Abstract
Respiratory syncytial virus (RSV) remains a leading global cause of infant mortality and adult morbidity. Infection, which recurs throughout life, elicits only short-lived immunity. The development of a safe and efficacious vaccine has, thus far, been elusive. Recent technological advances, however, have yielded promising RSV vaccine candidates that are based on solving atomic-level structures of surface glycoproteins interacting with neutralizing antibodies. The class I fusion glycoprotein, F, serves as the primary antigenic component of most vaccines, and is the target of the only licensed monoclonal antibody product used to reduce the frequency of severe disease in high-risk neonates. However, success of prior F-based vaccines has been limited by the lack of understanding how the conformational rearrangement between a metastable prefusion F (pre-F) and a stable postfusion F (post-F) affected the epitope content. Neutralizing epitopes reside on both conformations, but those specific to pre-F are far more potent than those previously identified and present on post-F. The solution of the pre-F structure and its subsequent characterization and stabilization illustrates the value of a structure-based approach to vaccine development, and provides hope that a safe and effective RSV vaccine is possible.
Keywords: respiratory syncytial virus, antigen, fusion, glycoprotein, vaccine, protein structure, neutralizing antibody, structure-based vaccine design
Introduction
Respiratory syncytial virus (RSV) is a pneumovirus in the family Paramyxoviridae. It has a single-stranded, negative-sense genome of about 15 kilobases with 10 gene start sites that encode 11 proteins. Three of those proteins are displayed on the viral envelope. The small hydrophobic (SH) protein is a pentameric ion channel; the putative attachment protein (G) is a heavily O-glycosylated mucin-like glycoprotein; and the fusion glycoprotein (F) is responsible for mediating viral entry through pH-independent membrane fusion from without. There are two major virus subtypes, A and B, largely defined by genetic variation in the G glycoprotein. Compared to other RNA viruses, RSV exhibits relatively little antigenic variation (1).
Viral replication occurs in the cell cytoplasm, in granules comprising the nucleoprotein (N), phosphoprotein (P), the M2-1 protein (a transcription processivity factor), and the polymerase (L) (1). Virus assembly occurs in cholesterol-rich membrane microdomains where the virus buds as filopodia-like filaments that can fuse with adjacent cells, resulting either in syncytia formation or the release of polymorphic virions of about 100-150 nm diameter when spherical or 10 microns in length when filamentous (2) (Figure 1).
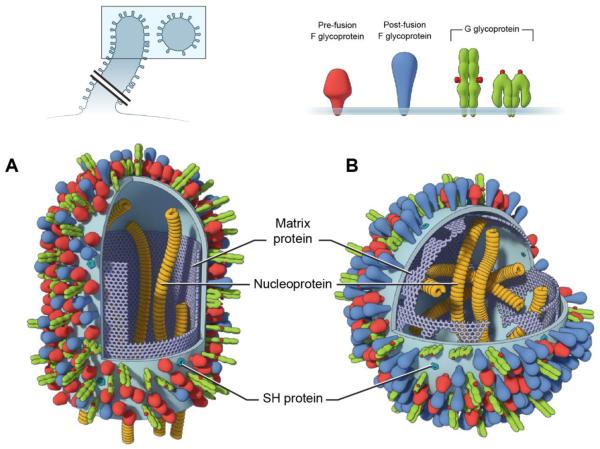
Figure 1. Schematic drawing of RSV particles.
RSV is an enveloped virus that buds from cells as filopodia-like filaments (A) that mediate cell-to-cell fusion or can be released as cell-free pleomorphic particles that range from filamentous to spherical (B). The shape of the virus particle is determined, in part, by the integrity of the matrix protein layer immediately under the membrane. This layer also influences the organization and stability of membrane-associated proteins. The F glycoprotein, in particular, is known to exist on the virion membrane in both metastable pre-F (functional) and stable post-F (non-functional) conformations. The pre-F (red) is thought to be more ordered and have a greater density in the filaments, while the post-F (blue) is more abundant in the spherical particles and continues to accumulate over time, as the matrix layer becomes fragmented. The conformational change in F is unidirectional ending in the nonfunctional post-F protein. The structure of G (green) is not known, but the N- and C-terminal glycan domains are envisioned to be extended or folded over, exposing the cysteine noose (highlighted in red) in the central conserved domain apically or laterally. The pentameric ion channel, SH, is present in the cholesterol-rich lipid microdomains in which virus assembly and budding occurs, but is in relatively low abundance on the surface of virus particles.
