Abstract
Brominated diphenyl ether (BDE)-47 is a prevalent flame retardant chemical found in human tissues and is linked to adverse pregnancy outcomes in humans. Because dysregulation of the prostaglandin pathway is implicated in adverse pregnancy outcomes, the present study investigates BDE-47 induction of prostaglandin synthesis in a human extravillous trophoblast cell line, HTR-8/SVneo, examining the hypothesis that BDE-47 increases generation of reactive oxygen species (ROS) to stimulate the prostaglandin response. Treatment with 20 μM BDE-47 significantly increased mRNA expression of prostaglandin-endoperoxide synthase 2 (PTGS2) at 4, 12 and 24 h, and 24-h treatment significantly increased cyclooxygenase (COX)-2 cellular protein expression and prostaglandin E2 (PGE2) concentration in culture medium. The BDE-47-stimulated PGE2 release was inhibited by the COX inhibitors indomethacin and NS398, implicating COX activity. Exposure to 20 μM BDE-47 significantly increased ROS generation as measured by carboxydichlorofluorescein fluorescence, and this response was blocked by cotreatment with the peroxyl radical scavenger (±)-α-tocopherol. (±)-α-Tocopherol cotreatment suppressed BDE-47-stimulated increases of PGE2 release without significant effects on COX-2 mRNA and protein expression, implicating a role for ROS in post-translational regulation of COX activity. Because prostaglandins regulate trophoblast functions necessary for placentation and pregnancy, further investigation is warranted of BDE-47 impacts on trophoblast responses.
Keywords: Polybrominated diphenyl ethers (PBDEs), HTR-8/SVneo cells, human placental cells, prostaglandins, α-tocopherol, cyclooxygenase (COX)-2
1. Introduction
Proper placental development is prerequisite for a successful pregnancy. Abnormal placentation contributes to the pathophysiology of adverse obstetrical complications such as preeclampsia (Brosens, 1977; Gerretsen et al., 1981; Robertson et al., 1967; Sheppard and Bonnar, 1976), intrauterine growth restriction (Gerretsen et al., 1981; Hustin et al., 1983; Labarrere and Althabe, 1987; Sheppard and Bonnar, 1981), spontaneous abortion (Hustin et al., 1990; Khong et al., 1987), preterm premature rupture of membranes (Kim et al., 2002), and preterm birth (Kim et al., 2003). Although the mechanisms responsible for improper placentation have not been fully elucidated, the role of impaired trophoblast invasion has been implicated (Zhou et al., 1997a).
The extravillous trophoblasts (EVTs) are a highly proliferative and migratory cell population that invades the decidual and myometrial segments of the spiral arteries, resulting in the reversible remodeling of the arterial wall architecture (Anton et al., 2012; Brosens et al., 1967; Pijnenborg et al., 1983; Pijnenborg et al., 1980). Trophoblast invasion is tightly regulated by a number of autocrine and paracrine factors including growth factors, growth factor-binding proteins, and proteoglycans (Chakraborty et al., 2002; Lala and Chakraborty, 2003). Recently, inflammatory mediators such cytokines and prostaglandins have been shown to play a role in the regulation of trophoblast function during first trimester of pregnancy (Biondi et al., 2006; Horita et al., 2007d; Jovanovic et al., 2010; Jovanovic and Vicovac, 2009; Nicola et al., 2005d).
Prostaglandins are small lipid molecules synthesized from membrane phospholipids in response to various physiological and pathological stimuli (Nicola et al., 2005d). Of these, prostaglandin E2 (PGE2) is one of the most extensively studied prostaglandins, and has been shown to play critical roles in processes required in successful pregnancy, for example, implantation (Psychoyos et al., 1995; Yee et al., 1993), immunoprotection of the semiallogenic conceptus (Parhar et al., 1988), and parturition (Keelan et al., 2003). Dysregulation of PGE2 production within the gestational compartment has been linked to adverse birth outcomes such as intrauterine growth restriction, preeclampsia and preterm birth (Germain et al., 1999; Ness and Sibai, 2006). Although it is not fully understood how dysregulated prostaglandin pathways lead to these adverse impacts, it is suggested that PGE2 regulates trophoblast cellular functions that are critical for successful placentation (Biondi et al., 2006; Horita et al., 2007a; Nicola et al., 2005a).
Polybrominated diphenyl ethers (PBDEs) are commercially produced synthetic flame retardants that have been used in textiles, plastics, building materials and insulation (Miller et al., 2009a). Because of PBDEs’ environmental persistence and toxicity, the US EPA has identified PBDEs as a priority human health concern (U.S. Environmental Protection Agency, 2006). Limited studies report reproductive toxicity of PBDEs during pregnancy. Rabbits orally exposed to PBDEs showed decreased gestation length (Breslin et al., 1989). In human studies, Main et al. reported a significantly higher risk of cryptorchidism for sons born to mothers with elevated PBDE levels in breast milk (Main et al., 2007). In addition, Chao et al. found that elevated levels of PBDEs in breast milk correlated with decreased infant birth weight, infant birth length, infant chest circumference and infant body mass index (Chao et al., 2007). Elevated levels of PBDEs in human umbilical cord blood have been correlated with preterm birth, low birth weight or stillbirth (Wu et al., 2010). Although these studies report associations between PBDE exposure and adverse birth outcomes, and PBDEs distribute to human placenta (Frederiksen et al., 2009), extraplacental membranes (Miller et al., 2009b), amniotic fluid (Miller et al., 2012), and umbilical cord blood (Frederiksen et al., 2009), studies of mechanisms by which PBDEs act on gestational tissues during pregnancy are limited. Specifically, we identified one study reporting that pre-exposure of placental explants to a PBDE mixture of congers 47, 99 and 100 enhanced placental pro-inflammatory response to heat-killed E. Coli, with increased PGE2 release and cyclooxygenase (COX)-2 expression (Peltier et al., 2012).
Our previous study showed that treatment with BDE-47, one of the most prevalent congeners found in human tissues (Hites, 2004), stimulates production of the proinflammatory cytokine IL-6 via a reactive oxygen species (ROS)-mediated mechanism in the first trimester EVT human placental cell line HTR-8/SVneo (Park et al., 2014b). Although inappropriate activation of prostaglandin pathways may lead to placental dysfunction, there is a paucity of reports on PBDE-stimulated prostaglandin release in first trimester placenta. Increased oxidative stress in placenta, possibly due to increased generation of ROS, has been observed in pathological pregnancies, and ROS have been implicated in the activation of inflammatory responses in gestational compartments (Buhimschi et al., 2003; Cindrova-Davies et al., 2007). Moreover, formation of ROS has been shown to modulate prostaglandin pathways in various experimental models including murine placenta (Basu, 1999; Davidge, 1998; Wentzel et al., 1999; White et al., 2002).
The present study examines the hypothesis that BDE-47 stimulates PGE2 production in human placental cells via a ROS-mediated mechanism. This work was performed with the HTR-8/SVneo cell line (Graham et al, 1993). The HTR-8/SVneo cell line was derived from first trimester placentae and has provided a useful cell culture model for studies of EVT cellular responses (Liu et al., 2012; Wang et al., 2012; Weber et al., 2013) and initial investigations of toxicant actions on EVTs (Park et al., 2014a; Tetz et al., 2013a).
2. Materials and Methods
2.1. Chemicals and assay kits
BDE-47 was purchased from AccuStandard (New Haven, CT, USA). Dimethyl sulfoxide (DMSO), tert-butyl hydroperoxide (TBHP), indomethacin, NS398, and (±)-α-tocopherol were purchased from Sigma Aldrich (St. Louis, MO, USA). Purchase of 6-carboxy dichlorodihydrofluorescein diacetate (carboxy-H2DCF-DA), Hoechst 33342 dye, RPMI 1640 medium, fetal bovine serum (FBS), OptiMem 1 reduced-serum medium, Hank’s balanced salt solution (HBSS), and 0.25% trypsin/EDTA solution and penicillin/streptomycin (P/S) were from Invitrogen Life Technologies (Carlsbad, CA, USA). The PGE2 ELISA kit and arachidonic acid were purchased from Cayman Chemical (Ann Arbor, MI, USA). QIAshredder, RNeasy mini plus kit, RT2 First Strand kit for reverse transcriptase reaction, RT2 qPCR SYBR Green/ROX Master Mix, and primers for human β-microglobulin, PTGS2, PTGES and HPGD were purchased from Qiagen (Valencia, CA, USA). The NP-40 substitute, IGEPAL CA-630, was purchased from United States Biological (Salem, MA). PhosStop protease inhibitor cocktail and complete mini protease inhibitor cocktail tablets were from Roche (Indianapolis, IN). Reducing Laemmli SDS sample buffer was purchased from Boston BioProducts (Ashland, MA). Memcode reversible protein staining kit and bicinchoninic acid (BCA) assay kit were from Thermo Scientific (Waltham, MA). Alkaline phosphatase-linked secondary antibody was purchased from Cell Signaling Technology (Beverly, MA). Enhanced chemifluorecence (ECF) substrate and PVDF membrane Hybond-P were purchased from GE Healthcare Life Sciences (Pittsburgh, PA).
