Abstract
Cytokines play crucial roles in coordinating the activities of innate and adaptive immune systems. In response to pathogen recognition, innate immune cells secrete cytokines that inform the adaptive immune system about the nature of the pathogen and instruct naïve T cells to differentiate into the appropriate T cell subtypes required to clear the infection. These include Interleukins, Interferons and other immune-regulatory cytokines that exhibit remarkable functional redundancy and pleiotropic effects. The focus of this review, however, is on the enigmatic Interleukin 12 (IL-12) family of cytokines. This family of cytokines plays crucial roles in shaping immune responses during antigen presentation and influence cell-fate decisions of differentiating naïve T cells. They also play essential roles in regulating functions of a variety of effector cells, making IL-12 family cytokines important therapeutic targets or agents in a number of inflammatory diseases, such as the CNS autoimmune diseases, uveitis and multiple sclerosis.
Keywords: Autoimmune disease, Cytokine therapy, IL-12 family cytokines, Regulatory B cells (Bregs and i35-Bregs), Adoptive B cell therapy
1. Introduction
Interleukin 12 (IL-12) family is comprised of 4 members, IL-12, IL-23, IL-27 and IL-35. IL-12, IL-23 and IL-27 are secreted by activated antigen presenting cells (APC) during antigen presentation to naïve T cells while IL-35 is a product of regulatory T and B cells [1–3]. They provide the bridge between innate and adaptive immune systems by priming naïve CD4+ T cells to differentiate into cytokine-producing T-helper subsets and memory T cells [4]. In addition to their influence on cell-fate decisions of differentiating lymphocytes, IL-12 cytokines regulate cellular pathways required for proper functioning of the immune system, with some members activating pro-inflammatory responses that confer protection against infection while others restrain unbridled immune responses that cause autoimmune diseases [1, 5, 6]. This review focuses on signaling pathways activated by IL-12 cytokines and their contribution to the development and regulation of CNS inflammatory diseases, with particular emphasis on ocular inflammatory diseases and to a lesser extent multiple sclerosis (MS). We highlight recent developments in the use of bioengineered IL-12 cytokines and autologous regulatory B cells for the treatment of CNS inflammatory diseases.
2. Interleukin 12 (IL-12) Cytokines
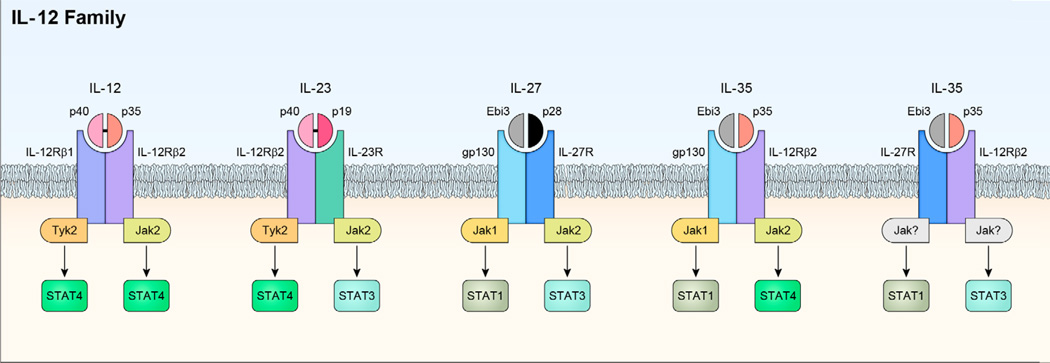
The IL-12 family of cytokines is comprised of IL-12 (IL-12p35/IL-12p40), IL-23 (IL-23p19/IL-12p40), IL-27 (IL-27p28/Ebi3) and IL-35 (IL-12p35/Ebi3) and has emerged as important regulators of host immunity [6, 7]. Each member is composed of α-subunit with a helical structure similar to type 1 cytokines like IL-6 and a β-subunit structurally related to the extracellular regions of Type 1 cytokine receptors (e.g. soluble IL-6 receptor) [6, 7]. The α subunits are IL-12p35, IL-23p19 and IL-27p28 while the β subunits are IL-12p40 and Ebi3 and co-expression of both chains is required for secretion of the bioactive cytokine [8]. It has been suggested that the production of each of the four heterodimeric cytokines might be limited by the expression of the α chain as IL-12 or IL-35 secretion is limited by availability of IL12p35 while secretion of IL-23 is mainly to tissues or cell types with high level expression of IL23p19 [8–12]. Chain-pairing promiscuity is a distinctive feature that accounts, in part, for the involvement of IL-12 cytokines in many aspects of host immunity. It is notable that the dimerization of an alpha chain with IL-12p40 (e.g. IL-12 or IL-23) generates IL-12 cytokines that promote inflammation and the development of chronic inflammatory diseases while dimerization with Ebi3 gives rise to members (e.g. IL-27 or IL-35) that suppress inflammation and mitigate autoimmune diseases (Figure 1). Another important attribute of IL-12 cytokines is that they mediate their biological activities by binding Janus kinases (JAKs) associated heterodimeric receptors and activating JAK-STAT signaling pathways [6]. Each IL-12 cytokine induces the recruitment and activation of specific members of the STAT (signal transducers and activators of transcription) family of transcription factors and this accounts for the unique, as well as, overlapping patterns of gene transcription induced by various IL-12 cytokines [5, 6, 13]. Although much is known about the molecular and functional characteristics of IL-12 and to a lesser extent, IL-23, very little is known about IL-27 or IL-35 and this is attributable to technical difficulties that have hampered the production of biologically active, native heterodimeric IL-27 or IL-35.
