Abstract
Anti-viral T- and B- cell responses play a critical role in suppressing HIV and SIV replication during chronic infection. However, these infections are rarely controlled by the host immune response, and most infected individuals need lifelong antiretroviral therapy (ART). Recent advances in our understanding of how anti-HIV immune responses are elicited and regulated prompted a surge of interest in harnessing these responses to reduce the HIV "residual disease" that is present in ART-treated HIV-infected individuals. Novel approaches that are currently explored include both conventional therapeutic vaccines (i.e., active immunization strategies using HIV-derived immunogens) as well as the use of checkpoint blockers such as anti-PD-1 antibodies. These approaches appear promising as key components of complex therapeutic strategies aimed at curing HIV infection.
Introduction
Since the discovery of HIV in 1983, the scientific community has worked diligently to generate a successful vaccine that affords sterilizing immunity against this infection. This effort has resulted in five large efficacy clinical trials, of which only one has shown a modest effect on preventing infection in low-risk individuals [**1]. The advent of anti-retroviral therapy (ART) has greatly enhanced viral control, decreased transmission rates, lowered AIDS-related morbidities, and improved the quality of life for HIV-infected individuals who can both access and tolerate ART. However, ART is a lifelong therapy that represents a major logistical burden to healthcare systems and can be associated with significant side effects and a series of non-AIDS related clinical complications that are referred to as “end-organ disease” [2]. All these limitations of ART are a result of the inability to eliminate the persistent reservoir of latently infected cells that lead to a rapid reemergence of viremia and disease progression if ART is interrupted [3,4]. Thus, there is a great need for the development of successful therapies, such as therapeutic vaccinations, that can decrease or eliminate this persistent reservoir and therefore reduce the need for lifelong ART. In this review we provide an overview of the current research efforts in the field of therapeutic vaccination for HIV infection and AIDS and the potential way forward for this approach as part of strategies to cure this infection.
ART alone does not eliminate the viral reservoirs and does not fully restore immune function
While ART is able to profoundly suppress viral replication, it does not eliminate the viral reservoir, and its treatment is associated with an incomplete restoration of the host immune system, particularly in those individuals that have initiated ART at later stages of the infection. In particular, studies have shown that while ART facilitates CD4 T cell reconstitution in the blood, there is only a limited improvement in the function of anti-HIV specific CD8 T cell responses [5,6]. More recently, Barouch and colleagues used the rhesus macaque model of SIV infection to demonstrate that initiation of ART as early as 3 days post infection was still unable to prevent the seeding of viral reservoirs following an intrarectal viral infection [**7]. This study also showed that early initiation of ART limited priming of anti-viral CD8 T cell responses such that when ART was interrupted and viral resurgence occurred, there were no SIV-specific CD8 T cells present to control viral replication. Structured treatment interruptions of ART have also been used as a therapeutic option to enhance anti-HIV immunity using the pulses of reemerging viremia as a source of antigen in both SIV-infected ART suppressed macaques [8,9] and HIV-infected humans [10-13], but this strategy proved to be unsuccessful with minimal effects on decreasing set-point viremia post-interruption. Thus, it is critical to develop therapies that profoundly increase the magnitude and function of anti-HIV immunity, which can facilitate long-term viral control in the absence of ART. Therapeutic vaccinations may play a significant role in achieving this due to both its feasibility and low costs.
