Abstract
Rationale
Accelerated arterial stiffening is a major complication of diabetes with no specific therapy available up to date.
Objective
The present study investigates the role of the osteogenic transcription factor Runx2 as a potential mediator and therapeutic target of aortic fibrosis and aortic stiffening in diabetes.
Methods and Results
Using a murine model of type 2 diabetes (db/db mice) we identify progressive structural aortic stiffening that precedes the onset of arterial hypertension. At the same time, Runx2 is aberrantly upregulated in the medial layer of db/db aortae as well as in thoracic aortic samples from type 2 diabetic patients. Vascular smooth muscle-specific overexpression of Runx2 in transgenic mice increases expression of its target genes, Col1a1 and Col1a2, leading to medial fibrosis and aortic stiffening. Interestingly, increased Runx2 expression per se is not sufficient to induce aortic calcification. Using in vivo and in vitro approaches, we further demonstrate that Runx2 expression in diabetes is regulated via a redox-sensitive pathway that involves a direct interaction of NF-κB with the Runx2 promoter.
Conclusion
In conclusion this study highlights Runx2 as a previously unrecognized inducer of vascular fibrosis in the setting of diabetes, promoting arterial stiffness irrespective of calcification.
Keywords: Arterial stiffness, diabetes mellitus, extracellular matrix, oxidative stress, smooth muscle cell
INTRODUCTION
Diabetes is a growing health problem throughout the world. The prevalence of diabetes was estimated to be 171 million people in the year 2000, and is predicted to more than double to over 366 million people by the year 20301. Largely unknown in the early 20th century, type 2 diabetes is now the 7th leading cause of death in the USA2.
Cardiovascular disease is the main cause of death in type 2 diabetes3. One of the mechanisms linking diabetes to increased cardiovascular risk may be accelerated arterial stiffening that is frequently observed in diabetic patients4. In fact, arterial stiffness predicts the development of cardiovascular disease and mortality in the general population and in type 2 diabetes5,6.
Arterial stiffening may occur as an adaptive response to hypertension, maintaining vascular mechanical stress homeostasis7. Conversely, the lack of Windkessel function associated with aortic stiffening, may generate elevated pulse pressure, thereby contributing to hypertension8. Due to the high prevalence of co-existent hypertension in the diabetic population (approximately 70%)9 it is difficult to discern whether aortic stiffening is primarily due to hypertension, or occurs as an independent pathology – and should therefore be diagnosed and treated independently. To this end, animal models may help study the relationship between arterial stiffness and hypertension.
The mechanical properties of the aorta, being a conduit vessel, are largely defined by its passive (structural) stiffness, which in turn is mainly determined by the extracellular matrix (ECM) components of the vessel wall10-12. Fibrillar collagen (type 1 and type 3) as well as elastin represent the major ECM proteins of the aorta. While elastin is a relatively inert protein, collagen is readily remodeled in response to various stimuli.
The osteogenic transcription factor Runx2 (Cbfa1) may qualify as a mediator of ECM remodeling and aortic stiffening in the context of diabetes. Runx2 was initially identified as an activator of osteoblast differentiation during embryonic development13. In postnatal life, however, it serves as a physiologic transcriptional regulator of bone matrix protein deposition (such as type I collagen) in osteogenic tissue14. More recently, Runx2 received attention in vascular biology, emerging as a marker of vascular smooth muscle cell (VSMC) trans-differentiation towards an osteogenic phenotype, and contributing to the active vascular calcification found in athero- and arteriosclerosis15-17. VSMC-specific knockout experiments demonstrated functional involvement of Runx2 in murine atherosclerotic calcification17. However, the immediate function of Runx2 in the vascular system – in particular as a potential regulator of matrix protein expression and arterial stiffness – is unknown.
This study tests the hypothesis that structural aortic stiffening occurs independently from arterial hypertension in a murine model of type 2 diabetes. We further use a VSMC-specific transgenic approach to investigate the potential of Runx2 to induce aberrant aortic matrix production, resulting in increased stiffness and hypertension. Finally, we delineate a novel signaling mechanism potentially leading to enhanced Runx2 expression in VSMC under diabetic conditions (ROS – NF-kB – Runx2 pathway).
METHODS
An expanded Methods section is available in the Online Supplement.
Animals
Male db/db mice (BKS.Cg-Dock7m +/+Leprdb/J) and their age-matched heterozygous non-diabetic controls (+/db) were purchased from the Jackson Laboratory (Bar Harbor, ME).
Transgenic mice with vascular smooth muscle cell (VSMC)-specific Runx2-overexpression (Runx2-smTg; C57Bl/6 background) were generated at our institution by pronuclear injection of a transgene cassette consisting of the entire coding region of mouse type 1 Runx2 driven by the 445 bp form of the SM22α promoter.
Pressure myography
Pressure myography was performed to directly assess the passive aortic mechanics according to adapted protocols18. In brief, murine thoracic aortae were explanted and placed on specially designed stainless steel cannulas. Subsequently the artery was pressurized from 0 to 180 mmHg in 18 mmHg increments, and the vessel’s outer diameter was simultaneously tracked by continuous computer video analysis.
RNA quantification
Total aortic RNA was isolated and processed for qRT-PCR using standard protocols and methods.
Histology, immunofluorescence, and in situ DHE staining
Standardized protocols were used, with details available in the Online Supplement.
Elastin imaging
Elastin autofluorescence was visualized in 30 μm thick aortic cross sections using confocal microscopy and 3D structure was reconstructed from 28 layers using ImageJ (National Institutes of Health, Bethesda, MD, USA). Elastin fragmentation was quantified in the histologic images using elastin fluorescence morphometric analysis (ImageJ).
Aortic collagen quantification
Total aortic collagen was quantified using a hydroxyproline-detection based assay (QuickZyme Total Collagen Assay; Quickzyme) as per manufacturer’s protocol.
Western blots
Standardized protocols were used, with details available in the Online Supplement.
Transfection of cultured cells
Transfection of human aortic smooth muscle cells (AoSMCs) was performed using Lipofectamine RNAiMAX (Invitrogen) reagent, and siRNA targeting Runx2 or NF-kB subunits RelA (p65) and Nfkb1 (p50) (all from Ambion).
Chromatin immunoprecipitation assay (ChIP)
AoSMC ChIP analysis was performed using the ChIP-IT High-Sensitivity Kit (Active Motif) as per manufacturer’s protocol.
NF-κB activation assay
In order to quantify cellular NF-κB activation in aortic tissue and cultured AoSMCs, nuclear extracts were obtained using the Nuclear Extract Kit (Active Motif) as per the manufacturer’s protocol. Subsequently NF-κB activation (p65 DNA-binding activity) was measured using the TransAM NF-κB activation Kit ELISA (Active Motif).