Figure 1.
The IL-12 cytokine family and its heterodimeric receptors. The family is comprised of four heterodimeric cytokines that share receptor components and activate the overlapped JAK/STAT pathways. Each IL-12 family cytokines is comprised of a helical alpha subunit (IL-12p19, IL-12 p35, IL-27p28) characteristic of the IL-6 superfamily and a beta subunit chain (IL-12p40, Ebi3) that shares homology with class I receptor chains. Janus kinases (Jak1, Jak2, Tyk2) are associated with the cytoplasmic tails of the receptors and binding of cognate receptors activate the Jaks and STATs (STAT1, STAT3, STAT4) resulting in transcription of targets genes that mediate biological activities. IL-35 appears to utilize unconventional receptors. In T cells it can utilize IL-12R 2/IL-12R 2, IL-12R 2/gp130 or gp130/gp130 and preferentially activate STAT1 and STAT4. In B cells, it utilizes IL-12R 2/IL-27 and activates STAT1 and STAT3. Jak utilization in response to IL-35 signaling in B cells has not been reported. IL27p28/IL12p40 is a novel bioengineered heterodimeric IL-12 cytokine generated by expression of IL-12p40 and IL-27p28 by use of a bicistronic vector. Jak utilization in response to ILp40/IL27p28 has not been reported and this cytokine inhibits gp130 and IL-27 signaling and does not activate STATs.
2.1. Interleukin 12 (IL-12)
Interleukin 12 (IL-12) is the first member of the family described [6]. It is comprised of the IL-12p35 and IL-12p40 subunits and co-expression of both subunits in the same cell is required to secrete the disulfide-linked bioactive IL12p70 cytokine [14]. Although it is secreted by a variety of hematopoietic cell types, the major physiological producers are antigen-presenting cells (APCs), such as DCs and macrophages. Binding of IL-12 to its high-affinity receptor (IL-12Rβ1/IL-12Rβ2) expressed on activated T cells, NK cells and DCs, activates TYK2 (tyrosine kinase 2), JAK2 and STAT pathways (Figure 1) [5, 6]. Although STAT1, STAT3 and STAT4 are activated to varying extents in vitro, physiological responses to IL-12 are mediated mainly through STAT4. IL-12 induces naïve CD4+ T cells to differentiate into Th1 cells, a T-helper subset that is implicated in the etiology of a number of human autoimmune diseases. High levels of IL-12 and Th1 cells are detected in the aqueous humor and vitreous of patients with autoimmune uveitis, suggesting a role for IL-12-induced expansion of Th1 cells in this group of sight-threatening intraocular inflammatory diseases [15]. Multiple sclerosis (MS) is another chronic CNS autoimmune disease which IL-12-induced expansion of Th1 cells is thought to play an important role [16, 17]. Similar to uveitis and MS, the levels of IL-12 and Th1 cells are elevated in the serum and synovial fluid of patients with rheumatoid arthritis (RA) and are correlated with disease activity [18]. Studies on experimental autoimmune uveoretinitis (EAU) the animal model of human uveitis and experimental autoimmune encephalomyelitis (EAE), the animal model of MS, also showed significant increases in IL-12 and Th1 levels, further underscoring the involvement of IL-12 in the pathogenesis of CNS autoimmune diseases [19, 20]. Collectively, these and other observations provide strong evidence that IL-12-induced differentiation of Th1 cells might be associated with the development of organ-specific autoimmune diseases. However, IL-12p40-deficient mice are resistant to EAE while IL-12p35−/− mice are susceptible, indicating redundancy of IL-12p40 in the development of autoimmune inflammation and suggesting that IL-23 (IL-12p40/IL-23p19) rather than IL-12, may be more important in developing autoimmune inflammation of the brain [21, 22]. On the other hand, the levels of IL-17 in the synovial fluid and joints of patients with RA are much higher than the levels of IFN-γ, suggesting that IL-17-producing Th17 cells may play a more important role in RA pathogenesis than IFN-γ-producing Th1 cells [18]. However, studies in the mouse have also shown that either a Th17 or a Th1 effector response can drive autoimmunity and that the dominant effector phenotype may depend on the conditions present during initial exposure to Ag, and/or type of Ag-presenting cells [23].
2.2. Interleukin 23 (IL-23)
A decade after the discovery of IL-12, homology search of the human DNA sequence database with a probe specific to the highly conserved “D” helical structure of IL-6-related cytokines led to identification of additional members of the IL-12 family. Interleukin-23 (IL-23) was discovered in 2003 and shares the IL-12p40 subunit with IL-12 but differs from IL-12 because of its unique IL-23p19 subunit [21, 24]. Similar to IL-12, co-expression of IL-12p40 and IL-23p19 subunits in the same cell is required to secrete the disulfide-linked bioactive IL-23 cytokine. Sharing the IL-12p40 subunit enables IL-12 and IL-23 to interact with the IL-12Rβ1 receptor subunit. The high affinity IL-23 receptor derives from the combination of IL-12Rβ1 with a unique IL-23 receptor subunit (IL-23R) and biologic effects of IL-23 on its target cells are mediated through activation of TYK2, JAK2, STAT3 and STAT4 (Figure 1) [21, 24]. Many innate immune cells including DCs, macrophages, B cells and endothelial cells produce IL-23 and the high affinity IL-23 receptor is expressed on activated T cells and immune cells including Th17 cells, γδ T cells, natural killer T (NKT) cells and innate lymphoid cells (ILCs) [22]. IL-23 prolongs the expression of type 17 signature cytokines (such as IL-17, IL-22 and GM-CSF) that induce tissue pathology and mediates chronic inflammation by promoting the survival and maintenance of Type 17 cells [22, 24]. Thus it is notable that during neuroinflammation, IL-23 produced by CNS-resident cells maintains the pathogenic capacity of CNS-invading T cells while resistance of IL-23p19- and p40-deficient mice to EAE correlates with marked reduction of encephalitogenic T cells [25, 26]. Similarly, IL-23 receptor expression on γδ T cells is implicated in immunopathogenic mechanisms of EAU [27] and it has been suggested that IL-23 plays an important role in birdshot retinochoroidopathy [28]. It is however disappointing that ustekinumab, an IL-12/23 p40 neutralizing antibody, was unsuccessful in a phase II clinical trials of patients with relapsing-remitting MS [29].