Protective anti-viral immunity is important for a therapeutic setting
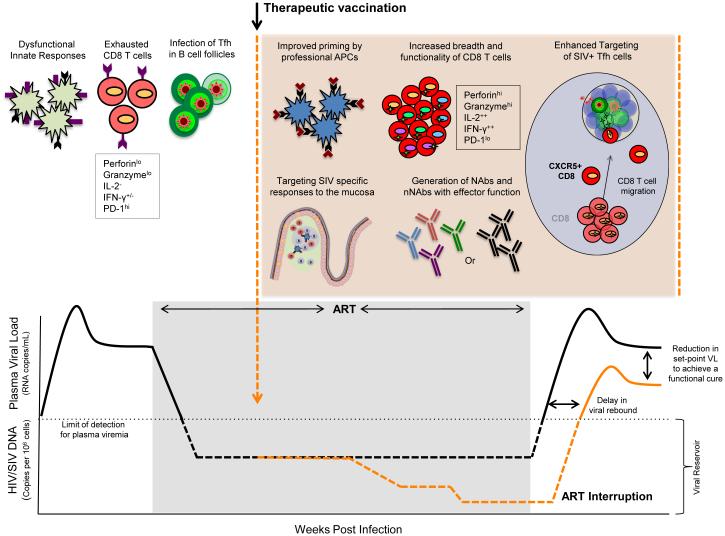
Therapeutic vaccines for HIV infection should aim to elicit anti-viral CD8 T cells (CTLs), CD4 T cells, and neutralizing antibody since these immune responses work in concert to control viral replication [14-17]. In addition to increasing the magnitude of these immune responses, it will be important to generate poly-functional T cells (capable of producing multiple cytokines and performing effector functions) (Fig. 1), as these HIV specific T cells have been shown to be associated with long-term non-progression [5,18,19]. It is also critical to generate broad cellular responses as HIV mutates very rapidly to escape immune pressure (Fig.1) [**20]. In addition, recent studies determined that T follicular helper cells (Tfh) constitute a significant source of virus production and contribute to the total viral reservoir [*21,*22,*23]. Since these cells reside in B cell follicles/germinal centers, it may be critical to generate CD8 T cells that can home to B cell follicles and exert immune pressure on these cells (Fig.1). The HIV-specific CD4 T cell response is also important for maintaining the functional CD8 T cell and B cell response. However, these HIV-specific CD4 T cells could also serve as potential targets for virus replication following ART interruption. Interestingly, CD4 T cells with cytolytic function have been shown to be associated with enhanced viral control [24,25], although it is yet to be demonstrated whether these responses can be primed by vaccination. The function of dendritic cells (DC) may be also critical for generating a protective cellular and humoral immune response, as chronic HIV/SIV infections are associated with impaired DC function (Fig.1) [26-30]. Thus, therapeutic vaccines may also need to use strategies such as adjuvants to enhance the function of innate immunity.
Figure 1.
Potential mechanisms of viral control by therapeutic vaccines. ART therapy has allowed for profound control of viral replication in HIV infected individuals, but is able to only partially restore immune function. Multiple arms of the immune response are compromised during chronic HIV infection and remain less functional under highly suppressive ART including; impaired innate responses, exhausted anti-viral CD8 T cell responses, and productive infection of CD4 T cells including Tfh cells, restricted mainly to the germinal centers of B cell follicles that are largely devoid of anti-viral CD8 T cells. Therapeutic vaccinations in combination with other immune based therapies have the potential to beneficially modulate multiple immune parameters that are crucial for long term control of viremia in the absence of ART and these include improving the function of professional APCs, expansion of highly functional CD8 T cells with broad specificity and the ability to infiltrate into sites of latent viral replication such as B cell follicles, restoration of mucosal homeostasis to diminish hyperimmune activation, and the induction of functional antibody response. These interventions may lead to a decrease in persistent viral reservoirs that may result in delayed viral resurgence and a significant reduction in viral set-point post ART interruption, facilitating host control of HIV. Keywords: APC, antigen presenting cells; Tfh, T follicular helper cells; Nab, neutralizing antibodies, nNAb, non-neutralizing antibodies.
Success of therapeutic vaccinations: lessons learned
A number of therapeutic vaccine modalities have been tested in humans to boost pre-existing immune responses to HIV by using multiple vectors such as DNA and viral vectors with and without adjuvants, DC based vaccines, or a combination of these [31]. Some studies have shown both a delay in the kinetics of viral rebound, a 0.5 to 1 log reduction in plasma viral load, but the clinical benefit of these positive results remain unknown. Furthermore, recent studies have sought to understand the therapeutic effects of a DNA vaccine under suppressive ART and have found little effect on reducing the HIV reservoir. While some of these vaccines induced reasonably strong anti-HIV T cell response they have shown little therapeutic benefit following ART interruption.