RelA (p65)/DNA binding assay
In order to analyze the NF-κB binding capacity of the predicted RelA (p65) binding motif within the Runx2 promoter we used a DNA-binding ELISA approach (TransAM Flexi NFκB p65 Kit; Active Motif) as previously described19
Luciferase assay
The functional relevance of direct NF-κB binding for the Runx2 promoter activity was tested by transfecting high glucose stimulated AoSMCs with SwitchGear GoClone luciferase constructs..
Statistics
Data are presented as mean ± SEM. For comparison of 2 groups Student’s t-test (two-tailed) was performed; multiple groups (≥3 groups) comparison was accomplished by ANOVA with Bonferroni’s post test. For pressure myography analysis two-way repeated measures ANOVA followed by Bonferroni’s post test was used. Normality and homoscedasticity was tested to ensure that parametric testing was appropriate. A value of p<0.05 (two-sided) was considered statistically significant.
RESULTS
Structural aortic stiffening precedes the onset of hypertension in db/db mice
In order to characterize the mechanical properties of db/db aortae we first performed ex vivo mechanical testing of thoracic aortic segments using pressure myography. Aortic tissue derived from 20 week-old hyperglycemic db/db mice (Online Figure I) exhibit significantly increased aortic stiffness compared to non-diabetic +/db controls (Figure 1A). Concomitantly, we found statistically significant elevations of systolic and mean blood pressure levels by non-invasive tail volume-pressure recording (Figure 1B). Importantly, pulse pressure, an indirect measure of aortic stiffness, was also increased in diabetic conditions (Figure 1B).
Figure 1. db/db mice exhibit increasing aortic stiffness that precedes blood pressure elevations.
(A) Passive pressure-diameter curves derived from pressure myography of 20-week old db/db and +/db aortae. Values are mean ± SEM. * p<0.05 vs +/db controls for pressure levels ≥ 36 mmHg. n=5/group. (B) Systolic, diastolic, mean and pulse pressure levels of 20-week old db/db mice and +/db controls. n=10/group. Values are mean ± SEM. * indicates p<0.05 (C) Pressure-diameter curves of 4,10, and 20 week-old db/db aortae. Values are mean ± SEM. * p<0.05 vs. db/db – 4wks (for pressure levels ≥ 36 mmHg); # p<0.05 vs. db/db – 10wks (for pressure levels ≥72 mmHg). (D) Systolic, diastolic, mean and pulse pressure levels of aging db/db mice. n=10/group. Values are mean ± SEM.
* indicates p<0.05.
Further, investigating the temporal course of passive aortic stiffening and its relation to the onset of hypertension in diabetic db/db mice, we performed serial pressure myography measurements. Starting our measurements at 4 weeks of age (when db/db mice were mildly hyperglycemic; Online Figure I), we found progressive aortic stiffening at 10 and 20 weeks of age (Figure 1C). Blood pressure levels monitored at corresponding time points showed statistically significant elevations of systolic, diastolic and mean blood pressure levels only at 20 weeks of age in db/db mice, but not at 10 weeks (Figures 1D). Pulse pressure was also only increased at 20 weeks of age (Figure 1D). Taken together these results suggest that db/db mice display progressive mechanical stiffening of the aorta prior to development of hypertension.
db/db mice exhibit enhanced medial collagen production and deposition associated with increased Runx2
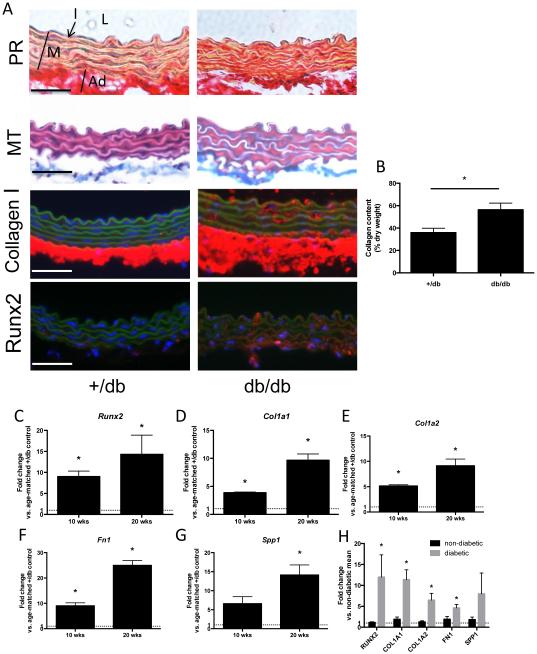
Passive aortic stiffness largely depends on the matrix composition within the vessel’s medial layer. Thus, we next focused on structural ECM alterations as the putative basis for the observed aortic stiffening in db/db mice. Histologic examination of aortic sections (by Picrosirius Red, Masson’s Trichrome; Figure 2A; as well as HE staining; Online Figure II) revealed increased collagen deposition in the media from db/db aortae compared to controls. Additional immunofluorescence analysis confirmed enhanced Collagen I expression in the aortic medial layer (Figure 2A). These observations were reflected by an increased collagen content measured in db/db aortae (Figure 2B).
Figure 2. Expression of Runx2 and target genes is increased in db/db aortae and in human aortae from type 2 diabetes patients.
(A) Aortic cross section from 20 week-old mice. Representative images of aortic cross section stained with Picrosirius Red (PR; collagen red, muscle yellow) and Masson’s Trichrome (MT; collagen blue, muscle red). Representative immunofluorescent images of aortic cross section stained for collagen I (red) or Runx2 (red). Green depicts the autofluorescence of the elastic lamella. Nuclei are Hoechst stained (blue). Original magnifications are 400x, scale bars 50 μm. In the upper left panel L indicates the aortic lumen, I points toward intimal layer, M identifies the aortic media and Ad the adventitia. (B) Total collagen content per aortic dry weight. n=4/group. * indicates p < 0.05. (C-G) Expression levels of Runx2 (C), Col1a1 (D) , Col1a2 (E), Fn1 (F), and Spp1 (G) in thoracic aortae of 10 weeks and 20 weeks old db/db mice compared to age-matched +/db controls. Values are expressed as fold changes relative to the mean expression level of +/db controls (=1; dotted line). * indicates p < 0.05 vs. +/db controls. n = 5/group (H) Expression levels of RUNX2, COL1A1, COL1A2, FN1 and SPP1 in thoracic aortae of diabetic patients (n=8) compared to non-diabetic patients (n=10). Values are expressed as fold changes relative to the mean expression level in non-diabetic patients (=1; dotted line). * indicates p < 0.05 vs. non-diabetic patients.
