH grants R01HL12808 and R01HL17896. W.A.C. is also supported by NIH grant R01NS15751. N.Z. is a Howard Hughes Medical Institute investigator.
Glossary
- Depolarization
A rapid shift of the charge inside the cell from negative to neutral or positive.
- Resting state
The state of a NaV or CaV channel under resting membrane potential. The resting state of the channel is characterized by a closed pore with low open probability and the S4 segments of the VSMs in inward, resting positions.
- Activated state
The state of a NaV or CaV channel during membrane depolarization. The channel is characterized by an open pore and the S4 segments of the VSMs in outward, activated positions.
- Pre-open state
A transitional state of a NaV or CaV channel under membrane depolarization. This state is characterized by activated VSMs with the S4 helix in outward, activated positions with the pore still closed, but poised to spring open.
- Inactivated state
The state adopted by a NaV or CaV channel during prolonged depolarization. Inactivated states are characterized by one or more VSMs in activated conformation but a blocked pore.
- Fast inactivation
Rapid inactivation of eukaryotic NaV channels within 1-2 milliseconds. The inactivation process involves a hinged-lid mechanism in which the pore is plugged by the intracellular linker between domains III and IV. This structural feature is not present in bacterial NaV channels. Fast inactivation is rapidly reversed with a few milliseconds upon repolarization.
- Slow inactivation
Slow inactivation of both prokaryotic and eukaryotic NaV channels over hundreds of milliseconds. The inactivation mechanism involves rearrangement of the pore. Slow inactivation is slowly reversed upon repolarization. Voltage-dependent inactivation of eukaryotic CaV channels is likely to share a similar mechanistic and structural basis.
- Selectivity filter
A constriction of an ion channel in the pore, which confers selective permeation of specific ions by the channel.
- Voltage-sensing module
A four transmembrane helical module that confers voltage sensitivity to voltage-gated ion channels.
- Gating charges
Positively charged amino acid residues (usually Arg) located at three-residue intervals along the S4 transmembrane segment in the VSM. The electrical field caused by the membrane potential exerts an electrostatic force on these positive charges. At the negative internal resting membrane potential, these positive gating charges are pulled inward, like the trigger of a cocked gun. Depolarization releases this electrostatic force, allowing the gating charges and the S4 segment to move outward rapidly, activate the VSM, and initiate the conformational changes that open the pore. Repolarization pulls the gating charges back into their inward, resting position where they are ready for activation again upon depolarization.
- Pore module
The S5 and S 6 transmembrane segments and intervening P loop that form the central pore of a voltage-gated ion channel where permeating ions are conducted.
- Activation gate
A structural element at the intracellular end of a voltage-gated ion channel that controls the opening and closure of the pore in response to VSM activation.
- “Knock-off” mechanism
A hypothetical ion conductance mechanism in which ionic repulsion triggered by simultaneous occupation of adjacent ion-binding sites facilitates the rapid and unidirectional movement of ions through the selectivity filter.
- Stepwise permeation
A rate-theory based ion permeation mechanism for CaV channel in which stepwise changes in the binding affinities of Ca2+ ions to multiple sites of the selectivity filter facilitate the high Ca2+ flux without repulsive interactions. The energy barriers are lower, and therefore conduction rates are faster, for stepwise movement from free solution to high-affinity ion binding sites than for a single jump from free solution to a high-affinity ion binding site.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hille B. Ionic Channels of Excitable Membranes. 3rd Ed Sinauer Associates Inc.; Sunderland, MA: 2001. [Google Scholar]
- 2.Tsien RW. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- 3.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catterall WA. The molecular basis of neuronal excitability. Science. 1984;223:653–661. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 6.Stühmer W. Structure-function studies of voltage-gated ion channels. Annu Rev Biophys Biophys Chem. 1991;20:65–78. doi: 10.1146/annurev.bb.20.060191.000433. [DOI] [PubMed] [Google Scholar]
- 7.Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 8.Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 9.Payandeh J, et al. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payandeh J, et al. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai CJ, et al. Two alternative conformations of a voltage-gated sodium channel. J Mol Biol. 2013;425:4074–4088. doi: 10.1016/j.jmb.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 13.McCusker EC, et al. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat Commun. 2012;3:1102. doi: 10.1038/ncomms2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaya D, et al. Voltage-gated sodium channel (NaV) protein dissection creates a set of functional pore-only proteins. Proc Natl Acad Sci U S A. 2011;108:12313–12318. doi: 10.1073/pnas.1106811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaya D, et al. Structure of a prokaryotic sodium channel pore reveals essential gating elements and an outer ion binding site common to eukaryotic channels. J Mol Biol. 2014;426:467–483. doi: 10.1016/j.jmb.