Abstract
In addition to disease diagnostics, there is a need for biomarkers to predict severity of cancer therapy side effects such as oral mucositis. Oral mucositis is an inflammatory lesion of oral mucosa caused by high dose chemotherapy and/or radiation that is especially prevalent during oral cancer treatment. We describe here a semiautomated, modular microfluidic immunoarray optimized for ultrasensitive detection of pro-inflammatory cytokines involved in pathobiology of oral mucositis. Our goal is methodology to identify risk of mucositis early in oral cancer treatment, before the onset of lesions. Biomarkers include tumor necrosis factor (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and C-reactive protein (CRP). Protein analytes were captured from serum in a capture chamber by 1 μm magnetic beads coated with antibodies and enzyme labels. Beads are then transported downstream to a detection chamber containing an 8-sensor array coated with glutathione-gold nanoparticles (GSH-AuNP) and a second set of antibodies to capture the beads with analyte proteins. In this first application of the immunoarray to 4-protein multiplexed measurements, ultralow detection limits of 10–40 fg mL−1 in 5 μL serum were achieved for simultaneous detection in 30 min. Mass detection limits were 2.5–10 zeptomol, as few as 1500 molecules. Accuracy and diagnostic utility of the arrays was demonstrated by correlation of levels of the 4 biomarker proteins in serum from head and neck cancer patients with results from standard ELISA. This approach may lead to rapid, low-cost estimates of projected risk for severity of oral mucositis in cancer patients to enable improved therapeutic management.
Keywords: Antibodies, Cytokines, Immunoassay, Inflammatory, Microfluidic, Oral mucositis
INTRODUCTION
High-dose chemotherapy and/or radiation utilized for treatment of cancer generates a complex inflammatory response in mucosal tissue called mucositis. The pathobiology is characterized by initial up-regulation of reactive oxygen species that can damage adjacent cells as well as cause activation of cell signaling pathways that escalate mucosal injury [1]. At the clinical level, oral mucositis is a frequent and debilitating complication of cancer therapies and occurs in more than 400,000 patients annually, with a mortality rate of 10% due to sepsis [1–3].
There has been an increasing trajectory of basic and clinical research directed to cancer treatment toxicity over the past 15 years. The contemporary pathobiologic model includes activation of transcription factor nuclear factor-κB (NF-κB) as a consequence of chemotherapy and radiotherapy that may contribute to development of mucositis [1–4]. Among many genes up-regulated by activation of NF-κB are those responsible for production of pro-inflammatory cytokines tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) [1–4]. Increased levels of these cytokines in tissues and peripheral blood coincide with non-hematological toxicity caused by radiation and chemotherapy and lead to tissue injury and cell apoptosis [4]. Measurements of NF-κB, TNF-α, IL-6 and IL-1β in the sera of rat models showed that concentrations of these proteins increase following administration of chemotherapeutic agents [2]. Experimental models show that drug-induced inhibition of the production of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β lessens the severity and frequency of mucositis [1,4]. High levels of C-reactive protein (CRP) have also been detected in sera of patients with radiation-induced mucositis [5,6]. For therapy side effects such as oral mucositis, multiplexed biomarker measurements could in future enable prediction of severity and frequency of mucositis based on personalized biomarker profiles determined at early stages of cancer treatment, before the onset of serious lesions.
We recently developed a prototype microfluidic electrochemical immunoarray that is 50–1000 times more sensitive and faster than traditional ELISA and bead-based assays using off-line capture of protein analytes on magnetic beads decorated with massive numbers of enzyme labels and antibodies [7, 8]. Massively labeled magnetic beads, a modular microfluidic platform, and nanostructured sensors enable rapid, ultrasensitive, simultaneous detection of biomarker proteins in small-volume serum samples (~5 μL). This system was effectively used to measure IL-6 and prostate specific antigen (PSA) in serum from prostate cancer patients [7] and IL-6, IL-8, vascular endothelial growth factor (VEGF) and VEGF-C in sera of oral cancer patients [8].