RSV infects all children by 3 years of age and about 60-70% by the end of the first year of life. In temperate climates the epidemics are seasonal, occurring annually during the winter months, while in tropical climates the seasonality is less distinct and infections can be detected year round. Globally, RSV is estimated to be second only to malaria as a cause of death due to a single pathogen in infants between 1 month and 1 year of age (3). Children in low and middle income countries suffer a disproportionate share of this mortality. In contrast, RSV carries relatively low mortality in high income countries, but is still the leading cause of hospitalization in children under 5 years of age (4). About 50% of those hospitalizations occur before 6 months and 50% occur between 6 months and 5 years, with a peak around 2-3 months of age. In older children and healthy adults hospitalization is rare, as infections are usually restricted to the upper airways, though often complicated by sinusitis and otitis. The elderly are more susceptible to severe RSV disease, particularly if they have underlying chronic heart or lung disease (4).
RSV has a tropism that is almost exclusively restricted to the respiratory tract. It primarily infects the ciliated epithelia of small bronchioles and type 1 pneumocytes in the alveoli (5). The clinical consequences of RSV’s infection of airway cells largely come as a result of airway obstruction, particularly in young infants, who are at highest risk of severe disease. Mechanical obstruction from sloughed epithelium, inflammatory cell debris, fibrin, mucous, and lymphoid aggregates as well as airway hypersensitivity result in restricted airflow, hypoxia, and wheezing (5).
Vaccine-enhanced disease and the criteria for a better vaccine
In the 1960’s a formalin-inactivated, alum-precipitated, whole-virus vaccine (FI-RSV) given intramuscularly (IM) resulted in enhanced disease, particularly in the youngest age cohort, immunized between 2 and 7 months of age (6). The vaccine did not reduce the frequency of infection and among infected children 80 percent required hospitalization and 2 died. In contrast, natural infection with RSV or its administration by IM or intranasal routes does not result in enhanced disease, but also does not protect from subsequent infection (7, 8). Therefore, an effective RSV vaccine will have to improve upon the immunity afforded by natural infection, and will have to avoid the deleterious responses induced by formalin-inactivated virus. The major characteristics of the immune response associated with the FI-RSV vaccine include: 1) poor antibody function (measured by neutralization (NT) or fusion inhibition) (9, 10), despite high antibody titers (detected by ELISA or complement fixation) (6); 2) high levels of lymphoproliferative activity in peripheral blood mononuclear cells (11) and tissue eosinophilia consistent with a Th2-biased immune response (12).
A primary goal for an RSV vaccine is to delay the age at which primary RSV infection occurs. In older children and adults, the major goal is to prevent infection of the lower airway and to reduce shedding from the upper airway. This translates into the following clinical objectives: 1) prevention of severe lower respiratory tract disease in young infants (i.e. prevent hospitalization in high income countries and prevent mortality and hospitalization in low and middle income countries), 2) prevent medically attended lower respiratory infection in children, 3) prevent excess mortality in the elderly, and 4) achieve ancillary benefits, including reduced incidence of childhood wheezing and otitis media and overall reduced morbidity in otherwise healthy children and adults. Immunological goals are to induce antibodies with high levels of neutralizing activity and, in antigen-naïve infants, avoid Th2-biased T cell responses and allergic inflammation.
In this brief review, we will focus on the antigenic and immunogenic properties of RSV surface proteins and antibody-mediated mechanisms of protection. While the pattern of vaccine-induced T cell responses are critical for safety, and CD8 T cells play an important role in viral clearance, space restrictions preclude an adequate discussion of approaches to elicit T cell-mediated immunity. In addition, we will restrict our comments to subunit vaccine approaches and individual viral proteins, and not discuss live-attenuated virus vaccines as recent reviews have already covered this topic in detail (13).
RSV F (Fusion) glycoprotein
F is a 574 amino acid (aa) class I fusion protein consisting of a 50 kilodalton (kDa) carboxy-terminal F1 fragment and a 20 kDa amino-terminal F2 fragment; making it a trimer of heterodimers. It is distinguished by two furin cleavage sites after aa109 and aa136 that liberate a 27aa glycopeptide and expose the hydrophobic fusion peptide at the F1 amino terminus. Other distinguishing features include a cysteine-rich region between the heptad repeats in F1 and two disulfide bonds (instead of one as in other paramyxovirus F proteins) connecting F1 and F2 between aa70 and aa212, and between aa37 and aa439. There are two N-linked glycosylation sites in F2 at aa27 and aa70, and only one in F1 at aa500. After removal of the 25 aa signal peptide and the 27 aa glycopeptide between F2 and F1, the remaining ectodomain of F consists of 472 aa. Only 25 amino acids in the F ectodomain differ between subtypes A and B.