The SIV/macaque model has been used to test the therapeutic potential of multiple vaccines to generate a strong anti-SIV T cell response and their influence on control of viral rebound after ART interruption. These include DNA vaccines with and without adjuvants [32], attenuated poxvirus vectors such as NYVAC [33] and MVA (Amara et al., unpublished results), and dendritic cells pulsed with autologous virus [34]. The majority of these studies performed vaccinations under ART, while a few studies were performed in the absence of ART. Notably, the majority of these vaccines induced SIV-specific CD4 and CD8 T cell responses with improved function as measured by the production of cytokines such as IFNγ, TNFα and IL-2, and the expression of cytolytic molecules such as granzyme B and Perforin (Fig.1). Earlier studies in which macaques were treated with ART very early (within the first few weeks) after SIV infection [35] revealed that ART alone provides some benefit in control of viremia after ART interruption similar to what has been observed in humans [13,36]. Some of these studies showed a modest effect (a log or lower) on control of reemerging viremia after ART interruption, with viral control maintained only for few months. A recent study by Fuller and colleagues [37] used a particle mediated delivery of DNA vaccine with and without lymphotoxin as an adjuvant and showed a significant reduction in viral burden in the blood and jejunum of SIV-infected ART suppressed macaques, and durable protection from viral rebound post treatment cessation. Interestingly, the enhanced viral control was associated with greater breadth of SIV-specific T cell response in the gut. Experiments are underway to test the therapeutic potential of CMV based viral vector that has recently been shown to effectively control pathogenic SIV infection in a preventive setting [38,**39].
A few studies have used dendritic cells (DC) presenting either the autologous virus or virus-derived peptides as therapeutic vaccines in macaques and humans. Andrieu and colleagues used repeated infusions of chemically-inactivated autologous virus pulsed DC as a therapeutic vaccine in the absence of ART in SIV-infected macaques and showed profound control of virus replication [40]. Impressively, they also showed that a similar approach could successfully control HIV replication in humans [41]. These studies established a proof-of-concept that presentation of autologous viral antigen by appropriately matured DC can induce protective immune responses that are capable of controlling HIV/SIV replication during chronic infection. Similarly, a recent study by Garcia et al showed that an efficient HIV-1 specific immune response could be generated through the use of an autologous monocyte-derived DC (MDDC) transfer [**42]. In addition, Levy Y and colleagues showed an effective HIV specific immune response elicited by vaccination with DC loaded HIV lipopeptides given to patients on suppressive ART. This study also showed a log lower plasma viral load post treatment interruption and induction of polyfunctional CD4 T cell responses [*43]. Thus, these proof-of-concept studies demonstrated that HIV-specific immune responses can be elicited by DC-based therapeutic vaccinations and suggest that therapeutic vaccinations should be explored as a combination therapy with other immune modulators under ART to achieve a “functional cure” (long-term control of virus replication in the absence of ART).
A number of potent broadly neutralizing antibodies (bNAbs) have been identified from HIV-infected individuals although the generation of bNAbs using traditional vaccine approaches has been elusive [44]. Recent studies by Barouch et al. and Shingai et al. demonstrated a proof-of-concept for the protective therapeutic benefit of passive immunizations with bNAbs [**45,**46]. Barouch and colleagues studied the efficacy of infusing a cocktail of monoclonal antibodies as well as a single infusion of a potent bNAb PGT121, both of which resulted in a rapid decline in plasma viremia to undetectable levels and a decrease in proviral DNA in the peripheral blood, lymph node, and gut mucosa of rhesus macaques infected with pathogenic chimeric Simian/Human Immunodeficiency Virus that contains the HIV env gene. The functionality of Gag-specific T cell responses were enhanced post antibody administration and although some animals rebounded concomitant with the waning of infused antibody, a few animals maintained long-term virologic control. Similarly, Shingai and colleagues tested either a single dose of 3BNC117 or 10-1074 (mAb specific for the CD4-binding site and the V3 region, respectively) or a combination of both antibodies and showed a precipitous decline in viral loads post infusion in SHIV infected macaques. Studies are currently being performed to test the potential of this passive bNAb infusion as an intervention to reduce the viral reservoir in ART-treated SHIV-infected macaques. Despite the impressive protective effect of passive NAb immunizations established by this study, long-term therapy would be impractical as a human intervention. Another approach to the generation of bNAbs is to totally circumvent “normal” immune responses and direct non-lymphoid cells to produce bNAbs in vivo using gene therapy. Vectored ImmunoProphylaxis (VIP) is a gene therapy method in which transgenes encoding bNAbs are delivered directly into muscle tissue where bNAbs are then produced [47-49]. Two recent animal studies by Baltimore and colleagues, and Johnson and colleagues demonstrated that VIP could generate modest titers of NAb that can effectively prevent against both an in vivo HIV infection in a humanized BLT (bone marrow-thymus-liver) HIV infection model and a simian immunodeficiency macaque model of infection [50,51].