2.3. Interleukin 27 (IL-27)
Interleukin 27 (IL-27) was first identified in 1996 from a subtractive hybridization screen of genes expressed in Epstein-Barr virus (EBV) transformed B cell lines [30, 31]. It is comprised of IL-27p28 and EBV-induced gene 3 (Ebi3). The IL-27p28 subunit was identified by a bioinformatics approach on the basis of its structural homology to α-helical cytokines of the IL-6 family and subsequently studies revealed that it is co-expressed with its heterodimeric partner, Ebi3 [31]. In contrast to IL-12 and IL-23, IL-27p28 and Ebi3 are not secreted as disulfide-linked dimer and the nature of the association between IL-27p28 and Ebi3 in vivo is uncertain. Thus, co-expression of Ebi3 and IL-27p28 subunits in the same cell may not be required for production of the bioactive IL-27 cytokine and may instead be secreted independently by various cell types. The IL-27 receptor (IL-27R) is comprised of the ubiquitously expressed gp130 protein and the WSX-1/TCCR and biologic effects of IL-27 are mediated through activation of JAK1, JAK2, TYK2, STAT1 and STAT3 [32]. Studies of Il27ra−/− mice revealed that one of the main functions of IL-27 is to limit the intensity and duration of T cell responses and inhibits Th1, Th2, and Th17 responses by suppressing CD28-mediated IL-2 production through SOCS3 (suppressor of cytokine signaling 3) [33, 34]. A number of reports have also shown that IL-27 limits autoimmune encephalomyelitis by suppressing the development of Th17 cells and inducing the expansion of a population of IL-10-secreting T cells [35–37]. In the immune privileged ocular tissues, IL-27 produced by retinal cells has also been shown to suppress uveitis and contribute to mechanisms of ocular immune privilege by inducing IL-10 and complement factor H [38–40].
2.4. Interleukin 35 (IL-35)
In the quest to identify potential pairing partners for the Ebi3 subunit, co-expression of IL-12p35 and Ebi3 led to the discovery of the novel IL-12p35/EbI3 heterodimer now named IL-35 [1, 30]. Initial studies indicated that the secretion of IL-35 is restricted to Foxp3+ Treg and treatment of naive T cells with IL-35 induces a regulatory population, called ‘iTR35 cells’, that mediates T cell suppression via IL-35 [41]. Furthermore, Treg cells also induce iTR35 cells in vivo under inflammatory conditions, which consequently, suppressed Trichuris muris infection and tumors in mice [41]. It is however notable that human Foxp3+ Tregs do not constitutively express IL-35 but can be induced to produce IL-35 by anergic dendritic cells characterized by cell surface expression of B7-H1 (CD274) and sialoadhesin (CD169) [42]. Nonetheless, IL-35 signaling in Tregs is mediated through unconventional receptors comprising IL-12Rβ2/gp130, IL-12Rβ2/IL-12Rβ2 or gp130/gp130. Although it is not clear which of these is the high affinity IL-35 receptor, binding of IL-35 to the receptor preferentially activates JAK1, JAK2, STAT1 and STAT4 [7, 43]. The restricted secretion of IL-35 in regulatory T cells was thought to be peculiar as other members of the IL-12 family are secreted by a variety of myeloid cell types and this led to the suggestion that IL-35 may have divergent functions from IL-12, IL-23 and IL-27. However, recent reports have now identified IL-35-producing regulatory B cells [44, 45]. Interestingly, the IL-35 receptor identified in B cells comprises of IL-12Rβ2 and IL-27Rα the analysis of IL-35 receptor usage in B cells did not examine whether IL-12Rβ2/IL-12Rβ2 or IL 27Rα/IL-27Rα homodimers are also utilized [45]. The analysis of gp130 utilization in B cells was also based on siRNA-mediated deletion of gp130 or antibody-mediated neutralization of gp130 and incontrovertible proof that IL-35 does not bind to gp130 will await similar analysis B cells from gp130−/− mice. IL-35 has also been shown to induce regulatory B cells that produce IL-10 (Bregs) and/or IL-35 (i35-Bregs), suggesting potential use of autologous regulatory B cells in regulating immune responses in health and disease [44, 45].