F is considered a major antigenic target for vaccine development for several reasons: 1) the strict requirement that F mediate virus entry; 2) the relative genetic and antigenic conservation of F; and 3) the established proof-of-principle that anti-F monoclonal antibody (mAb), palivizumab, can reduce severe RSV disease in high-risk infants. Prior efforts to develop subunit vaccines containing F have included: 1) F isolated from virions (Lederle-Praxis/Wyeth) (14-19), 2) a mixture of F, G, and N isolated from virions (Connaught/Sanofi) (20), and 3) an F/G fusion protein expressed in baculovirus (Wyeth) (21-23). The last product did not reach clinical evaluation because of concerning histological findings in cotton rats (23). The first two products, however, were found to be immunogenic, safe, and well tolerated in seropositive children and adults (including pregnant women). These promising data were tempered by the fact that the vaccine-elicited rise in serum NT activity was less than four-fold in most individuals and about four times lower than the rise in antibody binding activity to F as measured by ELISA. As a result, development of these products was abandoned in the early 2000s. More recently, new subunit F products have made it into clinical trials, including: 1) a baculovirus-expressed, uncleaved F that forms rosettes held together by the transmembrane domain (TM) (Novavax); 2) a Chinese hamster ovary (CHO) cell-expressed, uncleaved version of full length F (GSK); 3) a fully cleaved trimeric F in the GLA-SE adjuvant (MedImmune); and 4) a secreted version of wild-type F truncated upstream of the TM and expressed in a chimpanzee-derived adenovirus vector (PAN-Ad) together with N and M2-1 (Okairos/GSK). These products have been tested in healthy RSV-seropositive adults, but most of the data are not yet publically available. Only data from the Novavax rosetted F subunit have been published and show that, at the highest reported dose level of 60 mcg, there is about a 2-fold rise in NT activity and about a 10-fold rise in binding antibody to F (24).
Historically, there were two known major antigenic sites on F associated with NT activity. They were initially defined by binding to the murine mAbs 1129 (site II) (25) and 101F (site IV) (26). Site II is now better known as the target for palivizumab, the humanized murine monoclonal antibody licensed for passive prophylaxis in infants at high risk of severe disease. Another group of murine mAbs including 2F (27) and 131-2a (28) (site I, sometimes mapped to P389 by escape mutations) exhibit weak NT activity with a significant residual un-neutralized fraction. These sites were initially mapped with escape mutations and competition assays with F extracted from virions. The atomic structure of antigenic sites II and IV were then solved (29, 30) in complex with prototypic antibodies. Subsequently, the entire postfusion F (post-F) trimer in its stable 6-helix bundle conformation was solved (31, 32). It was evident that the known NT mAb-binding sites were present on the post-F structure. However, studies using RSVIG, an immunoglobulin product enriched for high RSV NT activity, showed that adsorbing the product with post-F did not remove NT activity from immunoglobulins elicited by natural infection, suggesting that there are other NT-sensitive targets on the prefusion form of F.
Prefusion F
Initial attempts at generating a soluble form of RSV pre-F were based on techniques that Jardetzky and Lamb had used to stabilize the pre-F conformation of the parainfluenza virus 5 (PIV5) F protein (33). They originally stabilized PIV5 pre-F by eliminating the protease site N-terminal to the fusion peptide and appending a GCN4 trimerization motif to the C-terminus of F, which served as a substitute for the transmembrane helices that would normally be present in the full-length protein. Unfortunately, both approaches failed to stabilize RSV F, although the addition of a T4 fibritin trimerization motif to the C-terminus of F did result in trimerization of the soluble F protein (30). The key to stabilizing RSV pre-F was the isolation of monoclonal antibodies that were specific to the prefusion state. One such monoclonal antibody, 5C4, was isolated from mice primed with plasmid DNA and boosted with recombinant adenovirus, both of which encoded full-length RSV F. Hybridomas from these mice were screened for strong NT activity and absence of binding to post-F. Two other pre-F-specific antibodies, AM22 and D25, were isolated from immortalized PBMCs obtained from a naturally infected human (34).
Co-expression of the pre-F-specific antibodies with foldon-appended RSV F led to homogenous preparations of pre-F complexes. The X-ray crystal structure of the D25 complex was determined at 3.6 Å resolution, revealing the long-sought, atomic-level detail of pre-F and a novel antigenic site at the membrane-distal apex targeted by all three pre-F-specific antibodies. This antigenic site, called site Ø (zero), is composed of residues 62-69 from the F2 subunit and solvent-exposed residues of the α4-helix from the F1 subunit. In post-F, the α4-helix is inverted 180° and, along with the rest of the F1 N-terminus, forms one long helix. This reorientation of α4 and the other elements at the F1 N-terminus and loss of proximity to the aa62-69 residues in F2 explain why site Ø mAbs selectively recognize pre-F and not post-F (35).