A very recent study by Farzan et al demonstrated the effectiveness of an adeno-associated virus vector (AAV) in providing durable protection from intra-rectal SHIV challenges [**52]. This immune strategy employed the use of a eCD4-Ig, a fusion of CD4-Ig and a CCR5-mimetic sulfopeptide, that binds only conserved regions of Env and efficiently neutralized 100% of a diverse panel of neutralization resistant strains of HIV-1, HIV-2, and SIV isolates, including those resistant to neutralization by CD4-binding site bNAbs VRC01, NIH45-46, and 3BNC117. The AAV vector stably expressed fully functional rhesus eCD4-Ig for greater than 40 weeks. This study suggests a possible new and effective HIV therapeutic that can direct the immune response to de novo generate stable and efficient protection against SHIV challenge. Challenges to these approaches will include immune responses generated against the adenoviral vector/bNAb and durability of the mimetic peptide long-term.
Combination approaches are needed to achieve a functional cure
While therapeutic vaccines have shown promise in effectively boosting anti-viral immune responses, major immune obstacles continue to persist during long-term ART therapy such as immune exhaustion, viral escape, and the persistence of long-lived viral reservoirs. These factors can cause resistance to current vaccine strategies and provide rationale for the use of combination strategies such as the addition of checkpoint inhibitors, cytokine therapies, and/or latency reversing agents (LRA’s). These immune modulators may be critical to the development of an optimal treatment regimen to achieve a functional cure.
During progressive HIV/SIV infection, increased expression of inhibitory/checkpoint receptors is associated with greater immune dysfunction. Persistent antigen exposure during chronic HIV/SIV infection leads to T cell exhaustion and immune dysfunction and is characterized by increased expression of inhibitory receptors such as PD-1 [53-55] and CTLA-4 [56-58] and progressive loss of important effector functions. Through a better understanding of the impact of these inhibitory mechanisms, it has been possible to develop novel immunotherapeutic strategies to reverse these immunological defects [58]. Studies using SIV infection in rhesus macaques have shown the relevance of targeting the inhibitory receptor PD-1 to boost the antiviral cellular immune response [59]. CTLA-4 expression was also found to be up-regulated on CD4 T cells in the lymphoid tissue during SIV-infection but anti-CTLA-4 blockade failed to show any effect on plasma viral load or survival for SIV-infected macaques treated at acute or chronic time points [57]. Some data also suggests that blockade of CTLA-4 in vivo in SIV infected macaques can lead to increased viral replication at mucosal sites [57,60]. There have also been conflicting studies as to whether CTLA-4 blockade can effectively expand SIV specific CTL responses. There are differences between the expression and function of PD-1 and CTLA-4 on antigen specific CD4 and CD8 T cells with CTLA-4 being integral for regulatory T cell function and more highly expressed on virus specific CD4 T cells than CD8 T cells [58]. As such, CTLA-4 blockade therapy might be more beneficial if preferentially used to increase CD4 T cell activation and latent viral reactivation in combination with PD-1 blockade to effectively enhance CTL function. Thus careful understanding of the dose, timing, and effects of checkpoint/inhibitory receptor blockade must be tested to harness these biologics for treatment of HIV under ART.