2.2. Cell Culture and treatment
The human first trimester extravillous trophoblast cell line HTR-8/SVneo was kindly provided by Dr. Charles S. Graham (Queen’s University, Kingston, ON, Canada). Cytotrophoblast cells isolated from first trimester human placentae were immortalized with simian virus 40 large T antigen to generate the HTR-8/SVneo cell line (Graham et al, 1993). Cells between passages 71 and 84 were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 humidified atmosphere. Cells were grown to 70–90% confluence before treatment. Cells were washed twice with OptiMem 1 containing 1% FBS and 1% P/S, and then acclimated with the medium for 1 h at 37 °C. From solutions of 5, 10, 15 and 20 mM BDE-47 in DMSO, exposure media containing 5, 10, 15 and 20 μM BDE-47 were made in OptiMem 1 containing 1% FBS and 1% P/S immediately prior to initiating the experiments. BDE-47 concentrations were selected to include concentrations relevant to human exposure (Doucet et al., 2009) and previously shown by us to increase generation of ROS in the HTR-8/SVneo cells (Park et al., 2014b). The final concentration of DMSO in medium was 0.7 % (v/v).
2.3. Carboxydichlorofluorescein assay
Stimulation of ROS generation was assessed using carboxydichlorofluorescein (cDCF) fluorescence in a variation of the dichlorofluorescein (DCF) assay. We used cDCF instead of DCF because the additional negative charges on cDCF improve cell retention of the probe. Because artifactual results can occur in the cDCF assay due to interactions with toxicants (Tetz et al., 2013b), we confirmed that there was no increased cDCF fluorescence by BDE-47 in cell-free medium (data not shown). The HTR-8/SVneo cells were seeded at a density of 2.4 × 105 cells per well in a 6-well plate and cultured for 24 h at 37 °C. Cells were washed once with OptiMem 1 medium containing 1% FBS and 1% P/S, and then were untreated (NT, non-treated controls), or were exposed to solvent control (DMSO 0.7% v/v), 15 μM BDE-47 or 20 μM BDE-47 for 4 h in the absence or presence of 20 μM (±)-α-tocopherol. Treatment with 100 μM tert-butyl hydroperoxide (TBHP) was included as a positive control (Vessey et al., 1992). After removal of the exposure media and rinsing with HBSS, cells were collected by treatment with 0.25% trypsin/EDTA solution for 2 min, washed twice by centrifugation and resuspension in HBSS, and then re-suspended in HBSS. After a 1-h incubation with 100 μM carboxy-H2DCF-DA in HBSS, the fluorescence intensity of 200,000 cells in a 96-well, black, clear-bottomed plate was measured using a Molecular Devices SpectraMax Gemini M2e plate reader at an excitation wavelength of 492 nm and emission wavelength of 522 nm.
2.4. Prostaglandin E2 assay
The HTR-8/SVneo cells were seeded at a density of 5 × 104 cells per well in a 24-well plate and cultured for 24 h at 37 °C. Cells were washed once with OptiMem1 medium containing 1% FBS and 1% P/S, and then exposed to 20 μM BDE-47 in the absence and presence of 10 μM indomethacin, a non-selective COX inhibitor, or 5 μM NS398, a COX-2-specific inhibitor. After a 24-h incubation, the culture medium was removed and cells were washed once with HBSS. Then, cells were incubated with 2.5 μM arachidonic acid in HBSS for 4 h at 37 °C. After the 4-h incubation, the concentration of PGE2 in culture medium was measured by sandwich ELISA following the manufacturer’s protocols. To probe ROS-mediated activation of prostaglandin pathways by BDE-47, HTR-8/SVneo cells were co-treated for 24 h with 20 μM (±)-α-tocopherol, a peroxyl radical scavenger. Concentrations of PGE2 in the medium were analyzed by ELISA as described above and expressed as pg/ml.
2.5. RNA extraction and quantitative real-time polymerase chain reaction
After a 24-h incubation with BDE-47, cell lysates were collected and homogenized using QIA shredder. Total RNA was extracted from homogenized lysates using a RNeasy mini plus kit, and cDNA was synthesized from 1 μg of total RNA using a RT2 First Strand Kit. The procedures were performed according to instructions of the manufacturer. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in a total volume of 25 μL containing 4 μL of cDNA template, 1 μL of a gene-specific primer (PTGS2, PTGES, HPGD), 12.5 μL of RT2 SYBR Green qPCR Master Mix, and 7.5 μL of nuclease-free H2O using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). A housekeeping gene, β-microglobulin, was co-amplified as an internal control. Analysis by qRT-PCR was performed with an initial denaturation step of 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, then 5 s at 60°C. At the end of each cycle, the fluorescence emitted by the SYBR Green was measured. After completion of the cycling process, samples were subjected to a temperature ramp (from 65°C to 95°C at 0.5°C/s) with continuous fluorescence monitoring for melting curve analysis. Signal intensities of target genes were quantified and normalized to the signal of β-microglobulin using Bio-Rad CFX manager software. The level of mRNA expression was presented as fold change compared to solvent controls.
2.6. Western blot
The HTR-8/SVneo cells were seeded at a density of 2.4 × 105 cells per well in a 6-well plate and cultured for 24 h at 37 °C. Cells were washed once with Optimem1 medium containing 1% FBS and 1% P/S, and then exposed to 20 μM BDE-47 in the absence or presence of 20 μM (±)-α-tocopherol. After a 24-h incubation, the culture medium was removed, and cells were washed twice with ice-cold dPBS, incubated with lysis buffer (0.5% IGEPAL, 250 mM NaCl, 50 mM tris-HCl, with a protease inhibitor tablets), and then scraped from the plates to collect cell lysates. After centrifugation of lysates, the supernatant was collected and stored at −80°C until analysis. Total protein was quantified by BCA assay. The protein samples were boiled in sample buffer, and then 75 μg protein was subjected to SDS-polyacrylamide gel electrophoresis followed by electrotransfer to a PVDF membrane. Transfer efficiency was confirmed by reversible membrane staining (Memcode or Ponceau). Membranes were blocked at room temperature for 1 h with 5% milk in Tris-buffered saline supplemented with 0.1% Tween (TBST; 20 mM Tris-HCl, 137 mM NaCl, pH 7.6). Membranes were probed with primary antibodies overnight at 4°C with agitation in 5% BSA TBST. Following washing with TBST under agitation for 3 min three times, membranes were incubated with alkaline phosphatase-conjugated secondary antibodies for 1 h at RT in 5% milk TBST. All antibodies were diluted at 1:2000. Bands were imaged after developing the blot with ECF for 5 min, and imaged on a Fujifilm Fluorescent Image Analyzer FLA-5000. Images shown are representative of 3 individual experiments. Densitometry was used to semi-quantitate data using Multi Gauge software (Fujifilm).
2.7. Statistical analysis
Statistical analysis was performed with Sigma Plot 11.0 software (Systat Software Inc., San Jose, CA, USA). After determining acceptable homogeneity of variance and normality (P<0.05), data were analyzed either by one-way analysis of variance (ANOVA) or two-way ANOVA. If significant effects were detected, the ANOVA was followed by Tukey post-hoc comparison of means. A P value <0.05 was considered statistically different. Data were expressed as means ± SEM. All experiments were repeated at least three times and all treatments were performed at least in triplicate in each experiment.