In the present paper, we adapt this analyte capture strategy to detect a panel of biomarkers associated with oral mucositis using a more advanced semi-automated on-line capture format. The device includes a protein capture chamber upstream of the 8-sensor detection chamber [9], and first delivers the sample to this capture chamber, where enzyme-antibodies decorated magnetic beads capture each target protein. Beads are washed under magnetic control and delivered to a nanostructured 8-sensor array decorated with a second set of antibodies that recognize and sort the magnetic bead-bound target proteins. After washing, the biomarker proteins are measured simultaneously by chemical activation of enzyme labels and electrochemical detection. We report here the first true multiplexed application of this technology to simultaneous ultrasensitive detection of 4 proteins.
Target proteins TNF-α, IL-6, IL-1β and CRP were selected for reported links to oral mucositis [1, 10, 11]. Herein, we demonstrate that the four proteins can be measured accurately, quickly, with high sensitivity using the novel on-line capture microfluidic immunoarray. Ultralow detection limits of 10–40 fg mL−1 were achieved for multiplex detection in 5 μL serum (2.5–10 zeptomol). Accuracy was verified by agreement of immunoassay results with ELISA values in oral cancer patients undergoing radiation therapy.
MATERIALS AND METHODS
Full experimental procedures and materials are reported in the Electronic Supplementary Material (ESM).
On-line capture and detection of target proteins
Screen printed carbon sensors in the array (Fig. 1 panel IIA) were coated with sequential layers of the polycation PDDA and 5 nm glutathione-coated gold nanoparticles (GSH-AuNPs) as previously reported [8]. Surface carboxyl groups on GSH-AuNPs were activated by freshly prepared EDC and NHSS to attach primary antibodies (Ab1) to the array through amidization [7, 8]. Arrays were then washed and incubated with 2% BSA in PBS for 1 hr to block non-specific binding (NSB) (see ESM Fig. S2). Multiple biotinylated HRPs and biotinylated antibodies (Ab2) were attached to 1 μm diameter streptavidin-coated super-paramagnetic particles (MPs) as previously described (see ESM Fig. S3) [8].
Fig. 1.
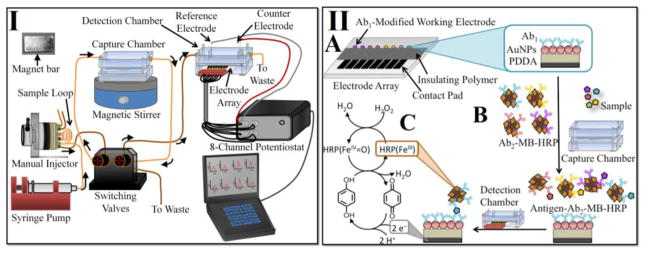
I. Modular microfluidic electrochemical immunoarray system. II. Immunoassay strategy for detecting the four protein analytes in serum on (A) the primary antibody (Ab1)-modified electrode surface. (B) On-line capture of protein analytes onto the secondary antibody (Ab2)-MP-HRP in the capture chamber to form protein-bead bioconjugates. (C) Amperometric signal generation by injecting a mixture of hydroquinone and H2O2 into the detection chamber.
The microfluidic system was configured and used with magnetic Ab2-MP-HRP detection beads and Ab1-decorated sensor arrays as previously reported [9] (Fig. 1 panel I and II). The capture and detection chamber was first subjected to a flow of PBS-T20 detergent for 4 mins to minimize NSB. 40 μL of Ab2-MP-HRP (1 mg mL−1 MPs) was dispersed in 120 μL of 20 mM PBS pH 7.4, loaded into a 100 μL sample loop and injected at 100 μL min−1 into the capture chamber. A magnet bar (N55-neodymium magnet) was then placed on top of the capture chamber to trap Ab2-MP-HRP prior to injection of the sample. Subsequently, biomarker protein standard (5 μL of protein analyte in 5-fold diluted calf serum) or patient sample was loaded into the sample loop and injected into the capture chamber. For multiplexed detection, a mixture of 20 μL of the Ab2-MP-HRP bioconjugate for each analyte was dispersed in 80 μL of PBS, loaded into the 100 μL sample loop and injected into the capture chamber, followed by injection of a mixture of the four standard target proteins diluted in calf serum for calibrations. Once the capture chamber was filled with sample, flow was stopped followed by the removal of the magnet bar to allow for incubation for 30 mins with stirring in the chamber to facilitate analyte capture. The resulting protein-Ab2-MP-HRP conjugates were flushed with PBS–Tween20 for 2 mins while the magnet is on top of the capture chamber. The excess unbound protein not trapped was thus washed off to waste. Flow was stopped, magnet bar removed and the protein–Ab2–MP–HRP was mixed with PBS-Tween20 for 1 minute. A valve was then switched to transport the protein–Ab2–MP–HRP to the detection chamber. When protein-Ab2-MP-HRP conjugates filled the detection chamber, flow was stopped for 15 min to capture of the conjugate beads on the Ab1-modified sensor surfaces. Buffer flow was then resumed to wash any unbound beads from the microfluidic channel for 4 min.