Even under complete ART suppression, immune reconstitution and the reestablishment of immune homeostasis is often incomplete [61,62]. One anatomic site at which immune reconstitution is particularly inefficient is the mucosal immune system [63-65]. The use of rIL-21 therapy shows great promise for restoring intestinal IL-17 producing CD4 T cells and improving mucosal barrier integrity, both of which are critical for the maintenance of systemic homeostasis [*66]. Moreover, inflammation and systemic immune activation persist during long-term ART and strongly correlate with non-AIDS morbidity and mortality, higher set-point viral loads, and acquisition of infection, but inflammation also drives an anti-viral immune response, particularly prompting a type I interferon response that participates in early innate control of infection. Sandler et al recently demonstrated the interesting dichotomy in modulating the type I interferon axis during SIV transmission and acute infection [**67]. Administration of a type I interferon receptor antagonist during transmission of SIV resulted in decreased anti-viral gene expression, increased loss of CD4 T cells, expansion of the SIV reservoir, and accelerated progression to AIDS. On the contrary, rIFN-α2a administered during SIV transmission and acute infection resulted in increased expression of anti-viral genes, limited systemic infection, and an overall benefit in viral control. However, continued administration of IFN-α2a during the acute stage of viremia resulted in detrimental consequences to viral control including IFN-1 desensitization, decreased anti-viral gene expression, and an increase in the SIV reservoir. As little as a 3 day delay in the expression of anti-viral genes after SIV infection resulted in accelerated disease progression. This highlights how tenuous anti-viral innate immune responses are very early after transmission and infection and the importance of defining the precise time to effectively modulate anti-viral innate immune responses. The use of cytokine-based therapies in combination with therapeutic vaccinations has the potential to augment viral control and facilitate the reestablishment of immune homeostasis during chronic SIV/HIV infection, but must be carefully administered at the correct time-point during the course of infection to effectively achieve a clinical benefit.
Arguably the greatest barrier to generation of a functional cure is the persistence of latently infected CD4 T cells. Biological agents aimed at “re-awakening” virus expression in latently infected cells, by interfering with signaling pathways thought to contribute to latency (latency reversing agents, LRA), may prove synergistic with immune interventions that directly enhance anti-viral immune function, thereby reducing the persistent reservoir under ART [68,**69]. Currently it is unclear what the optimal usage of these tools will be, but deciding the exact timing and treatment regimen in which vaccination and co-inhibitory blockade are administered in tandem with LRA during ART therapy, will allow for an efficient and productive anti-viral immune response that will “shock” and “kill” these latent viral reservoirs. Eliminating the latent viral reservoir is central to achieving a functional cure for HIV, as a lower viral burden post therapy interruption faced with an active functionally equipped immune response may inevitably provide host independent control of HIV.
Concluding remarks
A number of vaccine and checkpoint inhibitors modalities have been developed and tested over the past decade in a therapeutic setting during HIV and SIV infection, and some of these have been successful in inducing a strong anti-viral T cell immunity and modest effect on viral control. More recently, these approaches have been developed to treat the persistent virus reservoir under ART, possibly in combination with approaches to “kick” the latent virus out of the persistent viral reservoirs. We believe that the stage is set to combine multiple immune-based therapies, including therapeutic vaccines, to effectively target virus replication and viral reservoirs in further pre-clinical and clinical studies aimed at achieving a functional cure for HIV.
Highlights.
- Therapeutic vaccines can enhance anti-viral T cell immunity.
- Autologous virus-pulsed DC based vaccines provide a therapeutic benefit.
- Antibody based immunotherapy shows promise in controlling HIV replication.
- Combination approaches are needed to achieve a functional cure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Barouch DH, Michael NL. Accelerating HIV-1 Vaccine Efficacy Trials. Cell. 2014;159:969–972. doi: 10.1016/j.cell.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A consise review of current and future directions towards developing a safe and effective prophylactic HIV vaccine.