To stabilize the pre-F conformation, the structure of pre-F in complex with D25 was analyzed for mutations that would be predicted to maintain the F1 N-terminus in its D25-bound conformation. From more than 100 variants, three were identified as retaining binding capacity to the pre-F-specific antibodies. One variant (termed DS) was produced by substituting Ser155 and Ser290 with cysteines, resulting in a disulfide bond between the structurally labile N-terminus of F1 and the structurally rigid central region of F1. Another variant (termed Cav1) contained the “cavity-filling” mutations S190F and V207L, which were designed to fill cavities in the F1 N-terminus to both increase hydrophobic packing interactions and to make exposure of the hydrophobic residues to solvent unfavorable. The third variant contained an F488W mutation that interacted with and stabilized the hydrophobic fusion peptide. After testing combinations of these three stabilizing groups of mutations, it was determined that the disulfide bond together with the cavity-filling mutations produced a stable pre-F protein, termed DS-Cav1 (36).
The crystal structure of DS-Cav1 revealed an adopted conformation similar to the D25-bound conformation of RSV F. Additionally, DS-Cav1 bound with nanomolar affinity to the site Ø-directed antibodies. In mice and rhesus macaques, DS-Cav1 elicited NT activity more than 10-fold higher than that elicited by post-F. Although the immunized DS-Cav1 was derived from a subtype A strain (A2), it also elicited high NT activity against a subtype B strain (18537), although the titers were reduced 2-3 fold compared to subtype A neutralization. The cross-protection is not surprising given the >90% sequence identity in the RSV F ectodomains. Approximately 50% of the antibodies elicited by DS-Cav1 in macaques were pre-F-specific, and most of this activity could be competed with D25 (36). In addition to the potent antibodies specific for antigenic site Ø (D25, AM22, and 5C4) there have now been other pre-F-specific mAbs identified, such as MPE8 (37), that have higher neutralizing potency than mAbs to sites II and IV, which are present on both pre-F and post-F conformations (Figure 2). MPE8 is also unique in that it neutralizes multiple paramyxoviruses. Another feature shared between all paramyxoviruses is a disulfide bond between the F2 and F1 subunits analogous to the one between aa69 and aa212 in RSV. This universally conserved property of paramyxovirus fusion proteins suggests that it is important functionally. Furthermore, since this covalent link between F2 and F1 is part of antigenic site Ø, present at the apex of the prefusion F trimer, it seems likely that this region could be a neutralization-sensitive antigenic site on other paramyxoviruses. Whether this is a site of vulnerability that could be exploited against other viruses is a subject of ongoing research.
Figure 2. Surface representation of RSV F glycoprotein.
The RSV F glycoprotein exists in a prefusion (pre-F) conformation prior to an extensive rearrangement that occurs spontaneously or as part of the membrane fusion process that allows entry of the viral nucleocapsid into the target cell. Pre-F and post-F are approximately 11 nm and 16 nm high, and as shown in Figure 1, the density of pre-F and post-F vary depending on the age and condition of the virus. About 50% of the surface of pre-F is preserved in the post-F conformation. While only the pre-F conformation is functional, there are epitopes preserved on post-F (sites I, II, and IV) that are associated with NT activity. However, the most neutralization-sensitive sites recognized by the most potent neutralizing antibodies are only present on pre-F. These include site Ø (red) and at least two other sites (orange) that have not yet been given a numerical designation.
For subunit vaccine development, the pre- and postfusion proteins are attractive in that they display multiple epitopes that can elicit neutralizing antibodies. There may be some instances, however, when elicitation of an immune response focused on a single epitope or antigenic site is desired. Recently, Schief and colleagues computationally designed novel proteins that correctly displayed antigenic site II and bound to motavizumab with picomolar affinity (38). When these scaffolds were fused to a multivalent particle and injected into macaques they elicited neutralizing motavizumab-like antibodies, providing proof-of-principle for epitope-focused vaccine design. Although the elicited neutralizing antibody titers were lower than those elicited by prefusion F, these epitope-scaffold immunogens represent a promising approach to vaccine design that could be enhanced by displaying more neutralization-sensitive epitopes, such as antigenic site Ø.