Runx2 is known as a transcriptional regulator of collagen deposition in bone. Investigating the potential contribution of Runx2 in the pro-fibrotic response in db/db aortae, we detected increased protein expression of Runx2 that was largely confined to the medial layer of the aorta (Figure 2A). This was accompanied by significantly increased gene expression of Runx2 and several of its ECM target genes (Col1a1, Col1a2, Fn1 and Spp1) in 10- and 20-week-old db/db mice compared to age-matched controls (Figure 2C-G).
Aortic RUNX2 expression is increased in human type 2 diabetes patients
We sought to confirm the translational relevance of the increased aortic Runx2 expression found in our murine model. Therefore, we analyzed the gene expression in thoracic aortic samples that were collected during aorto-coronary bypass surgery (aortic plugs) from patients with type 2 diabetes (n=8; mean age 61.0 ± 2.9 years) and from non-diabetic patients (n=10; mean age 61.2 ± 4.1 years; p=n.s. vs. diabetic patients). All patients were male, Caucasian and non-smokers. Although the patients (diabetic and non-diabetic) taking part in this study were not systematically screened for vascular calcification as a predefined category there was no macroscopic evidence of gross thoracic aortic calcification that would complicate bypass anastomosis. We found RUNX2 as well as the target genes COL1A1, COL1A2 and FN1 to be significantly up-regulated in aortae from diabetic patients compared to non-diabetic patients (Figure 2H and Online Figure III).
Runx2 overexpression induces aortic media fibrosis, increased aortic stiffness and elevated pulse pressure
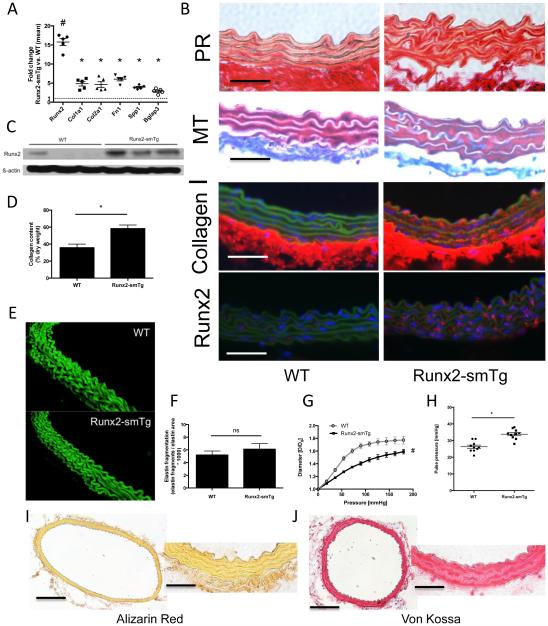
In order to discern the functional impact of enhanced medial Runx2 expression on aortic stiffness we generated Runx2-smTg mice expressing the entire coding region of murine Runx2 under control of the SM22α/transgelin promoter – thereby achieving vascular smooth muscle cell (VSMC)-specific overexpression of Runx2. VSMCs constitute the predominant cell type of the aortic medial layer that in turn largely defines the vessel’s mechanical stiffness. Runx2-smTg mice are viable and fertile and develop normally without any obvious phenotypic abnormalities. No differences in body weights were identified between Runx2-smTG mice and WT littermates in both genders (data not shown). Runx2-smTg mice exhibit uniform ~15-fold up-regulation of Runx2 gene expression that is similar to db/db mice compared to non-diabetic controls at 20 weeks of age. This is accompanied by significantly increased expression of matrix-related target genes (such as Col1a1, Col2a1, Fn1, Spp1, and Bglap3) (Figure 3A). Induction of Runx2 protein expression in the aortic media was confirmed via fluorescent immunohistochemistry (IHC) (Figure 3B) and Western blot (Figure 3C). According to its SM22α promoter-dependent regulation, Runx2 overexpression was not confined to the aorta but was comparably expressed in carotid and femoral arteries (Online Figure IV). We further analyzed the expression levels of the matrix genes that are not under direct control of Runx2 as a transcription factor, such as Col3a1, Col5a1, as well as Eln. Expectedly, those genes were not upregulated in Runx2-Tg aortae (Online Figure V,A). Similarly, diabetic aortae did not exhibit significant dysregulation of these aforementioned genes (Online Figure V,B).
Figure 3. VSMC-specific Runx2-overexpression (Runx2-smTg mice) induces aortic medial fibrosis, stiffness and hypertension.
(A) Expression levels of Runx2, Col1a1, Col1a2, Fn1, Spp1, and Bglap3 in thoracic aortae of 20 week-old Runx2-smTg mice compared to age-matched WT controls. Values are expressed as fold changes relative to the mean expression level in WT controls (=1; dotted line). # indicates p < 0.001 vs. WT controls, * indicates p < 0.05 vs. WT controls (n=5/group). (B) Aortic cross section from 20 week-old mice. Representative images of aortic cross section stained with Picrosirius Red (PR; collagen red, muscle yellow) and Masson’s Trichrome (MT; collagen blue, muscle red). Representative immunofluorescent images of aortic cross section stained for collagen I (red) or Runx2 (red). Green depicts the autofluorescence of the elastic lamella. Nuclei are Hoechst stained (blue). Original magnifications are 400x, scale bars 50 μm. (C) Aortic Runx2 protein expression in Runx2-smTg mice and WT controls. (D) Total collagen content per aortic dry weight. n=4/group. * indicates p < 0.05. (E) Representative images of aortic elastin lamellae (3D reconstruction of 28 individual layers). (F) Elastin fragmentation index quantified from 3 high power fields of 3 different aortas per group. (G) Aortic pressure-diameter curves from 20 weeks old mice. Values are mean ± SEM. # indicates p<0.05 vs. control for pressure levels ≥ 36 mmHg. (H) Pulse pressure derived from 20 week-old mice. * indicates p<0.05. n = 10/group (I,J) Representative images of thoracic aortic cross sections stained for calcium (Alizarin Red; I) and inorganic phosphate (von Kossa; J). Original magnifications are 100x (left panels; scale bars 200 μm) and 400x (right panels; scale bars 50 μm).
We next confirmed increased collagen expression histologically in the aortic medial layer of Runx2-smTg mice (Figure 3B, Online Figure VI), which was additionally reflected by increased total amounts of aortic collagen (Figure 3D). Further characterization of Runx2-smTg aortae revealed normally developed medial elastin lamellae without increased elastin breaks (Figures 3E,F, Supplemental Video) as well as normal appearance of the smooth muscle layer positively stained with SMA (Online Figure VII) without evidence of increased apoptosis (Online Figure VIII). Of note, Runx2-smTg mice exhibit normal blood glucose levels (Online Figure I).