- 2.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 3.Jilek BL, Zarr M, Sampah ME, Rabi SA, Bullen CK, Lai J, Shen L, Siliciano RF. A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat Med. 2012;18:446–451. doi: 10.1038/nm.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 5.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapogiannis BG, Henderson SL, Nigam P, Sharma S, Chennareddi L, Herndon JG, Robinson HL, Amara RR. Defective IL-2 production by HIV-1-specific CD4 and CD8 T cells in an adolescent/young adult cohort. AIDS Res Hum Retroviruses. 2006;22:272–282. doi: 10.1089/aid.2006.22.272. [DOI] [PubMed] [Google Scholar]
- 7**.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonstrates a critical finding in the field of HIV cure research, in establshing that reservoir seeding likely occurs earlier than 3 days after a mucosal pathogenic SIV challenge despite undetectable plasma viremia.
- 8.Lori F, Lisziewicz J. Structured treatment interruptions for the management of HIV infection. JAMA. 2001;286:2981–2987. doi: 10.1001/jama.286.23.2981. [DOI] [PubMed] [Google Scholar]
- 9.Nacsa J, Stanton J, Kunstman KJ, Tsai WP, Watkins DI, Wolinsky SM, Franchini G. Emergence of cytotoxic T lymphocyte escape mutants following antiretroviral treatment suspension in rhesus macaques infected with SIVmac251. Virology. 2003;305:210–218. doi: 10.1006/viro.2002.1753. [DOI] [PubMed] [Google Scholar]
- 10.Oxenius A, Price DA, Gunthard HF, Dawson SJ, Fagard C, Perrin L, Fischer M, Weber R, Plana M, Garcia F, et al. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci U S A. 2002;99:13747–13752. doi: 10.1073/pnas.202372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz GM, Wellons M, Brancato J, Vo HT, Zinn RL, Clarkson DE, Van Loon K, Bonhoeffer S, Miralles GD, Montefiori D, et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc Natl Acad Sci U S A. 2001;98:13288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lori F, Lewis MG, Xu J, Varga G, Zinn DE, Jr., Crabbs C, Wagner W, Greenhouse J, Silvera P, Yalley-Ogunro J, et al. Control of SIV rebound through structured treatment interruptions during early infection. Science. 2000;290:1591–1593. doi: 10.1126/science.290.5496.1591. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, Robbins GK, D'Aquila RT, Goulder PJ, Walker BD. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 14.Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B, Miller MD, Else J, Pandrea I, Estes JD, et al. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6:e1000747. doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller YM, Do DH, Boyer JD, Kader M, Mattapallil JJ, Lewis MG, Weiner DB, Katsikis PD. CD8+ cell depletion of SHIV89.6P-infected macaques induces CD4+ T cell proliferation that contributes to increased viral loads. J Immunol. 2009;183:5006–5012. doi: 10.4049/jimmunol.0900141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M, Jr., et al. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med. 2009;206:1575–1588. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 18.Betts MR, Krowka JF, Kepler TB, Davidian M, Christopherson C, Kwok S, Louie L, Eron J, Sheppard H, Frelinger JA. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res Hum Retroviruses. 1999;15:1219–1228. doi: 10.1089/088922299310313. [DOI] [PubMed] [Google Scholar]
- 19.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Details the requirement of a broad cytotoxic CD8 T cell response to clear latent HIV infection.
- 21*.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reports the significance of T follicular helper CD4 T cells in contributing to the persistant viral reservoir during chronic HIV infection and antiretroviral therapy.
- 22*.Xu Y, Weatherall C, Bailey M, Alcantara S, De Rose R, Estaquier J, Wilson K, Suzuki K, Corbeil J, Cooper DA, et al. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J Virol. 2013;87:3760–3773. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Establishes that a similar population of T follicular helper CD4 T cells are found in pigtail macaques infected during pathogenic SIV infection and may contribute to ongoing viral production.
- 23*.Mylvaganam GH, Velu V, Hong JJ, Sadagopal S, Kwa S, Basu R, Lawson B, Villinger F, Amara RR. Diminished viral control during simian immunodeficiency virus infection is associated with aberrant PD-1hi CD4 T cell enrichment in the lymphoid follicles of the rectal mucosa. J Immunol. 2014;193:4527–4536. doi: 10.4049/jimmunol.1401222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extends the infection of T follicular helper cells to PD-1hi CD4 T cells found in lymphoid follicles of the rectal mucosa, in addition to the lymph nodes of chronic SIV infected rhesus macaques.
