Abstract
Raw licorice roots represent heterogeneous materials obtained from mainly three Glycyrrhiza species. G. glabra, G. uralensis, and G. inflata exhibit marked metabolite differences in terms of flavanones (Fs), chalcones (Cs), and other phenolic constituents. The principal objective of this work was to develop complementary chemometric models for the metabolite profiling, classification, and quality control of authenticated licorice. A total of 51 commercial and macroscopically verified samples were DNA authenticated. Principal component analysis and canonical discriminant analysis were performed on 1H NMR spectra and area under the curve values obtained from UHPLC-UV chromatograms, respectively. The developed chemometric models enable the identification and classification of Glycyrrhiza species according to their composition in major Fs, Cs, and species specific phenolic compounds. Further key outcomes demonstrated that DNA authentication combined with chemometric analyses enabled the characterization of mixtures, hybrids, and species outliers. This study provides a new foundation for the botanical and chemical authentication, classification, and metabolomic characterization of crude licorice botanicals and derived materials. Collectively, the proposed methods offer a comprehensive approach for the quality control of licorice as one of the most widely used botanical dietary supplements.

In the United States herbal/dietary supplement market, consumers have presumably a 50% chance of choosing an authentic product containing both the correct species and characteristic bioactive compounds at their appropriate strength.1,2 Notably, the botanical authentication including the Latin binomial name is not always specified on the product, whether the botanical dietary supplement (BDS) is sold in the U.S., in the European Union, or in other market places. Traditionally, botanists rely upon morphological features to identify plants. However, the raw materials used to manufacture BDSs are typically ground to a powder, or otherwise processed, thereby hampering morphologic and macroscopic authentication. Investigators wishing to botanically identify these plant-derived materials, prior to any chemical or biological investigation, must turn to other intrinsic properties such as DNA barcoding, underlining genetic differences between species.3,4 DNA-based assays are not biased by the growth environment or the organ sampled and have become central to the identification of all types of biological samples including plant material.5,6 DNA barcoding encompasses the technique of identifying biological specimens using short DNA sequences from a standardized region of the nuclear and/or organelle genomes.4,7 On the other hand, metabolomic techniques for fingerprinting and profiling employing near-infrared (NIR) and Fourier transform infrared (FT-IR) spectroscopy, as well as chromatographic systems hyphenated with various detection modes (LC-UV, GC/LC-MS), and more recently NMR spectroscopy, have been successfully used for the chemical analysis and characterization of plant organs, as part of the quality control (QC) of medicinal plant products.3,8–10.
The global economic value of licorice (Glycyrrhiza sp., Leguminosae) is unquestionable, as evidenced by its multiple uses as a pharmaceutical agent, BDS, cosmetic, sweetener, food additive, flavor additive for tobacco, and confectionery food.11–13. BDSs containing licorice roots, rhizomes, and stolons are sourced globally from mainly three different species: Glycyrrhiza glabra L., G. uralensis Fisch. ex DC., and G. inflata Batalin. These species are listed in the pharmacopeias worldwide and have been employed in traditional remedies since the beginning of medicine.12 In the Chinese Gan-Cao14 and the Japanese Kampo medicines,15,16 the three Glycyrrhiza species are even considered as being equivalent and, thus, are combined and utilized without distinction. Similar concepts apply to other pharmacopeias.17,18
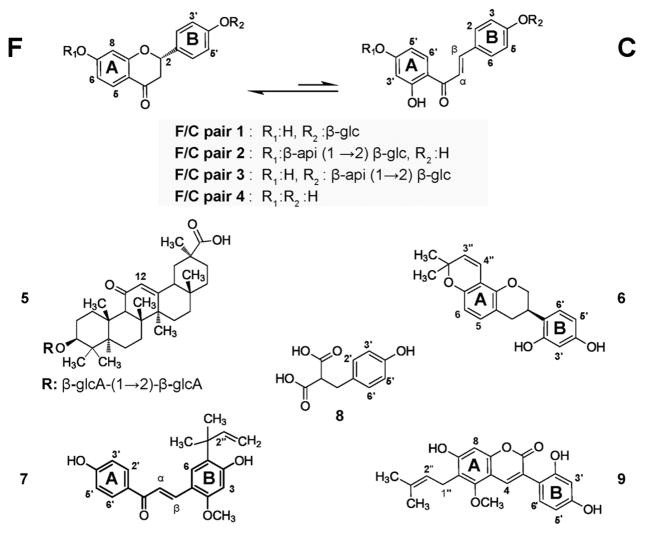
The traditional botanical authentication of commercial licorice is complex, as root powders from all three major Glycyrrhiza species share the same microscopic characteristics (Supporting Information S1).17 Moreover, the heterogeneity of the plant material, which may be due to contamination or purposeful combination with other Glycyrrhiza species, can depreciate the QC outcomes and impede all efforts aimed at their chemical standardization. Major secondary metabolites occurring in the roots and stolons of all three species are the glycosides of liquiritigenin (flavanones: 1-4F) and isoliquiritigenin (2′-hydroxychalcones: 1-4C), as well as glycyrrhizin (5), an oleanane-type triterpenoid saponin responsible for the sweet taste of licorice (Scheme 1). Prior research has shown that the roots of each of the three Glycyrrhiza species display unique chemical profiles, determined by the presence of species-specific metabolites (glabridin (6) for G. glabra, licochalcone A (7) for G. inflata, and glycycoumarin (9) for G. uralensis) and by the relative composition of major flavanone (F), chalcone (C), and triterpenoid compounds.16,19–24 Metabolomic analyses of licorice roots were initiated by Yang and co-workers,22 who performed 1H NMR multivariate analysis on botanically and macroscopically identified Glycyrrhiza species focusing on primary metabolites.22 Subsequently, Farag and co-workers23 combined LC-MS and 1H NMR metabolite profiling of botanically and macroscopically identified Glycyrrhiza species, using three sample acquisitions per species. Recently, we have demonstrated that both major Fs and their C isomers coexist in extracts from licorice roots and can be determined as pairs of isomers.20 In licorice, those major Fs and Cs are known to be biosynthetically related and chemically interchangeable, exemplifying the notion of dynamic residual complexity.25,26 Accordingly, each C in licorice extract has a corresponding F isomer, which together form an F/C pair (Scheme 1). Despite the widespread use of licorice, the levels of most of its metabolites, including the F/C pairs and the species-specific compounds, are generally not disclosed in commercial BDSs. In fact, the QC of pharmacopeial licorice relies mainly on the detection and quantitation of glycyrrhizin (5), a major constituent that is present in all three Glycyrrhiza species and, thus, is the least (species) specific marker.17,18 To improve this situation, modern and reliable QC methodologies that are more comprehensive and permit the simultaneous authentication, differentiation/classification, and chemical characterization of the pharmacopeial Glycyrrhiza species are needed.
Scheme 1. Major Metabolites Characterizing the EtOH Extracts of the Investigated Glycyrrhiza Speciesa.
aLiquiritin (1F) and isoliquiritin (1C) form the F/C pair 1, liquiritigenin-7-O-apiosylglucoside (2F) and licuraside (2C) define the F/C pair 2, liquiritin apioside (3F) together with isoliquiritin apioside (3C) form the F/C pair 3, and finally the aglycone liquiritigenin (4F) and its isomer isoliquiritigenin (4C) are the F/C pair 4. Glycyrrhizin (5) is the major common saponin. The phenolic acid, p-hydroxybenzylmalonic acid (8), was recently found in extracts from all three species.40 Glabridin (6), licochalcone A (7), and glycycoumarin (9) are specific metabolites from G. glabra, G. inflata, and G. uralensis, respectively.
The principal objective of this study was to perform a classification and metabolomic characterization of all three pharmacopeial Glycyrrhiza species used as “licorice” in BDSs. A total of 51 samples were acquired as root sticks, bulk powders, or capsules. All samples were authenticated by DNA barcoding, adjusting the method originally developed by Kondo and coworkers.27 Two orthogonal metabolomic techniques, untargeted 1D 1H NMR and targeted UHPLC-UV,20 were performed on crude extracts of each sample. The data obtained from these two analytical platforms were subjected to multivariate data analyses. All NMR spectra were analyzed through unsupervised principal component analysis (PCA) and supervised soft independent modeling of class analogy (SIMCA). Supervised canonical discriminant analysis (CDA) was employed to develop a classification model based on selected area under the curve (AUC) values from UHPLC-UV chromatograms, integrating the parameters linked to the F/C composition of each species. The second major objective was to demonstrate that DNA authentication is fundamental for the meaningful construction of chemometric models PCA/SIMCA and CDA, which collectively will enable the classification and characterization of various licorice samples sold as BDSs. Finally, our study proposes a comprehensive approach to the QC of licorice botanicals, combining botanical authentication with DNA analysis together with metabolomic and chemometric classification.
With regard to nomenclature, the contemporary literature uses the terms “metabolite profiling” and “metabolite/metabolic fingerprinting” widely and interchangeably.8 Both terms are used to describe more comprehensive studies of the (plant) metabolome.3,8 Considering that “metabolite profiling” is predominantly associated with the identification of key metabolites that lead to the differentiation of samples, its usage is preferred in this study.
RESULTS AND DISCUSSION
Authentication of Licorice Roots by DNA Barcoding
Commercially available licorice materials can be composed of a single Glycyrrhiza species, Glycyrrhiza hybrids, or a mixture of Glycyrrhiza species, but also mixtures of Glycyrrhiza species with other plants or contaminants. Over the past two decades, the international initiative, Consortium for the Barcode of Life (CBOL, http://barcoding.si.edu), has developed aims in global standards for DNA barcoding leading to species identification. With regard to plant barcoding, CBOL has sanctioned the use of two protein coding regions, matK and rbcl, as core DNA barcodes and two noncoding regions, nrITS and psbA-trnH, as supplemental markers.28 The matK, rbcL, and psbA-trnH loci are found in the chloroplast (cp) genome (cpDNA), while the nrITS locus is part of the nuclear DNA (nrDNA). The generation of DNA barcodes can generally be achieved from fresh and dried plant parts. Under certain circumstances,29–32 plant DNA can also be isolated from plant extracts, processed herbal drugs, and finished products such as herbal teas, tablets, and capsules, provided that industrial processing did not lead to extensive DNA degradation.
