Abstract
It has been suggested that acetaminophen (APAP)-protein adducts can be measured in circulation to diagnose APAP-induced liver injury. However, the full time course of plasma adducts has not been studied specifically in early-presenting overdose patients. In fact, surprisingly little work has been done on the metabolism of APAP after overdose in general.
We measured APAP, five APAP metabolites and APAP-protein adducts in plasma samples from early and late-presenting overdose patients, and APAP-protein adducts in culture medium from HepaRG cells.
In contrast to earlier rodents studies, we found that APAP-protein adducts were lower at early time points and peaked around the time of peak liver injury, suggesting that these adduct levels may take longer to become elevated or remain elevated than previously thought.
APAP and its major metabolites were elevated in plasma at early time points and rapidly decreased.
Although clinical measurement of APAP-protein adducts holds promise as a diagnostic tool, we suggest caution in its interpretation in very early-presenting patients. Our data also support the idea that sulfation is saturated even at low doses but glucuronidation has a much higher capacity, highlighting the importance of glucuronidation in APAP metabolism.
Keywords: Hepatotoxicity, Protein binding, Biomarkers, Glucuronidation, Sulfation
INTRODUCTION
At recommended doses, acetaminophen (APAP) is a safe and effective drug for the management of pain. However, overdose of APAP can cause severe acute liver injury and liver failure. It is responsible for almost 80,000 emergency department visits in the U.S. each year (Budnitz et al., 2011), as well as 26,000 hospitalizations and nearly 500 deaths (Nourjah et al., 2006). In fact, APAP hepatotoxicity is one of the most common etiologies of acute liver failure (Lee et al., 2012).
The metabolism and pharmacokinetics of APAP after therapeutic doses were first studied in detail in the 1960s; our understanding of APAP metabolism has grown considerably since that time (McGill and Jaeschke, 2013). The bioavailability of orally administered APAP is about 80%. The drug is rapidly absorbed from the lumen of the small intestine, achieving maximum plasma concentration within 0.5 – 1.5 h in fasted subjects. Glucuronidation and sulfation are the major elimination pathways. Sulfation is saturated even at therapeutic doses (Prescott, 1980; Clements et al., 1984), so glucuronidation is dominant. In total, about 50–70% of a pharmacological dose is glucuronidated, while 25–35% undergoes sulfation. A much smaller percentage of the dose (5–10%) is converted to the reactive intermediate N-acetyl-p-benzoquinone imine (NAPQI) by cytochrome P450 enzymes. NAPQI binds to the cysteine sulfhydryl group on glutathione (GSH) and can also bind to protein sulfhydryl groups, even after low doses (Heard et al., 2011; McGill et al. 2013). Most of the APAP-GSH is broken down to mercapturic acid and cysteine conjugates and the resulting APAP-N-acetylcysteine (APAP-NAC), APAP-cysteine (APAP-cys), and APAP-protein adducts can be measured in plasma and urine as surrogates of NAPQI formation. Any remaining APAP (< 5%) is excreted unchanged.
It has been suggested that APAP overdose can be rapidly diagnosed by measuring protein-derived APAP-cysteine adducts (hereafter referred to as “APAP-protein adducts”) in serum from both adults (Davern et al., 2006) and children (James et al., 2008), but some aspects of the time course and mechanism of release of these adducts have not yet been investigated in humans. In particular, time course experiments in mice suggest that the concentration of APAP-protein adducts in circulation increases rapidly after APAP treatment and peaks even before the onset of liver injury (McGill et al., 2013). However, the full time course of circulating adducts, beginning before the peak of injury, has not been characterized in APAP overdose patients. Also, despite our extensive knowledge of APAP metabolism after therapeutic doses, surprisingly few studies have specifically examined APAP metabolism after overdose.
To better understand the appearance of APAP-protein adducts in circulation and APAP metabolism after overdose, we collected plasma samples from patients with a recent history of APAP overdose. Patients were grouped as either early or late presenters on the basis of plasma APAP levels. We measured the five major metabolites of APAP, APAP-glucuronide (APAP-gluc), APAP-sulfate (APAP-sulf), APAP-GSH, free APAP-cys, and APAP-NAC, as well as APAP-protein adducts, in these samples using mass spectrometry. Our results suggest that APAP-protein adducts increase later than expected in humans and that clinical measurements of these adducts should be interpreted with caution. The data also reinforce the importance of glucuronidation in APAP metabolism.