- 24.Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, Ranasinghe S, Lindqvist M, Davis I, Lane K, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4:123ra125. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauduin MC, Yu Y, Barabasz A, Carville A, Piatak M, Lifson JD, Desrosiers RC, Johnson RP. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J Exp Med. 2006;203:2661–2672. doi: 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 27.Grassi F, Hosmalin A, McIlroy D, Calvez V, Debre P, Autran B. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS. 1999;13:759–766. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 28.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 29.Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, Meyer L, Oksenhendler E, Sinet M, Hosmalin A. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 30.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez LA, Arango T, Boyer J. Therapeutic and prophylactic DNA vaccines for HIV-1. Expert Opin Biol Ther. 2013;13:563–573. doi: 10.1517/14712598.2013.758709. [DOI] [PubMed] [Google Scholar]
- 32.Fuller DH, Rajakumar PA, Wu MS, McMahon CW, Shipley T, Fuller JT, Bazmi A, Trichel AM, Allen TM, Mothe B, et al. DNA immunization in combination with effective antiretroviral drug therapy controls viral rebound and prevents simian AIDS after treatment is discontinued. Virology. 2006;348:200–215. doi: 10.1016/j.virol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol. 2002;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 34.De Rose R, Fernandez CS, Smith MZ, Batten CJ, Alcantara S, Peut V, Rollman E, Loh L, Mason RD, Wilson K, et al. Control of viremia and prevention of AIDS following immunotherapy of SIV-infected macaques with peptide-pulsed blood. PLoS Pathog. 2008;4:e1000055. doi: 10.1371/journal.ppat.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, Chougnet C, Shearer G, Altman JD, Watkins D, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med. 2000;6:1140–1146. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- 36.Walker BD, Hirsch MS. Antiretroviral therapy in early HIV infection. N Engl J Med. 2013;368:279–281. doi: 10.1056/NEJMe1213734. [DOI] [PubMed] [Google Scholar]
- 37.Fuller DH, Rajakumar P, Che JW, Narendran A, Nyaundi J, Michael H, Yager EJ, Stagnar C, Wahlberg B, Taber R, et al. Therapeutic DNA vaccine induces broad T cell responses in the gut and sustained protection from viral rebound and AIDS in SIV-infected rhesus macaques. PLoS One. 2012;7:e33715. doi: 10.1371/journal.pone.0033715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Hansen SG, Piatak M, Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reports profound control of SIV infection in animals vaccinated with RhCMV/SIV vectors and subsquently challenged with SIVmac239 intravaginally, intrarectally, or intravenously. Rhesus macaques (RM) that showed protection had no measurable plasma or tissue-associated virus using ultrasensitive assays. RhCMV/SIV vectors elicited strong virus specific effector memory CD8 T cells capable of limiting SIV infection.
- 40.Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9:27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- 41.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 42**.Garcia F, Climent N, Guardo AC, Gil C, Leon A, Autran B, Lifson JD, Martinez-Picado J, Dalmau J, Clotet B, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5:166ra162. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- Proof of concept for the therapeutic administration of monocyte-derived dendritic cells (MD-DCs) pulsed with autologous inactivated whole HIV in patients on combination ART (cART). The vaccination proved safe, well tolerated, and elicited HIV-specific immune responses that inversely correlated with plasma viral load set-point post cessation of ART.
- 43*.Levy Y, Thiebaut R, Montes M, Lacabaratz C, Sloan L, King B, Perusat S, Harrod C, Cobb A, Roberts LK, et al. Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur J Immunol. 2014;44:2802–2810. doi: 10.1002/eji.201344433. [DOI] [PubMed] [Google Scholar]
- Demonstrates that ex vivo generation of dendritic cells (DC) loaded with HIV lipopeptides in patients on ART increased the breadth of the immune reponse, expansion of functional CD4 and CD8 T cells, and the breadth of cytokines in response to antigenic stimulation.