Multiloci DNA characterization of the three principal botanically identified Glycyrrhiza species (205 analyzed leaf samples) was initially developed by Kondo and co-workers in 2007.27 According to the molecular phylogeny set forth in this work, each Glycyrrhiza species or hybrid is characterized by different genotypes (Figure 1). The combination of nrITS, rbcL, matK, and psbA-trnH markers from each sample allows for the definition of different total genotypes (TGs), leading to the identification of each species or hybrid.27 Following this framework, G. uralensis is defined by four total genotypes (TG6–9), G. glabra has a total of two genotypes (TG2, TG3), and G. inflata comprises three genotypes (TG2, TG4, TG5). As the present study was dealing with partially processed raw material of below-ground plant parts, such as ground roots, root powders, and mixtures of root powders, some modifications were made to Kondo’s original protocol.27 Specifically, the nrITS “universal” primers designed by White and co-workers33 and employed by Kondo and co-workers27 were initially used for amplification. Owing to their universality, these nrITS primers can also amplify sequences belonging to other taxa, thus leading to interference, notably with the amplification of fungal DNA.34 To avoid such cross contaminations, Glycyrrhiza-specific internal nrITS primers were designed (see Experimental Section). Moreover, a second set of primers, which were 100% conserved between the three Glycyrrhiza species, were developed for commercial samples with degraded DNA, allowing the production of smaller amplicons (~200 bp).
Figure 1.
DNA barcoding data used for the authentication of Glycyrrhiza species and the determination of hybrids and mixtures. (A) Determination of Glycyrrhiza genotypes according to the combination of DNA markers (alleles) at four different loci (rbcL, matK, and psbA-trnH in the cpDNA and ITS in the nrDNA). (B) Comparative analysis of nucleotide sequence at specific sites of the ITS and psbA-trnH intergenic region contributing to the identification of the alleles I-2, I-3, and T-1 to T-4, respectively. Both A and B are information obtained from Kondo’s paper.27 (C) Comparative nucleotide sequences at the targeted sites indicated in B for both ITS and psbA-trnH, illustrating the differences between pure species (BC754), hybrid (BC758), and a mixture of Glycyrrhiza species (BC742). Each nucleotide is color-coded: A for adenine in green, T for thymine in red, C for cytosine in blue, and finally G for guanine in black.
Glycyrrhiza hybrids were identified by comparing the nucleotide sequence chromatograms of the nrITS locus (Figure 1C). Localized in the nucleus, this DNA marker is inherited from both parents. The occurrence of both I-2 and I-3 sequences for the nrITS has been called ADD genotype by Kondo and corresponds to “nucleotide additivities” often observed in hybrids between two species.27 In such a case, the nrITS DNA sequencing traces will show secondary peaks only at the variable species-specific nucleotides in both sequencing directions. For pure Glycyrrhiza hybrids, bidirectional DNA sequencing traces from cp loci will always show single peaks due to the chloroplast’s haploid genome (Figure 1C). In fact, it is generally accepted that angiosperm plastids are inherited maternally, although numerous reports have underlined that plastid inheritance pattern can be influenced by both maternal and paternal genotypes.35 This biparental inheritance has not been demonstrated yet for Glycyrrhiza species; thus, we conclude presently that the cpDNA sequences of hybrids should identify the maternal species (Table 3 in bold font). Specifically, Kondo and co-workers27 reported that the cpDNA markers R-2 and T-4 were located only in G. uralensis, whereas the markers M-2 and T-3 were found in G. inflata. This information was considered to specify the maternal species of hybrids.
Table 3.
DNA Authentication of Six Glycyrrhiza Hybrids (Root Sticks)
| sample code | DNA markers
|
TGc | claimed G. species | identified G. hybridsd | origin | |||
|---|---|---|---|---|---|---|---|---|
| ITSa,b | rbcL | matK | psbA-trnH | |||||
| BC750 | I-3/I-2 = ADD (14%) | R-1 | M-1 | T-2 | TG7 (cp) or TG3 (cp) | uralensis | G. uralensis × G. glabra | China, Xinjiang |
| BC752 | I-2/I-3 = ADD (12%) | R-1 | M-2 | T-3 | TG5 (cp) | inflata | G. uralensis × G. inf lata | China, Xinjiang |
| BC758 | I-3/I-2 = ADD (13%) | R-1 | M-2 | T-3 | TG5 (cp) | “Gan-cao” | G. uralensis × G. inf lata | China |
| BC772a | I-2/I-3 = ADD (≥86%) | R-1 | M-2 | T-3 | TG5 (cp) | inflata | G. uralensis × G. inf lata | China, Xinjiang |
| BC782c | I-2/I-3 = ADD (14%) | R-1 | M-2 | T-3 | TG5 (cp) | inflata | G. uralensis × G. inf lata | unknown |
| BC782d | I-2/I-3 = ADD (7%) | R-2 | M-1 | T-1 | TG8 (cp) | inflata | G. uralensis × G. inflata | unknown |
The occurrence of both I-2 and I-3 sequences for the ITS DNA marker is called ADD genotype and corresponds to “nucleotide additivities” often observed in hybrids between two species.27
The ITS ADD genotype is given as a ratio, with the primary genotype highlighted in bold, and the average secondary peak height ratio is given in parentheses.
Total genotype (TG) determined by the composition in DNA markers.
Genotype identified from the chloroplast DNA and corresponding to the putative maternal species (highlighted in bold).
In light of the undifferentiated pharmacopeial definitions of licorice, commercial BDSs are likely to represent mixtures of the three Glycyrrhiza species or even contain other plant species as adulterants. In order to evaluate the quality of the authentication and classification methods in the present study, in-house mixtures were prepared from powders of genetically uniform DNA-authenticated species. All mixtures were characterized by the proportional quantity (weight %) of each authenticated species (Table 4). The mixtures were DNA identified through the observations of secondary peaks on the nucleotide sequence chromatogram at variable species-specific nucleotides for all four DNA markers (Figure 1C). When mixing occurs between samples that differ at the species-specific nucleotides of chloroplast loci, the DNA sequencing traces for those nucleotides will show secondary peaks to some degree. Secondary peak height ratio, provided by Sequencher v5.2, was used to determine primary (1°) and secondary (2°) genotypes in both hybrid and mixture cases (Tables 3 and 4).
Table 4.
DNA Analysis of Commercial (Bulk Powders, Capsules) and in-House Glycyrrhiza Mixtures
| BC code | TG(s) detecteda | claimedb Glycyrrhiza species | DNA detected species | chemically identified species and relative abundancec | |
|---|---|---|---|---|---|
| commercial | 625 | TG5 (1°), TG7 (2°), possibly TG3 (2°) | G. glabra | G. inflata, G. uralensis, possibly G. glabra | G. inflata ≥ G. uralensis ≈ G. glabra |
| 686 | TG3 (1°), TG8 (2°) | G. glabra | G. glabra, G. uralensis | G. glabra > G. uralensis | |
| 741 | TG5 (1°), TG7 (2°), possibly TG3 (2°) | “licorice” | possibly all three G. species | G. uralensis > G. inflata | |
| 742 | TG5 (1°), TG8 and/or TG7 (2°), possibly TG3 and/or TG2 (2°) | possibly all three G. species | G. glabra > G. inflata ≈ G. uralensis | ||
| 744 | TG5 (1°), TG8 (2°), possibly TG3 (2°) | possibly all three G. species | G. uralensis ≈ G. glabra > G. inflata | ||
| 733 | TG3 (1°), TG9 (2°) | G. glabra | G. glabra, G. uralensis | G. glabra > G. uralensis | |
| 734 | TG2† (1°), TG3 (2°) | G. glabra, or G. inflata | mixture of G. glabra genotypes | ||
| 736 | TG5 (1°), TG8 (2°; 200 bp primers) | G. inflata, G. uralensis | G. inflata | ||
| in-housed | Mix 1 | TG3 and TG8 | TG3 50%, TG8 50% | G. glabra, G. uralensis | G. glabra ≈ G. uralensis |
| Mix 2 | TG3 and TG8 | TG3 5%, TG8 95% | G. glabra, G. uralensis | G. uralensis | |
| Mix 4 | TG3 and TG8 | TG3 1%, TG8 99% | G. glabra, G. uralensis | G. uralensis | |
| Mix 5 | TG3, TG5, and TG8 | TG5 33%, TG8 33%, TG3 33% | all three G. species | G. inflata ≥ G. glabra ≈ G. uralensis | |
| Mix 6 | TG3, TG5, and TG8 | TG5 70%, TG8 25%, TG3 5% | all three G. species | G. inflata > G. glabra ≈ G. uralensis | |
| Mix 8 | TG3, TG5, and TG8 | TG5 9%, TG8 90%, TG3 1% | all three G. species | G. uralensis > G. inflata | |
| Mix 10 | TG3 and TG5 | TG5 10%, TG3 90% | G. glabra, G. inflata | G. glabra ≫ G. inflata | |
Primary and secondary genotypes of mixtures were determined from secondary peak height ratio of markers/alleles that specify a particular species. Some total genotypes of commercial samples were considered “possibly” present since markers are shared across total genotypes.
For the commercial mixtures, the claimed species corresponds to the label indication.
Preponderant Glycyrrhiza species characterizing the mixture identified by both SIMCA and CDA classifications.
All in-house mixtures were prepared with DNA authenticated Glycyrrhiza species of known genotype. The percentage corresponds to the quantity of powder from each species mixed together prior to performing the DNA analysis.
Considering that the sample requirements for DNA extraction are rather small, the variability of DNA barcoding results is inevitably linked to the homogeneity of the powder mixtures. Thus, sampling representativeness has to be considered as a key factor when analyzing mixtures. Results obtained from the typical 50–100 mg of powdered sample might not represent an accurate composition of the one pound or larger batch, from which the sample was taken. In light of this variability, all DNA extractions from the prepared mixtures, amplifications, and sequencing were performed several times in order to avoid misinterpretation. With the genotype combination, and knowing that one species is characterized by at least two genotypes, it was possible to detect and identify Glycyrrhiza species, even when present at only ~1% w/w in the mixture.
Among the 51 acquired licorice samples, 11 (~21%) turned out to be misidentified Glycyrrhiza species; eight (~16%) were found to be mixtures of Glycyrrhiza species (Table 1); and the majority (37; 72%) were authenticated as being composed of one single Glycyrrhiza species (Table 2). The 11 misidentified samples comprised six hybrids (Tables 1 and 3) and three misidentified as G. inflata samples, which were authenticated as G. glabra (BC748), G. uralensis (BC629 and BC778), and one Glycyrrhiza mixture (BC736). Most of the mixtures were sold as BDS capsules or powders and were found in both U.S. and E.U. markets. Interestingly, their BDS label indicated either G. glabra or simply “licorice”, while lacking any species identification. Among the 37 samples containing single Glycyrrhiza species (Table 2), three were identified as being G. inflata (TG5), 13 belonged to the species G. uralensis (TG7–9), and 21 were G. glabra (TG2, 3). As Kondo and coworkers reported,27 the TG4 and TG6 genotypes are rare variants and, accordingly, were not detected in the present study. Interestingly, among the seven claimed G. inflata samples, only three were identified as pure G. inflata (TG5 genotype), while the others were found to be G. uralensis, a plant cultivated in the same region in China, or G. glabra, known to be also cultivated in Afghanistan, but also Glycyrrhiza hybrids (Tables 1 and 3). Moreover, when sold as BDSs, G. inflata was mostly found in mixtures with other Glycyrrhiza species. These observations collectively suggested that G. inflata is not readily available as pure single species and its sourcing requires working closely with local growers. G. inflata grows naturally in the northwest of China in the Xinjiang Uygur Autonomous Region36–38 and in Afghanistan.27 The relatively harsh natural geographical habitat, additionally subject to political tensions, combined with an increased likelihood of hybridization with G. uralensis might contribute to the difficulties in sourcing authentic G. inflata.