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagneris C, et al. Structural model of the open-closed-inactivated cycle of prokaryotic voltage-gated sodium channels. J Gen Physiol. 2015;145:5–16. doi: 10.1085/jgp.201411242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarov-Yarovoy V, et al. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci U S A. 2012;109:E93–102. doi: 10.1073/pnas.1118434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagneris C, et al. Role of the C-terminal domain in the structure and function of tetrameric sodium channels. Nat Commun. 2013;4:2465. doi: 10.1038/ncomms3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irie K, et al. The C-terminal helical bundle of the tetrameric prokaryotic sodium channel accelerates the inactivation rate. Nat Commun. 2012;3:793. doi: 10.1038/ncomms1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- 21.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 22.Yarov-Yarovoy V, et al. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K+ channels. Proc Natl Acad Sci U S A. 2006;103:7292–7297. doi: 10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vargas E, et al. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J Gen Physiol. 2012;140:587–594. doi: 10.1085/jgp.201210873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long SB, et al. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 26.Long SB, et al. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 27.Long SB, et al. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, et al. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat Struct Mol Biol. 2014;21:244–252. doi: 10.1038/nsmb.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952;116:497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilin YY, Ruben PC. Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem Biophys. 2001;35:171–190. doi: 10.1385/CBB:35:2:171. [DOI] [PubMed] [Google Scholar]
- 31.Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol. 1978;283:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, et al. A gating hinge in Na+ channels; a molecular switch for electrical signaling. Neuron. 2004;41:859–865. doi: 10.1016/s0896-6273(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 33.Pavlov E, et al. The pore, not cytoplasmic domains, underlies inactivation in a prokaryotic sodium channel. Biophys J. 2005;89:232–242. doi: 10.1529/biophysj.104.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, et al. Reversed voltage-dependent gating of a bacterial sodium channel with proline substitutions in the S6 transmembrane segment. Proc Natl Acad Sci U S A. 2004;101:17873–17878. doi: 10.1073/pnas.0408270101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971;59:599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972;59:637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975;66:535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulmschneider MB, et al. Molecular dynamics of ion transport through the open conformation of a bacterial voltage-gated sodium channel. Proc Natl Acad Sci U S A. 2013;110:6364–6369. doi: 10.1073/pnas.1214667110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, et al. Chemistry of ion coordination and hydration revealed by a potassium channel- Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti N, et al. Catalysis of Na+ permeation in the bacterial sodium channel NaVAb. Proc Natl Acad Sci U S A. 2013;110:11331–11336. doi: 10.1073/pnas.1309452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, et al. Analysis of the selectivity filter of the voltage-gated sodium channel NaVRh. Cell Res. 2013;23:409–422. doi: 10.1038/cr.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinemann SH, et al. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- 43.Ellinor PT, et al. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, et al. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 45.Yue L, et al. The cation selectivity filter of the bacterial sodium channel, NaChBac. J Gen Physiol. 2002;120:845–853. doi: 10.1085/jgp.20028699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang L, et al. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature. 2014;505:56–61. doi: 10.1038/nature12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mccleskey EW, Almers W. The Ca Channel in Skeletal-Muscle Is a Large Pore. Proc Nat Acad Sci U S A. 1985;82:7149–7153. doi: 10.1073/pnas.82.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almers W, McCleskey EW. The nonselective conductance due to calcium channels in frog muscle: calcium-selectivity in a single file pore. J Physiol. 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almers W, et al. A nonselective cation conductance in frog muscle membrane blocked by micromolar external Ca++ J Physiol. 1984;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- 51.Dang TX, McCleskey EW. Ion channel selectivity through stepwise changes in binding affinity. J Gen Physiol. 1998;111:185–193. doi: 10.1085/jgp.111.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev Physiol. 2003;65:133–159. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- 53.Ashcroft FM. Ion Channels and Disease. Academic Press; London: 2000. [Google Scholar]
- 54.Cox B, Gosling M. Ion Channel Drug Discovery. Royal Society of Chemistry; 2014. [Google Scholar]
- 55.Bagneris C, et al. Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism. Proc Natl Acad Sci U S A. 2014;111:8428–8433. doi: 10.1073/pnas.1406855111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catterall WA, Swanson TM. Structural Basis for Pharmacology of Voltage-Gated Sodium and Calcium Channels. Mol Pharmacol. 2015;88:141–150. doi: 10.1124/mol.114.097659. [DOI] [PMC free article] [PubMed] [Google Scholar]