METHODS
Patient enrollment and categorization
Patients presenting to the University of Kansas Hospital in Kansas City, Kansas were studied prospectively. The diagnosis of APAP overdose was made by a physician. All patients or next of kin were informed of the study purpose and protocol and signed a consent form. The criteria for study inclusion were reported history of APAP overdose, high plasma APAP levels, or evidence of liver injury (ALT > 1,000 U/L and prothrombin time ≥ 18s). All patients met at least two of these criteria. Patients were excluded if there was reasonable evidence that their liver injury was due to some other etiology (e.g. viral hepatitis, alcoholic hepatitis, etc.). Because APAP has a short half-life, the patients were grouped into early or late time points on the basis of plasma APAP concentration. Patients considered “early” had a reported history of APAP overdose and relatively high plasma APAP levels (> 25 µg/mL; the top of the therapeutic range) with little or no evidence of liver injury (< 100 U/L ALT) at the time of presentation, which is known to develop at later time points in humans (Singer et al., 1995; McGill et al., 2012). Patients considered “late” had lower plasma APAP levels (1–25 µg/mL), coupled with evidence of liver injury (ALT ≥ 100 U/L in the first study sample, peak ALT > 1,000 U/L and peak prothrombin time ≥ 18s). 25 µg/mL was chosen as our cutoff because it is at the top of the range of serum concentrations that is normally seen after therapeutic doses of APAP. Our assumption is that patients with higher levels likely exceeded the maximum recommended dose of APAP. All patients were treated with either oral or i.v. N-acetylcysteine (NAC). All study procedures were done in accordance with the Declaration of Helsinki and were approved by the internal review board (IRB) of the University of Kansas Medical Center.
Patient sample collection and handling
Blood samples were drawn in vacuum tubes dry-coated with heparin or EDTA. Plasma was obtained by centrifugation and aliquots were stored at −80°C for later analysis.
Biochemistry
Clinical parameters, including alanine aminotransferase (ALT), INR, bilirubin and creatinine, were measured and calculated in the clinical lab at the University of Kansas Hospital using standard methods and reagents. Lactate dehydrogenase (LDH) release was measured as previously described (Bajt et al., 2004).
HepaRG cells
HepaRG cells were obtained from Biopredic International (Rennes, France). The cells were grown and maintained as previously described (McGill et al., 2011). For time course experiments, the cells were washed with 1× PBS to remove the maintenance medium and treated with 20 mM APAP dissolved in DMSO-free Williams’ E medium containing 10% FBS, insulin and penicillin/streptomycin.
Analysis of APAP and metabolites in human plasma and HepaRG cells
Protein-derived APAP-cysteine adducts (APAP-protein adducts) were measured in HepaRG cells as previously described (McGill et al., 2011). Before measurement of APAP-protein adducts in plasma or cell culture medium, the samples were prepared as previously described (McGill et al., 2013). Briefly, low molecular weight compounds, including free APAP-cys, were removed by dialysis followed by filtration through size exclusion columns to obtain total plasma protein. The proteins were then digested overnight using a mixture of proteases, followed by precipitation of the proteases and filtration to ensure that the samples were clean enough for HPLC. A detailed description of the basic method for APAP-protein adduct measurement has recently been published (Cook et al., 2015) (Method 2).
For plasma samples, the following reference standards and deuterated internal standards were obtained from Toronto Research Chemicals Inc. (Toronto, ON, Canada): 4-acetamidophenyl β-D-glucuronide sodium salt (APAP-gluc, 98%), 4-acetaminophen sulfate potassium salt (APAP-sulf, 98%), 3-(N-acetyl-L-cystein-S-yl)-acetaminophen disodium salt (APAP-NAC, 95%), 3-cysteinylacetaminophen trifluoroacetic acid salt (APAP-cys, 95%), acetaminophen glutathione disodium salt (APAP-glut, 95%), acetaminophen-d4 (APAP-d4, 98% chemical purity, 99% isotopic purity), and 4-acetaminophen-d3 sulfate (APAP-d3-sulf, 98% chemical purity, 99% isotopic purity). APAP (analytical standard) and ammonium acetate (≥98%) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). LC-MS grade acetonitrile and methanol were obtained from Honeywell Burdick and Jackson (Morristown, NJ, USA). Glacial acetic acid was obtained from Spectrum Chemical Manufacturing Corp. (New Brunswick, NJ, USA). Formic acid (88%) was obtained from Fisher Scientific (Pittsburgh, PA, USA). Ultrapure water (18.2 MΩ) was obtained by passage of deionized water through a Milli-Q Plus filtration system (Millipore, Billerica, MA, USA).
Stock and working solutions of reference standards and internal standards were prepared in methanol-water (1:1, v/v). Reference standard working solutions of 0.01, 0.1, 1, and 10 µg/mL were prepared by serial dilution. Specified concentrations refer to the free acid of salt reference standards. Calibration standards and quality control (QC) samples were prepared concurrently with study samples by fortification of 10 µL of analyte-free, heparinized human plasma with reference standard working solutions. The calibration curve ranged from 0.01 to 100 µg/mL, and QC samples of 0.05, 0.5, 5, and 50 µg/mL were included for assessment of accuracy. If necessary, patient samples were diluted for quantification within the curve range.