The 8 sensors in the array and common Pt counter and Ag/AgCl reference electrodes were connected to a CHI 1040A multipotentiostat (see ESM Fig. S1B). To generate amperometric peaks, a mixture of 1 mM hydroquinone (HQ) mediator and 0.1 mM H2O2 was injected into the microfluidic device through the 100 μL sample loop to activate the HRP labels trapped on the sensors. Amperometric signals were measured with potential at −0.2 V vs. Ag/AgCl (0.14 M NaCl) [9]. The electron mediator (HQ) reduces activated ferryloxy-HRP, regenerating HRP. Signal is developed by reduction of oxidized mediator (benzoquinone) on the sensor surface (Fig. 1 panel IIC).
RESULTS
Multiplex Detection of IL-6, TNF-α, CRP, and IL-1β
The microfluidic array was first optimized separately for TNF-α, IL-6, IL-1β, and CRP in 5-fold diluted calf serum (see ESM Fig. S3). Amperometric responses for single biomarker proteins showed high sensitivity over 3 decades of concentration from pg mL−1 to the low fg mL−1 (see ESM Fig. S5 A, C, E, G). For multiplexed detection, primary antibodies for each protein were attached to two of the 8-sensors on the array. Individual HRP-labeled magnetic beads with antibodies for IL-6, TNF-α, CRP, and IL-1β were combined in PBS, and injected into the capture chamber, followed by injection of protein standards or samples. Binding studies related to the four protein analytes showed acceptably low cross-reactivity for all antibodies (see ESM Fig. S6).
Multiplexed detection (Fig. 2A, C, E, G) gave high sensitivity in the pg mL−1 to low fg mL−1 range. A linear relationship between amperometric signal and log protein concentration was found using mixed standards for fg mL−1 to low pg mL−1 levels with R2 values of 0.98 (± 0.02) (Fig. 2B, D, F, H). Relative standard deviations were 6 (± 3)%. Detection limits (DLs) measured as three standard deviations above the average control were 18 fg mL−1 for IL-6, 10 fg mL−1 for TNF-α, 15 fg mL−1 for CRP and 40 fg mL−1 for IL-1β for multiplex detection. The multiplexed DLs are lower by 50–100 times compared to standard ELISAs (see ESM Table S1).
Fig. 2.
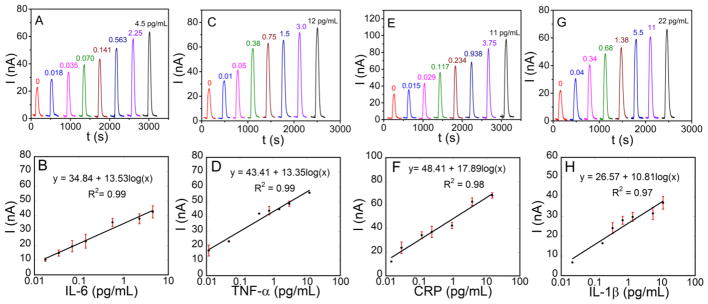
Amperometric responses for standard protein mixtures in 5-fold diluted calf serum for (A) IL-6, (C) TNF-α, (E) CRP, and (E) IL-1β, developed by injecting a mixture of 1 mM HQ and 0.1 mM H2O2 at −0.2 V vs. Ag/AgCl and the corresponding calibration plots for (B) IL-6, (D) TNF-α, (F) CRP, and (H) IL-β.