3. Current Strategies for Treatment of CNS Autoimmune Diseases
Therapeutic intervention in CNS autoimmune diseases, such as, uveitis and multiple sclerosis presents formidable challenges due to the need to prevent unbridled immune responses that can damage sensitive neuronal tissues. Data from the clinic and animal models form the basis of our current therapeutic strategies for the treatment of inflammatory and autoimmune diseases. These include: (i) inhibition of T-lymphocyte activity by a variety of humanized antibodies [Zenapax® or daclizumab (anti-IL2R); soluble TNF-α receptor antagonist (Etanercept or Enbrel®); Remicade® or Infliximab (anti-TNF-α); Thalidomide (degradation of TNF-α mRNA)] (ii) Blockage of T cell signal transduction pathways with Rapamycin (Rapimmune®, sirolimus) or FK-506 (Tacrolimus®) (iii) Targeting immunomodulatory molecules such as adhesion molecules (anti-LFA-1 (CD11a) or -ICAM-1 (CD54) (iv) Targeting co-stimulatory molecules (anti-CTLA4 and anti-CD40L) (v) Steroids and immunosuppressive (Corticosteroid, Cyclosporin A, Azathioprine, Cyclophosphamide, Chlorambucil, Methotrexate). Several excellent reviews have addressed these strategies of inhibiting T-lymphocyte functions. Here, we will discuss emerging therapeutic strategies based on the use of IL-12 cytokines and adoptive B cell therapy to target critical pathways in uveitis, a group of sight-threatening intraocular inflammatory diseases that includes Behcet’s disease, birdshot retinochoroidopathy, Vogt-Koyanagi-Harada’s, sympathetic ophthalmia, and ocular sarcoidosis. EAU is the animal model of human uveitis. It shares essential pathological features with human uveitis and serves as a useful experimental platform for testing the efficacy of new drugs and therapies for uveitis.
4. Targeting Th17 and Th17 pathways
There is now an emerging consensus of the critical role of Type 17 cells in etiology of several human autoimmune diseases. Prolonged stimulation of these cells promotes EAE, collagen-induced arthritis (CIA) and colitis while mice deficient in type 17 signature genes are relatively resistant to the development of these diseases [22]. The Type 17 cells include the Th17 subset, γ/δ T cells, natural killer T (NKT) cells and innate lymphoid cells (ILCs). They are characterized by expression of the transcription factor retinoic acid receptor-related orphan receptor (RORγt) and an inflammatory gene signature consisting of Il17a, Il17f, Il6, Csf2 (GM-CSF), Tnf, Ccl20, Ccl22, Il1r1 and Il23r [22]. Another common feature shared by Type 17 cells is their reliance of STAT3 for their development and STAT3 is required for the expression of IL-23, IL-23R and RORγt that play crucial roles in Th17 development, maturation and effector functions [46]. In fact, mice conditionally deficient in STAT3 in the CD4 T cell compartment (STAT3KO) cannot generate Th17 and do not develop EAU or EAE [47, 48]. Thus, RORγt and STAT3 are considered as potential targets that can be exploited in the development of therapy for suppressing CNS autoimmune diseases such as, uveitis and MS.
4.1. Targeting STAT3 Pathway
Genome-wide association studies (GWAS) suggest a link between aberrant regulation of STAT3 and susceptibility to MS [49]. Thus, small synthetic compounds that specifically target STAT3 are being developed for inhibiting STAT3 pathways and Th17 cells. One such compound, ORLL-NIH001, is a 406-kDa small chemical that has been used to suppress EAU by inhibiting the expansion of Th17 cells and down-regulating the expression of chemotactic proteins that mediate lymphocyte trafficking into the retina [50]. Importantly, ORLL-NIH001 suppressed EAU in mice that received the drug after EAU had been established, suggesting that ORLL-NIH001 may be used in treating pre-existing uveitis [50]. However, a drawback to therapeutic use of ORLL-NIH001 is its bioavailability, as frequent administration of the drug is required to mitigate uveitis. Another therapeutic strategy is to up-regulate cytoplasmic levels of endogenous inhibitors of STAT3 pathways. Suppressors of cytokine signaling (SOCS) are intracellular proteins that regulate the initiation, intensity and duration of cytokine responses by functioning as negative feedback regulators of STAT pathways [51, 52]. Two members of the SOCS family, SOCS1 and SOCS3, interact with and target activated JAKs and tyrosine-phosphorylated cytokine receptors for proteasome-mediated degradation. Thus, increasing their levels in vivo is an attractive therapeutic strategy for inhibiting STAT3 signaling [51, 53]. However, a major impediment to SOCS therapy has been the development of efficient methods for intracellular delivery of SOCS proteins. Cell-penetrating SOCS1 (MTS-SOCS1) and SOCS3 (MTS-SOCS3) proteins have been genetically engineered by fusing the mouse SOCS1 or SOCS3 cDNA to the cDNA sequence coding for a hydrophobic 12 AA sequence of the signal peptide of the Kaposi FGF4 protein [54]. Although the SOCS penetrating proteins inhibit IL-6-induced activation of STAT3 and suppress the expansion of the uveitogenic Th17 cells that mediate uveitis they have not been effective in suppressing uveitis, and this is in part due to their rapid clearance [55]. An alternative approach to SOCS protein therapy is the use of small peptides corresponding to the kinase inhibitory region (KIR) of SOCS1 or SOCS3 (SOCS-KIR). This 16–18 amino acid peptides are attached to lipophilic groups to promote penetration of the cell membrane [56, 57]. Orally administered SOCS mimetic peptides have been shown to antagonize STAT activation in vivo and inhibit expansion of encephalitogenic Th17 cells that mediate EAE [56, 57]. SOCS-KIR mimetics inhibit both lymphocyte and innate immune responses associated with EAE, suggesting that SOCS mimetics can be effective in suppressing human CNS inflammatory diseases. Because they readily cross the blood brain barrier, SOCS-KIR is considered to be more clinically efficacious than therapeutic antibodies that have difficulty crossing the blood-brain-barrier or blood-retina-barrier. Collectively, these studies suggest that targeting STAT3 pathways can be exploited for treating CNS autoimmune diseases.
