Abstract
Complexes formed between organic dyes and genetically encoded proteins combine the advantages of stable and tunable fluorescent molecules and targetable, biologically integrated labels. To overcome the challenges imposed by labeling with bright fluorescent dyes, a number of approaches now exploit chemical or environmental changes to control the properties of a bound dye, converting dyes from a weakly fluorescent state to a bright, easily detectable complex. Optimized, such approaches avoid the need for removal of unbound dyes, facilitate rapid and simple assays in cultured cells and enable hybrid labeling to function more robustly in living model organisms.
Graphical Abstract
Introduction
The emergence of new imaging approaches that interrogate cellular and organismal behavior with resolution and timescales previously inconceivable have placed new requirements on the performance of fluorescence labels used in these imaging approaches. In particular, brightness and photostability enhancements are required to provide increased resolution in methods such as stimulated emission depletion (STED) microscopy[1,2] and structured illumination microscopy (SIM) [3,4], both of which effectively oversample the specimen relative to conventional confocal and widefield microscopy to produce resolution gains. Localization microscopy methods such as photoactivation localization microscopy (PALM) [5] and stochastic optical reconstruction microscopy (STORM) [6] require high photon output molecules that can be precisely localized at a single molecule level. The use of these approaches in living cells, where molecules move (e.g. sptPALM) [7] require both sustained photon output and high brightness probes. The use of hybrid tagging approaches (i.e. a synthetic dye that labels a specific genetically encoded reporter) has been essential in establishing superresolution imaging as a viable biological imaging approach in conditions where the fluorescence brightness, photostability and photochemical properties of intrinsically fluorescent proteins are limiting.
Initial applications for many of these reagents and imaging approaches were based in cultured cell models. As the approaches for high-resolution and high-speed imaging expand, there is a persistent drive to move into more complex milieu[8,9]. This poses challenges for many labeling approaches that work in cell culture, because the removal of unbound probe is usually required to produce specific detectable labeling. To alleviate these challenges, chemical biologists have developed a series of reagents that consist of initially dark or quenched dyes that are activated upon target binding. Ideally, these reagents can provide very specific and selective activation upon target labeling, producing a low-background, high-contrast imaging approach that powers high-speed, high-resolution imaging in living animals.