To maintain equivalence in preparation, methanol-water (1:1, v/v) was used to bring all study samples (10-µL aliquots) and fortified calibration and QC samples to a total volume of 110 µL. Study samples, calibrators, and QC samples were then fortified with 40 µL of internal standard working solution containing 2.5 and 25 µg/mL of APAP-d4 and APAP-d3-sulf, respectively. Plasma proteins were precipitated by the addition of acetonitrile (600 µL), and each sample was vortex mixed for 30 s before 15 min of centrifugation at 1100 × g. Sample supernatants were transferred to clean tubes, placed in a 35 °C water bath, and evaporated to dryness under a 10 – 15 psi air stream in a Zymark TurboVap LV Evaporator. Sample residues were reconstituted in 500 µL of 0.1% aqueous formic acid, vortex mixed for 30 s, and centrifuged for 5 min at 1100 × g. The supernatants (200 µL) were then carefully removed and transferred to a autosampler vials and held at 5°C until the time of analysis. A 25 µL injection volume was used for analysis.
Sample analysis was performed on an Agilent 1200 Infinity Series HPLC system equipped with an Agilent Poroshell 120 EC-C18 column (2.1 × 100 mm ID, 2.7 µm particle size), which was interfaced with an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA) or on a Waters Acquity UPLC system equipped with a Waters C18 column and interfaced with a Waters Quattro Premier XE triple quadrupole mass spectrometer (Waters Corp., Milford, MA). Sample preparation and measurement were the same in both cases. Standard curves were used to quantify samples in every run. Chromatographic separation was achieved using 10 mM aqueous ammonium acetate, pH 3.5 (A) and methanol (B) at a flow rate of 0.25 mL/min. Mobile phase was maintained at 3% B for the first 6.0 min, increased linearly to 35% B at 9.0 min, maintained at 95% B from 9.1 to 12.0 min, decreased linearly to 3% B at 12.5 min, and then re-equilibrated at 3% B until 20.0 min. The column compartment was maintained at 40 °C, and prepared samples were stored in the autosampler at 5 °C during analysis. The mass spectrometer was operated in electrospray ionization mode with MRM data acquisition. APAP-sulf and APAP-d3-sulf were monitored in negative ionization mode; all other analytes and APAP-d4 were monitored in positive ionization mode. The following source conditions were applied: 350 °C gas temperature, 10 L/min gas flow, 30 psi nebulizer pressure, 350 °C sheath gas temperature, 9 L/min sheath gas flow, 3500 V capillary voltage, and 500 V nozzle voltage. Fragmentor voltage and collision energy were optimized for each MRM transition. The following MRM transitions (precursor m/z → product m/z) were monitored for quantification: 152.1→110.0 for APAP, 328.1→152.1 for APAP-gluc, 230.1→150.1 for APAP-sulf, 313.1→208.0 for APAP-NAC, 271.1→140.0 for APAP-cys, 457.1→140.0 for APAP-GSH, 156.1→114.1 for APAP-d4, and 233.1→153.1 for APAP-d3-sulf. Additional secondary transitions were monitored for qualitative confirmation of analyte presence. Data analysis was performed with MassHunter Workstation Quantitative Analysis software (v. B.04.00, Agilent Technologies, Santa Clara, CA). Calibration curves were constructed by plotting the analyte to internal standard chromatographic peak area ratio against the known analyte concentration in each calibration standard. APAP-d4 was used as the internal standard for APAP, and APAP-d3-sulf was used as the internal standard for all other analytes. Linear curve fits were applied for all analytes except APAP-NAC, for which a quadratic curve fit was used. Curves were weighted in proportion to the reciprocal of the squared analyte concentration.
Statistics
Normality was assessed using the Shapiro-Wilk test. For normally distributed data, significance was tested using the Student’s t-test or one-way analysis of variance (ANOVA) for experiments involving two or more groups, respectively. For non-normally distributed data, significance was tested using the Mann-Whitney U-test or the Kruskal-Wallis test for experiments involving two or more groups, respectively. Multiple comparisons were performed post-hoc using either Tukey’s test or Dunn’s test, depending upon whether the data were normal or non-normal, respectively. Fisher’s Exact test was used for categorical values.
RESULTS
To begin studying the time course of APAP-protein adducts and metabolism of APAP in overdose patients, we used mass spectrometry to measure APAP, five major APAP metabolites and APAP-protein adducts in plasma from nine APAP overdose patients who presented before the peak of injury as indicated by plasma ALT levels. APAP-GSH concentrations were below the lower limit of detection in most samples, indicating that most of this conjugate is quickly broken down to APAP-NAC and APAP-cys. However, we were able to detect and quantify all of the other metabolites. Consistent with previous results, we found that the major metabolite of APAP was APAP-gluc, followed by APAP-sulf, APAP-cys and finally by APAP-NAC (Fig. 1A,C). APAP and all four metabolites were high at the time of study admission, but rapidly decreased (Fig. 1 A,C,E). Surprisingly, although APAP-protein adduct concentrations were already detectable at the time of study admission, the peak of circulating adducts was not observed until later time points, around the time of peak ALT (Fig. 1F). This is different from what has been observed in mice (McGill et al., 2013).