4.2. Targeting RORγt and Th17 Developmental Pathway
There are three RAR-related orphan receptors (RORs), ROR-α, -β and -γ that are conserved across species, with each ROR gene exhibiting multiple isoforms generated by alternative promoter usage and splicing [58]. ROR-α and ROR-γ are expressed in a variety of tissues, including the thymus and brain while expression of ROR-β has thus far been detected mainly in the brain and retina. Two forms of ROR-γ are found in humans and mice, RORγ1 and RORγ2 or RORγt (originally identified in the thymus). RORγt and RORα direct the development of the Th17 subset and are required for Th17 generation [59]. Consequently, there is interest in developing small molecules that target RORγt and RORα for use in the treatment of human autoimmune diseases, including inflammatory bowel disease, psoriasis, rheumatoid arthritis, and, potentially, multiple sclerosis. Two drugs, Digoxin, a cardiac glycoside and SR1001, a derivative of the benzenesulphonamide drug T0901317, have recently been used to block the activities of RORα and RORγt. They prevent Th17 differentiation by inhibiting the expression of Th17 signature genes including IL23R, IL-17A, IL-17F and IL-22 [60, 61]. Most importantly, both drugs delayed the onset and reduced the severity of EAE. Thus, Digoxin and SR1001 are potential drugs that can be exploited for the treatment of uveitis and MS.
5. IL-12 Family Proteins as Therapeutic Agents
Promiscuous chain sharing is an important attribute that enables IL-12 family cytokines to participate in many aspects of host immunity. The shared structural features of the single chain IL-12 proteins and their capacity potential to interact with different molecular partners during the course of an immune response, allow different immune cells to interpret seemingly similar cytokine signals that might promote inflammation or immunologic tolerance. The individual IL-12 α or β subunits also possesses intrinsic biological activities that may be similar or distinct from their heterodimeric partners [45, 62, 63] and while this may promote desirable biological activities of the subunit protein, it may also inhibit and limit the therapeutic use of the heterodimeric cytokine. Moreover, subunits that form IL-27 and IL-35 are secreted independently and not covalently linked and factors that promote their binding or dissociation in vivo are not known, making it difficult to predict physiological effects of administering these cytokines. A major concern is that rapid dissociation and unpredictable re-association with alternative partners might generate heterodimers or homodimers with undesirable physiological outcomes. Nonetheless, single-chain α and β subunits, native or fusion IL-27 or IL-35 cytokines and novel fusokines might represent a new class of therapeutic cytokines.
5.1. Single-chain IL-12 family proteins
Besides forming heterodimers with either IL-12p35 or IL-23pl9, the IL-12p40 subunit is also secreted independently as a monomer or disulfide-linked homodimer [64, 65]. It is notable that both IL-12 p40 monomer and homodimer are detectable in the serum of C57BL/6 mice injected with Salmonella enteritidis LPS and the p40 homodimer constituted 20–40% of the total circulating p40 in the endotoxemic sera [66]. However, similar analysis of p40 homodimer levels in human serum has not been reported. Interestingly, the IL-12p40 homodimer also binds the IL-12 receptor and until recently the only function attributed to it was in antagonizing the IL-12 activity. In fact, IL-12p40 homodimer levels are elevated in MS patients and anti-IL-12p40 treatment has a protective effect on the neurological dysfunction, suggesting a role in pathogenesis of the disease [64, 67, 68]. However, administration of IL12p40 monoclonal antibody in a Phase II clinical trial did not block inflammation in MS patients [69]. The other IL-12 β subunit protein is Ebi3. Ebi3 knockout mice are deficient for both IL-27 and IL-35 and the additive effects on the loss of IL-27 and IL-35 signaling would be expected to influence the development of autoimmune inflammation. Indeed, EAE is enhanced in Ebi3-deficient C57BL6 mice and loss of Ebi3 is associated with increased Th17 and Th1 responses [70]. Furthermore, the BALB/c mouse strain is very resistant to EAU induction [71]. However, Ebi3−/− mice on a BALB/c background developed EAU which was characterized by optic neuritis, papilledema, retinal vasculitis, hemorrhage. Moreover, recombinant Ebi3 protein inhibited proliferation of the uveitogenic lymphocytes [45], suggesting that therapeutic administration of Ebi3 might be beneficial in uveitis. In context of therapeutic use of IL-12 cytokines, it is notable that pairing IL-12 α subunits with IL-12p40 generates inflammatory responses while pairing with Ebi3 is tolerogenic and suppresses inflammation. Among the IL-12 α subunit proteins, IL-23p19 is pro-inflammatory and blockade with IL-23p19-specific siRNA or neutralizing Abs is a viable therapeutic approach. On the other hand, IL-12p35 or IL-27p28 inhibited the expansion of uveitogenic and encephalitogenic T cells and suppressed EAU and EAE, respectively [62, 63]. Although neither subunit can by itself activate signal transduction, they both suppress proliferation by inhibiting STAT1, STAT3 and STAT4 activation by IL-27, IL-6 and IL-12, respectively [62, 63].