RSV G glycoprotein
Of the 11 proteins that comprise RSV, only F and G are targeted by neutralizing antibodies. Although F-directed antibodies are generally more potent and cross-protective, the G glycoprotein has a neutralization-sensitive epitope in the central conserved domain that makes it worth considering as a vaccine antigen. G is a 90 kDa type II integral membrane protein, and can mediate viral attachment to the host cell membrane either through interaction with heparan sulphate on proteoglycans or a more specific, yet undiscovered, receptor. While G is not necessary for entry, as G-deleted viruses can infect immortalized cells in vitro, albeit with diminished efficiency (39), G-deleted viruses are highly attenuated in vivo. In addition to its role in virus entry, G, which is the most variable RSV protein, dictates the virus subtype by virtue of its genetic variation. Variable sequences lie in the mucin domains at the amino and carboxy ends of the protein. These regions contribute to an unusual amino acid composition that includes ~30% serines and threonines, and ~10% prolines (40), similar to the mucin domain of Ebola GP. Like mucins, G is heavily O-glycosylated, providing a potential mechanism for evasion of host immunity. The central conserved domain of G is less glycosylated and has a CX3C motif in a cysteine noose that interacts with the fractalkine chemokine receptor (41) that has been shown to influence neutrophil migration and pulmonary inflammation in a murine model (42, 43).
Early studies in BALB/c mice showed immunization with a recombinant vaccinia virus expressing RSV G induced eosinophilia post-challenge (44), similar to the responses seen in mice immunized with FI-RSV (12). However, subsequent studies suggested the G-specific responses were species dependent (45), and determined by an I-Ad-restricted, Vβ14 CD4 T cell (46) that had a proclivity to produce IL-4 and IL-13 in addition to IFN-ɣ (47). Subsequent studies determined that neither the Vβ14 T cells nor G-specific responses were required in the murine model of FI-RSV to produce Th2 responses and eosinophilia (48, 49). Recent studies have shown that mice were vaccinated with either a G polypeptide encompassing the 131-2g binding domain (50), a recombinant influenza virus expressing conserved G peptides (51), or virus-like nanoparticle containing both RSV F and G (52) have diminished virus titers and pathology in the lungs post RSV challenge. No airway inflammation or pulmonary eosinophilia similar to that associated with FI-RSV immunized mice was observed with any of these vaccines. In addition, passive administration of the 131-2g monoclonal antibody (mAb) that targets the CX3C motif resulted in reduced pulmonary inflammation in mice following RSV challenge (53).
A phase I clinical trial evaluating a polypeptide from the central conserved region of G conjugated to a portion of the Streptococcal protein G was well tolerated and induced 2-fold or greater rise in NT activity in the majority of subjects (54). However, two individuals in a subsequent phase II study had type III hypersensitivity and raised concerns about using a G subunit vaccine in seronegative infants. However, given the promising data from recent murine studies, and the likely association of the inflammatory side effects in the clinical studies with the Streptococcal protein G conjugate component of the vaccine, the inclusion of the G glycoprotein into a RSV vaccine deserves consideration.
RSV SH Protein
The small hydrophobic (SH) protein, formerly known as 1A, is the third RSV surface glycoprotein. This 64 – 65 amino acid type II integral membrane protein forms a pentameric ion channel (55) that functions similarly to the M2 protein of influenza (56). Its role in the virus life cycle and eliciting immunity, however, are not well understood, although recent reports have provided some illumination (57). SH is important to viral replication, as a primary method for generating live-attenuated RSV is to delete SH. RSV lacking SH replicates productively in vivo but achieves ~1 log10 lower peak viral titers in mice (58, 59). SH may also play a role in prolonging the RSV life cycle and evading host immunity by inhibiting the TNF-α pathway that leads to apoptosis in infected cells. Additionally, functional SH is associated with activation of the NLRP3 inflammasome (60), resulting in IL-1β secretion. The potential for precipitating an inflammatory response, paucity of T cell epitopes, and the inability to induce neutralizing antibodies had left SH as a low priority vaccine antigen in the past. However, a recent report from Schepens et al. demonstrated that vaccination with the conserved extracellular domain of SH significantly reduced viral replication in RSV-challenged mice in an Fc dependent manner (61). Thus, alternative mechanisms of vaccine-elicited immunity, such as antibody-dependent cellular or complement-mediated cytotoxicity, could contribute to more conventional immune effector mechanisms such as NT activity and potentially improve the efficacy of an RSV vaccine.
Conclusions
Development of an effective RSV vaccine has remained elusive despite its ubiquitous prevalence and the severe disease burden imposed on infants, children, and the elderly. However, there has been steady, incremental progress on defining the immunological parameters associated with the FI-RSV vaccine-enhanced illness that can guide the regulatory process. In addition, recent breakthroughs have provided the atomic-level structure of the prefusion F trimer, a better understanding of the mechanisms and targets of neutralizing antibodies, and new candidate vaccines based on structure-guided antigen design of stabilized pre-F immunogens that elicit potent NT responses. These advances have motivated major investments in RSV vaccine development, as a safe and effective vaccine no longer merely holds promise but may actually be achievable in the near term.