Table 1.
Summary of DNA Authentication Results for the 51 Acquired Licorice Samples
| among the 51 acquired licorice samples | BC code | claimed species | DNA authentication results |
|---|---|---|---|
| 11 misidentified Glycyrrhiza species, 21.5% of all samples | 629 | G. inflata | G. uralensis |
| 778 | G. inflata | G. uralensis | |
| 748 | G. inflata | G. glabra | |
| 716 | G. glabra | G. uralensis | |
| 750 | G. uralensis | G. uralensis × G. glabraa | |
| 752 | G. inflata | G. uralensis × G. inf lataa | |
| 758 | Gan-cao | G. uralensis × G. inf lataa | |
| 772a | G. inflata | G. uralensis × G. inf lataa | |
| 782c | G. inflata | G. uralensis × G. inf lataa | |
| 782d | G. inflata | G. uralensisa × G. inflata | |
| 736 | G. glabra | mixture: G. inflata and G. uralensis and other plant species | |
| Eight samples acquired as “licorice” or G. glabra, but identified as mixtures of Glycyrrhiza species, 15.7% of all samples | 625 | G. glabra | mixture: G. inflata and G. uralensis or G. glabra |
| 686 | G. glabra | mixture: G. glabra and G. uralensis | |
| 741 | Licorice | mixture: G inflata with G. uralensis or G. glabra | |
| 742 | Licorice | mixture: 3 G. species are present | |
| 744 | Licorice | mixture 3 G. species are present | |
| 733 | G. glabra | mixture: G. glabra with G. uralensis | |
| 734 | G. glabra | mixture: G. glabra with potentially G. inflata | |
| 736 | G. glabra | mixture: G. inflata and G. uralensis and other plant species |
Genotype identified from the chloroplast DNA and corresponding to the maternal species (highlighted in bold).
Table 2.
DNA Authentication of 37 Samples Containing Single Glycyrrhiza Species
| Glycyrrzhiza species | code | DNA markers
|
TGa | claimed G. species | originb | |||
|---|---|---|---|---|---|---|---|---|
| ITS | rbcL | matK | psbA-trnH | |||||
| uralensis | 629 | I-3 | R-2 | M-1 | T-4 | TG9 | inflata | China |
| uralensis | 725 | uralensis | China | |||||
| uralensis | 751 | uralensis | Mongolia | |||||
| uralensis | 754 | uralensis | Mongolia | |||||
| uralensis | 755 | uralensis | Vietnam | |||||
| uralensis | 760 | Gan-cao | China | |||||
| uralensis | 778 | I-3 | R-2 | M-1 | T-1 | TG8 | inflata | China |
| uralensis | 716 | glabra | China | |||||
| uralensis | 624 | uralensis | China | |||||
| uralensis | 689 | uralensis | China | |||||
| uralensis | 759 | “Gan-cao” | China | |||||
| uralensis | 753 | I-3 | R-1 | M-1 | T-2 | TG7 | “Gan-cao” | Vietnam |
| uralensis | 756 | “Gan-cao” | China | |||||
| glabra | 289 | I-2 | R-1 | M-1 | T-2 | TG3 | glabra | unknown |
| glabra | 694 | Italy | ||||||
| glabra | 693 | Italy | ||||||
| glabra | 595 | IL, US | ||||||
| glabra | 727 | MA, US | ||||||
| glabra | 728 | MA, US | ||||||
| glabra | 729 | MA, US | ||||||
| glabra | 730 | WA, US | ||||||
| glabra | 731 | MA, US | ||||||
| glabra | 749 | Afghanistan | ||||||
| glabra | 743 | “licorice” | EU | |||||
| glabra | 745 | Iran | ||||||
| glabra | 757 | “Gan-cao” | China | |||||
| glabra | 747 | glabra var. glabra | Turkey, Nizip | |||||
| glabra | 748 | inflata | China, Xinjiang | |||||
| glabra | 044 | I-2 | R-1 | M-1 | T-1 | TG2c | glabra | unknown |
| glabra | 726 | I-2 | R-1 | M-1 | T-1d | TG2c | Egypt | |
| glabra | 732 | Egypt | ||||||
| glabra | 735 | Egypt | ||||||
| glabra | 695 | Egypt | ||||||
| glabra | 746 | glabra var. glabra | Turkey, Konya | |||||
| inflata | 711 | I-2 | R-1 | M-2 | T-3 | TG5 | inflata | China |
| inflata | 772b | I-2 | R-1 | M-2 | T-3 | TG5 | inflata | China, Xinjiang |
| inflata | 774 | I-2 | R-1 | M-2 | T-3 | TG5 | inflata | China, Xinjiang |
Total genotype (TG) determined by the composition of DNA markers.
Geographical origin of the sample when documented.
The “TG2” genotype can correspond to either G. inflata or G. glabra according to Kondo’s paper.27 “Gan-Cao”: Traditional Chinese medicine containing licorice.
With a novel “CTCTT” deletion.
In summary, DNA authentication was employed as an essential qualitative step of botanical verification and yielded three groups of samples: single species, hybrids, and mixtures of Glycyrrhiza species. Notably, and by a significant margin, DNA-based species identification does not suffice for the assessment of botanical quality, especially because bioactive compounds are mostly small metabolites rather than DNA molecules. Moreover, a given genotype does not necessarily determine the phenotype and overall plant metabolome. In addition, G. glabra and G. inflata samples share the same TG2 genotype (Figure 1, Table 2). The authentication of Glycyrrhiza species belonging to the TG2 genotype alone will, therefore, require the implementation of orthogonal methods based on metabolite profiling. The present results demonstrated that an initial DNA authentication step was still crucial for the selection of 37 samples containing single Glycyrrhiza species in order to perform the following chemometric analyses.
Chemometric Classification of DNA-Authenticated Glycyrrhiza Species
The DNA-authenticated samples were extracted with 95% EtOH using ASE 350 instrumentation. This reproducible and standardized extraction protocol allowed for a rapid processing of several samples simultaneously, which is a fundamental requirement of any comparative metabolite profiling study. As suggested previously,20 the use of EtOH as solvent yielded extracts enriched in phenolic metabolites and depleted in highly polar metabolites. Each licorice material was extracted twice, when possible, and each extract was analyzed in duplicate, in order to evaluate both sample representativeness and reproducibility of the chemical profiles.
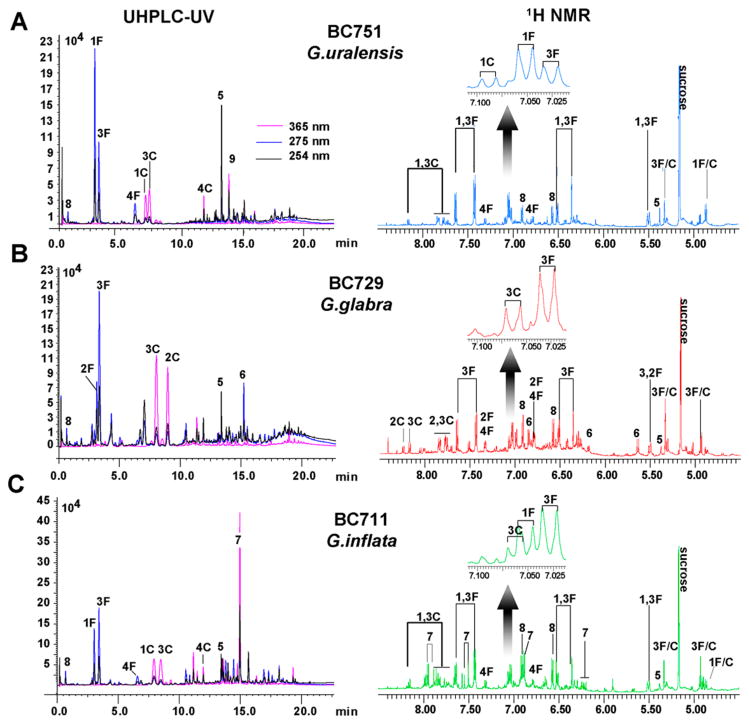
Two complementary analytical techniques were used for metabolite profiling: one spectroscopic, 1D 1H NMR, and one chromatographic, UHPLC-UV. The NMR technique offers a holistic view of the extract composition, allowing the simultaneous detection of UV-visible and -transparent metabolites. To enhance reproducibility, all spectra were acquired under quantitative conditions (qHNMR).39 The UHPLC-UV technique enabled a rather more targeted analysis of principal UV–visible metabolites such as the major F/C pairs (pairs 1–4), glycyrrhizin (5), glabridin (6), licochalcone A (7), the recently identified p-hydroxybenzylmalonic acid HBMA (8), and glycycoumarin (9).40 The major investigated F/C pairs were as follows: liquiritin (1F) and isoliquiritin (1C) form the first F/C pair (F/C pair 1); F/C pair 2 is defined by liquiritigenin 7-O-apiosylglucoside (2F) and licuraside (2C); liquiritin apioside (3F) and isoliquiritin apioside (3C) form F/C pair 3, and F/C pair 4 consists of liquiritigenin (4F) and isoliquiritigenin (4C). With regard to their analytical sensitivity,41 both 1H NMR and UHPLC-UV techniques will principally detect the more concentrated compounds (low μM range). Both analyses characterize and classify Glycyrrhiza extracts according to their major to moderately abundant metabolites (Figure 2).
Figure 2.
Representative UHPLC-UV chromatograms and 1H NMR spectra of extracts from individual Glycyrrhiza species. Comparative UHPLC-UV chromatograms and 1H NMR (4.50–8.50 ppm) spectra of representative extracts from G. uralensis (A), G. glabra (B), and G. inflata (C). The 1H NMR spectra were acquired under quantitative conditions in DMSO-d6 (+5% v/v D2O) at 600 MHz. The assignments were determined with the NMR spectra of reference standards (Supporting Information S12). The NMR spectra were directly subjected to PCA and SIMCA. The AUC values obtained for 1–4F and 1–4C from the chromatograms were used to determine the composition of each extract in major F/Cs in order to perform CDA. The numbers attributed to UHPLC-UV peaks and 1H NMR signals follow those listed in Table 5 and Scheme 1.