FIGURE 1.
Time courses of APAP, APAP metabolites, and APAP-protein adducts in early-presenting overdose patients. Plasma APAP, metabolites, and protein adducts were retrospectively measured in plasma from APAP overdose patients who presented at least two days before the peak of liver injury indicated by ALT. (A,C) Plasma concentrations of APAP and APAP metabolites from a representative patient. (B,D) Plasma levels of APAP-gluc and APAP-protein adducts from a representative patient. (E) Mean plasma levels of APAP-gluc and ALT in all patients presenting at least two days before the peak of liver injury. (F) Mean APAP-protein adducts and ALT in all patients presenting at least two days before the peak of liver injury. Data for multiple patients are expressed as mean ± SEM for n = 9. *p < 0.05.
We then sorted seventeen APAP overdose patients into early and late presenting groups. Patients were categorized on the basis of plasma APAP levels because it was difficult to ascertain the time of ingestion for many of the patients. APAP has a short circulating half-life, and liver injury develops relatively late after APAP overdose. Thus, patients presenting early after APAP ingestion with a reported history of overdose will have higher plasma APAP concentrations and little or no evidence of liver damage in the first sample obtained after study enrollment. On the other hand, patients with a reported history of APAP overdose who have lower plasma APAP but signs of liver injury in the first study sample are likely to be later presenters. When patient reported time of overdose was available, it was found to be consistent with our patient grouping. The time from overdose to hospital admission was known for three of the early-presenting patients (mean: 10 h, range: 5–16 h) and for ten of the late-presenting patients (mean: 83 h, range: 3–336 h). Although one patient in the latter group presented to the hospital within 3 h of the reported time of overdose, it is important to note that this particular patient did not enroll in our study for another 24 h. The remaining patients in the late group for whom this information is available presented much later. In every case, treatment with N-acetylcysteine (NAC) was immediately started. Although the time frame of APAP use was not available for all patients, the patients in the early group tended to be classified by the diagnosing physician as acute overdoses (duration of APAP use < 48 h) while the late group included a mix of acute and chronic (duration of APAP use > 48 h) cases. The average plasma APAP and ALT levels in the first plasma samples available after the time of study enrollment are shown in Figure 2. Other clinical and demographic information for these patients is listed in Table 1.
FIGURE 2.
Plasma APAP and ALT concentrations in the early and late groups. Plasma APAP and ALT were measured in the first sample available after study enrollment. (A) Plasma APAP. (B) Plasma ALT. Data expressed as mean ± SEM for 6 early patients and 11 late patients. *p < 0.05.
Table 1.
Patient Characteristics.
| Parameter | Early Group | Late Group | p-value |
|---|---|---|---|
| N | 6 | 11 | |
| Age (years)* | 31, 18 – 56 | 35, 19 – 46 | >0.5 |
| Sex (% female) | 50 | 73 | >0.5 |
| Survival | 5 / 6 | 11 / 11 | >0.5 |
| Admission INR*† | 1.2, 1.1 – 1.3 | 3.3, 1.6 – 5.7 | 0.018 |
| Admission Bilirubin (mg/dL)*† | 1.0, 0.50 – 1.3 | 3.9, 1.3 – 7.9 | 0.0090 |
| Admission Creatinine (mg/dL)*† | 1.0, 0.71 – 1.6 | 2.2, 0.72 – 5.7 | 0.062 |
Data are presented as mean, range.
When available.
We found that the average plasma concentration of APAP-sulf was not significantly different between our early and late patients (Fig. 3A). Moreover, the APAP-sulf levels in these overdose patients were not remarkably different from those previously reported in individuals receiving subtoxic or even just therapeutic doses of APAP (Gelotte et al., 2007; Prescott, 1980). On the other hand, APAP-gluc levels were much higher in the early patients (Fig. 3B) and were elevated approximately 20-fold compared with previously reported values for APAP-gluc after subtoxic doses. Together, these results are in agreement with the earlier findings that both sulfation and renal elimination of APAP-sulf are saturated after APAP ingestion and remain so until later time points (Prescott, 1980; Clements et al., 1984), while glucuronidation and APAP-gluc elimination are not so easily saturated even after overdose because the APAP-gluc levels in serum seem to scale with the concentration of APAP. Similar to APAP-sulf, the APAP-NAC and free APAP-cys breakdown products of APAP-GSH, were not significantly different between groups (Fig. 3C,D). Because one would expect a saturated metabolic pathway to plateau and the levels of its metabolite products to remain unchanged for some time, the latter finding is in agreement with the idea that the GSH pathway is also overwhelmed.
FIGURE 3.
Plasma APAP metabolite concentrations in the early and late groups. Plasma APAP metabolites were measured in the first sample available after study enrollment. (A) Plasma APAP-sulf. (B) Plasma APAP-gluc. (C) Plasma APAP-NAC. (D) Plasma free APAP-cys. Data expressed as mean ± SEM for 6 early patients and 11 late patients. *p < 0.05.