Mechanical testing revealed markedly enhanced stiffness of Runx2-smTg aortae as compared to C57Bl/6 controls (Figure 3G). At the hemodynamic level enhanced stiffness was paralleled by increased arterial pulse pressure levels (Figure 3H, Online Figure IX).
Given the known association between enhanced Runx2 expression and osteogenic trans-differentiation in vascular smooth muscle cells (VSMCs) we determined whether increased Runx2 expression per se was sufficient to induce medial calcification in vivo, potentially augmenting arterial stiffness. However, we were unable to detect any substantial deposition of calcium (by Alizarin Red staining; Figure 3I) or inorganic phosphate (by von Kossa staining; Figure 3J) in the thoracic aortae of the Runx2-transgenic mice. Further, we assessed whether subtle calcification precursors, such as microcalcifications or enhanced CD63-postive exosome/matrix vesicle formation as a first nidus for mineralization20, are present in Runx2-smTG aortae. Here, neither bisphosphonate-based microcalcification staining (OsteoSense) (Online Figure X) nor CD63 immunofluorescence (Online Figure XI),was able to detect differences between Runx2-smTG aortic sections compared to WT.
Antioxidant treatment reduces aortic Runx2 expression, medial fibrosis, aortic stiffness and pulse pressure in db/db mice
Diabetes is associated with increased generation of reactive oxygen species (ROS) in the vasculature. To determine if diabetes associated Runx2 expression is redox-sensitive, db/db mice were treated with the antioxidant agent TEMPOL between 10 and 20 weeks of age.
We performed in situ dihydroethidium (DHE) staining to monitor ROS formation in aortic sections. TEMPOL treatment resulted in a significant decrease in 2-hydroxyethidium generation in db/db mice, indicative of reduced oxidative stress (Figures 4A,B). TEMPOL, however, had no effect on blood glucose levels in db/db mice (Online Figure I).
Figure 4. Antioxidant treatment (TEMPOL) reduces aortic Runx2 expression, medial fibrosis, aortic stiffness and pulse pressure in db/db mice.
(A) In situ DHE staining of thoracic aortic section from 20 week-old db/db mice, with and without 10 weeks of prior TEMPOL treatment. ROS production was indicated by red fluorescence. Original magnification x400, scale bar 50 μm. (B) Average DHE intensity was quantified from 3 high power fields of 3 different aortas per group. * indicates p < 0.05 (C) Expression levels of Runx2, and its pro-fibrotic target genes Col1a1 and Col1a2 in thoracic aortae of 20 weeks old db/db mice with and without addition TEMPOL treatment. Values are expressed as fold changes relative to the mean expression level of +/db controls (=1; dotted line). * indicates p < 0.05. n = 5/group (D) Representative images of aortic cross section stained with Masson’s Trichrome (MT; collagen blue, muscle red). Representative immunofluorescent images of aortic cross section stained for collagen I (red) or Runx2 (red). Green depicts the autofluorescence of the elastic lamella. Nuclei are Hoechst stained (blue). Original magnifications are 400x, scale bars 50 μm. (E) Aortic Runx2 protein expression in db/db mice with and without TEMPOL treatment as well as in +/db controls. (F) Total collagen content per aortic dry weight. n=4/group. * indicates p < 0.05. (G) Aortic pressure-diameter curves from 20 weeks old db/db mice ± TEMPOL treatment. # indicates p<0.05 vs. control for pressure levels ≥ 90 mmHg. n= 5-7/group (H) Pulse pressure derived from 20 weeks old db/db mice with and without TEMPOL treatment. * indicates p<0.05. n=10/group
Gene expression analysis revealed that TEMPOL treatment dramatically attenuated Runx2 up-regulation in db/db mice (Figure 4C). This was accompanied by down-regulation of ECM target genes Col1a1 and Col1a2 (Figure 4C). We further detected decreased protein levels of Runx2 (by immunofluorescence and Western blot; Figures 4D,E) and collagen deposition (Figure 4D,F)in the aortic media of TEMPOL-treated db/db mice.
At the functional level, antioxidant treatment counteracted passive aortic stiffening as monitored by pressure myography, and reduced pulse pressure in db/db mice (Figure 4G,H; Online Figure XII).
Runx2 mediates a pro-fibrotic response of human aortic smooth muscle cells (AoSMC) to high glucose stimulation
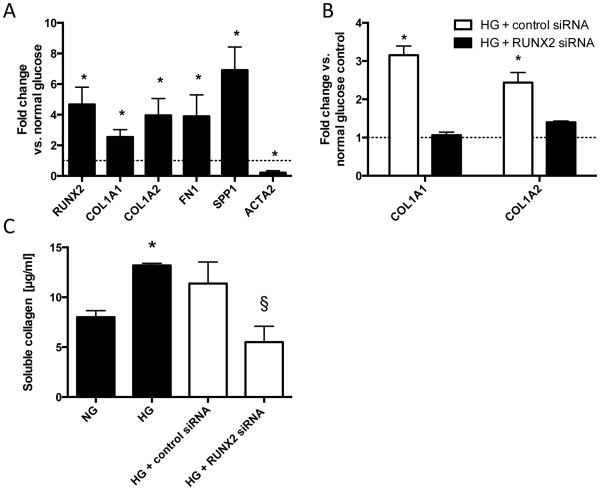
We investigated Runx2-dependent pro-fibrotic mechanisms in vitro by exposing human aortic smooth muscle cells (AoSMCs) to high glucose (HG) conditions. We found that HG (25 mM) increased the expression of RUNX2 and target genes including COL1A1, COL1A2, FN1 and SPP1. Also, ACTA2 – a SMC differentiation marker – was down-regulated in high glucose conditions (Figure 5A). Focusing on the requirement of Runx2 in mediating high glucose-induced ECM gene up-regulation, we found that siRNA-mediated knockdown of Runx2 abolished high glucose-induced up-regulation of COL1A1 and COL1A2 (Figure 5B). Further, siRNA-mediated knockdown of Runx2 eliminated high glucose-enhanced production of soluble collagen in the supernatant of AoSMCs (Figure 5C).
Figure 5. Runx2 mediates a pro-fibrotic response of human aortic smooth muscle cells (AoSMC) to high glucose stimulation.