3. Results
3.1. Effects of BDE-47 on mRNA expression of PTGS2, PTGES and HPGD
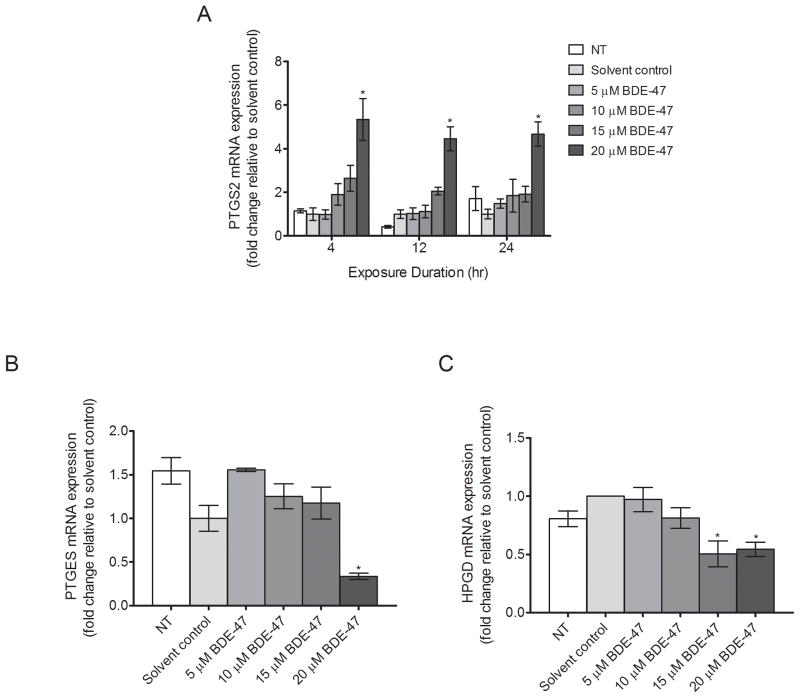
Expression of enzymes involved in prostaglandin synthesis and catabolism was measured at the mRNA level in HTR-8/SVneo cells exposed to BDE-47 concentrations ranging from 5 to 20 μM. The BDE-47 concentrations were selected based on prior findings that IL-6 and IL-8 release were stimulated with 15 and 20 μM BDE-47, and superoxide production was increased with 10, 15, 20 μM BDE-47 in HTR-8/SVneo cells (Park et al., 2014b). Treatment with 20 μM BDE-47, but not lower concentrations, significantly increased mRNA expression of PTGS2, the gene for COX-2, compared to the solvent control at 4, 12 and 24 h by 5.3-fold, 4.5-fold, and 4.7-fold, respectively (P<0.05, Fig. 1A). mRNA expression of PTGES, the gene for prostaglandin E synthase (PGES), was suppressed 66% with 20 μM BDE-47 treatment, but not lower BDE-47 concentrations, compared with solvent control after 24 h (P<0.05, Fig. 1B). In addition, mRNA expression of HPGD, the gene for the prostaglandin catabolic enzyme 15-hydroxyprostaglandin dehydrogenase, was reduced by 66% and 44% with 15 and 20 μM BDE-47, respectively, after 24 h (P<0.05, Fig. 1C), with no statistically significant treatment effects at lower BDE-47 concentrations. There were no statistically significant differences between non-treated controls and solvent controls at any time point.
Fig. 1.
Concentration-dependent effects of BDE-47 on mRNA expression of PTGS2, PTGES, and HPGD in HTR-8/SVneo cells. Cells received no treatment (non-treated control, NT), or were treated with solvent control (DMSO, 0.7% v/v) or BDE-47 (5, 10, 15 or 20 μM), and then mRNA expression of target genes was quantified by qRT-PCR. A) Time-course and concentration-dependent response of BDE-47 on PTGS2 mRNA expression after 4, 12, or 24 h. B) BDE-47 concentration-dependent effects on PTGES mRNA expression after 24 h. C) BDE-47 concentration-dependent effects on HPGD mRNA expression after 24 h. *P<0.05, significantly different compared to solvent control within each time point (A) or experiment (B and C).
3.2. Effects of BDE-47 on PGE2 release
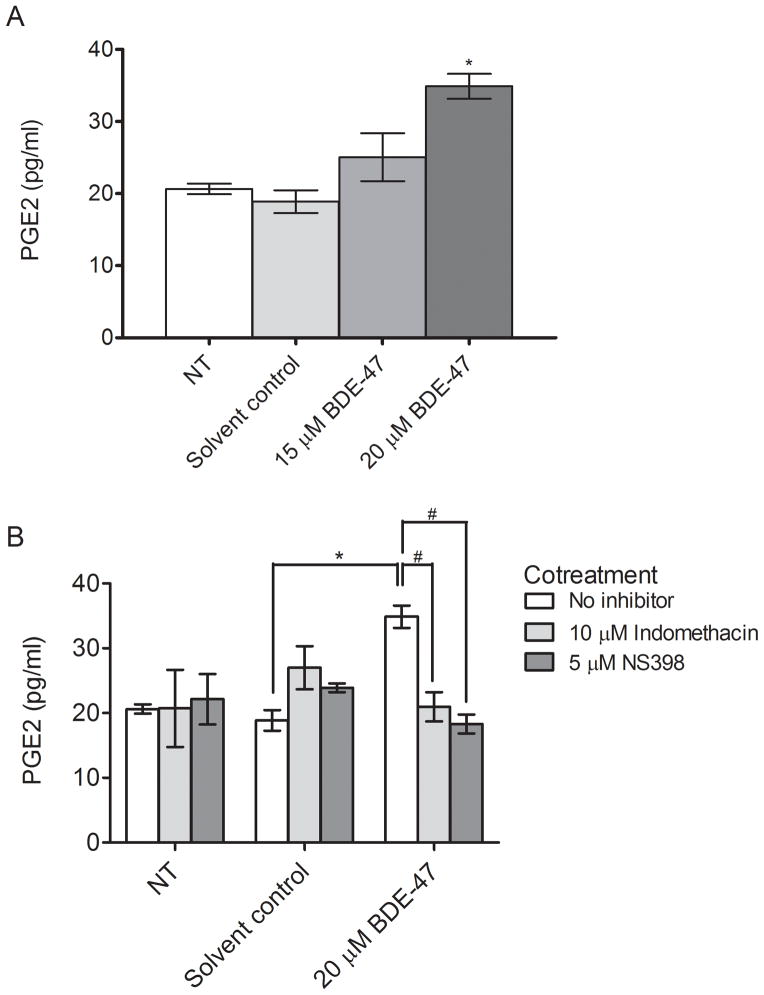
Because 20 μM BDE-47 induced a rapid increase of PTGS2 mRNA that was sustained for 24 h, we next evaluated stimulation of PGE2 release by 15 and 20 μM BDE-47. Treatment of HTR-8/SVneo cells for 24 h with 20 μM BDE-47 induced a significant 1.8-fold increase in PGE2 concentration in culture medium compared to the solvent control (P<0.05, Fig. 2A) whereas the effect at 15 μM BDE-47 was not statistically significant, in agreement with BDE-47-stimulated PTGS2 mRNA expression shown in Fig. 1A. Co-treatment for 24 h with 10 μM indomethacin, a nonspecific COX inhibitor, or 5 μM NS-398, a COX-2 specific inhibitor, resulted in the complete suppression of BDE-47-stimulated PGE2 release to the levels comparable of the solvent control (P<0.05, Fig. 2B), indicating that BDE-47-induced PGE2 release was dependent on COX activity. Notably, the NS-398-mediated PGE2 decrease was similar to the indomethacin-mediated PGE2 decrease, suggesting that BDE-47-stimulated PGE2 production is mainly dependent on COX-2 activity. There were no statistically significant differences between non-treated controls and solvent controls, nor did treatment with COX inhibitors alone or 15 μM BDE-47 significantly alter PGE2 release.
Fig. 2.
BDE-47 effects on COX activity in HTR-8/SVneo cells. COX activity was inferred by quantification of PGE2 in the culture medium from cells stimulated with exogenous arachidonic acid after BDE-47 treatment. A) PGE2 concentrations in medium of cells that were non-treated (NT, control), or treated with solvent control (DMSO, 0.7% v/v), 15 μM BDE-47, or 20 μM BDE-47 for 24 h. B) PGE2 concentrations in medium of cells that were non-treated (NT, control), or treated with solvent control (DMSO, 0.7% v/v) or 20 μM BDE-47 for 24 h in the absence or presence of the nonspecific cyclooxygenase (COX) inhibitor indomethacin or the COX-2 specific inhibitor NS 398. *P<0.05, significantly different compared to solvent control with no (±)-α-tocopherol cotreatment. #P<0.05, significantly different from each other.
3.3. Effects of (±)-α-tocopherol on BDE-47-stimulated ROS production
Fluorescence of cDCF was used to assess the effect of (±)-α-tocopherol on BDE-47-stimulated ROS production. Treatment with 20 μM BDE-47 increased cDCF fluorescence by 66% in the HTR-8/SVneo cells indicating increased generation of reactive species, and this BDE-47-stimulated response was blocked by (±)-α-tocopherol cotreatment (P<0.05, Table 1.). Treatment with 100 μM TBHP, included as a positive control, increased cDCF fluorescence by 176%. There were no statistically significant differences between non-treated controls, solvent controls, and (±)-α-tocopherol-treated groups, nor was the cDCF fluorescence observed with 15 μM BDE-47 statistically different from solvent controls.
Table 1.