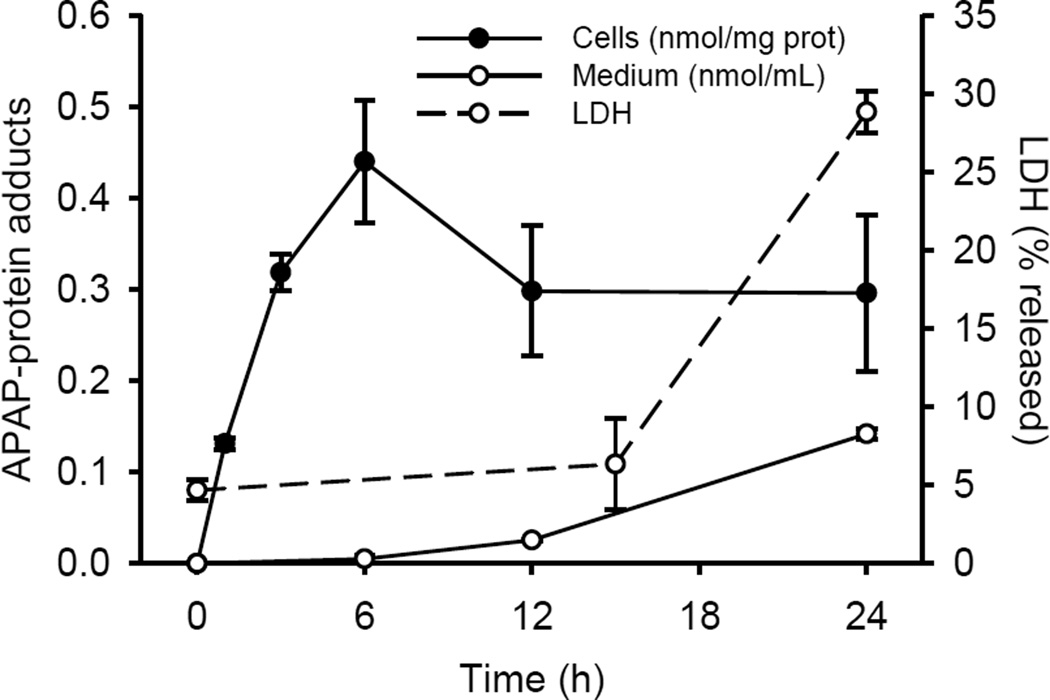
As we predicted from our time course data, protein adduct levels in plasma were much higher in the late-presenting group (Fig. 4). These data suggest that the appearance of peak levels of APAP-protein adducts may be delayed or evolve over hours or even days in humans after overdose, in contrast to rodents which have elevated plasma adducts well before any detectable increase in ALT (within 15–30 min after APAP treatment) (McGill et al., 2013). It is possible to argue that the lower plasma APAP-protein adducts level in the early group is due to earlier treatment with NAC, but the fact that APAP-NAC and free APAP-cys levels did not differ between groups suggest that this is unlikely to be the case. Moreover, these data are from the first available samples obtained after study enrollment. Generally, the patients were enrolled on the day of admission and therefore had not been receiving NAC for long. Furthermore, we also observed a late increase in APAP-protein adducts in culture medium from the human liver cell line HepaRG (Fig. 5), which also differs from what has been reported for primary mouse hepatocytes (McGill et al., 2013). Interestingly, however, the adduct concentration in the culture medium still increased before an increase in enzyme release (Fig. 5), consistent with the idea that adducts are released through a mechanism other than simply cell necrosis.
FIGURE 4.
Plasma APAP-protein adduct concentrations in the early and late groups. Plasma APAP-protein adducts were measured in the first sample available after study enrollment. Data expressed as mean ± SEM for 6 early patients and 11 late patients. *p < 0.05.
FIGURE 5.
Time course of APAP-protein adduct release and cell death in HepaRG cells. HepaRG cells were treated with 20 mM APAP for the indicated times. APAP-protein adducts were measured in both cell lysates and in the cell culture medium. Cell death was assessed by lactate dehydrogenase (LDH) release, as described in the methods section. Data are expressed as mean ± SEM for 3–6 independent experiments.
DISCUSSION
The metabolism and disposition of APAP in humans after therapeutic doses has been studied for quite some time. Recently, several groups have even succeeded with studies designed to explore the mechanisms of APAP hepatotoxicity in humans (Antoine et al., 2012; Antoniades et al., 2012; McGill et al., 2012; McGill et al., 2014a,b; Weerasinghe et al., 2014; Williams et al., 2014). One early advance in this area was the discovery that APAP-protein adducts can be detected in humans (Hinson et al., 1990; Webster et al., 1996; Muldrew et al., 2002). Since then, it has been suggested that the presence of APAP-protein adducts in serum can be used to diagnose APAP overdose (Davern et al., 2006; James et al., 2008). However, there are still gaps in our knowledge about the mechanism of appearance of APAP-protein adducts in serum and even the basic metabolism of APAP after overdose. It is clear that glucuronidation, sulfation, glutathione conjugation, and protein binding all occur in overdose patients, but the kinetics and toxicological significance of each is less well understood. Our goal with this study was to develop a better understanding of the full time course of APAP-protein adduct formation and APAP metabolism after overdose in humans. A summary of our findings has been shown schematically in figure 6.
