Introduction
The second generation antipsychotics (SGA) developed to treat schizophrenia, are associated with weight gain (1;2) and metabolic disease (3;4). Short- term administration has been shown to increase indices of metabolic impairment (fasting glucose, insulin and triglycerides) (5;6) while chronic SGA treatment increases the incidence of diabetes (7;8) and cardiovascular disease (9). Of concern is the observation that the most therapeutically effective agents are associated with the most severe adverse metabolic profiles. Olanzapine, a drug widely prescribed for schizophrenia and bipolar disorder in adults and children is associated with significant weight gain and metabolic abnormalities (1;10). Other SGAs, such as aripiprazole are thought to be associated with modest weight gain and fewer indices of metabolic disease (11).
The observed metabolic dysregulation is assumed a downstream effect of increased body weight due to SGA-associated changes in the central nervous system mechanisms regulating food intake and energy homeostasis. In vitro (12;13) and rodent studies (14;15) suggest that the SGAs may exert direct effects on tissue function independent of weight gain, raising questions as to whether metabolic dysregulation is solely a consequence of increased body adiposity. Our laboratory has recently demonstrated that short-term administration of SGAs alters metabolism, independent of weight gain. We administered olanzapine, aripiprazole or placebo to healthy control subjects (10 subjects in each interventional arm) for a 9-day period and conducted metabolic tests evaluating insulin sensitivity and hormonal secretion to a mixed nutrient challenge, prior to and following the interventions. Uniquely, this was an inpatient study in which subjects were permitted to eat ad libitum but were required to maintain their activity level in the hospital consistent with their free living level. We were able to demonstrate that both olanzapine and aripiprazole induce insulin resistance but only olanzapine elicits post-prandial metabolic dysregulation. These effects were shown to be independent of weight gain or changes in food intake (16).
Few studies have investigated detailed eating behaviors initially after drug administration and it is not known how and when the SGAs influence food intake, hunger and satiety during the early time period after starting treatment. This current paper presents the detailed results of the effect of two SGAs compared to placebo on eating behaviors and associations between food intake and weight variation which were not reported in the original paper. The study was approved by the Institutional Review Board of the University of Pennsylvania and all subjects gave their written informed consent.
Methods
For a detailed description of design, methodology and metabolic results see our earlier report (16). Methods for evaluation of hunger, fullness and food intake are explained here. Visual analog scales for ratings of hunger and fullness were completed daily immediately before and following each meal. The questions were phrased: “how hungry do you feel right now?” and “how full do you feel right now?” Each question had the numbers 1–9 listed underneath anchored by the descriptors, “not at all” and “extremely”.
Subjects were permitted to eat ad libitum while in the hospital. Meals were selected from a menu and the food was prepared in the metabolic kitchen of the Clinical and Translational Research Center (CTRC). On days 2 (pre- intervention) and 11 (post-intervention) of the 12-day inpatient stay, at each meal, subjects were given a menu composed of individual food items so that macronutrient composition could be accurately determined as previously described (17). Selected foods were provided in excess of normal portions but unknown to the subjects all food was weighed prior to and following breakfast, lunch, dinner and snack.
Results
As initially reported in our original paper, following the short-term administration of olanzapine, aripiprazole or placebo, change in weight after 9-days of olanzapine (0.76±0.42 kg) or aripiprazole (−0.46±0.31 kg) was not significantly different from the change after placebo (0.41±0.34 kg). Activity as indicated by the number of steps taken while in the 12-day study was not significantly different than the average taken over 5 days in a free living condition indicating that activity levels were maintained. No significant difference was found in the pre-post change in step number while on olanzapine (−910.5±636.36), or aripiprazole (−547.7±258.7) compared with the change in placebo (−334.2±234). The change in kcals ingested after olanzapine (363±380.6 kcal, t=0.39, P=0.70) or aripiprazole (−269.9±552.6 kcal, t=−0.64, P=0.53) was not different from placebo (162.1±339.7 kcal). Consistent with a lack of change in weight or food intake, we did not find any effect of olanzapine (Fig. 1a) or aripiprazole (Fig. 1b) compared to placebo (Fig. 1c) on subjective feelings of hunger, whether analyzed using repeated measures analysis of variance or total hunger over the 12 day period (Fig. 1d). Similarly, no effect of fullness was observed (Fig. 1e, f, g, h).
Figure 1.