An essential benefit to the use of fluorogenic protein labeling arises from the ability to control the time of dye addition[10]. These fluorogenic tagging approaches are well-suited to pulse-chase labeling, or differential labeling of distinct compartments using chemical modifications of the fluorogenic dye that are compartment-restricted. In addition, the stability and binding-dependent activation of these probes have generated unique applications in single molecule and superresolution imaging.
Fluorogenic protein labeling
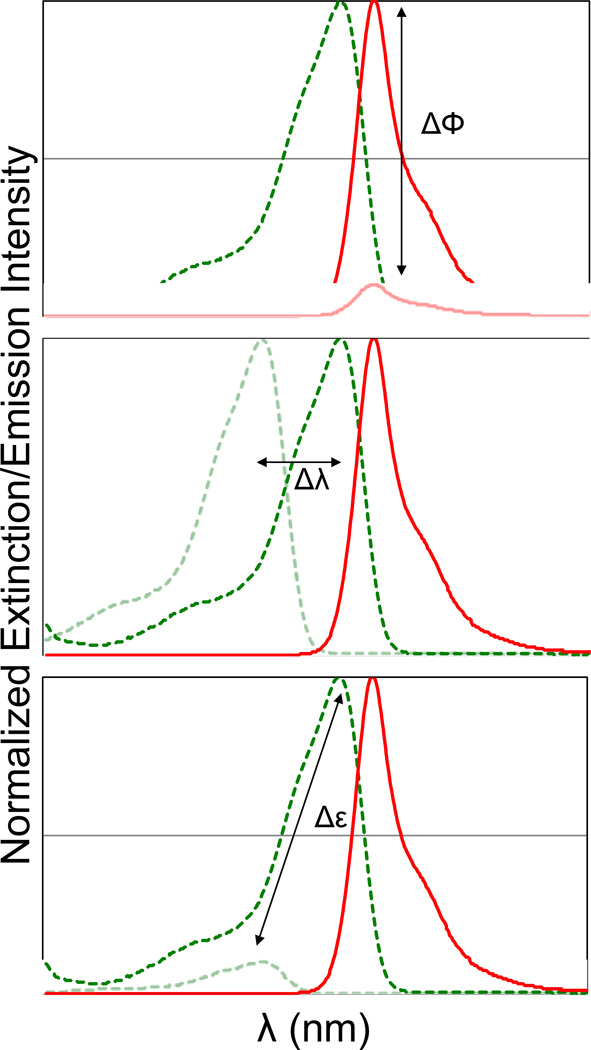
The overall goal of fluorogenic protein labeling is to develop a system that consists of a non-fluorescent dye and an apoprotein, where the binding of the dye results in a significant change in the fluorescence brightness[11,12]. An important metric for a fluorogenic labeling system is the “activation ratio” (AR), typically given as the fluorescence intensity of the fully bound dye divided by the fluorescence intensity of the unbound dye under the same excitation conditions. This definition allows three critical parameters to control the AR. Changes in the fluorescence quantum yield, spectral position, or dye extinction coefficient upon binding can be used to achieve a fluorogenic labeling effect. (Figure 1) To optimize the performance of a fluorogenic protein/peptide tag, the protein should be well folded in various cellular compartments, the dye should be freely cell permeant, and show low background within the cell in the absence of the expressed protein, and the interaction between the dye and protein should take place at a low concentration of dye, to ensure the highest activation ratio in a labeled cell. Fluorogenic labeling, because it takes place in the background of unremoved dye, will be directly affected by the ratio of free to bound dye in the specimen, so while the AR provides some useful information on the imaging contrast, the ultimate performance will depend on the AR and the excess concentration of dye required to achieve complete labeling. (Figure 2) From this perspective, it is important to have a fluorogenic system with a high AR that also functions effectively at low concentrations of added dye.
Figure 1. Spectroscopic changes utilized for fluorogen activation.
Binding of a dye to a protein domain can result in changes in quantum yield (top), excitation wavelength (middle) or extinction coefficient/excitation cross-section (bottom). Combinations of these properties will result in high activation ratios.
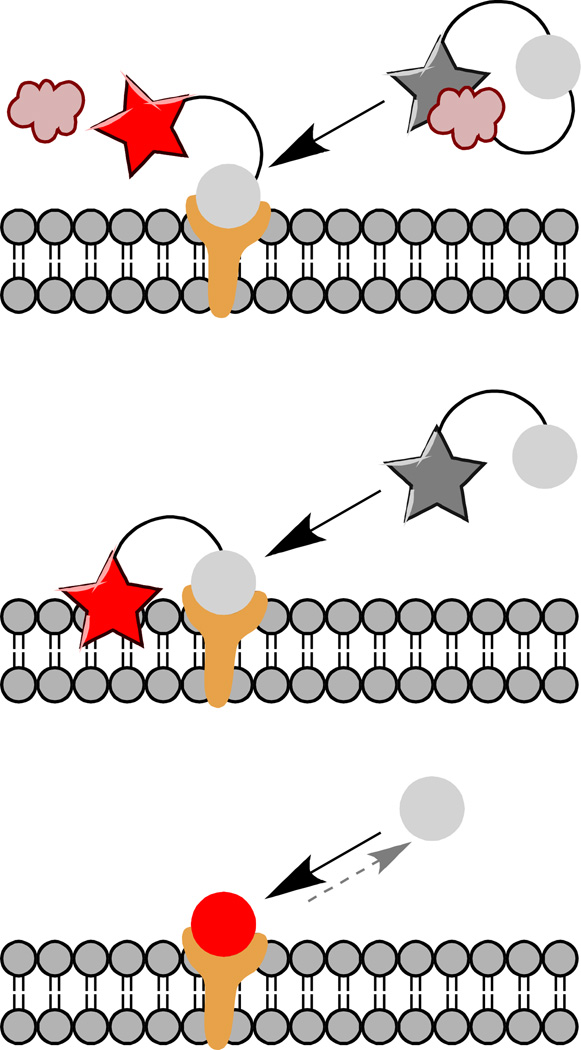
Figure 2. Fluorogen activation approaches.
Binding of the fluorogen to an expressed domain can be used to release a quenching group (top), to bring a dye near to an environment that activates it, for example a membrane or protein pocket (middle), or to directly bind the fluorogen dye in a fluorescent complex (bottom). Direct binding of the fluorogen to the fluorogen activating protein is reversible, unlike cleavage or covalent linkage, which is advantageous for some applications.