Quantification of reactive oxygen species production in HTR-8/SVneo cellsa
| Treatment | cDCF fluorescence intensity |
|---|---|
|
| |
| Non-treated control | 177.88 ± 5.22 |
| Solvent control | 178.79 ± 7.38 |
| 15 μM BDE-47 | 236.63 ± 16.60 |
| 20 μM BDE-47 | 296.81 ± 18.70* |
| 20 μM BDE-47+ 20 μM (±)-α-tocopherol | 183.88 ± 7.96# |
| 100 μM TBHP | 493.82 ± 40.47* |
| 20 μM (±)-α-tocopherol | 181.39 ± 6.97 |
HTR-8/SVneo cells were non-treated (non-treated control), or were treated with DMSO (0.7% v/v, solvent control), 15 or 20 μM BDE-47, or 100 μM tert-butyl hydroperoxide (TBHP, positive control) in the absence or presence of (±)-α-tocopherol for 4 h.
P<0.05, significantly different compared to solvent control.
P<0.05, significantly different compared to 20 μM BDE-47-treated group.
3.4. Effects of (±)-α-tocopherol treatment on BDE-47-stimulated PGE2 release
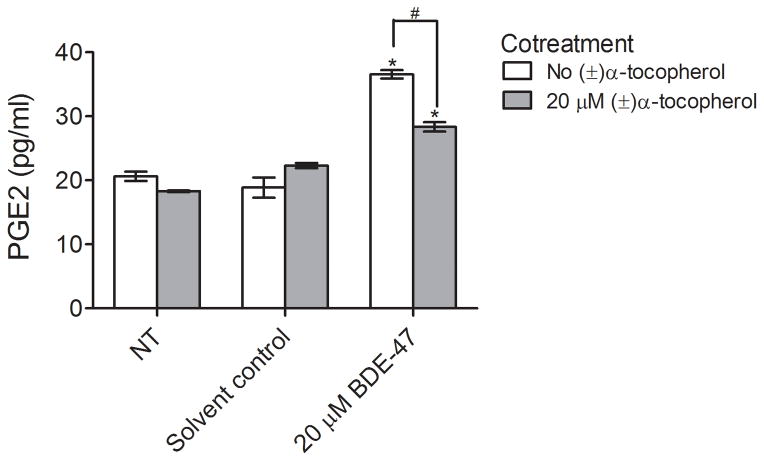
To investigate the role of ROS in BDE-47-induced PGE2 production, HTR-8/SVneo cells were co-treated with 20 μM BDE-47 and 20 μM (±)-α-tocopherol for 24 h. As shown in Fig. 3, (±)-α-tocopherol cotreatment reduced PGE2 release stimulated by 20 μM BDE-47, with PGE2 concentrations in culture medium decreased 22.5% compared to cultures exposed to BDE-47 without (±)-α-tocopherol pretreatment (Fig. 3; P<0.05). There were no statistically significant differences between non-treated controls and solvent controls, nor did treatment with (±)-α-tocopherol alone significantly alter PGE2 release.
Fig. 3.
(±)-α-Tocopherol effects on BDE-47-stimulated COX activity in HTR-8/SVneo cells. COX activity was inferred by quantification of PGE2 concentrations in the culture medium of cells stimulated with exogenous arachidonic acid after BDE-47 treatment. Cells received no BDE-47 treatment (non-treated control, NT), or were treated with solvent control (DMSO, 0.7% v/v) or 20 μM BDE-47 for 24 h in the absence or presence of 20 μM (±)-α-tocopherol. *P<0.05, significantly different compared to solvent control with no (±)-α-tocopherol cotreatment. #P<0.05, statistically significantly different from each other.
3.5. Effects of (±)-α-tocopherol treatment on COX-2 expression
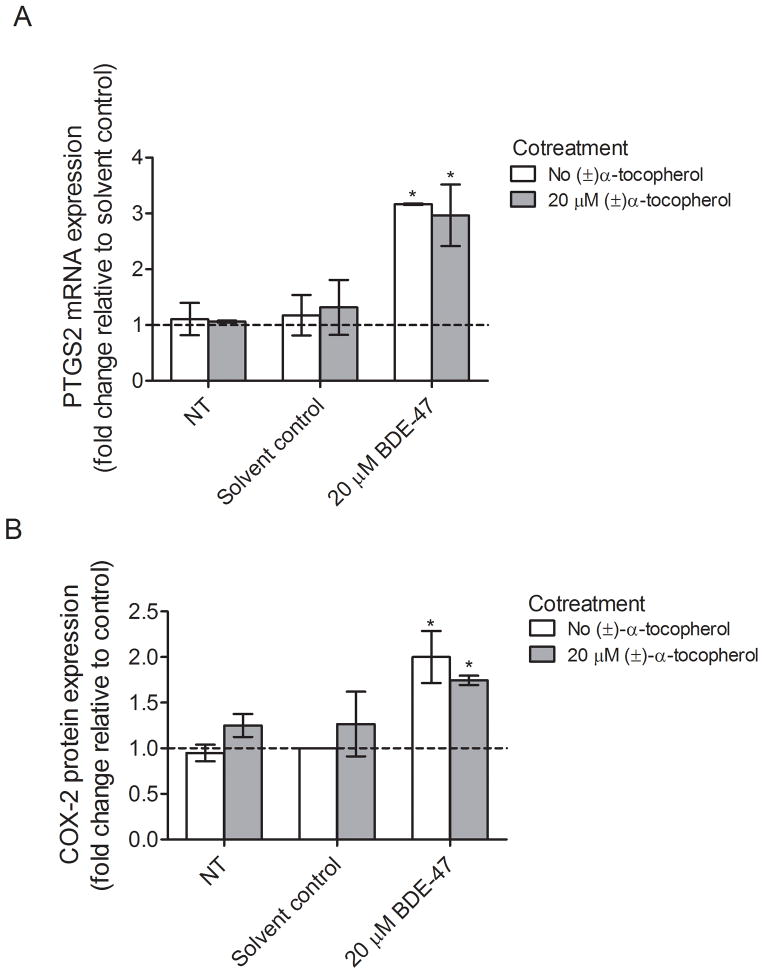
To test whether suppression of BDE-47-induced PGE2 release by (±)-α-tocopherol cotreatment stems from changes in mRNA or protein expression for COX-2, qRT-PCR or western blot were conducted. Although 24-h treatment with 20 μM BDE-47 induced a significant 3.2-fold increase in PTGS2 mRNA expression in HTR-8/SVneo cells compared with solvent control (P<0.05), the mRNA expression of PTGS2 was not significantly changed with (±)-α-tocopherol cotreatment compared to non-(±)-α-tocopherol-treated groups (Fig. 4), suggesting that ROS do not regulate COX-2 expression at the transcription level. Likewise, treatment for 24 h with 20 μM BDE-47 increased COX-2 protein abundance by 2.0-fold compared with control (Fig. 5B; P<0.05), but cotreatment with (±)-α-tocopherol did not significantly change BDE-47-stimulated COX-2 expression compared to non-(±)-α-tocopherol-treated groups, implicating that ROS do not regulate COX-2 expression at the translational level, either.
Fig. 4.
(±)-α-Tocopherol effects on BDE-47-stimulated PTGS2 mRNA expression and COX-2 protein expression in HTR-8/SVneo cells. Cells received no BDE-47 treatment (non-treated control, NT), or were treated with solvent control (DMSO, 0.7% v/v) or 20 μM BDE-47 for 24 h in the absence or presence of 20 μM (±)-α-tocopherol. A) Fold changes in PTGS2 mRNA expression relative to control (dashed line, Solvent control with no (±)-α-tocopherol cotreatment) *P<0.05, statistically significantly different compared to solvent control with no (±)-α-tocopherol cotreatment. B) Fold changes in COX-2 protein expression relative to control (dashed line, Solvent control with no (±)-α-tocopherol cotreatment). Densitometry data for COX-2 were normalized to the β-tubulin loading control. *P<0.05, statistically significantly different compared to control (dashed line, Solvent control with no (±)-α-tocopherol cotreatment).
4. Discussion
PGE2 is a pro-inflammatory mediator of critical trophoblast functions during placentation (Biondi et al., 2006; Horita et al., 2007a; Nicola et al., 2005a; Zhou et al., 1997a). The present study demonstrated that BDE-47, a prevalent flame retardant chemical in the environment and in human tissue samples, stimulated PGE2 release from a human first trimester EVT cell line, HTR-8/SVneo. In addition, we showed that treatment with BDE-47 resulted in differential expression of genes relevant to PGE2 pathways such as PTGS2, PGTES, and HPGD. Furthermore, we showed that BDE-47-stimulated PGE2 release was regulated by ROS formation in HTR-8/SVneo cells. The interaction of PBDEs and prostaglandin pathways in gestational tissues has not been extensively explored previously. Indeed, we found only one related previous study, which showed that pre-exposure of placental explants to a PBDE mixture of congers 47, 99 and 100 enhanced placental pro-inflammatory response to heat-killed E. Coli, with increased PGE2 release and COX-2 expression (Peltier et al., 2012).