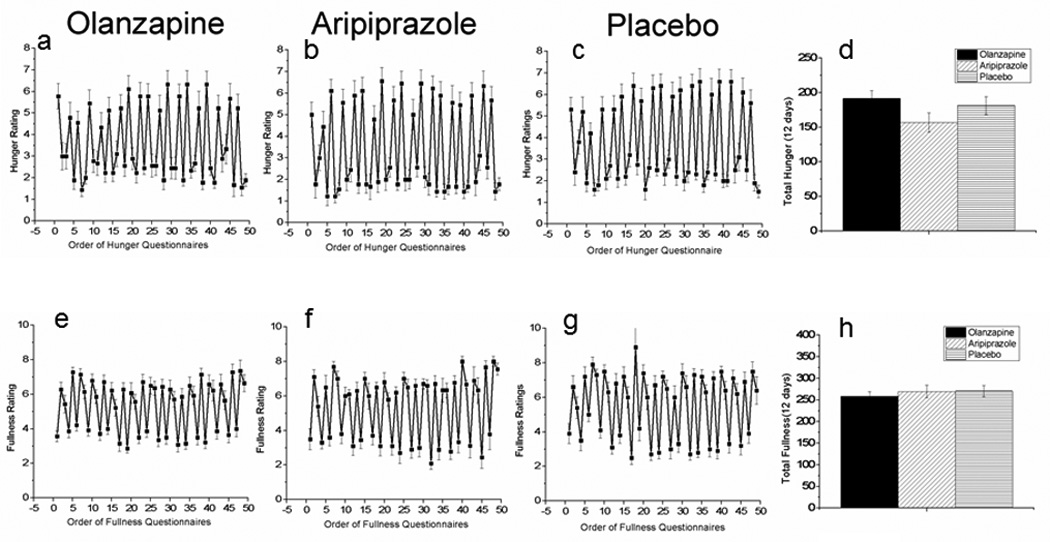
Hunger ratings during administration of olanzapine (a), aripiprazole (b) and placebo (c) over the course of the 12-day inpatient study. Fig. 1d illustrates the total hunger score over the 12-day period: daily fullness ratings during administration of olanzapine (e), aripiprazole (f) and placebo (g) over the course of the 12-day inpatient study. Fig. 1h illustrates the total fullness score over the 12-day period. Mean±S.E., n=10; olanzapine; n=10 aripiprazole: n=10 placebo.
The orexigenic hormone ghrelin and the satiety hormone leptin responses to the meal challenge were measured in a sub-group of participants from the larger study prior to and following the interventions. No significant differences were found in the change in ghrelin AUC after either olanzapine (−956.0±422.2 pg/ml/360 min, t=0.26, P=0.79) or aripiprazole (−856.4±458.4 pg/ml/360 min, t=0.4, P=0.69) when compared to placebo (−1118.7±445.0 pg/ml/360 min) (Fig. 2d). Similarly no change in post-prandial leptin AUC after olanzapine (13.5±7.8 ng/ml/360 min, t=0.49, P=0.63,) or aripiprazole (−4.1±2.9 ng/ml/360 min, t=1.3, P=0.21) compared to placebo (7.5±8.9 ng/ml/360 min, Fig. 2h)
Figure 2.
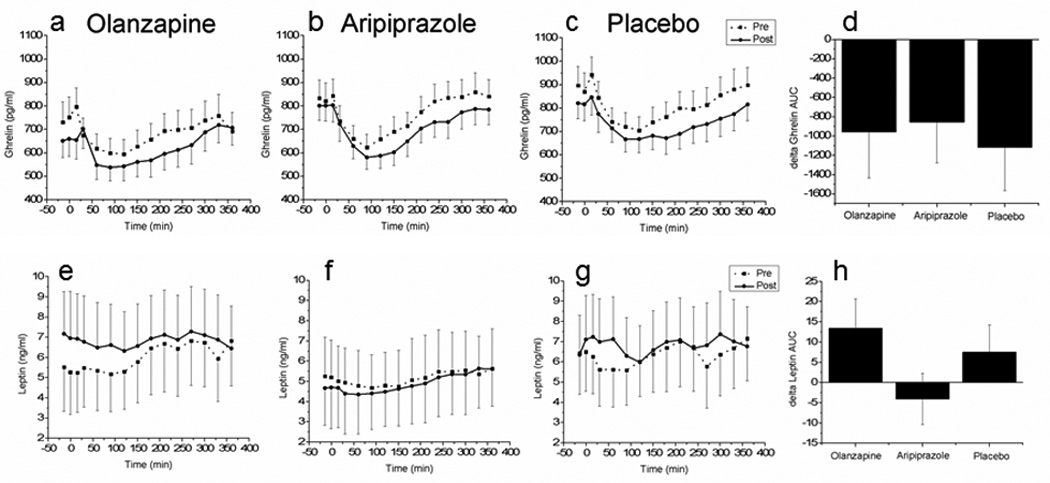
Post-prandial plasma ghrelin concentrations prior to (dashed line) and after (solid line) administration of olanzapine (a), aripiprazole (b) and placebo (c). Fig 3d illustrates the delta area under the curve (AUC) (post-intervention AUC- pre-intervention AUC). Post-prandial plasma leptin concentrations prior to (dashed line) and after (solid line) administration of olanzapine (a), aripiprazole (b) and placebo (c). Fig 3h illustrates the delta area under the curve (AUC) (post-intervention AUC- pre-intervention AUC). Mean±S. E., for leptin n=7, 9, 8 and ghrelin, n= 6,5,7 for olanzapine, aripiprazole and placebo, respectively.