(A) Expression of RUNX2 and targets COL1A1, COL1A2, FN1, SPP1 and SMC differentiation marker ACTA2 following high glucose (HG) treatment. * indicates p<0.05 vs normal glucose + osmotic control. (B) Expression of COL1A1 and COL1A2 under high glucose treatment with and without additional siRNA-mediated knockdown of Runx2. Values are expressed as fold changes relative to the mean expression level of normal glucose controls (=1; dotted line).* indicates p<0.05 vs. corresponding normal glucose condition and Runx2-knockdown condition. (C) Soluble collagen content in supernatant from HG treated cells ± additional Runx2 knockdown.* indicates p<0.05 vs. normal glucose control (+osmotic control); # indicates p<0.05 vs. HG + control siRNA. Data from n = 3 – 4 independent experiments.
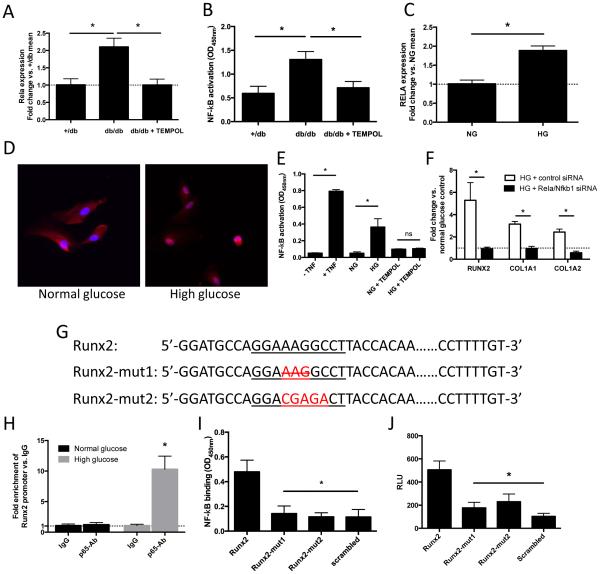
NF-κB is activated in db/db aortae and in AoSMCs under high-glucose conditions, and serves as a transcription factor for Runx2
Given that aortic tissue from db/db mice display elevated ROS production, we next investigated the role of the redox-sensitive transcription factor, NF-κB, in the enhanced expression of Runx2. Indeed, we found increased Rela expression (Figure 6A) as well as increased NF-κB activation (by RelA (p65)-DNA binding assay; Figure 6B) in db/db mice aortae, both of which are sensitive to antioxidant (TEMPOL) treatment. Similarly, we observed increased expression (Figure 6C) and nuclear translocation (Figure 6D) of RelA (p65) in AoSMCs in response to high glucose stimulation. Functionally, this resulted in increased NF-κB activity under high glucose stimulation that was reversible by antioxidant (TEMPOL) treatment (Figure 6E).
Figure 6. NF-κB is activated under high-glucose conditions in AoSMCs and induces transcription of Runx2.
(A) Rela expression levels in thoracic aortae of 20 weeks old +/db mice as well as db/db mice with and without addition TEMPOL treatment. Values are expressed as fold changes relative to the mean expression level of +/db controls (=1; dotted line). * indicates p < 0.05. n = 5/group. (B) NF-κB activity in nuclear extracts isolated from db/db aortae with and without prior TEMPOL treatment as well as +/db controls. * indicates p < 0.05. n = 5/group. (C) RELA expression levels following high glucose (HG) treatment. Values are expressed as fold changes relative to the mean expression level of normal glucose (NG) controls (=1; dotted line). * indicates p < 0.05. n = 3 independent experiments. (D) AoSMCs stained for RelA (p65) (red) under high glucose and normal glucose + osmotic control conditions. Nuclei are Hoechst stained (blue). (E) NF-κB activity in nuclear extracts isolated from AoSMCs following stimulation with TNF-a (positive control), high glucose (HG), and HG with additional TEMPOL treatment. * indicates p<0.05 vs. cells without TNF-a treatment, normal glucose + osmotic control (NG) or NG + TEMPOL, respectively. (F) Expression of RUNX2, COL1A1 and COL1A1 under high glucose treatment with and without additional siRNA mediated knockdown of the NF-kB subunits RelA (p65) and NF-kB (p50). Values are expressed as fold changes relative to the mean expression level of normal glucose (NG) controls (=1; dotted line).* indicates p<0.05 (G) Predicted NF-kB (RelA; p65) binding site (underlined) within the human Runx2 promoter (Runx2); custom mutants of the NF-kB binding site with deletion of a base triplet (Runx2-mut1) and mutation of 5 consecutive bases (Runx2-mut2). (H) Chromatin immunoprecipitation (ChIP) analysis of Runx2 promoter enrichment after anti-p65 antibody immunoprecipitation. Values are fold enrichment relative to IgG control. * indicates p < 0.05 vs. IgG control. (I) Quantification of NF-kB (RelA, p65) binding capacity of DNA oligos containing the predicted p65 binding motif located within the Runx2 promoter (Runx2), two custom mutants (Runx2-mut1; Runx2-mut2), or a scrambled sequence (negative control). Solid-phase bound oligos were incubated with nuclear extracts form high glucose stimulated AoSMCs. * indicates p<0.05 vs. Runx2. (J) Luciferase activity in high glucose stimulated AoSMCs transfected with the intact promoter of Runx2 (Runx2), Runx2 mutants (Runx2-mut1 and Runx2-mut2), or with a scrambled promoter (negative control). * indicates p<0.05 vs. Runx2. Data from n = 5 independent experiments.
Knockdown of the NF-κB subunits RelA (p65) and Nfkb1 (p50) via siRNA completely inhibited the high glucose-stimulated up-regulation of RUNX2, COL1A1 and COL1A2 in human AoSMCs (Figure 6F). To further delineate the mechanism behind NF-κB-dependent regulation of RUNX2, we searched for potential NF-κB binding sites within the human RUNX2 promoter using TRANSFAC and JASPAR core databases via the ConTra v2 web server (http://bioit.dmbr.ugent.be/contrav2/). We identified a potential RelA (p65) recognition motif (5’-GGAAAGGCCT-3’) 198 bp upstream of the transcription start site (TSS) (Figure 6G).
In order to verify binding of the RelA (p65) subunit to the Runx2 promoter, we performed a quantitative ChIP assay detecting complexes between specific proteins and DNA sequences. Here, following immunoprecipitation with an anti–p65 antibody we found significant (~10 fold) enrichment of the Runx2 promoter under high-glucose conditions relative to the IgG control (Figure 6H).