There are still many hurdles to overcome that are unique for each human target population. Direct immunization of infants <3 months of age, who are at highest risk of severe disease, is complicated by the presence of passively acquired maternal antibody, immaturity of antigen processing cells, lack of somatic hypermutation, and a generally higher rate of idiosyncratic adverse events, including apnea, that may confound the assessment of vaccine safety (62). In this age group, and even in older seronegative children, there will be the highest standard for vaccine safety because of the legacy of FI-RSV vaccine-enhanced disease. For these reasons, RSV-naïve infants and children will likely be the last group in which subunit RSV vaccines are evaluated. The greatest problem in all other target populations – pregnant women, the elderly, and older children – is pre-existing immunity. Although prior immunological priming from natural infection will avoid concerns about vaccine-enhanced illness, it also produces a B cell repertoire and T cell immune response pattern that may be difficult to change based on prior vaccine studies in these populations. The hope is that by using the stabilized pre-F as a vaccine antigen the neutralization-sensitive sites will be recognized by the precursor B cells capable of producing high potency neutralizing responses. Only large controlled studies in pre-immune humans will be able to answer questions about potency of vaccines containing stabilized pre-F. If significant boosting of NT activity in pre-immune subjects is possible, then a combined strategy may be most effective. This would consist of immunizing pregnant women to improve passive protection of infants and immunizing older children to reduce viral shedding and interrupt transmission to younger siblings. In addition, immunizing the elderly to increase NT activity may provide a direct benefit to this at-risk population.
The pre-F antigen could be produced as a subunit protein or expressed from a vector, a chimeric virus, or as part of a virus-like particle (VLP). Delivery of pre-F from a gene expressed in a vector or chimeric virus would have the advantage of eliciting not only pre-F-specific potent neutralizing antibodies, but also CD8 T cells. In addition, it would provide options for combined delivery of other RSV proteins if it was found that additional Fc-mediated antibody effector mechanisms or selected T cell responses provided improved immunity or safety. For example, internal proteins like N, M, and M2-1 are rich in T cell epitopes, and if delivered by recombinant vectors, would contribute to T cell-mediated immunity, just as including SH may add additional ADCC-mediated immunity.
The development of a novel pre-F vaccine antigen and identification of previously unknown pre-F-specific neutralization-sensitive epitopes is an example of how modern technologies can influence the historically empirical field of vaccinology. In this case, structural biology provided the insight to pursue a new approach to RSV vaccine development. The advances in human immunology, including human monoclonal antibody identification, sequencing technology to analyze the ontogeny of antibody responses and evolution of human pathogens, and ability to solve atomic level structures of vaccine targets have provided tools to guide the rational design of future vaccines (63). This convergence of technologies may eventually allow us to solve some of the long-standing vaccine development challenges for other difficult viruses in addition to RSV.
Highlights.
– There are multiple neutralizing antibody binding sites on F protein and at least one on G protein.
– The most potent neutralizing antibodies recognize sites exclusively on the prefusion conformation of F.
– mAbs with intermediate neutralizing potency recognize two sites present on both pre-F and post-F.
– F immunogens stabilized in a pre-F conformation improve prospects for an effective RSV vaccine.
Acknowledgments
B.S.G. and K.M. were supported by funding from the intramural program of the National Institute of Allergy and Infectious Diseases. J.S.M. was supported in part by NIAID grant 1R43AI112124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
B.S.G. and J.S.M are named inventors on patents describing mutations that stabilize RSV F in the prefusion conformation.
References and recommended reading
- 1.Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Current Topics in Microbiol and Immunol. 2013;372:3–38. doi: 10.1007/978-3-642-38919-1_1. * This review provides a comprehensive summary of the molecular biology and pathogenesis of RSV as well as a primer on the underlying mechansims of vaccine-enhanced illness observed during the FI-RSV vaccine trials of the 1960s.
- 2.Gower TL, Pastey MK, Peeples ME, Collins PL, McCurdy LH, Hart TK, et al. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J Virol. 2005;79(9):5326–36. doi: 10.1128/JVI.79.9.5326-5336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CB, Simoes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Current Topics in Microbiol and Immunol. 2013;372:39–57. doi: 10.1007/978-3-642-38919-1_2. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Modern Pathol. 2007;20(1):108–19. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 6.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 7.Belshe RB, Van Voris LP, Mufson MA. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982;145(3):311–9. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- 8.Wright PF, Belshe RB, Kim HW, Van Voris LP, Chanock RM. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infection and Immunity. 1982;37(1):397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy BR, Prince GA, Walsh EE, Kim HW, Parrott RH, Hemming VG, et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24(2):197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26(8):1595–7. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HW, Leikin SL, Arrobio J, Brandt CD, Chanock RM, Parrott RH. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976;10(1):75–8. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151(4):2032–40. [PubMed] [Google Scholar]
- 13.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Current Topics in Microbiol and Immunol. 2013;372:259–84. doi: 10.1007/978-3-642-38919-1_13. * This paper reviews the methods by which live-attenuated strains of RSV have been generated for research efforts and evaluates the most viable candidates for vaccine trials.