Discussion
The objective of this paper was to evaluate the acute effects of two SGAs on food intake and hunger in an inpatient setting where activity was controlled. We found no changes in subjective feelings of hunger or satiety, food intake or the appetite-related hormones leptin and ghrelin during 9 day inpatient administration of olanzapine or aripiprazole compared to placebo. The lack of change in hunger and food intake is consistent with the absence of weight gain despite drug-induced changes in post-prandial hormones and decreases in insulin sensitivity as we previously reported (16). The data presented here document the lack of an early change in eating behavior after SGA administration and suggest that in the initial period after SGA administration, the mechanisms mediating the metabolic impairments are independent of changes in food intake, and so would be expected to precede and possibly even contribute to any eventual changes in body weight.
A typical sequence of eating behavior under “real-life” circumstances would involve an increase in hunger precipitating an increase in food intake. Sustained increases in food intake in the absence of satiating mechanisms result in weight gain. While the effects of SGAs on weight gain, particularly olanzapine, are indisputable, documentation of increases in hunger is notably absent. Only two studies have measured hunger after olanzapine administration to healthy volunteers and neither found statistically significant increases (18;19). We measured hunger and fullness multiple times per day over the entire 12-day period and similarly, did not find any effect of either olanzapine or aripiprazole on feelings of hunger. Ghrelin, the only circulating hormone known to be associated with hunger (20) was unaffected by the interventions and was not correlated with hunger, food intake (data not shown) or change in weight .
Under our experimental conditions, we found no significant decreases in satiety as indicated by fullness ratings which remained equal across the three study conditions (Fig. 1). Both insulin and leptin are considered satiety hormones and theoretically contribute to long-term energy homeostasis in insulin sensitive individuals (22). The rapid onset of olanzapine-induced post-prandial hyperinsulinemia that we previously reported may have implications with regards to promoting weight gain through the anabolic actions of insulin and at the same time, initially contributing to satiety prior to induction of central insulin resistance (23). This could perhaps explain the lack of decrease in satiety during the early period of time after SGA administration. The effects of olanzapine on decreasing satiety and ultimately increasing food intake may only occur following an increase in body adiposity which is thought to lead to central insulin and leptin resistance.
Short-term administration of the SGAs to healthy volunteers has, in some studies, elicited small increases in body weight (18;19;21;24;25) but food intake itself has rarely been monitored. Only three studies monitored (18;19;21) both food intake and body weight. While increases in body weight were reported with all 3 studies (18;19;21), only one found a statistically significant increase in food intake but the measurement was based on consumption of a liquid breakfast in the absence of placebo control (19). In contrast to these studies, we conducted an inpatient study where activity was maintained constant to pre-study level. Consistent with the absence of weight gain, we found no differences in total food intake following administration of either SGA compared to placebo. However, we did find highly statistically significant correlations between an index of total food intake (sum of food consumed on pre- and post-intervention days) and change in weight for the group as a whole (R=0.55, P<0.002), placebo (R=0.83, P<0.001), and olanzapine (R=0.63, P<0.01). Only aripiprazole was not associated with a correlation between change in weight and the index of total food intake.
Overall the data presented here, as well as in our earlier publication(16), indicate that under conditions where activity is controlled, the SGAs olanzapine and aripiprazole are associated with metabolic changes in healthy subjects that occur prior to changes in hunger, satiety, food intake and appetite related hormones and in the absence of weight gain. The temporal separation of the metabolic disturbances and behavioral effects suggests that there are differential mechanisms mediating SGA induced changes in metabolism and food intake, which may be a later consequence to subsequent changes in body weight. However, one limitation that must be acknowledged is the possibility that clinical populations may respond differently than this healthy control group.
Acknowledgements
This study was supported by NIH grants DK084383 (KLT), core services were provided by the University of Pennsylvania Diabetes Research Center sponsored by NIH DK19525 and CTRC facilities from NIH UL1-RR-024134.