Fluorogen activation mechanisms
FRET Quenching
Because many of the earliest hybrid tagging approaches involved ligating a protein to a chemically modified substrate, it was recognized that coupling of an energy transfer acceptor at a short distance could result in efficient quenching of the donor. Coupling to the tagging motif through chemistry that releases the linked quencher results in efficient reactivation of the fluorescent dye[13,14]. For dyes that are efficiently quenched by the proximal donor, the reactivation can typically bring 10-fold or higher activation. Although the distance-dependence in the Förster relation would predict 1000-fold quenching for a dye pair held at ~1/3 the Förster radius, the orientation dependence for dyes on a short linker alters this efficiency, and only moderate fluorescence activation has been achieved using these approaches (typically ~100-fold maximum activation).
Direct
Because the electronic ground and excited states of conjugated molecules have differences in their rigidity and charge distribution, many show sensitivity to the local environment that can be exploited for fluorogen activation. Polarity sensing dyes are typically those that have differences in the charge distribution between ground and excited states, and respond to solvent stabilization of one or the other states with a spectral shift. Many polarity sensing dyes are “push-pull” or “donor-acceptor” chromphores, which have an electron donating group electronically coupled to the acceptor group. Viscosity sensing dyes, on the other hand, typically have some vibration or rotational mode in the excited state that can couple directly to the ground electronic state, resulting in internal conversion and relaxation without fluorescence. High viscosity or conformational constraints can provide significant barriers to these relaxation modes, and significantly activate the fluorescence. Finally, excited state charge transfer (electron or proton) can allow direct relaxation, and chemical modification or binding to the charge donor (or acceptor) group can activate the fluorescence directly. While this method has primarily been used for direct chemical sensing (e.g. BAPTA based Ca2+ indicators[15,16]), some recent approaches have shown this to be useful for direct fluorogenic labeling of proteins with suitable ligands[2,17].
While each of these methods have been used for “activity-based” labeling, where the construct is targeted to enzymes to assess activity in a living cell or animal, those applications are beyond the scope of this review, and have been reviewed elsewhere. In this work, we focus on using fusion-tags to label specific proteins for imaging applications.
Fluorogen activating proteins/peptides
Apart from activity-based probes, most fluorogens are activated by a dye-binding or dye-anchoring domain genetically fused to the target of interest. The target can be a cellular protein, which is labeled and expressed in live cells directly[18], or the protein can be fused to a targeting domain, such as an affibody[19] or a scFv[20], which is expressed recombinantly and then used to label endogenous proteins on cells or in tissues. For effective protein labeling, the fluorogenic dye should be bound and activated at the expressed protein target. Figure 2 shows the various ways in which fluorogens can be activated by their target proteins. Covalent labeling can chemically transform the dye, creating a conjugated structure or removing a quencher to activate the detectable fluorescent probe at the labeled site (e.g. [21]). Once converted, however, these dyes are always “on”. Covalent linking of an environmentally sensitive dye to a protein of interest, allows local interrogation of the chemical environment, and selective activation of the dye in the proper environment. Targeting of pH sensors can be used to assess protein localization to acidic compartments such as endosomes[22], and targeting of lipid probes can be used to detect membrane associated proteins fluorogenically, as recently demonstrated with SNAP-tag directed Nile Red[23].
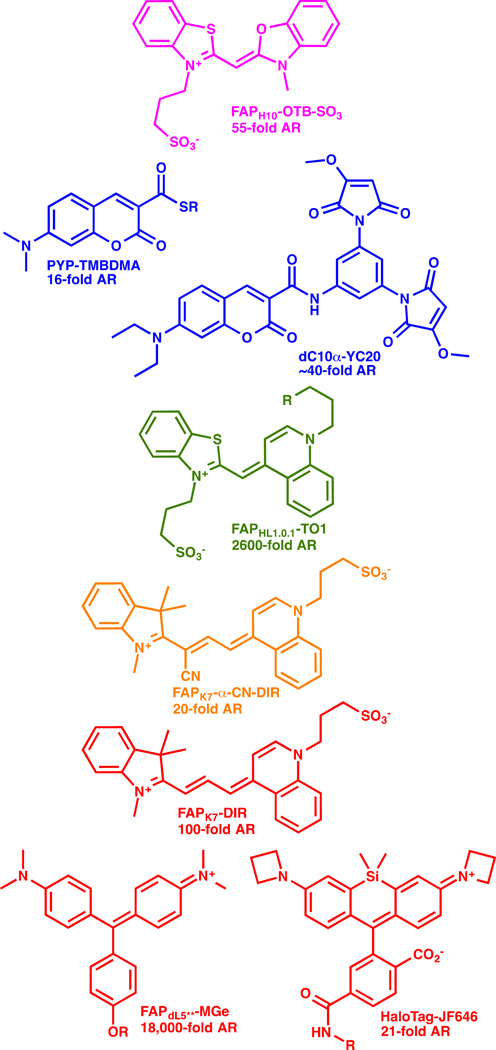