- 14.Paradiso PR, Hildreth SW, Hogerman DA, Speelman DJ, Lewin EB, Oren J, et al. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24 to 48 months old. Pediatr Infect Dis J. 1994;13(9):792–8. doi: 10.1097/00006454-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Tristram DA, Welliver RC, Hogerman DA, Hildreth SW, Paradiso P. Second-year surveillance of recipients of a respiratory syncytial virus (RSV) F protein subunit vaccine, PFP-1: evaluation of antibody persistence and possible disease enhancement. Vaccine. 1994;12(6):551–6. doi: 10.1016/0264-410x(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 16.Falsey AR, Walsh EE. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine. 1997;15(10):1130–2. doi: 10.1016/s0264-410x(97)00002-9. [DOI] [PubMed] [Google Scholar]
- 17.Falsey AR, Walsh EE. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine. 1996;14(13):1214–8. doi: 10.1016/s0264-410x(96)00030-8. [DOI] [PubMed] [Google Scholar]
- 18.Piedra PA, Cron SG, Jewell A, Hamblett N, McBride R, Palacio MA, et al. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine. 2003;21(19-20):2448–60. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 19.Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21(24):3465–7. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 20.Langley JM, Sales V, McGeer A, Guasparini R, Predy G, Meekison W, et al. A dose-ranging study of a subunit Respiratory Syncytial Virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults > or =65 years of age. Vaccine. 2009;27(42):5913–9. doi: 10.1016/j.vaccine.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Homa FL, Brideau RJ, Lehman DJ, Thomsen DR, Olmsted RA, Wathen MW. Development of a novel subunit vaccine that protects cotton rats against both human respiratory syncytial virus and human parainfluenza virus type 3. J Gen Virol. 1993;74(Pt 9):1995–9. doi: 10.1099/0022-1317-74-9-1995. [DOI] [PubMed] [Google Scholar]
- 22.Oien NL, Brideau RJ, Thomsen DR, Homa FL, Wathen MW. Vaccination with a heterologous respiratory syncytial virus chimeric FG glycoprotein demonstrates significant subgroup cross-reactivity. Vaccine. 1993;11(10):1040–8. doi: 10.1016/0264-410x(93)90131-g. [DOI] [PubMed] [Google Scholar]
- 23.Connors M, Collins PL, Firestone CY, Sotnikov AV, Waitze A, Davis AR, et al. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia--RSV recombinants or RSV. Vaccine. 1992;10(7):475–84. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 24.Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, et al. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31(3):524–32. doi: 10.1016/j.vaccine.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63(7):2941–50. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SJ, Schmidt A, Beil EJ, Day ND, Branigan PJ, Liu C, et al. Characterization of the epitope for anti-human respiratory syncytial virus F protein monoclonal antibody 101F using synthetic peptides and genetic approaches. J Gen Virol. 2007;88(Pt 10):2719–23. doi: 10.1099/vir.0.82753-0. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Barreno B, Palomo C, Penas C, Delgado T, Perez-Brena P, Melero JA. Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J Virol. 1989;63(2):925–32. doi: 10.1128/jvi.63.2.925-932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson LJ, Bingham P, Hierholzer JC. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol. 1988;62(11):4232–8. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLellan JS, Chen M, Chang JS, Yang Y, Kim A, Graham BS, et al. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J Virol. 2010;84(23):12236–44. doi: 10.1128/JVI.01579-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol. 2010;17(2):248–50. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85(15):7788–96. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A. 2011;108(23):9619–24. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin HS, Wen X, Paterson RG, Lamb RA, Jardetzky TS. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439(7072):38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CM, Lukens MV, et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nature Med. 2010;16(1):123–8. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–7. doi: 10.1126/science.1234914. ** This paper describes the first crystal structure of a stablized prefusion form of the RSV F glycoprotein in complex with a potent neutralizing antibody that binds a novel neutralization-sensitive antigenic site (Ø) at the apex of the F trimer.
- 36.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–8. doi: 10.1126/science.1243283. ** This paper reports the structure-based design of a stabilized prefusion RSV F glycoprotein trimer, which induces potent neutralizing activity in mice and non-human primates.