5.2. Development of Novel Therapeutic IL-12 Family Cytokines
Because the three alpha subunits (IL-12p35, IL-23p19 and IL-27p28) are structurally related, each can conceivably pair with either of the structurally homologous β subunits (IL-12p40 and Ebi3). In fact, this is the basis for the shared usage of IL-12p40 by IL-12 and IL-23 and similar sharing of Ebi3 by IL-27 and IL-35. Thus, although there are currently four known members in the family, the predictable range of combinations is six (Table 1) and exploiting the propensity for promiscuous chain sharing might be of therapeutic value. For example, we took advantage of the fact that IL-27p28 antagonizes gp130-mediated signaling [72] while IL-12p40 inhibits IL-12-induced inflammatory responses [73] to genetically engineer a novel IL-27p28/IL-12p40 heterodimeric cytokine [62]. In vitro, the IL-27p28/IL-12p40 cytokine inhibited signaling downstream of IL-12Rβ1/gp130 receptor and antagonized the differentiation and expansion of Th1 and Th17 cells. In vivo, it suppressed EAU by inhibiting inflammatory responses of uveitogenic Th1 and Th17 cells while promoting the expansion of IL-10-producing regulatory T cells (Foxp3+ Tregs) [62]. It is conceivable that the inhibition of Th1 and Th17 cells contributed to skewing of the immune response in towards immunosuppressive Treg-mediated responses. This important proof-of-concept study underscores the feasibility of producing additional novel IL-12 family cytokines and fusokines that may form the basis of a new generation of therapeutic cytokines.
TABLE I.
Promiscuous chain-pairing of the 3 alpha and the 2 beta subunits, predicts six possible heterodimeric proteins in the IL-12 family. The top row indicates alpha subunits and the left-most column represents beta subunits. A novel IL-12 cytokine with the pairing of IL-12p40/IL-27p28 was recently bioengineered and shown to suppress ocular inflammation. Whether bioengineered pairing of IL-23p19 and Ebi3 would also exhibit biological activity awaits validation.
| Subunits | IL-12p35 | IL-23p19 | IL-27p28 |
|---|---|---|---|
| IL-12p40 | IL-12 native | IL-23 native | IL-27p28/IL-12p40 Bioengineered |
| Ebi3 | IL-35 native | IL-23p19/Ebi3 ? | IL-27 native |
5.3. Interleukin 35 (IL-35) and Adoptive B cell Therapy
Despite the immunoregulatory effects of IL-27, it also possesses immunostimulatory functions in vivo and whether or not it activates immunostimulatory or immunoregulatory functions is thought to depend on the cytokine milieu in vivo [7, 74]. In view of the ambiguity surrounding physiological functions of IL-27, our comments here are on the therapeutic use of the other immunoregulatory IL-12 cytokine, IL-35. Although IL-35 was originally thought to be exclusively produced by Treg cells [1], subsequent studies revealed that it also induces the development of a novel IL-35-producing regulatory T cell population named, iTr35 [41]. Two recent studies have now shown that B cells also produce IL-35 and that the IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases [44, 45]. Mice with loss of IL-35 expression in B cells could not recover from the demyelinating EAE but were markedly resistant to Salmonella enterica serovar Typhimurium infection [44]. In the EAU model, treatment of mice with IL-35 conferred protection from uveitis and mice that lack IL-35 or are defective in IL-35-signaling develop severe uveitis with reduced capacity to produce regulatory B cells that produce IL-10 (Bregs) of IL-35 (i35-Bregs) [45]. Importantly, IL-35 induces the expansion of Bregs in vivo and promotes the conversion of Bregs into i35-Breg [45] and ex-vivo generated Bregs suppressed uveitis by inhibiting pathogenic Th17/Th1 cells while promoting Tregs expansion [45]. IL-35 also induces the conversion of human B-cells into Bregs, suggesting that this function of IL-35 is evolutionarily conserved between humans and mice. Thus, autologous tolerogenic B cell therapy may provide a novel approach for treatment of uveitis and other organ-specific autoimmune diseases with potentially less undesirable off-target effects compared to steroids.
Conclusion
In this review, we have summarized results of recent studies showing that IL-35 and the transfer of ex-vivo generated Bregs and i35-Bregs can be used to treat uveitis, an organ-specific autoimmune disease of the CNS. We have also discussed the use of the novel bioengineered IL-27p28/IL-12p40 cytokine and the IL-12 single-chain protein, IL-27p28, to treat autoimmune uveitis (Table II). The finding that IL-12p35 and Ebi3 inhibit lymphocyte proliferation but cannot induce expansion of Breg or Treg cells, suggests that they have functions independent of IL-35 and that they can be used to modulate host defense and anti-tumor immunity without the danger of inducing Breg-mediated sterilizing immunity. Thus, native or bioengineered IL-12 cytokines, as well as, their single chain subunits may constitute a new class of therapeutic cytokines. In the context of exploiting bioengineering of novel IL-12 cytokines as a viable therapeutic strategy, it is of interest that IL-12 α subunits are structurally related to the extracellular region of IL-6 and IL-6 related cytokines, such as IL-11, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic Factor (CNTF), and cardiotrophin-1. This suggests that the formation of novel fusokines, based on combination of Ebi3 and each member of the IL-6-related cytokines, might lead to the development of additional biologics that would expand the repertoire of therapeutic IL-12/IL-6 fusokines. Finally, it is important to appreciate that IL-12 cytokines are unique in being one of the few heterodimeric cytokines in nature with remarkable influence on innate and adaptive immune responses and understanding immunobiology of IL-12 cytokines would undoubtedly provide valuable knowledge about cytokines that can be exploited therapeutically. In addition, the structurally similarities between their alpha and beta subunits coupled with their propensity for promiscuous chain-sharing raise some important and intriguing questions. Are there additional α/β subunit combinations that exist in vivo and are they biologically active? Why are the α/β subunits of the two pro-inflammatory members secreted as disulfide-linked heterodimeric proteins while the two immunosuppressive members seem to be secreted as single chains that associate non-covalently in the extracellular milieu? What are the evolutionary and functional implications? What factors regulate the stability of the non-covalently linked IL-27 (p28 and Ebi3) or IL-35 (p35 and Ebi3) heterodimer or their dissociation leading to termination of their inhibitory activities? It is likely that answers to these questions will not only be surprising but would open exciting new avenues of research leading to the development of therapeutics based on the enigmatic IL-12 family of cytokines.