Alternative proteins directly activate the fluorogen dye upon binding (e.g. [11,12,24]). Binding of fluorescent molecules to a specific stereoelectronic environment inside a protein can activate the fluorescence directly without any chemical modification of the protein or the dye itself. An assortment of dye structures have been used as fluorogens, and these include dyes that are inherently rotationally relaxed in the excited state[12,25–27], those that are enhanced by solvent polarity[28], and those that are activated by anchoring in the protein environment, potentially through altered spirolactone formation[24,29]. Some of these fluorogens are shown in Figure 3, with the associated AR values reported for these in the optimal fluorogen activating protein. These dyes are typically weakly or completely non-fluroescent in aqueous media, and activation ratios of up to ~20,000 have been reported with MG-binding scFv based activating proteins, with equilibrium dissociation constants in the low pM range[30]. Many of these FAP-fluorogen pairs function at the cell surface, but so far, only MGe-FAP[18], aminocoumarin-PYP[28] and the SiR-SNAP[29] and SiR-halo ligands (and analogous azetidine containing JF646 ligands[24]) complexed to their target proteins function as fluorogens robustly within living cells. These challenges are three-fold: dye penetration across the plasma membrane requires a compact, low-polarity fluorogen; the protein targeting domain must fold robustly at the target site in the cell; and the dye must not activate nonspecifically within living cells, even at sites of high accumulation (e.g. mitochondria or DNA in the nucleus). Modifications to the fluorogens can potentially reduce these interactions, and improve the function within living cells[24,31].
Figure 3. Fluorogen dye structures spanning the visible spectral range.
Fluorogenic dyes of various structure and spectral properties have been demonstrated for labeling of living cells. The dye structure is shown, along with the activating protein (as named in the cited references), and the reported activation ratio (AR). References: OTB-SO3 [25]; TMBDMA [28]; YC20 [21]; TO1 [12]; α-CN-DIR [27]; DIR [26]; MG [30]; JF646 [24].
Applications
An essential advantage of a high-affinity, high AR fluorogenic labeling approach is the ability to selectively and rapidly label protein in living, behaving cells and organisms, without any need for washing away of unbound dye for quantitative detection. Although labeling inside the cell is practical, the timescale for labeling is still on the order of minutes, since dye permeation through the plasma membrane is required[18,28]. A number of applications for rapid fluorogen activation have evolved in the past two years that exploit the rapid, no-wash labeling of surface displayed proteins for quantitative analysis of protein plasma membrane abundance and protein trafficking.
Malachite green itself is a cell-permeant, cationic dye that shows significant fluorescent activation in cells, as well as some growth suppression when incubated with growing yeast. Substitution of the base phenyl ring with a phenolic ether allowed preparation of various dye analogs with low nonspecific and tailored cell permeability properties, allowing chemical discrimination between cell surface and intracellular fractions of membrane proteins[12]. Recently, Yan et al. [31] prepared bis-sulfonated versions of this malachite green chromophore, and showed that they had improved cell exclusion, further reduced nonspecific activation on dying cells, and rapid labeling kinetics (kon ~105 M−1s−1) for FAP-labeled proteins in the plasma membrane. Using dye addition and flow cytometric analysis, labeling is complete within <30 sec, and results are in quantitative agreement with surface-selective antibody labeling approaches for measurement of G-protein coupled receptor (GPCR) desensitization. This approach substantially reduces the variability seen with antibody labeling approaches, and potentially opens the door to very high-throughput analysis of cell surface abundance of proteins in high throughput flow cytometry[32–34].