To further corroborate these findings we tested the RelA (p65) binding capacity of the predicted RelA (p65) recognition motif within the Runx2 promoter using a quantitative protein / solid-phase-oligonucleotide binding assay in high glucose stimulated AoSMCs (TransAM assay). Immobilized oligos containing the predicted RelA (p65) binding site were able to capture significantly more RelA (p65) protein compared to corresponding oligos with various sequence mutations (Figure 6G, Online Table I) of the RelA (p65) binding motif (Figure 6I).
Finally, luciferase reporter constructs containing either the intact Runx2 promoter or mutations of the predicted RelA (p65) binding site (Figure 6G, Online Table II) demonstrated that this binding site is critical for NF-κB-mediated activation of the Runx2 promoter in high-glucose stimulated AoSMCs (Figure 6J).
DISCUSSION
Arterial stiffness has long been recognized as a feature of arterial aging21. Over the last decade, arterial stiffness has received increasing attention in cardiovascular medicine, emerging as an independent risk factor for cardiovascular disease and mortality5. This insight is particularly relevant in diabetic populations, which exhibit accelerated arterial stiffening and carry some of the highest risks of death from cardiovascular disease6. Thus, there is a pressing need to characterize the mechanisms leading to arterial stiffness in diabetes – which may then help to establish targets for prophylactic or therapeutic intervention.
In the realm of clinical medicine, diabetes is inextricably linked to arterial hypertension9. Hypertension per se induces aortic stiffening, both acutely (by increasing the aortic diameter and thereby shifting the load from distensible elastin to rigid collagen) and chronically (through active structural vascular remodeling processes)7,11. Therefore, from a patho-mechanistic point of view, it is essential to elucidate whether structural aortic stiffening in diabetes is primarily a function of concomitant hypertension or constitutes a distinct pathology – that may then require separate treatment. We addressed this question with the help of a murine model of diabetes, longitudinally screening aortic mechanical stiffness and blood pressure levels of diabetic db/db mice at different ages. As the stiffness of conduit arteries is largely determined by their passive, structural properties10-12 we performed ex vivo pressure myography to directly test the aortic mechanical properties under physiologically relevant loading conditions. We found that structural aortic stiffening in diabetic mice preceded the onset of arterial hypertension (Figure 1). In particular, aortic stiffening occurred prior to augmentation of pulse pressure (i.e., the difference between systolic and diastolic blood pressure), which is frequently used as an indirect marker of aortic stiffness22. These findings demonstrate that passive aortic stiffening may occur independently of hypertension in this model of diabetes, and are consistent with previous studies that highlight early aortic stiffness in association with decreased nitric oxide (NO) bioavailability that precedes arterial hypertension in a murine model of diet-induced obesity23.
Delineating the structural basis for the observed passive aortic stiffening we found enhanced medial fibrosis to be a prominent feature of aged db/db aortae (Figure 2A). This observation is consistent with enhanced aortic collagen deposition found in other models of diabetes24-26.
We next investigated the potential role of the transcription factor Runx2 as a central regulator of aberrant ECM accumulation and aortic stiffness in the setting of diabetes. While not significantly expressed in the vasculature under normal conditions, Runx2 has recently emerged as a marker of pathologic osteogenic VSMC trans-differentiation in the context of vascular calcification27,28. However, the immediate transcriptional potential of Runx2 to induce vascular fibrosis resulting in arterial stiffness had never before been addressed experimentally.
Gene expression analysis revealed that Runx2 as well as ECM target genes were progressively up-regulated in aging db/db animals compared to age-matched +/db controls (Figures 2C-G). Further, we detected markedly up-regulated expression of Runx2 protein in the aortic media of 20 week old diabetic db/db mice as analogously reported in a streptozotocin-induced model of diabetes29. Importantly, consistent with these animal model-derived data we also found increased expression of RUNX2 as well as “downstream” genes COL1A1, COL1A2 and FN1 in thoracic aortic samples from type 2 diabetic patients (Figure 2H).
In order to assess the functional impact of Runx2 on vascular matrix homeostasis, we generated Runx2-smTg mice expressing the entire coding region of murine Runx2 under control of the SM22α promoter – achieving vascular smooth muscle cell-specific overexpression of Runx230,31. This approach enabled us to dissect direct downstream effects of increased Runx2 function in the VSMC-dominated aortic media. Runx2 overexpression histologically translated into marked collagen I accumulation in the aortic media (Figure 3). These structural alterations accompanied significantly increased material stiffness of the thoracic aorta as well as increased arterial blood/pulse pressure levels.
Although we did not study Runx2 expression during embryonic development of Runx2-smTg mice directly it is known that the SM22α promoter – regulating Runx2 expression in our mouse model – exhibits embryonic aortic activation (as early as E9.0)31. Consequently, we cannot exclude developmental abnormalities in our model per se. Therefore, it is important to exclude structural developmental defects of the aorta that may result in increased aortic stiffness unrelated to the pro-fibrotic mechanisms under investigation. In contrast to the collagen matrix that readily undergoes lifelong remodeling in response to various stimuli, elastic fibers, being another major structural determinant of the mechanical properties of conduit arteries, are almost exclusively assembled during tissue development and function for the entire lifespan of the organism with low potential for postnatal repair11. Therefore, elastin function is particularly susceptible to developmental defects. In this regard we were unable to detect any irregularities of the medial elastin architecture in the Runx2-smTg by histologic elastin 3D reconstruction and elastin break quantification (Figure 3E,F).
Further, in vitro experiments demonstrated that high glucose conditions lead to Runx2-dependent enhanced collagen production in human AoSMCs (Figure 5). Taken together, the data identify Runx2 as an inducer of aberrant medial fibrosis and aortic stiffening in the context of diabetes.
Interestingly, Runx2-overexpression per se did not induce enhanced mineralization/calcification in the aortic specimens taken from Runx2-smTg mice (Figures 3I,J; Online Figures X and XI). This was somewhat unexpected, as Runx2 is not only considered a marker of osteogenic VSMC trans-differentiation in calcifying vascular disorders15,17,28, but also was shown to be of mechanistic significance for atherosclerotic calcification in a murine Runx2 knockout model17. Thus, Runx2 may be necessary but not sufficient to promote vascular calcification, underlining the complex, multi-factorial nature of vascular calcification processes32,33. In this context Runx2-smTg aortae do not exhibit increased elastin breakdown (Figure 3E,F) or VSMC apoptosis (Online Figure VIII), both factors associated with vascular (medial) calcification32,33.