TABLE II.
It summarizes of IL-12 family cytokine and individual subunit biological activities during immune response.
| IL-12 Subunits and Cytokines | Biological Activity |
|---|---|
| IL-27p28 | Antagonizes IL-6 and IL-27 Inhibits induction of Th17 and Th1 cells |
| IL-27p28/IL-12p40 | Antagonizes IL-6 and IL-12 Inhibits induction of Th17 and Th1 cells |
| IL-12p40 | Antagonizes IL-12 Inhibits induction of Th1 cells |
| IL-12p40/IL-12p40 | Antagonizes IL-12 Inhibits induction of Th1 cells |
| IL-27 | Promotes Tr1 induction Inhibits Th17 expansion |
| IL-35 | Inhibits Th1 and Th17 induction Inhibits Th1 expansion Converts Tregs into iTr35 regulatory cells |
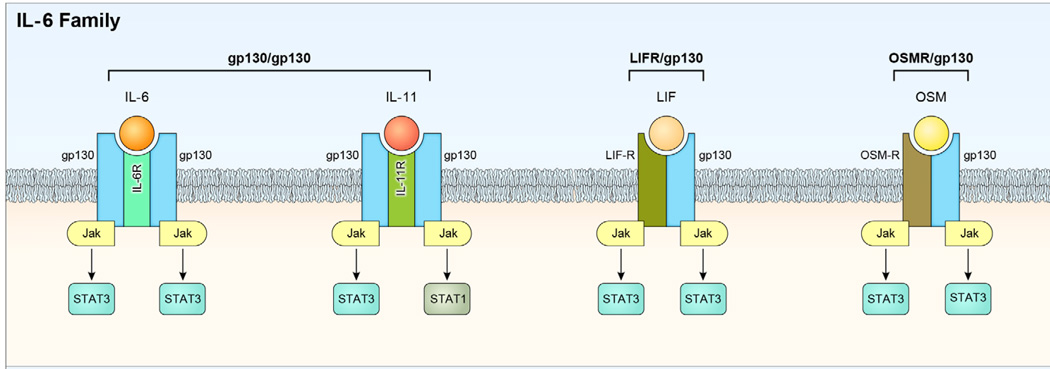
Figure 2.
IL-6 superfamily share gp130 receptor subunit. Cytokines in the IL-6 superfamily include IL-6, ciliary neurotrophic factor (CNTF), oncostatin M (OSM), IL-11, Leukemia inhibitory factor (LIF) and they each bind to a receptor comprising of a common gp130 subunit and a cytokine-specific co-receptor (IL-6R, CNTFR, LIFR, OSMR, IL-11R). Signaling through the gp130 homodimer results in the phosphorylation of Janus kinases and preferential activation of STAT3 and to a lesser extent STAT1 and STAT5. IL-6R, IL-11R, and CNTFR are not necessarily membrane-anchored. and secretion of soluble receptor components may be a common attribute of all members. The soluble receptors are capable of ligand binding and signal transduction through gp130-containing dimers in a process referred to as trans-signaling.
Highlights.
We describe a regulatory B cell (Breg) population that produces IL-35 (i35-Breg)
Interleukin 35 induces in-vivo and ex-vivo the IL-35-producing B cell (i35-Breg)
IL-35 induces regulatory B cells that suppress CNS autoimmune disease
Ex-vivo generated Bregs and i35 Bregs suppress ocular inflammation and uveitis
Treatment of experimental uveitis with a novel IL-27p28/IL-12p40 Fusokine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 2.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM. Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp. 2006;279:101–109. discussion 9–13, 216-9. [PubMed] [Google Scholar]
- 5.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nature reviews. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 6.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 7.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 9.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 10.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 12.Babik JM, Adams E, Tone Y, Fairchild PJ, Tone M, Waldmann H. Expression of murine IL-12 is regulated by translational control of the p35 subunit. J Immunol. 1999;162:4069–4078. [PubMed] [Google Scholar]
- 13.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 14.Jalah R, Rosati M, Ganneru B, Pilkington GR, Valentin A, Kulkarni V, et al. The p40 subunit of interleukin (IL)-12 promotes stabilization and export of the p35 subunit: implications for improved IL-12 cytokine production. J Biol Chem. 2013;288:6763–6776. doi: 10.1074/jbc.M112.436675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.el-Shabrawi Y, Livir-Rallatos C, Christen W, Baltatzis S, Foster CS. High levels of interleukin-12 in the aqueous humor and vitreous of patients with uveitis. Ophthalmology. 1998;105:1659–1663. doi: 10.1016/S0161-6420(98)99035-2. [DOI] [PubMed] [Google Scholar]
- 16.Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc Natl Acad Sci U S A. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comabella M, Balashov K, Issazadeh S, Smith D, Weiner HL, Khoury SJ. Elevated interleukin-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J Clin Invest. 1998;102:671–678. doi: 10.1172/JCI3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pope RM, Shahrara S. Possible roles of IL-12-family cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 9:252–256. doi: 10.1038/nrrheum.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bright JJ, Musuro BF, Du C, Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J Neuroimmunol. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- 20.Tarrant TK, Silver PB, Chan CC, Wiggert B, Caspi RR. Endogenous IL-12 is required for induction and expression of experimental autoimmune uveitis. Journal of immunology (Baltimore, Md. 1998;161:122–127. [PubMed] [Google Scholar]
- 21.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 22.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008 doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 25.Becher B, Durell BG, Noelle RJ. IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J Clin Invest. 2003;112:1186–1191. doi: 10.1172/JCI19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang D, Zuo A, Shao H, Born WK, O'Brien RL, Kaplan HJ, et al. IL-23 receptor expression on gammadelta T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J Immunol. 191:1118–1125. doi: 10.4049/jimmunol.1300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang P, Foster CS. Interleukin 21, interleukin 23, and transforming growth factor beta1 in HLA-A29-associated birdshot retinochoroidopathy. Am J Ophthalmol. 