PGE2 production is mainly regulated by substrate availability (arachidonic acid) and the activity of COX, the rate limiting step in PGE2 production (Beharka et al., 2002; Shanmugam et al., 2006). Because each treatment group was supplemented with exogenous arachidonic acid in the present study, stimulated PGE2 production is not affected by substrate availability but may be a reflection of increased COX activity (Hayek et al., 1994; Hayek et al., 1997). Suppression of PGE2 release by co-treatment with COX inhibitors confirmed that BDE47-induced PGE2 production was dependent on COX activity. Because treatment with NS-398, a COX-2-specific inhibitor, was sufficient to completely suppress BDE-47-stimulated PGE2 release, it is suggested that BDE-47-mediated PGE2 production was mainly dependent on COX-2 activity in HTR-8/SVneo cells.
Stimulated PGE2 release could result from changes in mRNA transcription or protein synthesis (Beharka et al., 2002). Our results showed that mRNA expression of PTGS2 was highly induced by BDE-47 treatment in HTR-8/SVneo cells whereas mRNA expression of PTGES and HPGD was reduced. Stimulated PTGS2 expression is consistent with the increased PGE2 release we observed, supporting the hypothesis that increased gene transcription may contribute to the increased COX activity. Decreased HPGD expression may also contribute to the increased PGE2 concentrations in medium, due to reduced conversion of PGE2 to inactive metabolites (Tai et al., 2006). Because PGES plays a role in the final step of PGE2 synthesis by converting PGH2 to PGE2, decreased PTGES mRNA expression is inconsistent with our findings of elevated PGE2 concentrations. However, western blot analysis of PGES protein showed that PGES protein abundance was not affected by BDE-47 treatment, suggesting that PGES protein remains at a level with sufficient activity for PGE2 production even in the circumstance of decreased PGES mRNA (Supplementary Fig. 2). An alternative explanation may involve isoforms of PGES, because there are three different PGES isotypes including cytosolic PGES (cPGES) and two membrane-bound PGES (mPGES-1 and mPGES-2) (Samuelsson et al., 2007). Of these isoforms, cPGES and mPGES-2 are constitutively expressed, whereas mPGES-1 is mainly an induced isoform (Samuelsson et al., 2007). Although the present study only measured mRNA expression of inducible mPGES-1 (PTGES), constitutively expressed cPGES and mPGES-2 would convert PGH2 produced by COX to PGE2. Moreover, the rate of PGE2 synthesis is mainly dependent on COX activity (Beharka et al., 2002; Shanmugam et al., 2006).
The present study provides new information that ROS play a role in regulation of BDE-47-mediated prostaglandin pathways in HTR-8/SVneo cells. Our finding that cotreatment with the antioxidant (±)-α-tocopherol suppressed BDE-47-stimulated ROS production and PGE2 release suggests that ROS likely play a key role in regulating BDE-47 stimulated PGE2 release from HTR-8/SVneo cells. Our results are in agreement with previous findings that α-tocopherol diminished ROS-stimulated placental PGF2α and thromboxane B2 (TXB2), as well as lipoperoxide levels (White et al., 2002). Interestingly, (±)-α-tocopherol cotreatment led to reduced PGE2 release without changing its expression, implicating post-translational regulation of COX activity by ROS. This explanation is consistent with previous reports that α-tocopherol inhibits PGE2 production and COX activity with no effect on the expression of COX in murine macrophages and in Caco2 cells (Jiang et al., 2000; O’Leary et al., 2004; Wu et al., 1998).
Vitamin E (tocopherols and tocotrienols) is an effective biological antioxidant and lipid peroxide chain-breaking free radical scavenger (Wu et al., 1998). It is reported that COX activity requires the presence of oxidant hydroperoxides (Hemler and Lands, 1980; Kulmacz and Wang, 1995; Smith et al., 1992). Therefore, it has been proposed that vitamin E may attenuate COX activity by scavenging the oxidant hydroperoxides necessary for COX activation (Wu et al., 2001). Increased lipid peroxidation by BDE-47 treatment in vitro is consistent with this mechanism (He et al., 2008; Shao et al., 2008); however, we did not measure lipid peroxidation in the present study. Another proposed mechanism involves nitric oxide (NO) and peroxynitrite (ONOO) regulation of COX activity (Wu et al., 2001). Specifically, NO and ONOO stimulate COX activity without affecting COX expression (Salvemini et al., 1995; Wu et al., 2001), and vitamin E reduces COX activity in murine macrophages by decreasing NO and ONOO production (Wu et al., 2001). Production of NO, ONOO, and NO synthase activity were reported in human first trimester primary trophoblasts, first trimester trophoblast cell lines, term primary trophoblasts, and term placenta (Al-Hijji et al., 2003; Asagiri et al., 2003; Dash et al., 2003). Because NO can combine with superoxide to form ONOO (Wu et al., 2001), our previous report of increased superoxide production by BDE-47 in HTR-8/SVneo cells (Park et al., 2014b) is consistent with the potential production of ONOO in BDE-47-treated HTR-8/SVneo cells. However, further study will be needed to measure NO and ONOO levels in HTR-8/SVneo cells stimulated by BDE-47 to test the roles of NO and ONOO on COX activity in human trophoblasts.
Sakamoto et al. suggested an alternative explanation to post-translational modulation of COX activity by vitamin E (Sakamoto et al., 1993). They reported that PGE2 production stimulated by phorbol 12-myristate 13-acetate or A-23187 was inhibited by intraperitoneal injection of vitamin E via suppression of phospholipase A2 (PLA2) activity and the subsequent decrease in arachidonic acid release (Sakamoto et al., 1991; Sakamoto et al., 1993). The latter mechanism may be relevant to our findings because we observed augmented PGE2 production with endogenous arachidonic acid in BDE-47-treated HTR-8/SVneo cells compared to controls without exogenous arachidonic acid supplementation (data not shown). However, we used an experimental approach that supplemented the cell culture medium with exogenous arachidonic acid because the observed PGE2 levels were close to the limit of detection in the assay otherwise. Therefore, the effect of vitamin E on PLA2 activity and subsequent arachidonic acid release was not tested in the present study because arachidonic acid was not limited in our experimental setting. Because prostaglandin production involves multiple step-wise reactions, we suggest that multiple mechanisms, not a single mechanism, may contribute to modulation of COX activity mediated by vitamin E. Besides, (±)-α-tocopherol treatment was not able to suppress BDE-47-stimulated PGE2 release completely, supporting additional mechanisms. Further study will be needed to better understand the mechanisms for modulatory effects by vitamin E on COX activity.
COX-2 is induced in response to various stimuli including oxidative stress, pro-inflammatory cytokines, growth factors, oncogenes and tumor promoters while negatively regulated by glucocorticoids, interleukin (IL)-4, IL-13, and IL-10 (Surh et al., 2004). The precise molecular mechanism underlying COX-2 expression is not fully elucidated, but roles of cellular signaling pathways mediated via kinases such as mitogen-activated protein kinases (MAPKs), protein kinase C (PKC), phosphatidyl-ionositol-3-kinases (PI3K), Akt/PKB are reported (Surh, 2003). In addition, the promoter region for COX-2 gene contains binding sites for various transcription factors such as nuclear factor kappa B (NF-kB), nuclear factor for IL-6 (NF-IL6), nuclear factor of activated T-cells (NFAT), cAMP response element-binding protein (CREB), activating protein (AP)-2, and specificity protein (SP)-1 (Dannenberg et al., 2001; Kosaka et al., 1994; Shao et al., 2000). Therefore, activation of kinase signaling pathways and transcription factors, either alone or in combination, results in increased COX-2 expression. This may explain why COX expression is not entirely dependent on ROS formation in the present study. Although there are limited studies on PBDE effects on cellular signaling pathways, it is reported that a commercial PBDE mixture DE-71 and PBDE congeners such as BDE-47, 77, 99, and 209 stimulates PKC translocation, PKC phosphorylation, and ERK phosphorylation in vitro (Fan et al., 2010; Li et al., 2012; Madia et al., 2004). Further investigation on the interactions among kinase signaling pathways, transcription factors, and prostaglandin pathways will lead us toward a better understanding of the mechanisms associated with PGE2 production and COX expression stimulated by BDE-47 in gestational compartments.