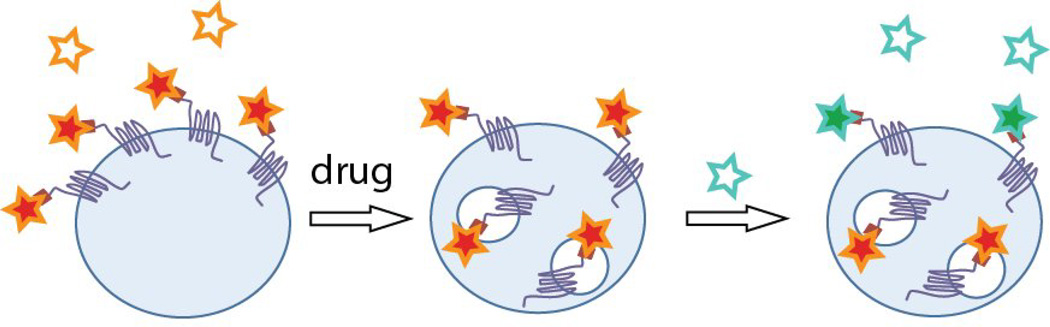
Fluorogens based on sulfonated thiazole orange analogs were shown to be cell excluded, and suitable for labeling FAP-tagged proteins at the plasma membrane with rapid labeling kinetics[35]. This fluorogen could be linked to a variety of dye acceptors[36], including pH sensors and far-red energy transfer acceptors, producing multicolor labeling reagents. Grover et al. prepared a pH senstitive analog of the TO1 dye, which reported in real-time on changes in GPCR endocytosis and intracellular acidification of the endosomes[37]. Fisher et al. prepared a pH insensitive analog of this dye, and used it as a two-color pulse-chase labeling reagent. By pre-labeling receptors with the low-affinity TO1-2p-Cy5 fluorogen, treating with an agonist, and then labeling with the higher-affinity TO1-2p, internalized receptors retained red-shifted fluorescence (488 nm excitation/670 nm emission) and residual surface receptors were displaced with the higher-affinity dye, (488 excitation/530 nm emission) (Figure 4). The assay was shown to function robustly for a wide variety of receptors and agonists[38].
Figure 4. Fluorogen labeling for protein trafficking at the membrane.
Pulse-treat-chase labeling of cell-surface receptors with exchangeable fluorogens of two resolvable colors results in differential coding of proteins protected (from exchange) in endosomes and those still exposed at the plasma membrane.
The simplicity of a noncovalent interaction to activate a fluorogen leads to an interesting and useful feature of this labeling approach. Regardless of expression level, a sparse, optically resolvable subset of target sites can be labeled by using a dye concentration that is considerably below the saturation concentration. Over time, this population can be analyzed at a single molecule level, revealing single particle trajectories or superresolution reconstructions of intracellular structures[39]. Although currently available constructs have slow dissociation kinetics, selection and optimization of these properties may enable rapid and dynamic superresolution imaging in living cells. At the surface, stochastic labeling of FAP-tagged FcεRI was used to show that diffusion properties of these receptors did not change upon binding of cytokinergic (activating) IgE molecules, illustrating a different mode of activation than that obtained with normal IgE crosslinking for activation of the receptor[40]. The sparse labeling is compatible with continuous application of low dye concentration, so there is a persistent, low density of labeled receptors throughout the experiment. This allows for on-the-fly changes in the media, which can be used to test “before” and “after” challenges in the same cell, for example addition of a biological ligand, while examining the same population of receptors.
Conclusions
The utility of fluorogens as a chemical-genetic protein labeling method is now established as a general approach that can function at various intracellular sites and in various experimental approaches. Extension to living model organisms has lagged behind the applications in cells, but remains a very exciting future direction[41]. For these in-vivo applications, the optimal fluorogens will need to be coupled with highly active, highly robust fluorogen activating proteins that are functional in various intracellular compartments. Future applications that integrate acute fluorogenic labeling with chemical sensing may provide powerful new measurements of living systems and their dynamic changes.
Highlights.
Protein-dye complexes that activate fluorescence allow no-wash protein labeling.
Quencher removal, environmental change and direct binding can activate fluorescence.
Modifications of the chromophore and linker can influence fluorogen performance.
Pulse-chase and order-of-addition experiments can reveal dynamic cellular changes.