- 37.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501(7467):439–43. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 38.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, et al. Proof of principle for epitope-focused vaccine design. Nature. 2014;507(7491):201–6. doi: 10.1038/nature12966. * This report describes a novel computational approach to designing a scaffold protein for antigenic site II (the palivizumab epitope) on RSV F that could potentially be applied as a general strategy for the design of vaccines against a broad array of pathogens.
- 39.Teng MN, Collins PL. The central conserved cystine noose of the attachment G protein of human respiratory syncytial virus is not required for efficient viral infection in vitro or in vivo. J Virol. 2002;76(12):6164–71. doi: 10.1128/JVI.76.12.6164-6171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wertz GW, Collins PL, Huang Y, Gruber C, Levine S, Ball LA. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985;82(12):4075–9. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2(8):732–8. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 42.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. J Virol. 2003;77(18):9831–44. doi: 10.1128/JVI.77.18.9831-9844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tripp RA, Dakhama A, Jones LP, Barskey A, Gelfand EW, Anderson LJ. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. J Virol. 2003;77(11):6580–4. doi: 10.1128/JVI.77.11.6580-6584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4(4):493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 45.Hussell T, Georgiou A, Sparer TE, Matthews S, Pala P, Openshaw PJ. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol. 1998;161(11):6215–22. [PubMed] [Google Scholar]
- 46.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15(4):637–46. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 47.Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, et al. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168(6):2944–52. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- 48.Johnson TR, Teng MN, Collins PL, Graham BS. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J Virol. 2004;78(11):6024–32. doi: 10.1128/JVI.78.11.6024-6032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson TR, Varga SM, Braciale TJ, Graham BS. Vbeta14(+) T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J Virol. 2004;78(16):8753–60. doi: 10.1128/JVI.78.16.8753-8760.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, et al. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol. 2010;84(2):1148–57. doi: 10.1128/JVI.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YN, Suk Hwang H, Kim MC, Lee YT, Cho MK, Kwon YM, et al. Recombinant influenza virus carrying the conserved domain of respiratory syncytial virus (RSV) G protein confers protection against RSV without inflammatory disease. Virology. 2015;476:217–25. doi: 10.1016/j.virol.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jorquera PA, Choi Y, Oakley KE, Powell TJ, Boyd JG, Palath N, et al. Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease. PLoS ONE. 2013;8(9):e74905. doi: 10.1371/journal.pone.0074905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi Y, Mason CS, Jones LP, Crabtree J, Jorquera PA, Tripp RA. Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C CX3CR1 binding and cross-neutralize RSV A and B strains. Viral Immunol. 2012;25(3):193–203. doi: 10.1089/vim.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power UF, Nguyen TN, Rietveld E, de Swart RL, Groen J, Osterhaus AD, et al. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J Infect Dis. 2001;184(11):1456–60. doi: 10.1086/324426. [DOI] [PubMed] [Google Scholar]
- 55.Gan SW, Ng L, Lin X, Gong X, Torres J. Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain. Protein Sci. 2008;17(5):813–20. doi: 10.1110/ps.073366208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cady SD, Luo W, Hu F, Hong M. Structure and function of the influenza A M2 proton channel. Biochemistry. 2009;48(31):7356–64. doi: 10.1021/bi9008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol. 2007;81(15):8361–6. doi: 10.1128/JVI.02717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bukreyev A, Whitehead SS, Murphy BR, Collins PL. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71(12):8973–82. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, et al. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci U S A. 1997;94(25):13961–6. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Triantafilou K, Kar S, Vakakis E, Kotecha S, Triantafilou M. Human respiratory syncytial virus viroporin SH: a viral recognition pathway used by the host to signal inflammasome activation. Thorax. 2013;68(1):66–75. doi: 10.1136/thoraxjnl-2012-202182. [DOI] [PubMed] [Google Scholar]
- 61.Schepens B, Sedeyn K, Vande Ginste L, De Baets S, Schotsaert M, Roose K, et al. Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO Mol Med. 2014;6(11):1436–54. doi: 10.15252/emmm.201404005. * This paper demonstrates how immunization with the extracellular domain of RSV SH protein induces antibody-mediated, Fc-dependent, neutralization-independent immunity in mice.
- 62.Malloy AM, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Current topics in Microbiol and Immunol. 2013;372:211–31. doi: 10.1007/978-3-642-38919-1_11. [DOI] [PubMed] [Google Scholar]
- 63.Graham BS. Advances in antiviral vaccine development. Immunological Reviews. 2013;255(1):230–42. doi: 10.1111/imr.12098. * This is a review of how recent technological advances have influenced the field of vaccinology and made it more feasible to apply rationale vaccine design approaches to future development efforts.