NMR-Based Metabolomic Analysis
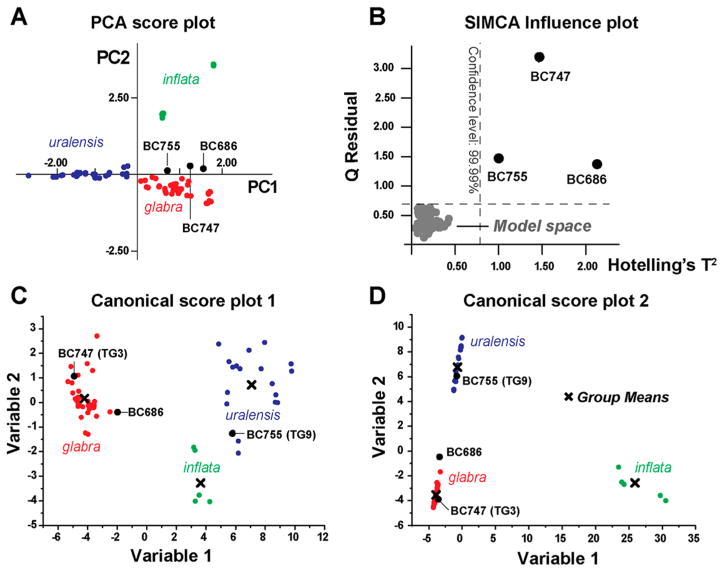
Multivariate statistical analysis with PCA has been performed previously with either 1H NMR spectra or MS data for the differentiation of botanically and macroscopically authenticated Glycyrrhiza species.22–24 However, as demonstrated above, macroscopic identification is insufficient to detect Glycyrrhiza hybrids. All licorice roots/rhizomes are similar macroscopically and microscopically. A potential botanical misidentification, in particular with G. inflata samples, could lead to misinterpretation or erroneous classification. Hence, our PCA analyses included only DNA-authenticated samples containing single Glycyrrhiza species. This statistical analysis was chosen for its capacity to group spectra according to their similarities without using the knowledge of sample class. Accordingly, PCA performed with 1H NMR spectra generated an unbiased clustering among chemically similar samples and produced an untargeted classification of the plant extracts. The PCA was performed with the AMIX software, applying rectangular bucketing on processed qHNMR spectra, and a final Pareto scaling, giving weight to minor signals.42 The first PCA result was obtained considering the full NMR spectrum (Supporting Information S2) and excluding only the solvent signals (DMSO-d5, HOD, EtOH residue), the sugar region, and the proline signals.40 The second PCA used only the downfield region from 4.50 to 8.50 ppm, without the sucrose anomeric proton signal at 5.169 ppm (d, J = 3.75 Hz).40 Both PCA models excluded the chemical fluctuation linked to the presence of sucrose and proline, as their concentrations are known to be related to the conditions of cultivation, drought, and time of harvest.43,44 Therefore, those metabolites were regarded as nonspecific for the distinction of Glycyrrhiza species. Interestingly, the score and loading plots obtained with both models (full spectrum vs downfield region) led to similar clustering results (Supporting Information S3–4), suggesting that the high-field NMR signals were less discriminant. Using the downfield region only, PC1 and PC2 together accounted for 58.2% of the metabolomic variance, compared to 47.2% when considering the full NMR spectrum. In light of these observations, the PCA model associated with the NMR downfield region was selected. The score plot in Figure 3A shows three distinct clusters, corresponding to the three DNA-authenticated species, and distributed over three opposite regions. By comparing the NMR signals (variables as buckets) in the loading plots in Figure 3B with the results in the score plot, discriminating variables were identified as being responsible for the sample differentiation. As expected, the signal corresponding to the omnipresent licorice compound 5 (H-12, s, at 5.38 ppm) was not discriminant and did not lead to species differentiation. Without considering the anomeric protons of the glycosidic moiety, and due to extensive signal overlap, the differentiation of 1F and 3F in the crude extracts required attention to detail (Table 5, Figure 2). However, the H-3′/5′ proton signals were slightly shifted, with the protons of resonating at 7.052 ppm vs those of 3F at 7.028 ppm. The anomeric protons from the glycosidic moiety of the major F/C pairs, as well as the aromatic signals of 8 (buckets at 6.56 and 6.92 ppm), clearly contributed to the differentiation between G. glabra and G. inflata on one hand vs G. uralensis on the other. The anomeric glucose proton of F/C pair 1 was included in the bucket at 4.88 ppm (d, J = 7.46 Hz), whereas the anomeric proton from the apiose moiety of the F/C pair 3 was found at 5.32 ppm (d, J = 1.20 Hz). Regarding the identification of discriminative variables, this led to the conclusion that NMR signals corresponding to the F/C pair 1 and to compound 4F (buckets at 5.48, 6.48, and 7.40 ppm) determined the group formed by G. uralensis extracts. The major variables enabling the distinction of G. glabra samples were signals from the apiose anomeric proton, the F/C pair 2 (bucket centered at 8.24 ppm of 2C), the signal from 3F (bucket centered at 7.00 ppm), and signals from glabridin (6) derivatives (Table 5, Figures 2 and 3). Finally, the distinct aromatic signals corresponding to 7 led to the clustering of G. inflata samples. As shown in the score plot, this species displayed a unique 1H NMR chemical profile, forming a group independent from the close clustering observed between G. glabra and G. uralensis. Interestingly, both 6 and 7 were clearly detectable by NMR and UHPLC-UV, thereby suggesting their relative abundance in the corresponding Glycyrrhiza extracts (Figure 2). In contrast, the 1H NMR conditions did not lead to the detection of the G. uralensis-specific compound, glycycoumarin (9). This observation can be explained by two factors: the 1H NMR spectrum of 9 does not offer characteristic isolated signals within the complex spectrum of G. uralensis extract, and 9 is present at a lower level compared to 6 and 7.
Figure 3.
PCA using quantitative 1H NMR (qHNMR) spectra of DNA-authenticated Glycyrrhiza species. PCA score plot (A) obtained from the 1H NMR spectra of all DNA-authenticated Glycyrrhiza species and considering only the downfield region from 4.50 to 8.50 ppm. The two major PCs together explained 58.2% of the total metabolomic variance. The three species were differentiated according to their major secondary metabolites. Their discriminant chemical shifts (δ in ppm) are shown on the 2D loading plot (B). The latter represents (B) a total of 84 buckets of 0.04 ppm width and explained the major trends, in terms of NMR signals, leading to samples clustering in the score plot (A). G. glabra samples from Iran and Afghanistan, on one hand, and samples from Egypt, on the other hand, formed the two extremities of the G. glabra cluster, as seen in (A).
Table 5.
Characteristic Downfield NMR Data of Major Detectable Metabolites in Glycyrrhiza Extracts (DMSO-d6 with 5% v/v D2O and 2.05 mM of Calibrant, at 600 MHz)
| compound | no. | position | δH, mult. (J in Hz)a |
|---|---|---|---|
| liquiritin | 1F | H-3′/5′ | 7.052, XX′ type (8.58/2.46/0.31) |
| liquiritin apioside | 3F | H-3′/5′ | 7.028, XX′ type (8.58/2.46/0.31) |
| liquiritin and liquiritin apioside | 1/3F | H-5 | 7.637, d (8.67/0.16) |
| H-2′/6′ | 7.446, AA′ type (8.58/2.46/0.31) | ||
| H-6 | 6.511, dd (8.67/2.21) | ||
| H-8 | 6.354, d (2.21) | ||
| H-2 | 5.507, dd (12.84/2.91) | ||
| liquiritin-isoliquiritin | 1F, 1C | 1″-O-Glc | 4.936, d (7.46) |
| liquiritin apioside and isoliquiritin apioside | 3F/3C | 1″-O-Glc | 4.957, d (7.75) |
| 1″-O-Api | 5.328, d (1.20) | ||
| isoliquiritin and isoliquiritin apioside | 1/2C | H-6′ | 8.166, dd (8.93/0.53) |
| H-2/6 | 7.838, AA′ type (8.70/2.04/0.18) | ||
| H-β | 7.830, d (15.44) | ||
| H-α | 7.740, d (15.44) | ||
| H-3/5 | 7.063, XX′ type (8.70/2.04/0.18) | ||
| H-3′ | 6.296, d (2.33) | ||
| licuraside | 2C | H-6′ | 8.240, d (9.02) |
| H-3/5 | 6.848, XX′ type (8.51/2.72/0.15) | ||
| H-5′ | 6.598, dd (9.02/2.41) | ||
| H-3′ | 6.547, d (2.41) | ||
| liquiritigenin-7-O-apiosylglucoside | 2F | H-2′/6′ | 7.340 AA′ type (8.14/ 1.81/0.33) |
| H-3′/5′ | 6.795 XX′ type (8.14/1.81/0.33) | ||
| liquiritigenin | 4F | H-2′/6′ | 7.332 AA′ type (8.41/2.63/0.33) |
| H-3′/5′ | 6.822, XX′ type (8.41/2.63/0.33) | ||
| H-6 | 6.486, dd (8.68/2.18) | ||
| H-8 | 6.315, d (2.18) | ||
| glycyrrhizin | 5 | H-12 | 5.381, s |
| glabridin | 6 | H-6′ | 6.848, d (8.21) |
| H-5 | 6.827, d (8.32) | ||
| H-4″ | 6.574, d (9.89) | ||
| H-3″ | 5.635, d (9.89) | ||
| H-3′ | 6.313, d (2.42) | ||
| H-6 | 6.268, d (8.21) | ||
| H-5′ | 6.183, dd (8.32/2.42) | ||
| licochalcone A | 7 | H-2′/6′ | 7.949, AA′ type (8.59/2.43/0.27) |
| H-β | 7.870, d (15.54) | ||
| H-α | 7.557, d (15.54) | ||
| H-3 | 6.526, s | ||
| H-3′/5′ | 6.887, XX′ type (8.59/2.43/0.27) | ||
| H-6 | 7.526, s | ||
| H-2″ | 6.221, dd (17.49/ 10.62) | ||
| HBMA | 8 | H-2′/6′ | 6.911, AA′ type (8.33/2.65/0.32) |
| H-3′/5′ | 6.574, XX′ type (8.33/2.65/0.32) |
Exact values obtained through 1H iterative full-spin analysis (HiFSA) using Perch NMR software (v.2011.1, PERCH Solutions Ltd., Kuopio, Finland).
Using PCA, the authenticated samples with TG2 genotype were chemically identified as G. glabra. Within the cluster formed by the G. glabra samples, three groups were observed. Samples containing proportionally more 3F, while depleted in 6, were closest to the G. uralensis group on the score plot. When indicated, these samples (BC695, BC726) originated from Egypt. G. glabra samples from Iran and Afghanistan clustered at the other extremity of this species group (Figure 3). Such observations were in agreement with the results obtained by Farag and co-workers, who also observed that G. glabra samples from Egypt clustered apart from those originating from Syria and Afghanistan.23 Further analyses of the qHNMR spectra suggested that samples BC745, BC749, and BC757 were characterized by a higher proportion of the F/C pair 2, with proportionally more Cs than Fs compared to other G. glabra samples (Supporting Information S5).