FIGURE 6.
Schematic of APAP metabolism and APAP-protein adducts release. At therapeutic doses, roughly a third of the dose is eliminated by sulfation while two-thirds is eliminated by glucuronidation. After overdose, sulfation is saturated and a much higher percentage is glucuronidated. Furthermore, our data suggest that APAP-protein adducts are released more slowly in humans than in mice.
Kinetics of plasma APAP-protein adducts after overdose
In mice, APAP-protein adducts rapidly appear in plasma after APAP treatment (McGill et al., 2013). Although it is important to keep in mind that APAP studies in mice are usually done under fasting conditions to deplete GSH more quickly and this could affect the results, it has also been shown APAP-protein adducts are detectable in serum from humans as well as mice even after low doses which do not cause injury (Heard et al., 2011; McGill et al., 2013). Despite earlier suggestion that APAP-protein adducts are released by cell lysis (Davern et al., 2006; James et al., 2009), it is clear from these studies that liver injury is not required for the appearance of adducts in circulation. Furthermore, APAP-protein adducts form rapidly in human hepatocytes after APAP treatment (Xie et al., 2014). Thus, it would seem likely that plasma adducts would increase early in humans after APAP overdose even before the injury begins. If true, then the rapid determination of APAP-protein adducts upon patient presentation using a point-of-care assay could hold considerable clinical value for the early diagnosis of APAP-induced liver injury. However, the detailed time courses of circulating APAP-protein adducts had not previously been measured specifically in a cohort of early-presenting patients who went on to develop high ALT and other signs of severe liver injury. Although individual early-presenting patients have sometimes been included, most work to date has focused on later time points in order to determine the half-life of these adducts in circulation (James et al., 2009; Heard et al., 2011). Perhaps the most interesting finding from our data is that plasma APAP-protein adducts may rise slowly in humans after overdose. This is consistent with the earlier observation that APAP-protein adduct peak levels correlate well with ALT (James et al., 2009). Based on this, it is possible that APAP overdose patients presenting early will have low plasma APAP-protein adduct concentrations. If treatment decisions are made solely on the basis of a rapid point-of-care assay performed at the time of presentation, such low levels may prevent the proper treatment of some patients. Thus, circulating adduct levels should be interpreted carefully. Additionally, because plasma APAP and its metabolites are higher at early time points, we suggest that the plasma level of the parent drug or a metabolite should also be measured in patients with suspected overdose in order to confirm a negative result from adduct measurement. If both adducts and the APAP or APAP metabolite levels are low, then the result is likely to be a true negative. Although this approach may not aid diagnosis in every possible case (Bebarta et al., 2014), it is likely to be effective in most cases. The results of previous work that suggested that serum APAP levels (i.e. the Rumack-Matthews nomogram) are more useful for diagnosis than APAP-protein adducts at early time points are in agreement with this idea (James et al., 2008).
Little is known about the mechanisms of APAP-protein adduct release into circulation. It has been shown that APAP-protein adducts appear in the medium of primary mouse hepatocytes cultured in the absence of extracellular protein and before the onset of cell injury, suggesting that adducts are actively secreted out of hepatocytes into the extracellular space, not simply passively released during cell death. However, the actual route of secretion is unknown. Furthermore, although it is clear that the release of APAP-protein adducts into circulation does not require cell death (Heard et al., 2011; McGill et al., 2013), there is evidence that cell death can further increase APAP-protein adduct levels (McGill et al., 2013). Thus, it seems that the mechanism of adduct release is complex. Additional study is needed to better understand this phenomenon.
Attempts have been made to develop models to determine the time and dose of an APAP overdose (Remien et al., 2012). Although it was suggested that APAP-protein adduct levels in serum would likely not improve these models (Remien et al., 2012), our data showing a clear decrease in APAP and its metabolites while adduct levels were increasing (Fig. 1) may indicate that the relative amounts of metabolites and adducts can provide some indication of the timing of overdose relative to presentation on their own.