Our findings implicate PGE2 as a potential target of PBDE exposure. A few studies indicate that PGE2 regulates trophoblast cellular functions in vitro. For example, PGE2 promoted migration of HTR-8/SVneo cells (Horita et al., 2007a; Nicola et al., 2005a) and the stimulated migration was suppressed by COX-2 inhibition. In contrast, Biondi et al. (2006) showed that PGE2 suppressed the proliferation and migration of HTR-8/SVneo cells. These contradictory results may be due to different experimental conditions (media, serum concentration, exposure duration, cell density, etc.) generating divergent responses to the same stimuli. Regardless of these inconsistencies, these few reports implicate that PGE2 may play a role in regulating trophoblast cellular function and that dysregulation of PGE2 production at the gestational compartment may affect trophoblast invasion and migration that are critical for proper placentation (Pijnenborg et al., 1983; Pijnenborg et al., 1980). Moreover, dysregulation of PGE2 production within the gestational compartment has been linked to adverse birth outcomes such as intrauterine growth restriction, preeclampsia and preterm birth (Germain et al., 1999; Ness and Sibai, 2006). Because improper placentation is associated with adverse obstetrical complications (Brosens, 1977; Hustin et al., 1983; Kim et al., 2003), further investigation will be needed to ascertain the potential relevance of BDE-47 stimulation of PGE2 on trophoblast invasion and placental function.
BDE-47 concentrations used in our study range from 5 to 20 μM. Correcting for adsorption onto plastic, estimated at 73% (Barber et al., 2006; Mundy et al., 2004), the corrected concentrations of BDE-47 in culture medium in this study are estimated to range from 1.34 μM to 5.4 μM. Because concentrations of PBDEs in human placentae have been reported as high as ~8 μM (Doucet et al., 2009), the effects observed in the present study with 20 μM BDE-47 in the prepared exposure medium may have relevance for human exposures, albeit at the high end of the exposure range. However, 20 μM was the only effective concentration in most end points except that 15 μM BDE-47 significantly suppressed HPGD mRNA expression (Fig. 1C), failing to show concentration-dependent responses. In the present study, we used DMSO at a final concentration of 0.7% to deliver BDE-47 to the cell cultures. Although previous reports used lower DMSO concentrations to deliver similar or higher concentrations of BDE-47 to cell cultures (Shao et al., 2008; Yan et al., 2011), we found that BDE-47 precipitated out over time in cultures at final DMSO concentrations below 0.7% in our laboratory. We also observed anti-inflammatory effects of higher DMSO concentrations (0.75–1%) (Park et al., 2014b), limiting the maximum concentration of DMSO and BDE-47 by 0.7% and 20 μM, respectively, in our study.
We have to be cautious in interpreting our results because overproduction of PGE2 alone may not accurately represent the response of trophoblast cells during an inflammatory state nor the impact of BDE-47 exposure on trophoblast cellular function in vivo. Although roles of PGE2 have been implicated in regulating trophoblast function (Biondi et al., 2006; Horita et al., 2007d; Nicola et al., 2005d), there are complex interactions between trophoblasts and a number of autocrine and paracrine factors such as growth factors, growth factor-binding proteins, proteoglycans, other cytokines/chemokines, integrins, adhesion and proteolytic molecules during trophoblast invasion and placentation (Anton et al., 2012; Chakraborty et al., 2002; Lala and Chakraborty, 2003). Moreover, the results of in vitro experiments using a transformed cell line may not accurately reflect responses of primary extravillous trophoblast cells. It has been reported that HTR-8/SVneo cells have a similar phenotype compared to their primary counterparts (Biondi et al., 2006; Graham et al., 1993; Jovanović et al., 2010). For example, HTR-8/SVneo cells retain migratory capability and express specific placental trophoblast markers including HLA-G, cytokeratin-7, and α5β1 integrin up to passage number 105 (Biondi et al., 2006; Khan et al., 2011). However, it has been reported that HTR-8/SVneo cells may have a different transcriptomic and epigenetic profile compared to primary extravillous trophoblast cells (Bilban et al., 2010; Novakovic et al., 2011). To address this issue, further investigation using primary trophoblasts or placental tissues will be needed to validate the potential relevance of our results to pregnancy.
Despite these limitations, our findings suggest potential adverse impacts of PBDE exposure during pregnancy. Invasion of EVTs into maternal spiral arteries is a key event during placentation (Brosens et al., 1967; Pijnenborg et al., 1983; Pijnenborg et al., 1980), and impaired EVT invasion has been attributed to pathologies of adverse birth outcomes with the evidence of abnormal placentation (Zhou et al., 1997a; Zhou et al., 1997d). The present study used HTR-8/SVneo, a human first trimester EVT cell line as a model to study the effects of BDE-47 treatment. Because PGE2 has been shown to regulate EVT proliferation, migration, and invasion during first trimester of pregnancy (Biondi et al., 2006; Horita et al., 2007d; Nicola et al., 2005d), overproduction of PGE2 in HTR-8/SVneo cells by BDE-47 suggests that BDE-47 exposure may disrupt trophoblast cellular function, leading to improper trophoblast invasion and abnormal placentation, thereby potentially contributing to adverse obstetrical outcomes. Ongoing research in our laboratory on the effects of PBDEs on trophoblast cellular function will lead us toward a better understanding of the mechanisms and relevant risks associated with PBDE exposures during pregnancy.
5. Conclusions
In conclusion, this is the first study to show that treatment with BDE-47, a predominant flame retardant chemical found in human tissues, stimulated expression of COX-2, leading to increased conversion of arachidonic acid to PGE2 in human first trimester placental cells. In addition, (±)-α-tocopherol cotreatment reduced BDE-47-stimulated PGE2 release without affecting mRNA and protein expression of COX-2, implicating post-translational regulation of COX activity by ROS. Because dysregulation of PGE2 has been implicated in improper trophoblast invasion and placental dysfunction, and associated with adverse birth outcomes, further investigation of the impact of BDE-47 on trophoblast function is warranted.
Supplementary Material
Highlights.
BDE-47 stimulated PGE2 release and COX-2 expression.
BDE-47 resulted in differential expression of genes relevant to PGE2 pathways.
(±)-α-tocopherol suppressed BDE-47-stimulated increases of PGE2 without affecting COX-2 mRNA and protein expression.
Acknowledgments
This work was supported by a grant to RLC (R01 ES014860), a project in the Superfund Research Program PROTECT Center to RL-C (P42 ES017198), and the Center for Lifestage Exposure and Adult Disease (P30 ES017885) from the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH).