In conclusion, the PCA analysis performed with 1H NMR data enabled the metabolomic differentiation/classification of DNA-authenticated Glycyrrhiza species according to their composition in major F/C pairs and the presence of the detectable species-specific metabolites 6 and 7.
UHPLC-UV-Based Metabolomic Analysis
As a second approach, an orthogonal UHPLC-UV analysis was performed to determine whether all Glycyrrhiza extracts can be classified according to their composition in major F/C pairs, solely (Figure 4, Supporting Information S6), or whether their classification requires the additional consideration of species-specific metabolites. For this purpose, a methodology using only the AUC ratios of selected metabolites was developed. The AUC values for each F and for compound 6 were taken at 275 nm, whereas the AUCs for each C and for compounds 7 and 9 were measured at 360 nm. Each AUC value was divided by the total calculated AUC at each wavelength (Supporting Information S7). The total F/C ratio was then determined by dividing the total AUC275 nm by the total AUC360 nm. The results obtained for the 37 authenticated species demonstrated that they all displayed a unique F/C composition (Figure 4A). The F/C pairs 2 and 3 clearly characterized G. glabra extracts, whereas the F/C pairs 1 and 3 predominated in all the G. uralensis and G. inflata extracts. With regard to their major F/C pairs, G. uralensis extracts were similar to G. inflata. However, the total F/C ratio clearly indicated that G. glabra and G. inflata extracts have proportionally more C than F compared to G. uralensis samples (Figure 2, Figure 4A). Interestingly, the calculated ratios for samples originating from Iran and Afghanistan (BC745, BC749) revealed that the F/C pair 2 represented 50% of the total calculated F/C pairs, compared to the other G. glabra samples (28 ± 11%). Moreover the total F/C ratio determined for the species from Iran and Afghanistan was found to be the lowest (0.53 ± 0.03) of all G. glabra samples (0.74 ± 0.16). In conclusion, both the composition in major F/C pairs and the calculated F/C ratios can contribute to the authentication of Glycyrrhiza species.
Figure 4.
Comparative F/C composition and CDA of Glycyrrhiza species. Box charts (A) represent the proportion of each major F/C pair among all four considered F/C pairs and for each authenticated Glycyrrhiza species. The proportion was calculated from the AUC ratios at 275 nm (for Fs) and 360 nm (for Cs). Results were obtained with all EtOH extracts and expressed as mean ± standard deviation for each F/C pair. For the G. glabra samples, the box chart in gray represents the F/C composition of samples from Iran and Afghanistan, for which the F/C pair 2 represents 50% of all four F/C pairs. (B) The score plot 1 results from the CDA initially performed with the calculated F/C ratios and proportions of F/C pairs. The canonical score plot 2 in C was obtained after adding the AUC ratios of 6, 7, and 9 to the table used to generate the score plot 1.
The ratios representing the composition in F/C pairs 1–3, combined with the total F/C ratio, were utilized to perform a statistical classification by CDA (Figure 4B, Supporting Information S8). This multivariate analysis is used to distinguish sets of observations (e.g., extracts from authenticated Glycyrrhiza species) and allocate new observations to previously defined groups (e.g., species). Notably, such a method based on AUC ratios is independent from the retention time and from the status of the UV lamp. Therefore, UHPLC-UV-based CDA can integrate AUC ratios acquired at different times, on different columns and instruments, and does not rely on the generation of calibration curves, collectively promoting the universality of the proposed classification method.
The first canonical score plot (Figure 4B) confirmed the box chart results (Figure 4A). The integration of the AUC ratios from 5 taken at 254 nm ([AUC glycyrrhizin]/[total AUC]) did not alter nor optimize the classification results, thereby confirming again that the ubiquitous Glycyrrhiza metabolite 5 does not allow the distinction of Glycyrrhiza species. The canonical score plot 1 (Figure 4B) demonstrated the heterogeneity within all G. uralensis extracts regarding their F/C composition. These observations complement the results of Kondo and co-workers,19 who also noted the content variation of the F/C pair 1, as well as compounds 4F and 5, in their DNA-authenticated samples of G. uralensis. All G. glabra samples clustered independently of their geographical origins.
Recently Yu and co-workers45 demonstrated that the presence of 1F and 1C is genetically determined in G. uralensis, whereas their respective concentrations together with the abundance of 4F, 4C, and 5 are determined by environmental pressure. Despite the fact that the absolute concentrations of all principal F and C isomers have been shown to greatly fluctuate between samples,21,45–47 we demonstrate here that the proportionality between major F and C, as well as the composition in F/C pairs, remained stable between various samples of the same species. Hence, the F/C composition is unique for each of the three principal Glycyrrhiza species and can be considered as being determined genetically. This is the first study to show that pharmacopeial Glycyrrhiza species can be classified according to the proportionality of their major F/C pairs, regardless of the absolute concentration of each individual F and C.
In a subsequent step, the AUC ratios obtained for the species-specific compounds 6, 7, and 9 were added to the classification table, and a second CDA was performed (Figure 4C). The canonical score plot 2 offered an improved clustering of Glycyrrhiza species, thereby fostering their differentiation. Both canonical score plots were utilized for further identification and classification of Glycyrrhiza mixtures, hybrids, and outliers.
SIMCA and CDA for the Characterization of Outliers, Hybrids, and Mixtures
The second major objective of this study was to utilize the newly developed chemometric models for the classification and characterization of commercial licorice mixtures and Glycyrrhiza hybrids, in addition to the identification of potential outliers. The PCA result obtained with the downfield 1H NMR region (84 buckets) was validated to build a classification model by SIMCA. By analyzing the influence plot, the SIMCA classification indicated whether a sample/spectrum belonged to the Glycyrrhiza model (model space). In the influence plot, the y-axis represents the calculated residual variances (Q residual) between the analyzed spectrum and the model space, whereas the x-axis indicates Hotelling’s T2 scores, representing the distance of the analyzed spectrum to the model center. The score plots revealed the position of the sample relative to each species cluster (Figures 5–7A and B). The AUC ratios calculated from the UHPLC-UV data were incorporated into each CDA table in order to classify these samples according to their F/C composition (score plot 1, Figures 5–7C), with and without considering species-specific compounds (score plot 2, Figures 5–6D). The CDA classification results automatically attributed each observation/sample within a predetermined group, whereas the canonical score plots reflected the overall chemical composition and trends.
Figure 5.
SIMCA and CDA characterization of Glycyrrhiza outliers. Classification results for Glycyrrhiza outliers with (A) the PCA score plot and (B) the SIMCA influence plot. The Q residuals plotted on the y-axis of B represent the calculated residual variance of the considered spectra from the model space. The influence plot revealed that samples BC686, BC755, and BC747 are outliers: they are outside the model space, above each confidence line (Supporting Information S9). (C and D) Canonical discriminant plots for the classification of Glycyrrhiza outliers. The targeted CDA classification considers only the F/C composition (C) and the presence of species markers (D) and, therefore, did not identify outliers, but rather confirmed the species identity of each sample.
Figure 7.
SIMCA and CDA classification of mixtures. (A, B) PCA score plots from SIMCA classification results obtained for in-house (A) and commercial (B) mixtures. The canonical score plots (C) for in-house and (D) for commercial mixtures demonstrated that the F/C composition determined for each sample led to the identification of the preponderant Glycyrrhiza species. Samples containing >50% w/w of a given species were classified within the same group species (mix 2, 4, 8, and 10). Samples BC733, BC734, and BC742 contained >50% of G. glabra. Mix 5, Mix 6, and BC625 were similarly classified in both PCA and CDA score plots, suggesting that >30% w/w of the BC625 mixture is composed of G. inflata. According to this chemometric classification, BC741 seemed to contain >50% w/w of G. uralensis and ≤30% w/w of G. inflata, whereas BC744 would contain ≥50% w/w of G. uralensis, then ≤50% of G. glabra, and <30% w/w of G. inflata (Supporting Information S11).
Figure 6.
SIMCA and CDA classification of DNA-authenticated hybrids. (A, B) Classification results for Glycyrrhiza hybrids by 1H NMR/SIMCA analysis. The PCA score plot indicates that BC752 and BC758 displayed a 1H NMR profile equivalent to the samples in the G. uralensis group, whereas BC750 was more closely related to G. glabra, and BC782c/d as well as BC772a clustered in the group formed by G. inflata (Supporting Information S10). (C, D) Classification score plots of Glycyrrhiza hybrids by CDA using the F/C composition (C) and considering additionally species-specific compounds (D).
Three Species Outliers Were Identified by 1H NMR/SIMCA
The samples BC747, BC755, and BC686 were DNA authenticated as being G. glabra, G. uralensis, and a mixture of G. glabra and G. uralensis, respectively, as shown in Figure 5 as well as Tables 2 and 4. According to the SIMCA classification, all three samples fell outside the model space, above both confidence lines, and, thus, were characterized as outliers (Figure 5B). Both canonical score plots, either when using solely the information from their F/C composition or the species-specific compounds, confirmed the DNA identification of each sample (Figures 5C and D). This outcome illustrates that both chemometric models are complementary: CDA can confirm the species identity, whereas SIMCA can facilitate the identification of species outliers with unusual chemical profiles.
Further analyses of qHNMR spectra and UHPLC-UV chromatograms highlighted the occurrence of major chemical differences compared to the other confirmed Glycyrrhiza species (Supporting Information S9). For example, G. uralensis sample BC755 did not contain any detectable polar primary metabolites such as sucrose, while 4F was found to be particularly abundant. G. glabra sample BC747 was characterized by a high concentration of glycyroside [formononetin-7-O-apiosyl-(1→2)-glucoside], with a proportionally lower concentration of the F/C pairs 2 and 3. The Glycyrrhiza mixture BC686 was characterized by a remarkably high concentration of phloretic acid and 5, while being proportionally depleted in both Fs and Cs.
SIMCA and CDA Classification of Glycyrrhiza Hybrids
The SIMCA and CDA plots in Figure 6 demonstrate that all DNA-authenticated hybrids had distinct metabolite profiles. The G. uralensis × G. inflata hybrid, BC782c, had an F/C composition comparable to G. glabra and, together with the hybrids BC782d, contained compound 7, which is G. inflata specific. Inversely, BC752, BC758, displayed an F/C composition comparable to G. uralensis, but compound 7 was not detected, and compound 9 was only found in BC752. The sample BC750 determined to be a G. glabra × G. uralensis hybrid was classified within the G. glabra group due to its characteristic F/C composition and despite the absence of 6. The SIMCA results indicated that samples BC782c and d, BC772a, and BC750 displayed significant off-model space components due to minor differences in their NMR signals, which were not present in the NMR spectra of other Glycyrrhiza samples within the model space. Nevertheless, prior DNA authentication was found to be a prerequisite to make both chemometric models suitable for the characterization of Glycyrrhiza hybrids.