Importance of glucuronidation after overdose
It has been known for some time that sulfation is saturated by APAP even after doses in the therapeutic range (Prescott, 1980; Clements et al., 1984; Gelotte et al., 2007). Our data support this finding in overdose patients. At therapeutic doses, about 1/3 of the drug undergoes sulfation, while roughly 2/3 is glucuronidated. The remainder is either converted to NAPQI and binds to GSH or proteins, or is excreted unchanged. Though it is common to cite these percentages in manuscripts even when referring to overdose patients, our results show that glucuronidation plays an even larger role after overdose due to saturation of the sulfation pathway. It seems that glucuronidation is not easily saturated even after overdose. While the APAP-sulf concentrations measured in our patients did not differ much from what has previously been reported after therapeutic doses, the APAP-gluc levels were much higher. It is commonly thought that both of these phase II elimination pathways, along with glutathionylation, are saturated after APAP overdose and that the APAP is then converted to its reactive metabolite (McClain et al., 1999; Chun et al., 2009; Srivastava et al., 2010). However, it is now clear that the reactive metabolite forms and binds to proteins even after therapeutic doses at which glucuronidation and glutathionylation are certainly not saturated (Heard et al., 2011; McGill et al., 2013). The latter strongly suggests that saturation of these pathways is not necessary for NAPQI formation to occur. Nevertheless, glucuronidation is quite important.
The significance of the glucuronidation pathway of APAP elimination has been studied in both rodents and humans, though the results have been controversial. Glucuronidation-deficient Gunn rats have been shown to be much more sensitive to the toxicity of APAP than other rat strains (de Morais and Wells, 1989). Consistent with this, early experiments with low doses in humans revealed that individuals with Gilbert’s syndrome do not glucuronidate APAP as well and have higher concentrations of APAP-GSH in urine (de Morais et al., 1992). However, these results are in conflict with an earlier experiment involving Gilbert’s syndrome patients that yielded opposite results (Ullrich et al., 1987). The difference in findings between these studies may have been due to differences in dose normalization and route of exposure, although later work using an experimental design similar to that used in the no-effect study provided support for the idea that Gilbert’s patients may be at increased risk of APAP hepatotoxicity (Esteban and Pérez-Mateo, 1999). More recently, Court et al. (2013) found that liver samples from humans with polymorphisms in the 3’ untranslated region of UGT1A, particularly rs8330, had higher acetaminophen glucuronidation activity. Importantly, they also found that there were fewer individuals with these alleles among patients with acute liver failure caused by unintentional APAP overdose than among those with acute liver failure from other etiologies (Court et al., 2013), which may suggest that a greater ability to glucuronidate APAP can protect against the hepatotoxicity caused by overdose. Overall, there is a strong case that glucuronidation is the most important route of elimination of APAP, such that Gilbert’s syndrome patients are likely to be more susceptible to APAP toxicity while patients who are better at glucuronidation are less likely to develop APAP-induced liver injury. Our data support this conclusion.
Potential weaknesses
Our grouping of patients based on serum APAP and ALT levels is a potential weakness of this study. Although our grouping was consistent with the available information for time from overdose to time of presentation, we did not have complete data. Furthermore, we did not collect data concerning the time from overdose to NAC treatment or other basic patient information, such as the dose of APAP taken, which could affect the metabolite and adduct levels that we measured. This should be kept in mind when interpreting the results presented in this manuscript.
Conclusions
Our data suggest that plasma APAP-protein adducts increase slowly in humans in contrast to mice, and this may have important implications for the rapid clinical measurement of APAP-protein adducts in early-presenting patients. Moreover, our data confirm that the sulfation pathway is quickly saturated after exposure to APAP, but indicate that glucuronidation is a much higher capacity system. Overall, however, the results of this study provide additional support for the idea that glucuronidation is the most critical elimination pathway for APAP.
ACKNOWLEDGEMENTS
We thank Biopredic International (Rennes, France) for providing HepaRG cells.
DISCLOSURE
This work was supported in part by a pilot grant from the KUMC Liver Center (to H.J.), by the National Institutes of Health grants R01 DK070195 and R01 AA12916 (to H.J.), and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health and from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to M.R.M.) from the National Institute of Environmental Health Sciences.