Assay validation using human serum samples
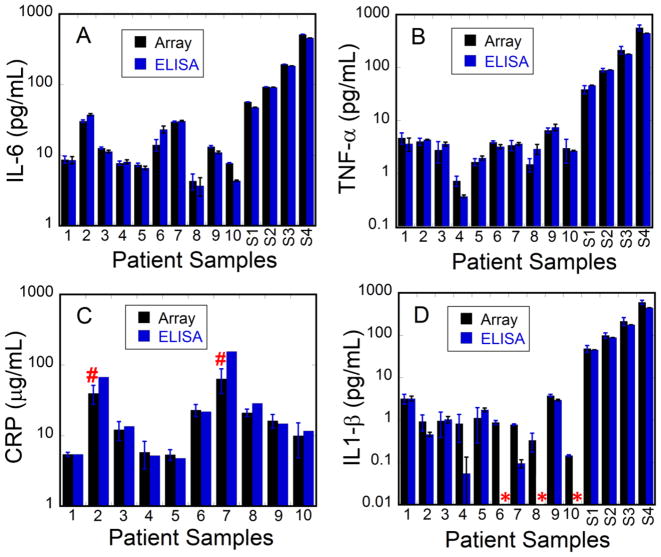
The microfluidic array was used to measure IL-6, TNF-α, and IL-1β simultaneously in ten serum samples from head and neck cancer patients undergoing radiation therapy. CRP was measured separately due to its very high concentration in oral cancer patient serum. For CRP, only 8 of the samples were analyzed as two of them had CRP levels well above our linear range after standard dilution, and amounts of sample were limited. In addition, CRP ELISA results were from a single sample run because of limited sample amounts. Serum samples collected on day 14 of radiation (1, 2, 3), day 35 of radiation (4, 5, 6, 7) and 21 days post-radiation (8, 9, 10) were used (see ESM Table S2). Samples (5 μL) were diluted 30-fold in PBS prior to assay to bring concentrations into the linear range of the calibrations. Several patient samples were spiked with different concentrations of standards to augment accuracy and analytical recovery assessments. Good correlation of our results with those obtained from standard single-protein ELISA measurements are illustrated using bar graphs (Fig. 3), and by linear correlation plots of immunoarray results vs. single protein ELISAs. For this small sample set (≤10), linear plots resulted in slopes and R2-values close to 1 and intercepts near 0 for all target proteins suggesting relatively good correlation for all the biomarker proteins (see ESM Fig. S7 and Table S3). The correlation plot slope for CRP (0.78) is smaller than that of other proteins. This may be due to high levels of the protein in the μg mL−1 range requiring 1 million-fold serial dilutions that may generate volumetric errors, in addition to the fact that only one ELISA assay measurement for CRP was obtained for each sample. Nevertheless, as with all target proteins, CRP microfluidic immunoassay results gave self-consistent values for individual samples that were similar to ELISA for most samples (Fig. 3C).
Fig. 3.
Immunoarray and ELISA assay results from serum samples of cancer patients for (A) IL-6, (B) TNF-α, (C) CRP, and (D) IL-1β. S1–S4 corresponds to patient samples spiked with 50, 100, 200, and 500 pg mL-1 respectively. (*) corresponds to values below the detection limits of ELISA and (#) corresponds to values above the dynamic range of the microfluidic immunoarray.
DISCUSSION
Results demonstrate the ability of the microfluidic immunorray to rapidly measure four proteins in 5 μL of serum simultaneously. Massively labeled magnetic beads with 300,000 enzyme labels and 40,000 antibodies provided very high sensitivity. Detection limits of 10–40 fg mL−1 for serum (mass detection limits 2.5–10 zeptomol) were achieved for multiplexed detection. Detection limits are 10–1000 times better than those of ELISA or commercial multiplexed bead-based assays.