Analysis of Licorice Mixtures via in-House Mixture Validation
In order to better understand the results achievable with commercial mixtures (Figure 7), licorice mixtures of known composition (Table 4) were analyzed by SIMCA and CDA. Both chemometric models revealed that mixtures were classified according to their most abundant species. When G. uralensis (Mix 1, 2, and 4) and G. glabra (Mix 1, 8, and 10) were present at >50% w/w in the mixture, and G. inflata was present at >30% w/w (Mix 5 and 6), the samples fell within or close to their corresponding species groups. G. inflata displayed a sufficiently unique chemical profile to be detected chemically at a lower percentage in a mixture (30% vs 50%). On both PCA and CDA score plots, the comparison of the positions obtained for in-house mixtures with those of commercial mixtures enabled the identification of the second most preponderant species. Samples BC625, Mix 5, and Mix 6 could be classified similarly, revealing that the first most abundant species in BC625 is G. inflata, the second is G. glabra, closely followed by G. uralensis (Figure 7, Table 4). Samples BC741 and BC744 were classified similarly to Mix 8 and Mix 1, respectively. Therefore, the most abundant species in BC741 was found to be G. uralensis in both samples (>50% w/w), followed by G. inflata and G. glabra. Samples BC733 and BC734 were also classified as G. glabra. Sample BC734 was identified as a mixture of G. glabra genotypes (TG2 and TG3), whereas BC733 contained >50% w/w of G. glabra (TG3). Interestingly, the mislabeled sample BC736 was considered as chemically close to G. inflata by both statistical models and contained a remarkable amount of 7. Notably, this sample was sold in the U.S. market without containing any of the recognized U.S. pharmacopeial Glycyrrhiza species, i.e., G. glabra and G. uralensis.17,18
Summary and Outlook
In conclusion, this study proposes a metabolomic approach for the classification and characterization of three Glycyrrhiza species, which are widely accepted source plants for the production of BDSs. Complementary multivariate analyses adapted to two analytical platforms, NMR and LC, were utilized for this purpose. To ensure high specificity, only DNA-verified samples containing a single species were employed to build the classification models.
Offering an unbiased and holistic analytical overview of the licorice metabolite composition, 1H NMR spectra were utilized for PCA and SIMCA and led to the untargeted classification of licorice samples. In parallel, UHPLC-UV was employed for LC-based classification, taking advantage of its inherently targeted characteristics, aimed at UV–visible compounds. The outcomes show that results obtained from chromatographic systems are less reproducible than those obtained by NMR, which is evidently due to the variations associated with retention time and intensity of UV absorbance. To overcome such limitations, an LC method using only the AUC ratios was developed. This led to the establishment of a CDA method that enabled a targeted classification of Glycyrrhiza species according to their unique F/C composition with and without considering species-specific compounds.
The newly developed chemometric models, which were built in agreement with the initial botanical authentication by DNA analysis, enabled the classification of licorice botanicals, confirmed the metabolite profiles of misidentified Glycyrrhiza species, and facilitated the metabolomic characterization of mixtures, species outliers, and hybrids. This approach is in line with the view of Harnly and co-workers that “the more we know about a sample, the easier it is to develop an accurate assay”.48 Hence, the present study provides the first comprehensive method that combines the use of botanical authentication by DNA barcoding prior to extraction, with orthogonal metabolomic analyses after extraction, for the rigorous classification and characterization of Glycyrrhiza species.
The present results also accentuate the existence of marked chemical differences between Glycyrrhiza species in terms of their major secondary metabolites. Various F/C pairs were identified as species markers in addition to the accepted species-specific glabridin, licochalcone A, and glycycoumarin. As expected, glycyrrhizin (5) is inadequate for species distinction. From a phytochemical point of view, variations in pharmacological/biological activities and potential herbal–drug interactions can be expected, not only for each species but also for their mixtures and hybrids.19,20,23 When licorice is used for medicinal purposes and in light of the distinct properties of its metabolites, it is important that the exact species is selected.19,23 However, studies comparing the biological properties of authenticated Glycyrrhiza species are rare.49,50 At the same time, this kind of study design is paramount for the adequate chemical and biological standardization.
In light of these observations and the results obtained herein, pharmacopeial and other monographs supporting the QC of “licorice” should consider a metabolomic characterization associated with the identification and quantitation of several key markers51 rather than the (quantitative) determination of a single ubiquitous Glycyrrhiza constituent, such as glycyrrhizin (5).
The present study also highlights the importance of building integrative botanical authentication measures. These should include DNA analysis of the raw plant material as well as metabolomic characterization of the extracts, which collectively can enhance the quality control process of botanicals. From a general point of view, the main role of DNA analysis is the botanical authentication of raw plant material, upstream of the industrial BDS production process, whereas NMR- and LC-based metabolomic analyses should primarily be implemented as a QC measure throughout the extraction and further processing chain for the assessment of the chemical composition. Hence, the QC of licorice botanicals requires that the data acquired from both botanical and analytical chemistry platforms are merged. In addition, classical methods employed for the botanical authentication of licorice raw material should be complemented by DNA analysis.
Considering the chemical diversity and complexity of Glycyrrhiza constituents, metabolomic and chemometric approaches that assess the compositional characteristics in terms of bioactive markers are more suitable for the QC of licorice extracts. To this end, the present study offers guidance for the implementation of a more comprehensive QC of pharmacopeial licorice materials and may inspire the development of more specific standardization protocols that enhance the integrity of widely used licorice preparations.
EXPERIMENTAL SECTION
General Experimental Procedures
All DNA preparations were checked using a Thermo Scientific NanoDrop 1000 spectrophotometer (Waltham, MA, USA). Polymerase chain reactions (PCRs) were performed with Big Dye Terminator v3.1 chemistry, and the amplicons were electrophoresed on an ABI 3730xl DNA analyzer (Life Technologies, Carlsbad, CA, USA). DNA sequence chromatograms were edited and analyzed with Sequencher v5.2 (Gene Codes, Ann Arbor, MI, USA). All ground Glycyrrhiza samples were extracted with an Accelerated Solvent Extraction (ASE 350) from Dionex Corporation (Sunnyvale, CA, USA). The extracts were dried using a Thermo-Fischer Savant SC250 EXP speed vacuum equipped with an RVT4104 refrigerator vapor trap. Freeze-drying was performed on a Labconco Freezone 4.5 (Kansas City, MO, USA). A precise Mettler Toledo XS105 dual range analytical balance was employed to prepare extracts for UHPLC and qHNMR analyses. The UHPLC analyses were performed on a Shimadzu UPLC, equipped with a Kinetex XB-C18 (2.1 × 5.0 mm, 1.7 μm, 00B-4498-AN, Phenomenex) column, and using a diode array detector (DAD, Shimadzu SPD-M20-A). The autosampler temperature was set at 4 °C, and the column oven temperature at 40 °C. Postrun data analyses were done with the Shimadzu LabSolution software package. CDA was performed with the OriginPro 9.1 software (Northampton, MA, USA). Samples for NMR analyses were prepared with a Pressure-Lock gas syringe (VICI Precision Sampling Inc., Baton Rouge, LA, USA) and calibrated glass pipets (cat. no: 2-000-200, Drummond Scientific, Broomall, PA, USA). Standard NMR tubes of 3 mm, 7 in., were from Norell (part no. S-3-HT-7, Norell Inc., Landisville, NJ, USA). NMR spectra were acquired on a Bruker AVANCE 600.13 MHz spectrometer equipped with a 5 mm TXI cryoprobe. Off-line data processing was performed using the Topsin 3.0.b.8 NMR software package. Multivariate data analyses, PCA, and SIMCA were performed with the AMIX statistics 3.9.14 software, both from Bruker Biospin Corporation (Karlsruhe, Germany).
Reagents
All chemicals and reagents including HPLC-grade solvents were obtained from Fischer Scientific (Hanover Park, IL, USA). Licorice standards glycyrrhizin, glabridin, and licochalcone A were obtained from Sigma-Aldrich (St. Louis, MO, USA). Glycycoumarin was purchased from BioBioPha Co., Ltd. (Kunming Institute of Botany, China). For NMR acquisition, DMSO-d6 (99.9% D) was purchased from Cambridge Isotope Laboratories Inc. (Andover, MA, USA). The PCR-grade tubes, tips, and most biological reagents used for DNA authentication, including the DNeasy Plant mini kit, were acquired from Qiagen (Valencia, CA, USA) and/or Thermo-Fisher Scientific and Beckman Coulter (Indianapolis, IN, USA). The BeadBug microtube homogenizer was purchased from Benchmark Scientific, Inc. (Edison, NJ, USA). The bovine serum albumin, BSA, was obtained from New Englands Biolabs (Ipswich, MA, USA). Seakem LE agarose for gel electrophoresis was purchased from Lonza (Rockland, ME, USA). The Ambion RNase-free water was acquired from Life Technologies.
Plant Material
A total of 51 licorice samples sold as bulk root powders, sticks, or capsules were either purchased online and from Chicago metropolitan area stores or acquired through our collaborative network. Sampling of BDS capsules was restricted to dried preparations (powdered plant tissue in gelatin capsules). To protect the manufacturer’s identity, the samples were reported with internal Botanical Center codes (Tables 1–4). Samples BC629, BC693–694, BC711, BC746–747, BC749, BC751–755, BC772, BC774, and BC778 were botanically/macroscopically verified prior to inclusion.
DNA Isolation
Tissue for DNA authentication was sampled and washed twice with 100% EtOH using aseptic techniques under sterile conditions, before homogenization to remove any loose debris or contaminants on the surface of the sampled tissue or powder. Total DNA was extracted with the DNeasy plant mini kit following the manufacturer’s recommendations with some modifications. For each sample, total DNA was extracted from approximately 50–100 mg of tissue. Tissue was disrupted with the BeadBug microtube homogenizer with 3 mM triple-pure zirconium beads. Concentration and purity of DNA (260/280 nm) were checked using NanoDrop.