High-quality fluorogen imaging in organisms will reveal new biological processes.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health R01EB017268 and R21MH100612.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW, et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320(5873):246–249. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 2.Lukinavicius G, Reymond L, D'Este E, Masharina A, Gottfert F, Ta H, Guther A, Fournier M, Rizzo S, Waldmann H, Blaukopf C, et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nature methods. 2014;11(7):731–733. doi: 10.1038/nmeth.2972. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson MG, et al. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. Journal of microscopy. 2000;198(Pt 2):82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson MG, et al. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(37):13081–13086. doi: 10.1073/pnas.0406877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 6.Jones SA, Shim SH, He J, Zhuang X, et al. Fast, three-dimensional super-resolution imaging of live cells. Nature methods. 2011;8(6):499–508. doi: 10.1038/nmeth.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J, et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nature methods. 2008;5(2):155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 8.Ji N, Sato TR, Betzig E, et al. Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(1):22–27. doi: 10.1073/pnas.1109202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Planchon TA, Gao L, Milkie DE, Davidson MW, Galbraith JA, Galbraith CG, Betzig E, et al. Rapid three-dimensional isotropic imaging of living cells using bessel beam plane illumination. Nature methods. 2011;8(5):417–423. doi: 10.1038/nmeth.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosinsky GE, Gaietta GM, Hand G, Deerinck TJ, Han A, Mackey M, Adams SR, Bouwer J, Tsien RY, Ellisman MH, et al. Tetracysteine genetic tags complexed with biarsenical ligands as a tool for investigating gap junction structure and dynamics. Cell communication & adhesion. 2003;10(4–6):181–186. doi: 10.1080/cac.10.4-6.181.186. [DOI] [PubMed] [Google Scholar]
- 11.Martin BR, Giepmans BN, Adams SR, Tsien RY, et al. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nature biotechnology. 2005;23(10):1308–1314. doi: 10.1038/nbt1136. [DOI] [PubMed] [Google Scholar]
- 12. Szent-Gyorgyi C, Schmidt BF, Creeger Y, Fisher GW, Zakel KL, Adler S, Fitzpatrick JA, Woolford CA, Yan Q, Vasilev KV, Berget PB, et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nature biotechnology. 2008;26(2):235–240. doi: 10.1038/nbt1368. This paper outlined the selection and characterization of initial scFv derived fluorogen activating proteins that showed specific molecular recognition and activation for malachite green and thiazole orange derived fluorogens.
- 13. Jing C, Cornish VW, et al. A fluorogenic tmp-tag for high signal-to-background intracellular live cell imaging. ACS chemical biology. 2013;8(8):1704–1712. doi: 10.1021/cb300657r. Use of a quenched variant of the covalent TMP-tag ligand and eDHFR resulted in quencher release upon covalent ligation to the fusion protein.
- 14. Sun X, Zhang A, Baker B, Sun L, Howard A, Buswell J, Maurel D, Masharina A, Johnsson K, Noren CJ, Xu MQ, et al. Development of snap-tag fluorogenic probes for wash-free fluorescence imaging. Chembiochem. 2011;12(14):2217–2226. doi: 10.1002/cbic.201100173. A series of quenched benzylguanine linked dyes were evaluated for their SNAPtag binding-mediated fluorescence activation, and activation ratios of >50 were obtained.
- 15.Minta A, Kao JP, Tsien RY, et al. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. The Journal of biological chemistry. 1989;264(14):8171–8178. [PubMed] [Google Scholar]
- 16.Tsien RY, et al. New calcium indicators and buffers with high selectivity against magnesium and protons: Design, synthesis, and properties of prototype structures. Biochemistry. 1980;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 17. Ondrus AE, Lee HL, Iwanaga S, Parsons WH, Andresen BM, Moerner WE, Du Bois J, et al. Fluorescent saxitoxins for live cell imaging of single voltage-gated sodium ion channels beyond the optical diffraction limit. Chemistry & biology. 2012;19(7):902–912. doi: 10.1016/j.chembiol.2012.05.021. Fluorescently labeled toxin molecules showed fluorescence activation upon binding their biological receptor, and were used for superresolution imaging by stochastic collision and activation.
- 18. Telmer CA, Verma R, Teng H, Andreko S, Law L, Bruchez MP, et al. Rapid, specific, no-wash, far-red fluorogen activation in subcellular compartments by targeted fluorogen activating proteins. ACS chemical biology. 2015 doi: 10.1021/cb500957k. A series of malachite green binding fluorogen activating proteins were evaluated for their function in living cells in various compartments. One clone, the dL5** protein, showed robust expression, high signal levels, and improved photostablity relative to a mCerulean fusion protein.
- 19. Wang Y, Telmer CA, Schmidt BF, Franke JD, Ort S, Arndt-Jovin DJ, Bruchez MP, et al. Fluorogen activating protein-affibody probes: Modular, no-wash measurement of epidermal growth factor receptors. Bioconjugate chemistry. 2015;26(1):137–144. doi: 10.1021/bc500525b. A recombinant reagent that combined fluorogen activating proteins with affibodies for target recognition was used to label endogneous EGFR on tumor cells. This reagent enabled fluorogenic labeling even in the presence of excess protein, suggesting some EGFR-binding mediated fluorogen activation.