Diabetes is generally associated with increased abundance of reactive oxygen species (ROS) in the cardiovascular system34. We found increased ROS (superoxide) production in aortic sections from db/db mice (Figures 4A,B), consistent with other reports35,36. Aberrant ROS-mediated signaling contributes to pathologic vascular remodeling in various diseases (e.g. atherosclerosis, arteriosclerosis, hypertension and abdominal aortic aneurysm)37, and moreover has been shown to induce Runx2 expression in vitro38. A recent study reported the utility of the antioxidant TEMPOL in preventing age-related arterial stiffening39. In line with those findings we demonstrated that 10 weeks of anti-oxidant treatment (TEMPOL) reduces ROS abundance in the aortic wall of db/db mice, and reduces expression of Runx2 and the downstream collagen isoforms Col1a1 and Col1a2 (Figure 4C). Functionally, this results in decreased aortic stiffness and reduced pulse pressure in 20 week-old db/db mice (Figures 4G,H). Although TEMPOL is known to reduce blood pressure via multiple functional, vaso-dilatatory mechanisms (including interference with NO metabolism and through sympatholytic properties)40 this study provides evidence for TEMPOL’s additional anti-hypertensive effects via modulation of diabetic arterial remodeling.
In contrast to a previous report35, we did not find a significant effect of TEMPOL treatment on blood glucose levels in db/db mice, possibly due to the lower dose used in the present study (1 mmol/l vs. 2 mmol/l).
In order to elucidate a mechanistic link between increased ROS abundance in the diabetic aorta and the enhanced expression of Runx2, we focused on the role of the oxidative stress-sensitive transcription factor, NF-κB41,42. Indeed, previous studies have demonstrated increased aortic NF-κB expression and activation in diabetic mouse models43,44 and indicated that hyperglycemia can activate NF-κB in vascular smooth muscle cells45-47. In line with those data we find that NF-κB expression and activation is increased in a redox-sensitive fashion in db/db aortae (Figures 6A,B) as well as in human aortic SMCs under high glucose conditions (Figures 6C,D).
In silico analysis revealed a potential RelA (p65) binding site within the human Runx2 promoter, suggesting that Runx2 might be a direct transcriptionally-activated target of NF-κB. Through an NF-κB binding assay, we demonstrated that RelA (p65) binds to the predicted binding site within the Runx2 promoter (Figure 6I). Luciferase reporter constructs containing mutations of the RelA (p65) binding site further confirm the functional relevance of the NF-κB – Runx2 interaction for the Runx2 promoter activity (Figure 6J).
There is ample evidence that vascular and systemic inflammation are associated with (or precede) increased arterial stiffness48-51. In light of the present study, the NF-κB – Runx2 interaction might provide a fundamental molecular mechanism linking inflammatory processes with arterial fibrosis/stiffening and hypertension.
In conclusion, the present study identifies the transcription factor Runx2, which is significantly expressed in human type 2 diabetic aortae, as an inducer of aortic fibrosis and stiffening. Of note, Runx2 may exert its effects on arterial stiffness independently of calcification processes. We delineate a redox-sensitive pathway of high glucose-induced Runx2 expression via a direct interaction of the NF-κB subunit RelA (p65) with the human Runx2 promoter (Online Figure XIII). Given that Runx2 may have no physiologic role in the vasculature, the results from our study highlight the ROS – NF-kB – Runx2 pathway as a target to counteract pathologic aortic collagen deposition and stiffening in the context of diabetes. Moreover, the characterized direct NF-κB – Runx2 interaction may be of mechanistic importance in numerous additional pathologies that involve inflammatory remodeling of the collagen matrix.
Supplementary Material
Novelty and Significance.
What Is Known?
Arterial stiffness is significantly increased in patients with diabetes mellitus (DM) and contributes to high cardiovascular morbidity and mortality.
Collagen content is increased in the blood vessels of patients with DM.
RUNX2 regulates extracellular matrix deposition in osteogenic tissue.
RUNX2 also regulates vascular calcification processes.
What New Information Does This Article Contribute?
Aortic fibrosis and stiffness precedes the onset of hypertension in a mouse model (db/db) of DM.
RUNX2 is upregulated under high glucose conditions in vitro and in vivo via a redox-sensitive activation of NF-κB.
RUNX2 induces aortic medial fibrosis and thereby increases aortic stiffness.
Vascular smooth muscle cell (VSMC) -specific overexpression of RUNX2 is insufficient to induce vascular calcification in vivo.
Arterial stiffening is a significant complication of DM with no specific therapy available to date. The present study identifies the osteogenic transcription factor Runx2 as an inducer of arterial fibrosis and stiffness. We find RUNX2 expression to be upregulated in VSMCs under high glucose conditions in vitro and in vivo and delineate a redox-sensitive mechanism of Runx2 upregulation with NF-κB serving as a transcription factor of Runx2. Interestingly, although RUNX2 has been previously identified as a marker and regulator of vascular calcification, we demonstrate that VSMC-specific overexpression of RUNX2 per se is unable to induce calcification in vivo. The results of this study reveal a novel mechanism of arterial stiffening with RUNX2 as a pro-fibrotic vascular regulator. The findings might provide new targets for therapeutical intervention in patients with DM.
ACKNOWLEDGEMENTS
We would like to thank Michelle Ramseier, Brian Deng (Stanford University), Vladana Vukojevic, Albert Busch, Yuhuang Li, Hong Jin, Alexandra Bäcklund, Silvia Aldi, Ljubica Perisic, Joelle Magné, Valentina Paloschi, Fariba Foroogh, Shohreh Maleki, Olivera Werngren, Peter Gustafsson, Fanny Saidoune (Karolinska Institute), as well as Katrin Schiebel (University of Erlangen, School of Medicine) for expert assistance.
SOURCES OF FUNDING
This work was supported by research grants from the NIH (1R01HL105299 to P.S. Tsao), the Deutsche Forschungsgemeinschaft (RA 2179/1-1 to U. Raaz, He 6855/1-1 to J.K. Hennigs, AD 492/1-1 to M. Adam), the Boehringer Ingelheim Fonds and the International Scholarship for Medical Students, Medical School of the University Erlangen, Germany (both to I. N. Schellinger) and the Stanford Cardiovascular Institute (to J.M. Spin).