Primers
Primer sequences were obtained directly from the literature or designed from licorice GenBank accessions. New modified primers were designed to produce larger amplicons than obtained by Kondo and co-workers,27 leading to a better quality of sequence for our Sanger DNA sequencing methods. Samples that did not initially amplify due to excessive DNA degradation required the use of primers that made smaller amplicons (<200 bp). Two sets of primers from Kondo and co-workers27 (rbcL and matK), as well as custom-designed primers for ITS and psbA-trnH, were utilized in PCR for smaller amplicons. An initial PCR testing of the ITS primers designed by White and co-workers33 (ITS5 5′-GGAAGTAAAAGTCGTAACAAGG-3′ and ITS4 5′-TCCTCCGCTTATTGATATGC-3′) showed some fungal interference due to rDNA sequence homology between fungi and plants. More plant-specific primers were designed based on the partial 5.8S alignment originally put forth by Oliveira de Miranda and co-workers34 with extra GenBank ITS1–5.8S–ITS2 accessions covering some of the major fungi and plant families (including licorice). The ITS primers designed from our alignment lay just internal to the primers by White and co-workers33 and were ITS-Fint 5′-TCGATGCCTTGCAAGCAGT-3′ and ITS-Rint 5′-AGAGCCCAAACTCAGTGGA-3′. The ITS-Fint and ITS-Rint primers make a PCR product of 585 bp in all three Glycyrrhiza species, whereas primers designed by White and co-workers33 made a PCR product of 729 bp. For the three chloroplast loci matK, rbcL, and psbA-trnH, the forward and reverse primers used, and the expected PCR product size for all species are as follows: matkF1 5′-GTGTCAGATATACGAATACC-3′ and matKR2 5′-TCCTTGAAGCCAGAATGG-3′ (430 bp for G. glabra and uralensis; 424 bp for G. inf lata TG5); rbcLF1 5′-CGCGCTCTACGTCTTGAGGA-3′ and rbcLR1 5′-GCGTGAATATGATCTCCACCAGA-3′ (593 bp); psbA-trnHF1 5′-ACGGGAATTGAACCCGCGCA-3′ and psbA-trnHR1 5′-CATATGACTTCACAATGTA-3′ (239 bp). We also used the psbA-trnH primers psbA3′f 5′-GTTATGCATGAACGTAATGCTC-3′ (Sang and coworkers52) with trnHf 5′-CGCGCATGGTGGATTCACAATCC-3′ (434 bp, Tate & Simpson53), and trnH (GUG) 5′-ACTGCCTTGATCCACTTGGC-3′ with psbA 5′-CGAAGCTCCATCTACAAATGG-3′ (362 bp, Hamilton54). Primers designed for degraded DNA samples were trnHF200 5′-ATCCACTTGGCTACATTCGC-3′ and trnHR200 5′-GTAATACATATGACTTCACAATG-3′ (198 bp); ITSYRM-F 5′-CGTGAACCATCGAGTCTTTGAACG-3′ and ITSYRM-R 5′-CTCAGTTTTGAGCCAACCGTG-3′ (180 bp); ITSY-F 5′-CGCTGAATGCGCCAAGGAAC-3′ and ITSY-R 5′-GTTCTTCATCGATGCAAGAGCC-3′ (141 bp). Primers were purchased from Integrated DNA Technologies.
PCR and Sequencing
Samples were PCR-amplified and bidirectionally sequenced for all four loci ITS, rbcL, matK, and psbA-trnH. Individual amplifications took place in a 30 μL volume containing 3 μL of Qiagen 10× PCR buffer (with 15 mM MgCl2), 0.8 μL of Promega deoxynucleotides (dNTPs, at 10 mM each), 0.2 μL of Qiagen HotstarTaq polymerase (5 U/μL), 2.5 μL of each primer (5 μM), and 1 μL of total DNA (5–50 ng/μL). The solutions were completed with up to 30 μL with Ambion RNase-free water. Approximately 25 ng of total DNA was added to each PCR. The addition of 300 ng/μL BSA greatly enhanced amplification of samples that repeatedly yielded impure DNA based on a low 260/280 nm ratio (<1.5). A PCR reagent master mix was made for each primer pair depending on the number of samples to amplify, and water controls (no template) were included in all PCR reactions for each primer pair. The general PCR cycling conditions were as follows: 94 °C for 15 min; 45 cycles: 94 °C for 20 s, 58 °C for 30 s, 72 °C for 50 s; 72 °C for 8 min and hold at 4 °C. PCR reactions that produced single clear bands were purified with the Qiagen PCR purification kit. The concentration and purity of each PCR product were determined using NanoDrop. Amplified PCR products were diluted to 20 ng/μL and cycle sequenced utilizing ABI Big Dye Terminator v3.1 chemistry, purified with the Agencourt CleanSEQ kit (Beckman Coulter), and then electrophoresed on an ABI 3730xl DNA analyzer, all according to manufacturer recommendations. All PCR primers were used as cycle sequencing primers. DNA sequence chromatograms were edited and analyzed with Sequencher v5.2. The PCR conditions for psbA-trnH were reformatted for total DNA preparations that showed poor results with the general PCR conditions.
GenBank Accessions for Primer Design and Reference
G. uralensis ITS (AB280738), matK (AB280741), rbcL (AB012126), G. glabra psbA-trnH (AB280745), rbcL (AB012126), G. inflata rbcL (AB012127), G. lepidota ITS (U50758 and U50759) rbcL (AB126685).
Sample Extraction
Each licorice sample was extracted using 2 g of material for 40 mL of 95% EtOH (USP 190 proof) utilizing the ASE 350 setup at 80 °C, for 30 min of static time at 1500 psi, for a total of 45 min of extraction. The extractions were performed in duplicate, when possible. After extraction, the plant material was washed with 10 mL of the same solvent. Each extract was first concentrated, then freeze-dried. Prior to NMR analysis, samples were dried under vacuum for 24 h in the presence of P2O5.
UHPLC-UV Analyses
Solutions of licorice extract were prepared at 10 mg/mL in HPLC-grade MeOH (2 μL injection). The Kinetex column was eluted with a gradient composed of H2O/0.1% formic acid (A) and MeCN/0.1% formic acid (B) as follows: 8% to 11% B in 2 min, and during 30 s, to 13% B at 4 min, to 15.5% B at 8 min, to 36% at 13 min, and during 30 s, to 80% B at 21 min, and during 1 min, back to 8% B at 23 min (flow rate: 0.7 mL/min). Under these conditions, the retention times (tR) of the licorice compounds were as follows: HBMA (8) 0.6 min, liquiritin (1F) 2.77 min, liquiritigenin 7-O-apiosylglucoside (2F) 3.08 min, liquiritin apioside (3F) 3.25 min, liquiritigenin (4F) 5.82 min, isoliquiritin (1C) 6.76 min, isoliquiritin apioside (3C) 7.11 min, licuraside (2C) 7.70 min, isoliquiritigenin (4C) 10.99 min, glycyrrhizin (5) 13.47 min, glabridin (6) 15.13 min, licochalcone A (7) 14.87 min, glycycoumarin (9) 13.77 min. All Fs and Cs as well as compound 8 were identified and/or isolated as previously described.20,26,40 Each extract was analyzed in duplicate during the same UHPLC sequence. Some extracts were analyzed a second time after 6 months in order to confirm the robustness of the classification method.
1H NMR Acquisition
NMR data acquisitions were performed using 3,5-dinitrobenzoic acid (DNBA, Fluka, TraceCERT, purity P = 99.54% w/w lot no. BCBH8381 V) as internal calibrant (IC). The IC stock solution was prepared at 6.15 mM in a mixture of D2O/DMSO-d6 (1.5:8.5). NMR samples of crude extracts were prepared by precisely weighing 7–8 mg of each extract, followed by the addition of 200 μL of DMSO-d6 and 100 μL of IC stock solution (2.05 mM final concentration). From this solution, a 200 μL aliquot was transferred with calibrated glass pipets into 3 mm standard NMR tubes. The 1D 1H NMR spectra were acquired at 298 K under quantitative conditions (qHNMR) using a 90° excitation pulse experiment (Bruker pulprog: zg). The 90° pulse width for each sample was determined by prorating the measured 360° pulse width (p90 = 1/4 × p360). The probe was frequency tuned and impedance matched before each acquisition. For each sample, 18 scans (ns) and 4 dummy scans (ds) were recorded with the following parameters: pulse width (P1) of typically 9.25 μs (90°), spectral width of 28 ppm, relaxation delay (D1) of 60 s, receiver gain (RG) set to 256. The total duration of each 1H NMR acquisition was 23 min.
1H NMR Multivariate Data Analysis: PCA and SIMCA
Each acquired qHNMR spectrum was first processed with TopSpin 3.0.b.8. The 1H NMR chemical shifts (δ) were expressed in ppm with reference to the residual solvent signal of DMSO-d6, i.e., DMSO-d5, set to 2.500 ppm. The following processing scheme was applied: a mild Lorentzian-to-Gaussian window function (line broadening = −0.3 Hz, Gaussian factor = 0.05) was applied, followed by two zero fillings before fast Fourier transformation. After manual phasing, a fifth-order polynomial baseline correction was applied. Using the AMIX software (version 3.9.14), and prior to any statistical analysis, the spectrum baselines were adjusted. Spectral intensities were reduced to integrated regions, referred to as buckets of equal width (0.04 ppm) within the region of 8.50 to 0.50 ppm and within the region of 8.50 to 4.50 ppm (downfield region). In the resulting bucket tables, all rows were scaled to the total intensity, and a Pareto scaling was applied for the columns preceding PCA. The regions between 4.00 and 3.00 ppm corresponding to the sugar region, the sucrose anomeric resonance at 5.169 ppm (doublet J = 3.75 Hz), the proline signals (from 1.60 to 2.12 ppm),40 and the residual HOD (singlet at 3.149 ppm), D2O (singlet at 3.567 ppm), EtOH (triplet at 1.043 ppm, J = 7.01 Hz), and DMSO-d5 (2.500 ppm) signals were removed from the bucket table prior to multivariate analyses. PCA using the full-spectrum bucket table (143 buckets) was performed with a total of 83 spectra. A total of 16 principal components (PCs) were selected to explain 95.1% of the variance, and the confidence level was set at 99.99% in order to include the authenticated G. inflata samples. The PCA utilizing only the downfield region contained 84 buckets, and 15 PCs were selected to explain 95.7% of the total variance. The confidence level was maintained at 99.99%. From this PCA model, cross-validation and test set validation were performed to build the SIMCA classification model.
UHPLC-UV Multivariate Data Analysis: CDA
For each UHPLC-UV chromatogram, the AUC values were taken at 275 nm for all major Fs (1–4F) and at 360 nm for all major Cs (1–4C). For each F/C pair, the AUC values were added, divided by the sum of all considered AUCs taken at 275 and at 360 nm (Supporting Information S7,8), and expressed as a percentage of all considered major F/C pairs. Finally, the total F/C ratio was determined by dividing the sum of all AUCs taken at 275 nm (major Fs) by the sum of all AUCs considered at 360 nm (for major Cs): [sum of AUC 275 nm]/[sum of AUC 360 nm]. The percentages of major F/C pairs 1–3 and the total F/C ratio were utilized to perform a classification by CDA. The first canonical score plot was constructed using only the ratios reflecting the F/C composition of each extract. The second score plot considered additionally the AUC ratios from the species-specific metabolites: glabridin ([AUC glabridin 275 nm]/[sum of considered AUC 275 nm]), licochalcone A ([AUC licochalcone A 360 nm]/[sum of considered AUC 360 nm]), and eventually glycycoumarin ([AUC glycycoumarin 360 nm]/[sum of considered AUC 360 nm]). The evaluation of the classifier was performed with the results obtained from independent acquisition time and generated from the same extracts. In both instances, prior probabilities were proportional to each group size. A linear discriminant function was selected. The error rate for the classification and cross-validation of training data was 0%.