- 20.Saunders MJ, Block E, Sorkin A, Waggoner AS, Bruchez MP, et al. A bifunctional converter: Fluorescein quenching scfv/fluorogen activating protein for photostability and improved signal to noise in fluorescence experiments. Bioconjugate chemistry. 2014;25(8):1556–1564. doi: 10.1021/bc500273n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Clouthier CM, Tsao K, Strmiskova M, Lachance H, Keillor JW, et al. Coumarin-based fluorogenic probes for no-wash protein labeling. Angewandte Chemie. 2014;53(50):13785–13788. doi: 10.1002/anie.201408015. Using the intrinsic quenching ability of maleimide groups, this group identified an optimized dimaleimide that was selectively activated by a dicysteine containing alpha helix domain, and not activated by excess glutathione.
- 22.Benink H, McDougall M, Klaubert D, Los G, et al. Direct ph measurements by using subcellular targeting of 5(and 6-) carboxyseminaphthorhodafluor in mammalian cells. BioTechniques. 2009;47(3):769–774. doi: 10.2144/000113220. [DOI] [PubMed] [Google Scholar]
- 23. Prifti E, Reymond L, Umebayashi M, Hovius R, Riezman H, Johnsson K, et al. A fluorogenic probe for snap-tagged plasma membrane proteins based on the solvatochromic molecule nile red. ACS chemical biology. 2014;9(3):606–612. doi: 10.1021/cb400819c. A membrane probe was linked to the benzylguanine ligand and used to label SNAP-tag modified surface proteins. Optimized linker length allowed the probe to be used as a no-wash labeling method for membrane proteins with low background signal.
- 24. Grimm JB, English BP, Chen J, Slaughter JP, Zhang Z, Revyakin A, Patel R, Macklin JJ, Normanno D, Singer RH, Lionnet T, et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nature methods. 2015;12(3):244–250. doi: 10.1038/nmeth.3256. Azetidine modifications of rhodamine and coumarin fluorophores was shown to signficantly improve their fluorescence properties (brightness and photostability). Interestingly, the azetidinyl modified SiR dye with the halotag ligand showed significantly improved fluorogenicity.
- 25.Zanotti KJ, Silva GL, Creeger Y, Robertson KL, Waggoner AS, Berget PB, Armitage BA, et al. Blue fluorescent dye-protein complexes based on fluorogenic cyanine dyes and single chain antibody fragments. Organic & biomolecular chemistry. 2011;9(4):1012–1020. doi: 10.1039/c0ob00444h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozhalici-Unal H, Pow CL, Marks SA, Jesper LD, Silva GL, Shank NI, Jones EW, Burnette JM, 3rd, Berget PB, Armitage BA, et al. A rainbow of fluoromodules: A promiscuous scfv protein binds to and activates a diverse set of fluorogenic cyanine dyes. Journal of the American Chemical Society. 2008;130(38):12620–12621. doi: 10.1021/ja805042p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shank NI, Zanotti KJ, Lanni F, Berget PB, Armitage BA, et al. Enhanced photostability of genetically encodable fluoromodules based on fluorogenic cyanine dyes and a promiscuous protein partner. Journal of the American Chemical Society. 2009;131(36):12960–12969. doi: 10.1021/ja9016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hori Y, Norinobu T, Sato M, Arita K, Shirakawa M, Kikuchi K, et al. Development of fluorogenic probes for quick no-wash live-cell imaging of intracellular proteins. Journal of the American Chemical Society. 2013;135(33):12360–12365. doi: 10.1021/ja405745v. The polarity-sensitive dimethylaminocoumarin fluorophore is activated when linked covalently to the photoactive yellow protein, producing a rapid labeling inside living cells via a covalent thioester linkage.
- 29. Lukinavicius G, Umezawa K, Olivier N, Honigmann A, Yang G, Plass T, Mueller V, Reymond L, Correa IR, Jr, Luo ZG, Schultz C, et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nature chemistry. 2013;5(2):132–139. doi: 10.1038/nchem.1546. A silane-bridged rhodamine fluorophore was developed and illustrated for intracellular and superresolution imaging. This dye showed some fluorogenic activation, and the authors hypothesized it was due to equilibrium between the spirolactone form and the ring-opened chromophore.