156:400 e2–406 e2. doi: 10.1016/j.ajo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 30.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 32.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. Journal of immunology (Baltimore, Md. 2004;173:715–720. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 33.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, et al. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. Journal of immunology (Baltimore, Md. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 34.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. Journal of immunology (Baltimore, Md. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 36.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 37.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature immunology. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 38.Amadi-Obi A, Yu CR, Dambuza I, Kim SH, Marrero B, Egwuagu CE. Interleukin 27 induces the expression of complement factor H (CFH) in the retina. PLoS One. 7:e45801. doi: 10.1371/journal.pone.0045801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 40.Lee YS, Amadi-Obi A, Yu CR, Egwuagu CE. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, et al. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. Eur J Immunol. 2010;40:321–329. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- 43.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 47.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakkula E, Leppa V, Sulonen AM, Varilo T, Kallio S, Kemppinen A, et al. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am J Hum Genet. 86:285–291. doi: 10.1016/j.ajhg.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu CR, Lee YS, Mahdi RM, Surendran N, Egwuagu CE. Therapeutic Targeting of STAT3 (Signal Transducers and Activators of Transcription 3) Pathway Inhibits Experimental Autoimmune Uveitis. PLoS One. 7:e29742. doi: 10.1371/journal.pone.0029742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egwuagu CE. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine. 2009;47:149–156. doi: 10.1016/j.cyto.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 53.Egwuagu CE, Larkin Iii J. Therapeutic targeting of STAT pathways in CNS autoimmune diseases. JAKSTAT. 2:e24134. doi: 10.4161/jkst.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 55.Yu CR, Hayashi K, Lee YS, Mahdi RM, Shen DF, Chan CC, et al. Suppressor of Cytokine Signaling 1 (SOCS1) Mitigates Anterior Uveitis and Confers Protection Against Ocular HSV-1 Infection. Inflammation. doi: 10.1007/s10753-014-9962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Storek J, Dawson MA, Lim LC, Burman BE, Stevens-Ayers T, Viganego F, et al. Efficacy of donor vaccination before hematopoietic cell transplantation and recipient vaccination both before and early after transplantation. Bone Marrow Transplant. 2004;33:337–346. doi: 10.1038/sj.bmt.1704336. [DOI] [PubMed] [Google Scholar]
- 57.Waiboci LW, Ahmed CM, Mujtaba MG, Flowers LO, Martin JP, Haider MI, et al. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J Immunol. 2007;178:5058–5068. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]
- 58.Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 59.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem. 287:36012–36021. doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brahmachari S, Pahan K. Role of cytokine p40 family in multiple sclerosis. Minerva Med. 2008;99:105–118. [PMC free article] [PubMed] [Google Scholar]
- 65.Heinzel FP, Hujer AM, Ahmed FN, Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- 66.Shigehara K, Shijubo N, Ohmichi M, Kamiguchi K, Takahashi R, Morita-Ichimura S, et al. Increased circulating interleukin-12 (IL-12) p40 in pulmonary sarcoidosis. Clin Exp Immunol. 2003;132:152–157. doi: 10.1046/j.1365-2249.2003.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Boxel-Dezaire AH, Hoff SC, van Oosten BW, Verweij CL, Drager AM, Ader HJ, et al. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol. 1999;45:695–703. doi: 10.1002/1531-8249(199906)45:6<695::aid-ana3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 68.Fassbender K, Ragoschke A, Rossol S, Schwartz A, Mielke O, Paulig A, et al. Increased release of interleukin-12p40 in MS: association with intracerebral inflammation. Neurology. 1998;51:753–758. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]
- 69.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 70.Liu JQ, Liu Z, Zhang X, Shi Y, Talebian F, Carl JW, Jr, et al. Increased Th17 and regulatory T cell responses in EBV-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. J Immunol. 188:3099–3106. doi: 10.4049/jimmunol.1100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–3112. [PubMed] [Google Scholar]
- 72.Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gillessen S, Carvajal D, Ling P, Podlaski FJ, Stremlo DL, Familletti PC, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 74.Nagai H, Oniki S, Fujiwara S, Xu M, Mizoguchi I, Yoshimoto T, et al. Antitumor activities of interleukin-27 on melanoma. Endocr Metab Immune Disord Drug Targets. 10:41–46. doi: 10.2174/187153010790827920. [DOI] [PubMed] [Google Scholar]