- 44.Montefiori DC, Morris L, Ferrari G, Mascola JR. Neutralizing and other antiviral antibodies in HIV-1 infection and vaccination. Curr Opin HIV AIDS. 2007;2:169–176. doi: 10.1097/COH.0b013e3280ef691e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Implicates the potential therapeutic use of monoclonal antibody treatments to suppress viremia. Administration of a cocktail of HIV-specific antibodies and/or a single monoclonal PGT121 resulted in a rapid decline in plasma viremia to undetectable levels in pathogenic SHIV infected rhesus macaques. Durability of control persisted in a few animals despite undetectable serum levels of infused antibody. These data suggest the potential use of monoclonal antibody therapy in HIV infected individuals.
- 46**.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Jr., Lifson JD, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonstrates the therapeutic efficacy of antibody-mediated immunotherapy in both potently blocking virus acquistion and suppressing plasma viremia in SHIV infected rhesus macaques (RM). Macaques were adminstered a single dose of either 3BNC117 or 10-1074 (monoclonal antibodies specific for the CD4-binding site and the V3 region, respectively) or in combination. These results highlight the potential use of antibody immunotherapy in HIV infected individuals.
- 47.Yang L, Wang P. Passive immunization against HIV/AIDS by antibody gene transfer. Viruses. 2014;6:428–447. doi: 10.3390/v6020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ondondo BO. The influence of delivery vectors on HIV vaccine efficacy. Front Microbiol. 2014;5:439. doi: 10.3389/fmicb.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Gils MJ, Sanders RW. Broadly neutralizing antibodies against HIV-1: templates for a vaccine. Virology. 2013;435:46–56. doi: 10.1016/j.virol.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proof of concept that rhesus macaques (RM) can be immunized with a stablily expressed adeno-associated virus vector (AAV) eCD4-Ig, a fusion of CD4-Ig with a CCR5-mimetic sulfopeptide that affords protection from several repeated infectious SHIV-AD8 challenges and provides neutralization against previous neutralization resistant HIV-1, HIV-2, and SIV isolates.
- 53.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 55.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 57.Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep. 2011;8:4–11. doi: 10.1007/s11904-010-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117:5582–5590. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 62.Lori F, Maserati R, Lisziewicz J, Minoli L. Immune reconstitution and control of HIV. HIV Clin Trials. 2004;5:170–182. doi: 10.1310/QHMQ-6B3J-3D92-RC4E. [DOI] [PubMed] [Google Scholar]
- 63.Mavigner M, Cazabat M, Dubois M, L'Faqihi FE, Requena M, Pasquier C, Klopp P, Amar J, Alric L, Barange K, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. 2012;122:62–69. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheth PM, Chege D, Shin LY, Huibner S, Yue FY, Loutfy M, Halpenny R, Persad D, Kovacs C, Chun TW, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. 2008;1:382–388. doi: 10.1038/mi.2008.23. [DOI] [PubMed] [Google Scholar]
- 65.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 3 doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, McGary CS, Rogers KA, Else JG, Silvestri G, et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog. 2013;9:e1003471. doi: 10.1371/journal.ppat.1003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Establishes the potential therapeutic use of recombinant IL-21 treatment in restoring mucosal homeostasis and reducing systemic inflammation during chronic HIV infection.
- 67**.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonstrates the dynamics of the type I interferon response during transmission and acute SIV infection and the profound effects timing of the type I interferon induced innate response has on disease progression.
- 68.Siliciano JD, Siliciano RF. Recent developments in the search for a cure for HIV-1 infection: targeting the latent reservoir for HIV-1. J Allergy Clin Immunol. 2014;134:12–19. doi: 10.1016/j.jaci.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 69**.Dahabieh MS, Battivelli E, Verdin E. Understanding HIV latency: the road to an HIV cure. Annu Rev Med. 2015;66:407–421. doi: 10.1146/annurev-med-092112-152941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An extensive review discussing the molecular mechanisms that contribute to HIV latency and possible therapeutic options aimed at eradicating HIV infection.