Abbreviations
- BDE-47
brominated diphenyl ether-47
- carboxy-H2DCF-DA
6-carboxy dichlorodihydrofluorescein diacetate
- COX-2
cyclooxygenase-2
- cPGES
cytosolic prostaglandin E synthase
- DCF
dichlorofluorescein
- cDCF
carboxydichlorofluorescein
- DMSO
dimethyl sulfoxide
- HPGD
gene for 15-hydroxyprostaglandin dehydrogenase
- mPGES-1
membrane-bound prostaglandin E synthase-1
- mPGES-2
membrane-bound prostaglandin E synthase-2
- NO
nitric oxide
- ONOO
peroxynitrite
- PBDE
polybrominated diphenyl ether
- PGE2
prostaglandin E2
- PGES
prostaglandin E synthase
- PLA2
Phospholipase A2
- PTGES
gene for prostaglandin E synthase
- PTGS2
gene for prostaglandin-endoperoxide synthase 2 or COX-2
- ROS
reactive oxygen species
- TBHP
tert-butyl hydroperoxide
- TXB2
thromboxane B2
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hijji J, Andolf E, Laurini R, Batra S. Nitric oxide synthase activity in human trophoblast, term placenta and pregnant myometrium. Reproductive biology and endocrinology: RB&E. 2003;1:51. doi: 10.1186/1477-7827-1-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L, Brown AG, Parry S, Elovitz MA. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: Possible mechanisms of first trimester placental dysfunction. Human reproduction. 2012;27(1):61–72. doi: 10.1093/humrep/der362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagiri K, Nakatsuka M, Konishi H, Noguchi S, Takata M, Habara T, Kudo T. Involvement of peroxynitrite in lps-induced apoptosis of trophoblasts. The journal of obstetrics and gynaecology research. 2003;29(1):49–55. doi: 10.1046/j.1341-8076.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Barber JL, Walsh MJ, Hewitt R, Jones KC, Martin FL. Low-dose treatment with polybrominated diphenyl ethers (pbdes) induce altered characteristics in mcf-7 cells. Mutagenesis. 2006;21(5):351–60. doi: 10.1093/mutage/gel038. [DOI] [PubMed] [Google Scholar]
- Basu S. Oxidative injury induced cyclooxygenase activation in experimental hepatotoxicity. Biochemical and biophysical research communications. 1999;254(3):764–7. doi: 10.1006/bbrc.1998.9956. [DOI] [PubMed] [Google Scholar]
- Beharka AA, Wu D, Serafini M, Meydani SN. Mechanism of vitamin e inhibition of cyclooxygenase activity in macrophages from old mice: Role of peroxynitrite. Free radical biology & medicine. 2002;32(6):503–11. doi: 10.1016/s0891-5849(01)00817-6. [DOI] [PubMed] [Google Scholar]
- Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, Wagner O, Knofler M. Trophoblast invasion: Assessment of cellular models using gene expression signatures. Placenta. 2010;31(11):989–96. doi: 10.1016/j.placenta.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Biondi C, Ferretti ME, Pavan B, Lunghi L, Gravina B, Nicoloso MS, Vesce F, Baldassarre G. Prostaglandin e2 inhibits proliferation and migration of htr-8/svneo cells, a human trophoblast-derived cell line. Placenta. 2006;27(6–7):592–601. doi: 10.1016/j.placenta.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Breslin WJ, Kirk HD, Zimmer MA. Teratogenic evaluation of a polybromodiphenyl oxide mixture in new zealand white rabbits following oral exposure. Fundamental and applied toxicology: official journal of the Society of Toxicology. 1989;12(1):151–7. doi: 10.1016/0272-0590(89)90070-5. [DOI] [PubMed] [Google Scholar]
- Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. The Journal of pathology and bacteriology. 1967;93(2):569–79. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clinics in obstetrics and gynaecology. 1977;4(3):573–93. [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of n-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. American journal of obstetrics and gynecology. 2003;188(1):203–8. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Gleeson LM, McKinnon T, Lala PK. Regulation of human trophoblast migration and invasiveness. Canadian journal of physiology and pharmacology. 2002;80(2):116–24. doi: 10.1139/y02-016. [DOI] [PubMed] [Google Scholar]
- Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Levels of polybrominated diphenyl ethers (pbdes) in breast milk from central taiwan and their relation to infant birth outcome and maternal menstruation effects. Environment international. 2007;33(2):239–45. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. The American journal of pathology. 2007;171(4):1168–79. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: A pharmacological target for the prevention of cancer. The lancet oncology. 2001;2(9):544–51. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- Dash PR, Cartwright JE, Baker PN, Johnstone AP, Whitley GS. Nitric oxide protects human extravillous trophoblast cells from apoptosis by a cyclic gmp-dependent mechanism and independently of caspase 3 nitrosylation. Experimental cell research. 2003;287(2):314–24. doi: 10.1016/s0014-4827(03)00156-3. [DOI] [PubMed] [Google Scholar]
- Davidge ST. Oxidative stress and altered endothelial cell function in preeclampsia. Seminars in reproductive endocrinology. 1998;16(1):65–73. doi: 10.1055/s-2007-1016254. [DOI] [PubMed] [Google Scholar]
- Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent organic pollutant residues in human fetal liver and placenta from greater montreal, quebec: A longitudinal study from 1998 through 2006. Environ Health Perspect. 2009;117(4):605–10. doi: 10.1289/ehp.0800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Besas J, Kodavanti PR. Changes in mitogen-activated protein kinase in cerebellar granule neurons by polybrominated diphenyl ethers and polychlorinated biphenyls. Toxicology and applied pharmacology. 2010;245(1):1–8. doi: 10.1016/j.taap.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to pbdes--a review of levels and sources. Int J Hyg Environ Health. 2009;212(2):109–34. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: Placental pathology and clinical correlation. Obstet Gynecol. 1999;94(2):284–9. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. British journal of obstetrics and gynaecology. 1981;88(9):876–81. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Hayek MG, Meydani SN, Meydani M, Blumberg JB. Age differences in eicosanoid production of mouse splenocytes: Effects on mitogen-induced t-cell proliferation. Journal of gerontology. 1994;49(5):B197–207. doi: 10.1093/geronj/49.5.b197. [DOI] [PubMed] [Google Scholar]
- Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. Journal of immunology. 1997;159(5):2445–51. [PubMed] [Google Scholar]
- He P, He W, Wang A, Xia T, Xu B, Zhang M, Chen X. Pbde-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008;29(1):124–9. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Lands WE. Evidence for a peroxide-initiated free radical mechanism of prostaglandin biosynthesis. The Journal of biological chemistry. 1980;255(13):6253–61. [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environmental science & technology. 2004;38(4):945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U. Induction of prostaglandin e2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, htr-8/svneo. Human reproduction. 2007a;22(7):1801–9. doi: 10.1093/humrep/dem125. [DOI] [PubMed] [Google Scholar]
- Horita H, Kuroda E, Hachisuga T, Kashimura M, Yamashita U. Induction of prostaglandin e2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, htr-8/svneo. Human reproduction. 2007d;22(7):1801–9. doi: 10.1093/humrep/dem125. [DOI] [PubMed] [Google Scholar]
- Hustin J, Foidart JM, Lambotte R. Maternal vascular lesions in pre-eclampsia and intrauterine growth retardation: Light microscopy and immunofluorescence. Placenta. 1983;4(Spec No):489–98. [PubMed] [Google Scholar]
- Hustin J, Jauniaux E, Schaaps JP. Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. 1990;11(6):477–86. doi: 10.1016/s0143-4004(05)80193-6. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11494–9. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (cxcl8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (mmp)2 and mmp9 and integrins alpha5 and beta1. Reproduction. 2010;139(4):789–98. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in htr-8/svneo cell line. Placenta. 2009;30(4):320–8. doi: 10.1016/j.placenta.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel vegfr-2-binding antagonist for the human extravillous trophoblast. Molecular endocrinology. 2011;25(8):1431–43. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong TY, Liddell HS, Robertson WB. Defective haemochorial placentation as a cause of miscarriage: A preliminary study. British journal of obstetrics and gynaecology. 1987;94(7):649–55. doi: 10.1111/j.1471-0528.1987.tb03169.x. [DOI] [PubMed] [Google Scholar]
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2003;189(4):1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. American journal of obstetrics and gynecology. 2002;187(5):1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T. Characterization of the human gene (ptgs2) encoding prostaglandin-endoperoxide synthase 2. European journal of biochemistry/FEBS. 1994;221(3):889–97. doi: 10.1111/j.1432-1033.1994.tb18804.x. [DOI] [PubMed] [Google Scholar]
- Kulmacz RJ, Wang LH. Comparison of hydroperoxide initiator requirements for the cyclooxygenase activities of prostaglandin h synthase-1 and -2. The Journal of biological chemistry. 1995;270(41):24019–23. doi: 10.1074/jbc.270.41.24019. [DOI] [PubMed] [Google Scholar]
- Labarrere CA, Althabe OH. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational-age infants. British journal of obstetrics and gynaecology. 1987;94(11):1113–6. doi: 10.1111/j.1471-0528.1987.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: Possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24(6):575–87. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Li ZH, Liu XY, Wang N, Chen JS, Chen YH, Huang JT, Su CH, Xie F, Yu B, Chen DJ. Effects of decabrominated diphenyl ether (pbde-209) in regulation of growth and apoptosis of breast, ovarian, and cervical cancer cells. Environmental health perspectives. 2012;120(4):541–6. doi: 10.1289/ehp.1104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZK, Liu HY, Fang WN, Yang Y, Wang HM, Peng JP. Insulin-like growth factor binding protein 7 modulates estrogen-induced trophoblast proliferation and invasion in htr-8 and jeg-3 cells. Cell Biochem Biophys. 2012;63(1):73–84. doi: 10.1007/s12013-012-9342-5. [DOI] [PubMed] [Google Scholar]
- Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant pbde-99 and of the pcb aroclor 1254 in human astrocytoma cells. Toxicology letters. 2004;154(1–2):11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebaek NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environmental health perspectives. 2007;115(10):1519–26. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated diphenyl ethers in human gestational membranes from women in southeast michigan. Environmental science & technology. 2009a;43(9):3042–6. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated diphenyl ethers in human gestational membranes from women in southeast michigan. Environmental science & technology. 2009b;43(9):3042–6. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Domino SE, Batterman SA, Loch-Caruso R. Concentrations and speciation of polybrominated diphenyl ethers in human amniotic fluid. Sci Total Environ. 2012;417–418:294–8. doi: 10.1016/j.scitotenv.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM, Crofton KM, DeVito MJ. Accumulation of pbde-47 in primary cultures of rat neocortical cells. Toxicol Sci. 2004;82(1):164–9. doi: 10.1093/toxsci/kfh239. [DOI] [PubMed] [Google Scholar]
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. American journal of obstetrics and gynecology. 2006;195(1):40–9. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Nicola C, Timoshenko AV, Dixon SJ, Lala PK, Chakraborty C. Ep1 receptor-mediated migration of the first trimester human extravillous trophoblast: The role of intracellular calcium and calpain. J Clin Endocrinol Metab. 2005a;90(8):4736–46. doi: 10.1210/jc.2005-0413. [DOI] [PubMed] [Google Scholar]
- Nicola C, Timoshenko AV, Dixon SJ, Lala PK, Chakraborty C. Ep1 receptor-mediated migration of the first trimester human extravillous trophoblast: The role of intracellular calcium and calpain. The Journal of clinical endocrinology and metabolism. 2005d;90(8):4736–46. doi: 10.1210/jc.2005-0413. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Gordon L, Wong NC, Moffett A, Manuelpillai U, Craig JM, Sharkey A, Saffery R. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: Implications and opportunities for understanding trophoblast function. Molecular human reproduction. 2011;17(6):344–53. doi: 10.1093/molehr/gar005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary KA, de Pascual-Teresa S, Needs PW, Bao YP, O’Brien NM, Williamson G. Effect of flavonoids and vitamin e on cyclooxygenase-2 (cox-2) transcription. Mutation research. 2004;551(1–2):245–54. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Parhar RS, Kennedy TG, Lala PK. Suppression of lymphocyte alloreactivity by early gestational human decidua. I. Characterization of suppressor cells and suppressor molecules. Cellular immunology. 1988;116(2):392–410. doi: 10.1016/0008-8749(88)90240-7. [DOI] [PubMed] [Google Scholar]
- Park HR, Kamau PW, Korte C, Loch-Caruso R. Tetrabromobisphenol a activates inflammatory pathways in human first trimester extravillous trophoblasts in vitro. Reprod Toxicol. 2014a;50:154–62. doi: 10.1016/j.reprotox.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Kamau PW, Loch-Caruso R. Involvement of reactive oxygen species in brominated diphenyl ether-47-induced inflammatory cytokine release from human extravillous trophoblasts in vitro. Toxicology and applied pharmacology. 2014b;274(2):283–92. doi: 10.1016/j.taap.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Klimova NG, Arita Y, Gurzenda EM, Murthy A, Chawala K, Lerner V, Richardson J, Hanna N. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta. 2012;33(9):745–9. doi: 10.1016/j.placenta.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4(4):397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1(1):3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- Psychoyos A, Nikas G, Gravanis A. The role of prostaglandins in blastocyst implantation. Human reproduction. 1995;10(Suppl 2):30–42. doi: 10.1093/humrep/10.suppl_2.30. [DOI] [PubMed] [Google Scholar]
- Robertson WB, Brosens I, Dixon HG. The pathological response of the vessels of the placental bed to hypertensive pregnancy. The Journal of pathology and bacteriology. 1967;93(2):581–92. doi: 10.1002/path.1700930219. [DOI] [PubMed] [Google Scholar]
- Sakamoto W, Fujie K, Handa H, Nishihira J, Mino M. Vitamin e inhibits pge2 and o2- production in rat peritoneal macrophages. Biochimica et biophysica acta. 1991;1074(2):251–5. doi: 10.1016/0304-4165(91)90160-i. [DOI] [PubMed] [Google Scholar]
- Sakamoto W, Fujie K, Nishihira J, Handa H. Effect of vitamin e on arachidonic acid-release in rat peritoneal macrophages. Biochimica et biophysica acta. 1993;1170(3):296–300. doi: 10.1016/0005-2760(93)90013-y. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Settle SL, Masferrer JL, Seibert K, Currie MG, Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. British journal of pharmacology. 1995;114(6):1171–8. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin e synthase-1: A novel therapeutic target. Pharmacological reviews. 2007;59(3):207–24. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- Shanmugam N, Todorov IT, Nair I, Omori K, Reddy MA, Natarajan R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (ager), and in islets from diabetic mice. Diabetologia. 2006;49(1):100–7. doi: 10.1007/s00125-005-0065-7. [DOI] [PubMed] [Google Scholar]
- Shao J, Sheng H, Inoue H, Morrow JD, DuBois RN. Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. The Journal of biological chemistry. 2000;275(43):33951–6. doi: 10.1074/jbc.M002324200. [DOI] [PubMed] [Google Scholar]
- Shao J, White CC, Dabrowski MJ, Kavanagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in bde 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol Sci. 2008;101(1):81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. British journal of obstetrics and gynaecology. 1981;88(7):695–705. doi: 10.1111/j.1471-0528.1981.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Sheppard BL, Bonnar J. The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. British journal of obstetrics and gynaecology. 1976;83(12):948–59. doi: 10.1111/j.1471-0528.1976.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Smith WL, Eling TE, Kulmacz RJ, Marnett LJ, Tsai A. Tyrosyl radicals and their role in hydroperoxide-dependent activation and inactivation of prostaglandin endoperoxide synthase. Biochemistry. 1992;31(1):3–7. doi: 10.1021/bi00116a001. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature reviews Cancer. 2003;3(10):768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Na HK, Lee SS. Transcription factors and mitogen-activated protein kinases as molecular targets for chemoprevention with anti-inflammatory phytochemicals. Bio Factors. 2004;21(1–4):103–8. doi: 10.1002/biof.552210119. [DOI] [PubMed] [Google Scholar]
- Tai HH, Cho H, Tong M, Ding Y. Nad+-linked 15-hydroxyprostaglandin dehydrogenase: Structure and biological functions. Current pharmaceutical design. 2006;12(8):955–62. doi: 10.2174/138161206776055958. [DOI] [PubMed] [Google Scholar]
- Tetz LM, Cheng AA, Korte CS, Giese RW, Wang P, Harris C, Meeker JD, Loch-Caruso R. Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicology and applied pharmacology. 2013a;268(1):47–54. doi: 10.1016/j.taap.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz LM, Kamau PW, Cheng AA, Meeker JD, Loch-Caruso R. Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant-stimulated cellular production of reactive oxidant species. J Pharmacol Toxicol Methods. 2013b;67(2):56–60. doi: 10.1016/j.vascn.2013.01.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency; Office of Pollution Prevention & Toxics, editor Polybrominated diphenyl ethers (pbdes) project plan. 2006. [Google Scholar]
- Vessey DA, Lee KH, Blacker KL. Characterization of the oxidative stress initiated in cultured human keratinocytes by treatment with peroxides. The Journal of investigative dermatology. 1992;99(6):859–63. doi: 10.1111/1523-1747.ep12614831. [DOI] [PubMed] [Google Scholar]
- Wang D, Song W, Na Q. The emerging roles of placenta-specific micrornas in regulating trophoblast proliferation during the first trimester. Aust N Z J Obstet Gynaecol. 2012;52(6):565–70. doi: 10.1111/j.1479-828X.2012.01481.x. [DOI] [PubMed] [Google Scholar]
- Weber M, Knoefler I, Schleussner E, Markert UR, Fitzgerald JS. Htr8/svneo cells display trophoblast progenitor cell-like characteristics indicative of self-renewal, repopulation activity, and expression of “stemness-” associated transcription factors. Biomed Res Int. 2013;2013:243649. doi: 10.1155/2013/243649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel P, Welsh N, Eriksson UJ. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered prostaglandin e2 levels in rat embryos exposed to a diabetic environment. Diabetes. 1999;48(4):813–20. doi: 10.2337/diabetes.48.4.813. [DOI] [PubMed] [Google Scholar]
- White V, Jawerbaum A, Sinner D, Pustovrh C, Capobianco E, Gonzalez E. Oxidative stress and altered prostanoid production in the placenta of streptozotocin-induced diabetic rats. Reproduction, fertility, and development. 2002;14(1–2):117–23. doi: 10.1071/rd01032. [DOI] [PubMed] [Google Scholar]
- Wu D, Hayek MG, Meydani S. Vitamin e and macrophage cyclooxygenase regulation in the aged. The Journal of nutrition. 2001;131(2):382S–8S. doi: 10.1093/jn/131.2.382S. [DOI] [PubMed] [Google Scholar]
- Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Age-associated increase in pge2 synthesis and cox activity in murine macrophages is reversed by vitamin e. The American journal of physiology. 1998;275(3 Pt 1):C661–8. doi: 10.1152/ajpcell.1998.275.3.C661. [DOI] [PubMed] [Google Scholar]
- Wu K, Xu X, Liu J, Guo Y, Li Y, Huo X. Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from guiyu, china. Environmental science & technology. 2010;44(2):813–9. doi: 10.1021/es9024518. [DOI] [PubMed] [Google Scholar]
- Yan C, Huang D, Zhang Y. The involvement of ros overproduction and mitochondrial dysfunction in pbde-47-induced apoptosis on jurkat cells. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2011;63(5):413–7. doi: 10.1016/j.etp.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Yee GM, Squires PM, Cejic SS, Kennedy TG. Lipid mediators of implantation and decidualization. Journal of lipid mediators. 1993;6(1–3):525–34. [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? The Journal of clinical investigation. 1997a;99(9):2152–64. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? The Journal of clinical investigation. 1997d;99(9):2139–51. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.