References
- 1.Allison D, Mentore J, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 2.Parsons B, Allison DB, Loebel A, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110(1–3):103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Meyer JM, Davis VG, McEvoy JP, et al. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE Schizophrenia Trial phase 1. Schizophr Res. 2008;103(1–3):104–109. doi: 10.1016/j.schres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haupt D, Newcomer J. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosomatic Research. 2002;53:925–933. doi: 10.1016/s0022-3999(02)00471-3. [DOI] [PubMed] [Google Scholar]
- 5.Henderson D, Cagliero E, Copeland P, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62(1):19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Birkenaes AB, Birkeland KI, Engh JA, et al. Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. J Clin Psychopharmacol. 2008;28(2):132–137. doi: 10.1097/JCP.0b013e318166c4f7. [DOI] [PubMed] [Google Scholar]
- 7.Bobo WV, Cooper WO, Stein CM, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70(10):1067–1075. doi: 10.1001/jamapsychiatry.2013.2053. [DOI] [PubMed] [Google Scholar]
- 8.Erickson SC, Le L, Zakharyan A, et al. New-onset treatment-dependent diabetes mellitus and hyperlipidemia associated with atypical antipsychotic use in older adults without schizophrenia or bipolar disorder. J Am Geriatr Soc. 2012;60(3):474–479. doi: 10.1111/j.1532-5415.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 9.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna P, Komossa K, Rummel-Kluge C, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2013;2:CD006569. doi: 10.1002/14651858.CD006569.pub4. [DOI] [PubMed] [Google Scholar]
- 12.Albaugh VL, Vary TC, Ilkayeva O, et al. Atypical Antipsychotics Rapidly and Inappropriately Switch Peripheral Fuel Utilization to Lipids, Impairing Metabolic Flexibility in Rodents. Schizophr Bull. 2010;38(1):153–166. doi: 10.1093/schbul/sbq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DE, Yamazaki H, Ward KM, et al. Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes. 2005;54(5):1552–1558. doi: 10.2337/diabetes.54.5.1552. [DOI] [PubMed] [Google Scholar]
- 14.Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32(2):289–297. doi: 10.1038/sj.npp.1301209. [DOI] [PubMed] [Google Scholar]
- 15.Hahn M, Chintoh A, Giacca A, et al. Atypical antipsychotics and effects of muscarinic, serotonergic, dopaminergic and histaminergic receptor binding on insulin secretion in vivo: an animal model. Schizophr Res. 2011;131(1–3):90–95. doi: 10.1016/j.schres.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–3240. doi: 10.2337/db13-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teff KL, Petrova M, Havel PJ, Townsend RR. 48-h glucose infusion in humans: effect on hormonal responses, hunger and food intake. Physiol Behav. 2007;90(5):733–743. doi: 10.1016/j.physbeh.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roerig JL, Mitchell JE, de Zwaan M, et al. A comparison of the effects of olanzapine and risperidone versus placebo on eating behaviors. J Clin Psychopharmacol. 2005;25(5):413–418. doi: 10.1097/01.jcp.0000177549.36585.29. [DOI] [PubMed] [Google Scholar]
- 19.Mathews J, Newcomer JW, Mathews JR, et al. Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry. 2012;69(12):1226–1237. doi: 10.1001/archgenpsychiatry.2012.934. [DOI] [PubMed] [Google Scholar]
- 20.Wren A, Seal L, Cohen M, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86(12):5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 21.Fountaine RJ, Taylor AE, Mancuso JP, et al. Increased food intake and energy expenditure following administration of olanzapine to healthy men. Obesity (Silver Spring) 2010;18(8):1646–1651. doi: 10.1038/oby.2010.6. [DOI] [PubMed] [Google Scholar]
- 22.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91(2):389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan KK, Woods SC, Seeley RJ. Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metab. 2012;15(2):137–149. doi: 10.1016/j.cmet.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacher J, Mossaheb N, Spindelegger C, et al. Effects of Olanzapine and Ziprasidone on Glucose Tolerance in Healthy Volunteers. Neuropsychopharmacology. 2008;33(7):1633–1641. doi: 10.1038/sj.npp.1301541. [DOI] [PubMed] [Google Scholar]
- 25.Sowell M, Mukhopadhyay N, Cavazzoni P, et al. Evaluation of insulin sensitivity in healthy volunteers treated with olanzapine, risperidone, or placebo: a prospective, randomized study using the two-step hyperinsulinemic, euglycemic clamp. J Clin Endocrinol Metab. 2003;88(12):5875–5880. doi: 10.1210/jc.2002-021884. [DOI] [PubMed] [Google Scholar]