- 30.Szent-Gyorgyi C, Stanfield RL, Andreko S, Dempsey A, Ahmed M, Capek S, Waggoner AS, Wilson IA, Bruchez MP, et al. Malachite green mediates homodimerization of antibody vl domains to form a fluorescent ternary complex with singular symmetric interfaces. Journal of molecular biology. 2013;425(22):4595–4613. doi: 10.1016/j.jmb.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan Q, Schmidt BF, Perkins LA, Naganbabu M, Saurabh S, Andreko SK, Bruchez MP, et al. Near-instant surface-selective fluorogenic protein quantification using sulfonated triarylmethane dyes and fluorogen activating proteins. Organic & biomolecular chemistry. 2015;13(7):2078–2086. doi: 10.1039/c4ob02309a. Modifications to the linker of a fluorogenic chromophore (inclusion of two sulfonates) substantially alters the fluorescence quantum yield and the nonspecific interactions, improving the overall performance for whole-cell surface assays.
- 32.Holleran JP, Glover ML, Peters KW, Bertrand CA, Watkins SC, Jarvik JW, Frizzell RA, et al. Pharmacological rescue of the mutant cystic fibrosis transmembrane conductance regulator (cftr) detected by use of a novel fluorescence platform. Molecular medicine. 2012;18:685–696. doi: 10.2119/molmed.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Tapia PH, Fisher GW, Simons PC, Strouse JJ, Foutz T, Waggoner AS, Jarvik J, Sklar LA, et al. Discovery of regulators of receptor internalization with high-throughput flow cytometry. Molecular pharmacology. 2012;82(4):645–657. doi: 10.1124/mol.112.079897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Tapia PH, Fisher GW, Waggoner AS, Jarvik J, Sklar LA, et al. High-throughput flow cytometry compatible biosensor based on fluorogen activating protein technology. Cytometry A. 2013;83(2):220–226. doi: 10.1002/cyto.a.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher GW, Adler SA, Fuhrman MH, Waggoner AS, Bruchez MP, Jarvik JW, et al. Detection and quantification of beta2ar internalization in living cells using fap-based biosensor technology. Journal of biomolecular screening. 2010;15(6):703–709. doi: 10.1177/1087057110370892. [DOI] [PubMed] [Google Scholar]
- 36.Pham HH, Szent-Gyorgyi C, Brotherton WL, Schmidt BF, Zanotti KJ, Waggoner AS, Armitage BA, et al. Bichromophoric dyes for wavelength shifting of dye-protein fluoromodules. Organic & biomolecular chemistry. 2015 doi: 10.1039/c4ob02522a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grover A, Schmidt BF, Salter RD, Watkins SC, Waggoner AS, Bruchez MP, et al. Genetically encoded ph sensor for tracking surface proteins through endocytosis. Angewandte Chemie. 2012;51(20):4838–4842. doi: 10.1002/anie.201108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fisher GW, Fuhrman MH, Adler SA, Szent-Gyorgyi C, Waggoner AS, Jarvik JW, et al. Self-checking cell-based assays for gpcr desensitization and resensitization. Journal of biomolecular screening. 2014;19(8):1220–1226. doi: 10.1177/1087057114534299. Use of TO1 and a tandem TO1-Cy5 dye with lower affinity for the cognate fluorogen activating protein allowed surface-restricted exchange of fluorescence signal. The resulting assay for receptor internalization has one channel that decreases while the other channel increases, improving the accuracy and reliability of the results.
- 39.Yan Q, Schwartz SL, Maji S, Huang F, Szent-Gyorgyi C, Lidke DS, Lidke KA, Bruchez MP, et al. Localization microscopy using noncovalent fluorogen activation by genetically encoded fluorogen-activating proteins. Chemphyschem : a European journal of chemical physics and physical chemistry. 2014;15(4):687–695. doi: 10.1002/cphc.201300757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwartz SL, Yan Q, Telmer CA, Lidke KA, Bruchez MP, Lidke DS, et al. Fluorogen-activating proteins provide tunable labeling densities for tracking fcepsilonri independent of ige. ACS chemical biology. 2015;10(2):539–546. doi: 10.1021/cb5005146. Using low labeling concentrations of MG fluorogens, a sparse subset of IgE receptors were analyzed by single particle tracking with and without bound IgE.
- 41.Yang G, de Castro Reis F, Sundukova M, Pimpinella S, Asaro A, Castaldi L, Batti L, Bilbao D, Reymond L, Johnsson K, Heppenstall PA, et al. Genetic targeting of chemical indicators in vivo. Nature methods. 2015;12(2):137–139. doi: 10.1038/nmeth.3207. [DOI] [PubMed] [Google Scholar]