REFERENCES
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56(5):1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, Wagner B, Barnardo A, Pomplun S, Auzinger G, Bernal W, Heaton N, Vergani D, Thursz MR, Wendon J. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56(2):735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80(2):343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bebarta VS, Shiner DC, Varney SM. A case of moderate liver enzyme elevation after acute acetaminophen overdose despite undetectable acetaminophen level and normal initial liver enzymes. Am J Ther. 2014;21(3):e82–e84. doi: 10.1097/MJT.0b013e31824714a8. [DOI] [PubMed] [Google Scholar]
- Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40(6):585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43(4):342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- Clements JA, Critchley JA, Prescott LF. The role of sulphate conjugation in the metabolism and disposition of oral and intravenous paracetamol in man. Br J Clin Pharmacol. 1984;18(4):481–485. doi: 10.1111/j.1365-2125.1984.tb02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SF, King AD, Chang Y, Murray GJ, Norris HR, Dart RC, Green JL, Curry SC, Rollins DE, Wilkins DG. Quantification of a biomarker of acetaminophen protein adducts in human serum by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: clinical and animal model applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;985C:131–141. doi: 10.1016/j.jchromb.2015.01.028. [DOI] [PubMed] [Google Scholar]
- Court MH, Freytsis M, Wang X, Peter I, Guillemette C, Hazarika S, Duan SX, Greenblatt DJ, Lee WM Acute Liver Failure Study Group. The UDP-glucuronosyltransferase (UGT) 1A polymorphism c.2042C>:G (rs8330) is associated with increased human liver acetaminophen glucuronidation, increased UGT1A exon 5a/5b splice variant mRNA ratio, and decreased risk of unintentional acetaminophen-induced acute liver failure. J Pharmacol Exp Ther. 2013;345(2):297–307. doi: 10.1124/jpet.112.202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130(3):687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- de Morais SM, Uetrecht JP, Wells PG. Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert’s syndrome. Gastroenterology. 1992;102(2):577–586. doi: 10.1016/0016-5085(92)90106-9. [DOI] [PubMed] [Google Scholar]
- de Morais SM, Wells PG. Enhanced acetaminophen toxicity in rats with bilirubin glucuronyl transferase deficiency. Hepatology. 1989;10(2):163–167. doi: 10.1002/hep.1840100207. [DOI] [PubMed] [Google Scholar]
- Esteban A, Pérez-Mateo M. Heterogeneity of paracetamol metabolism in Gilbert’s syndrome. Eur J Drug Metab Pharmacokinet. 1999;24(1):9–13. doi: 10.1007/BF03190005. [DOI] [PubMed] [Google Scholar]
- Gelotte CK, Auiler JF, Lynch JM, Temple AR, Slattery JT. Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clin Pharmacol Ther. 2007;81(6):840–848. doi: 10.1038/sj.clpt.6100121. [DOI] [PubMed] [Google Scholar]
- Heard KJ, Green JL, James LP, Judge BS, Zolot L, Rhyee S, Dart RC. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011;11:20. doi: 10.1186/1471-230X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, Benson RW, Dalhoff K, Loft S, Poulsen HE. Mechanism of paracetamol toxicity. Lancet. 1990;335(8691):732. doi: 10.1016/0140-6736(90)90851-u. [DOI] [PubMed] [Google Scholar]
- James LP, Capparelli EV, Simpson PM, Letzig L, Roberts D, Hinson JA, Kearns GL, Blumer JL, Sullivan JE Network of Pediatric Pharmacology Research Units, National Institutes of Child Health and Human Development. Acetaminophen-associated hepatic injury: evalutation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84(6):684–690. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37(8):1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33(1):36–45. doi: 10.1055/s-0032-1301733. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Price S, Barve S, Devalarja R, Shedlofsky S. Acetaminophen hepatotoxicity: an update. Curr Gastroenterol Rep. 1999;1(1):42–49. doi: 10.1007/s11894-999-0086-3. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30(9):2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269(3):240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Li F, Sharpe MR, Williams CD, Curry SC, Ma X, Jaeschke H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch Toxicol. 2014a;88(2):391–401. doi: 10.1007/s00204-013-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Staggs VS, Sharpe MR, Lee WM, Jaeschke H Acute Liver Failure Study Group. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 2014b;60(4):1336–1345. doi: 10.1002/hep.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53(3):974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30(4):446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (paracetamol)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15(6):398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S–298S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remien CH, Adler FR, Waddoups L, Box TD, Sussman NL. Mathematical modeling of liver injury and dysfunction after acetaminophen overdose: early discrimination between survival and death. Hepatology. 2012;56(2):727–734. doi: 10.1002/hep.25656. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Carracio TR, Mofenson HC. The temporal profile of increased transaminase levels in patients with acetaminophen-induced liver dysfunction. Ann Emerg Med. 1995;26(1):49–53. doi: 10.1016/s0196-0644(95)70237-7. [DOI] [PubMed] [Google Scholar]
- Ullrich D, Sieg A, Blume R, Bock KW, Schröter W, Bircher J. Normal pathways for glucuronidation, sulphation and oxidation of paracetamol in Gilbert’s syndrome. Eur J Clin Invest. 1987;17(3):237–240. doi: 10.1111/j.1365-2362.1987.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Maggs JL, Antoine DJ, Williams DP, Smith DA, Park BK. Role of reactive metabolites in drug-induced hepatotoxicity. Handb Exp Pharmacol. 2010;196:165–194. doi: 10.1007/978-3-642-00663-0_7. [DOI] [PubMed] [Google Scholar]
- Webster PA, Roberts DW, Benson RW, Kearns GL. Acetaminophen toxicity in children: diagnostic confirmation using a specific antigenic biomarker. J Clin Pharmacol. 1996;36(5):397–402. doi: 10.1002/j.1552-4604.1996.tb05025.x. [DOI] [PubMed] [Google Scholar]
- Weerasinghe SV, Jang YJ, Fontana RJ, Omary MB. Carbamoyl phosphate synthetase-1 is a rapid turnover biomarker in mouse and human acute liver injury. Am J Physiol Gastrointest Liver Physiol. 2014;307(3):G355–G364. doi: 10.1152/ajpgi.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275(2):122–133. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014;279(3):266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]