Nonstandard Abbreviations and Acronyms
- AoSMCS
aortic smooth muscle cells
- COL1A1
collagen, type 1, alpha 1
- COL1A2
collagen, type 1, alpha 2
- DHE
dihydroethidium
- ECM
extracellular matrix
- FN1
fibronectin 1
- HEK293
human embryonic kidney 293 cells
- HG
high glucose
- IHC
immunohistochemistry
- NF-κB
nuclear factor kappa beta
- NG
normal glucose
- NO
nitric oxide
- ROS
reactive oxygen species
- Runx2
runt-related transcription factor 2
- SPP1
secreted phosphoprotein 1
- TNF
tumor necrosis factor
- TSS
transcription start site
- VSCM
vascular smooth muscle cells
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. U.S. Department of Helath and Human Services; Atlanta, GA: 2014. [Google Scholar]
- 3.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jonsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyorala K, Raz I, Schernthaner G, Volpe M, Wood D. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 4.Stehouwer C, Henry R, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–39. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank K. Aortic Pulse-Wave Velocity and Its Relationship to Mortality in Diabetes and Glucose Intolerance: An Integrated Index of Vascular Function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 7.Hoefer I, den Adel B, Daemen M. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. 2013;99:276–83. doi: 10.1093/cvr/cvt089. [DOI] [PubMed] [Google Scholar]
- 8.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–22. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Lago R, Singh P, Nesto R. Diabetes and hypertension. Nat Clin Pract Endocrinol Metab. 2007;3:667. doi: 10.1038/ncpendmet0638. [DOI] [PubMed] [Google Scholar]
- 10.WOLINSKY H, GLAGOV S. Structural Basis for the Static Mechanical Properties of the Aortic Media. Circulation Research. 1964;14:400–413. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- 11.Wagenseil J, Mecham R. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–89. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagenseil J, Mecham R. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012;5:264–73. doi: 10.1007/s12265-012-9349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 14.Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–36. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steitz SA, Speer MY, Curinga G, Yang H-Y, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth Muscle Cell Phenotypic Transition Associated With Calcification: Upregulation of Cbfa1 and Downregulation of Smooth Muscle Lineage Markers. Circulation Research. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 16.Trion A, van der Laarse A. Vascular smooth muscle cells and calcification in atherosclerosis. Am Heart J. 2004;147:808–14. doi: 10.1016/j.ahj.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Byon C, Yuan K, Chen J, Mao X, Heath J, Javed A, Zhang K, Anderson P, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–52. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin M, Le V, Wagenseil J. Mechanical testing of mouse carotid arteries: from newborn to adult. J Vis Exp. 2012;60:3733. doi: 10.3791/3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle JJ, Johns M, Kampfer T, Nguyen AT, Game L, Schaer DJ, Mason JC, Haskard DO. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res. 2012;110:20–33. doi: 10.1161/CIRCRESAHA.111.247577. [DOI] [PubMed] [Google Scholar]
- 20.Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT, Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–23. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 21.O'Rourke M, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 22.Safar M, Levy B, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–9. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 23.Weisbrod R, Shiang T, Al Sayah L, Fry J, Bajpai S, Reinhart-King C, Lob H, Santhanam L, Mitchell G, Cohen R, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–10. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy GK. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004;68:132–42. doi: 10.1016/j.mvr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Song W, Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: relation to vessel size. Cardiovasc Diabetol. 2006;5:3. doi: 10.1186/1475-2840-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Zhong M, Miao Y, Ma X, Gong H, Tan H, Zhang Y, Zhang W. Impaired elastic properties of the aorta in fat-fed, streptozotocin-treated rats. Vascular remodeling in diabetic arteries. Cardiology. 2009;114:107–13. doi: 10.1159/000219211. [DOI] [PubMed] [Google Scholar]
- 27.Engelse MA, Neele JM, Bronckers AL, Pannekoek H, de Vries CJ. Vascular calcification: expression patterns of the osteoblast-specific gene core binding factor alpha-1 and the protective factor matrix gla protein in human atherogenesis. Cardiovasc Res. 2001;52:281–9. doi: 10.1016/s0008-6363(01)00375-3. [DOI] [PubMed] [Google Scholar]
- 28.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–94. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 29.Heath J, Sun Y, Yuan K, Bradley W, Litovsky S, Dell'Italia L, Chatham J, Wu H, Chen Y. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res. 2014;114:1094–102. doi: 10.1161/CIRCRESAHA.114.302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solway J, Seltzer J, Samaha FF, Kim S, Alger LE, Niu Q, Morrisey EE, Ip HS, Parmacek MS. Structure and expression of a smooth muscle cell-specific gene, SM22 alpha. J Biol Chem. 1995;270:13460–9. doi: 10.1074/jbc.270.22.13460. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–59. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sage A, Tintut Y, Demer L. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–36. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515–25. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–92. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 35.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–82. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 36.Wong W, Tian X, Xu A, Ng C, Lee H, Chen Z, Au C, Yao X, Huang Y. Angiotensin II type 1 receptor-dependent oxidative stress mediates endothelial dysfunction in type 2 diabetic mice. Antioxid Redox Signal. 2010;13:757–68. doi: 10.1089/ars.2009.2831. [DOI] [PubMed] [Google Scholar]
- 37.Raaz U, Toh R, Maegdefessel L, Adam M, Nakagami F, Emrich F, Spin J, Tsao P. Hemodynamic regulation of reactive oxygen species: implications for vascular diseases. Antioxid Redox Signal. 2014;20:914–28. doi: 10.1089/ars.2013.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byon C, Javed A, Dai Q, Kappes J, Clemens T, Darley-Usmar V, McDonald J, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–27. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell. 2014;13:576–8. doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilcox C, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–69. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–37. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 42.Morgan M, Liu Z. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–15. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58:1229–36. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diab Vasc Dis Res. 2014;11:92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 45.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–64. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 46.Hattori Y, Hattori S, Sato N, Kasai K. High-glucose-induced nuclear factor kappaB activation in vascular smooth muscle cells. Cardiovasc Res. 2000;46:188–97. doi: 10.1016/s0008-6363(99)00425-3. [DOI] [PubMed] [Google Scholar]
- 47.Jeong IK, Oh da H, Park SJ, Kang JH, Kim S, Lee MS, Kim MJ, Hwang YC, Ahn KJ, Chung HY, Chae MK, Yoo HJ. Inhibition of NF-kappaB prevents high glucose-induced proliferation and plasminogen activator inhibitor-1 expression in vascular smooth muscle cells. Exp Mol Med. 2011;43:684–92. doi: 10.3858/emm.2011.43.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booth AD, Wallace S, McEniery CM, Yasmin,Brown J, Jayne DR, Wilkinson IB. Inflammation and arterial stiffness in systemic vasculitis: a model of vascular inflammation. Arthritis Rheum. 2004;50:581–8. doi: 10.1002/art.20002. [DOI] [PubMed] [Google Scholar]
- 49.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 50.Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, Crow MK, Sammaritano L, Levine DM, Shankar BA, Moeller E, Salmon JE. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005;46:194–9. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 51.Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei medical journal. 2012;53:258–61. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.