Supplementary Material
Acknowledgments
The authors are grateful to A. Massarotto from Nature Med (Italy), Drs. K. Spelman from Herb Pharm (OR, USA), Dr. S. Gafner (American Botanical Council, USA), and Dr. A. Schinkovitz (Angers, France), as well as Drs. M. Farag and W. Ludger (Leibniz-Institut für Pflanzenbiochemie, Germany), and Dr. G. Topcu (Bezmialem Vakif University, Turkey) for kindly providing various licorice samples. The authors are thankful to Dr. L. Zhao (Lanzhou Institute of Chemical Physics CAS) for collecting wild G. inflata samples. The authors also acknowledge M. Coven from Vitality Works (NM, USA) for providing GMP-grade licorice extracts. C.S. is particularly grateful to Dr. J. Herrou for his constant support and thoughtful advice in the course of the research project. C.S. also wishes to acknowledge Dr. D. D. Soejarto and T. Burton for their guidance in microscopic analysis. The authors are grateful to the DNA service facility of the UIC Research Resources Center (UIC RRC), in particular its director, Dr. S. Green, for excellent DNA analytical support. The authors wish to acknowledge Dr. B. Ramirez for his support at the NMR facility of the UIC Center for Structural Biology (UIC, CSB). The construction of this facility was funded by NIH grant P41 GM068944, awarded by NIGMS/NIH (PI: Dr. P. G. W. Gettins). Financial support for this work was provided through grants P50AT000155 by NCCIH and ODS, as well as RC2AT005899 by NCCIH of the NIH.
Footnotes
Notes
The authors declare no competing financial interest.
Microscopic analyses of Glycyrrhiza powder; 1H NMR spectra subjected to PCA; comparative PCA results including the statistical properties; UHPLC-UV chromatograms, calculation method for the determination of AUC ratios, and the resulting workbook table; SIMCA properties and calculated distances for the classification of mixtures, hybrids, and outliers; as well as 1H NMR data of characteristic licorice compounds. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jnat-prod.5b00342.
References
- 1.Betz JM, Fisher KD, Saldanha LG, Coates PM. Anal Bioanal Chem. 2007;389:19–25. doi: 10.1007/s00216-007-1342-8. [DOI] [PubMed] [Google Scholar]
- 2.Cordell GA, Colvard MD. J Nat Prod. 2012;75:514–525. doi: 10.1021/np200803m. [DOI] [PubMed] [Google Scholar]
- 3.Smillie TJ, Khan IA. Clin Pharmacol Ther. 2010;87:175–186. doi: 10.1038/clpt.2009.287. [DOI] [PubMed] [Google Scholar]
- 4.Techen N, Parveen I, Pan Z, Khan IA. Curr Opin Biotechnol. 2014;25:103–110. doi: 10.1016/j.copbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Cao H, But PPH, Shaw PC. J Syst Evol. 2011;49:271–283. [Google Scholar]
- 6.Chen S, Pang X, Song J, Shi L, Yao H, Han J, Leon C. Biotechnol Adv. 2014;32:1237–1244. doi: 10.1016/j.biotechadv.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Hebert PDN, Gregory TR. Syst Biol. 2005;54:852–859. doi: 10.1080/10635150500354886. [DOI] [PubMed] [Google Scholar]
- 8.Van der Kooy F, Maltese F, Choi YH, Kim HK, Verpoorte R. Planta Med. 2009;75:763–775. doi: 10.1055/s-0029-1185450. [DOI] [PubMed] [Google Scholar]
- 9.Gad HA, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Phytochem Anal. 2013;24:1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- 10.Petrakis EA, Cagliani LR, Polissiou MG, Consonni R. Food Chem. 2015;173:890–896. doi: 10.1016/j.foodchem.2014.10.107. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Sudo H. Plant Biotechnol. 2009;26:101–104. [Google Scholar]
- 12.Asl MN, Hosseinzadeh H. Phytother Res. 2008;724:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore C, Eisenhut M, Ragazzi E, Zanchin G, Armanini D. J Ethnopharmacol. 2005;99:317–324. doi: 10.1016/j.jep.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Ye M. J Chromatogr A. 2009;1216:1954–1969. doi: 10.1016/j.chroma.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 15.Nomura T, Fukai T, Akiyama T. Pure Appl Chem. 2002;74:1199–1206. [Google Scholar]
- 16.Nomura T, Fukai T. Prog Chem Org Nat Prod. 1998;73:1–158. doi: 10.1007/978-3-7091-6480-8_1. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO Monographs on Selected Medicinal Plants. Vol. 1. World Health Organization; Geneva: 1999. pp. 183–194. [Google Scholar]
- 18.United States Pharmacopeia. [accessed Jan 6, 2015];2622: Dietary Supplements: Licorice. http://www.pharmacopeia.cn/v29240/usp29nf24s0_m45050.html.
- 19.Kondo K, Shiba M, Nakamura R, Morota T, Shoyama Y. Biol Pharm Bull. 2007;30:1271–1277. doi: 10.1248/bpb.30.1271. [DOI] [PubMed] [Google Scholar]
- 20.Simmler C, Jones T, Anderson JR, Nikolić DC, van Breemen RB, Soejarto DD, Chen SN, Pauli GF. Phytochem Anal. 2014;25:378–388. doi: 10.1002/pca.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S, Cao J, Qiu F, Kong W, Yang S, Yang M. Phytochem Anal. 2013;24:527–533. doi: 10.1002/pca.2427. [DOI] [PubMed] [Google Scholar]
- 22.Yang SO, Hyun SH, Kim SH, Kim HS, Lee JH, Whang WK, Lee MW, Choi HK. Bull Korean Chem Soc. 2010;31:825–828. [Google Scholar]
- 23.Farag MA, Porzel A, Wessjohann LA. Phytochemistry. 2012;76:60–72. doi: 10.1016/j.phytochem.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Tao W, Duan J, Zhao R, Li X, Yan H, Li J, Guo S, Yang N, Tang Y. Food Chem. 2013;141:1681–1689. doi: 10.1016/j.foodchem.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 25.Ralston L, Subramanian S, Matsuno M, Yu O. Plant Physiol. 2005;137:1375–1388. doi: 10.1104/pp.104.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, Jones T, Soejarto DD, Chen SN, Pauli GF. J Agric Food Chem. 2013;61:2146–2157. doi: 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo K, Shiba M, Yamaji H, Morota T, Zhengmin C, Huixia P, Shoyama Y. Biol Pharm Bull. 2007;30:1497–1502. doi: 10.1248/bpb.30.1497. [DOI] [PubMed] [Google Scholar]
- 28.Consortium for the Barcode of Life, C. Plant Barcode Region Proposals — Review Report. http://www.barcoding.si.edu/plant_working_group.html.
- 29.Baker DA. J AOAC Int. 2012;95:1023–1034. doi: 10.5740/jaoacint.11-261. [DOI] [PubMed] [Google Scholar]
- 30.Ausubel JH. Proc Natl Acad Sci U S A. 2009;106:12569–12570. doi: 10.1073/pnas.0906757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoeckle MY, Gamble CC, Kirpekar R, Young G, Ahmed S, Little DP. Sci Rep. 2011;1:42. doi: 10.1038/srep00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little DP. Genome. 2014;57:1–4. [Google Scholar]
- 33.White T, Bruns T, Lee S, Taylor J. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- 34.Oliveira de Miranda V, Martins VG, Furlan A, Bacci M. Braz Arch Biol Technol. 2010;53:141–152. [Google Scholar]
- 35.Zhang Q, Sodmergen J Plant Res. 2010;123:201–206. doi: 10.1007/s10265-009-0291-z. [DOI] [PubMed] [Google Scholar]
- 36.Pan B, Zhang Y. Sci China, Ser D: Earth Sci. 2002;45:174–179. [Google Scholar]
- 37.Zhang YM, Chen YN, Pan BR. J Arid Environ. 2005;63:772–784. [Google Scholar]
- 38.Yamamoto Y, Tani T. J Trad Med. 2006;23:27–35. [Google Scholar]
- 39.Pauli GF, Chen S, Simmler C, Lankin DC, Gödecke T, Jaki BU, Friesen JB, McAlpine JB, Napolitano JG. J Med Chem. 2014;57:9220–9231. doi: 10.1021/jm500734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmler C, Nikolić D, Lankin D, Yu Y, Friesen J, van Breemen RB, Lecomte A, Le Quémener C, Audo G, Pauli G. J Nat Prod. 2014;77:1806–1816. doi: 10.1021/np5001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmler C, Napolitano JG, McAlpine JB, Chen SN, Pauli GF. Curr Opin Biotechnol. 2014;25:51–59. doi: 10.1016/j.copbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heyman H, Meyer J. S Afr J Bot. 2012;82:21–32. [Google Scholar]
- 43.Pinheiro C, Passarinho JA, Ricardo CP. J Plant Physiol. 2004;161:1203–1210. doi: 10.1016/j.jplph.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C. Front Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu F, Wang Q, Wei S, Wang D, Fang Y, Liu F, Zhao Z, Hou J, Wang W. Biol Pharm Bull. 2015;38:75–81. doi: 10.1248/bpb.b14-00574. [DOI] [PubMed] [Google Scholar]
- 46.Rauchensteiner F, Matsumura Y, Yamamoto Y, Yamaji S, Tani T. J Pharm Biomed Anal. 2005;38:594–600. doi: 10.1016/j.jpba.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 47.Kojoma M, Hayashi S, Shibata T, Yamamoto Y, Sekizaki H. Biol Pharm Bull. 2011;34:1334–1337. doi: 10.1248/bpb.34.1334. [DOI] [PubMed] [Google Scholar]
- 48.Harnly JM, Luthria D, Chen P. J AOAC Int. 2012;95:1579–1587. doi: 10.5740/jaoacint.12-096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hajirahimkhan A, Simmler C, Jeffery A, Yuan Y, Nikolić D, Chen S, Dietz BM, Pauli GF, van Breemen RB, Bolton JL. PLoS One. 2013;8:e67947. doi: 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunlap T, Wang S, Simmler C, Chen S-N, Pauli GF, Dietz B, Bolton JL. Chem Res Toxicol. 2015:150716145843000. doi: 10.1021/acs.chemrestox.5b00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilia AR. J Ethnopharmacol. 2014;158:487–494. doi: 10.1016/j.jep.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 52.Sang T, Crawford D, Stuessy T. Am J Bot. 1997;84:1120–1136. [PubMed] [Google Scholar]
- 53.Tate JA, Simpson BB. Syst Botany. 2003;28:723–737. [Google Scholar]
- 54.Hamilton MB. Mol Ecol. 1999;8:513–525. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.