Sensitivities of immunoarray assays obtained from the slope of the calibration curves ranged from 4.18–7.07 (nA/cm2) (mL/pg protein) (see ESM Table S1). Assay time (30 min) and minimal sample volume (5 μL) are additional factors highlighting potential utility of these microfluidic arrays in the clinic. The method is general and can be adapted to other small panels of protein. Accuracy of the assay in complex mixtures was verified by good correlations between immunoarray results and ELISAs for human patient serum levels. Compared to ELISA, our system is inexpensive ($6 per array vs > $300 per ELISA kit), requires small sample volume (5 μL vs >100 μL), shorter assay times (30 mins vs >3 hrs) and have multiplexing capability (up to 4 proteins simultaneously vs, one protein for ELISA) [8]. In terms of sample volume, our assay requires 5 μL sample for a 4-protein multiplexed assay in 30 min. vs. >400 μL for the 4 ELISA assays in >12 hrs. The levels of proteins in spiked samples demonstrate the analytical accuracy of the immunoassay with % recovery ~ 100%. The ability of the arrays to accurately detect individual biomarkers in serum that contains many hundreds of potentially interfering proteins demonstrates high selectivity of the immunoassay.
Preliminary correlation analysis of patient sample protein levels assayed in our work vs. standard metrics of oral mucositis severity suggested a strong correlation with IL-6, but results are inconclusive due to the limited number of samples. An ongoing related study by some of us (RVL, LAC, DEP) utilizing a wider range of samples established tentative correlations with IL-6 and CRP as biomarkers for oral mucositis (unpublished data).
Supplementary Material
Acknowledgments
This work was supported financially by Grant No. EB014586 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), NIH. Serum samples were obtained in a study supported by grant K23DE016946 from the National Institute of Dental and Craniofacial Research and grant M01RR06192 from the National Center for Research Resources. We thank Colleen Holewa for initial assay development of TNF-α.
References
- 1.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–84. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 2.Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DMK. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol. 2008;62:33–41. doi: 10.1007/s00280-007-0570-0. [DOI] [PubMed] [Google Scholar]
- 3.Peterson D, Srivastava R, Lalla R. Oral mucosal injury in oncology patients: perspectives on maturation of a field. Oral Dis. 2013 doi: 10.1111/odi.12167. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Morales-Rojas T, Viera N, Morón-Medina A, Alvarez CJ, Alvarez A. Proinflammatory cytokines during the initial phase of oral mucositis in patients with acute lymphoblastic leukaemia. Int J Paediatr Dent. 2012;22:191–6. doi: 10.1111/j.1365-263X.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- 5.Ki Y, Kim W, Nam J, Kim D, Park D, Kim D. C-reactive protein levels and radiation-induced mucositis in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;75:393–8. doi: 10.1016/j.ijrobp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed FF, Poon I, Zhang L, Elliott L, Hodson ID, Sagar SM, Wright J. Acute-phase response reactants as objective biomarkers of radiation-induced mucositis in head and neck cancer. Head Neck. 2012;34:985–93. doi: 10.1002/hed.21848. [DOI] [PubMed] [Google Scholar]
- 7.Chikkaveeraiah BV, Mani V, Patel V, Gutkind JS, Rusling JF. Microfluidic electrochemical immunoarray for ultrasensitive detection of two cancer biomarker proteins in serum. Biosens Bioelectron. 2011;26:4477–83. doi: 10.1016/j.bios.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra R, Patel V, Chikkaveeraiah BV, Munge BS, Cheong SC, Zain RB, Abraham MT, Dey DK, Gutkind JS, Rusling JF. Ultrasensitive detection of cancer biomarkers in the clinic by use of a nanostructured microfluidic array. Anal Chem. 2012;84:6249–55. doi: 10.1021/ac301392g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otieno BA, Krause CE, Latus A, Chikkaveeraiah BV, Faria RC, Rusling JF. On-line protein capture on magnetic beads for ultrasensitive microfluidic immunoassays of cancer biomarkers. Biosens Bioelectron. 2014;53:268–274. doi: 10.1016/j.bios.2013.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brailo V, Vucicevic-Boras V, Lukac J, Biocina-Lukenda D, Zilic-Alajbeg I, Milenovic A, Balija M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med Oral Patol Oral Cir Bucal. 2012;17:10–5. doi: 10.4317/medoral.17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jablonska E, Piotrowski L, Grabowska Z. Serum Levels of IL-1β, IL-6, TNF-α, sTNF-RI and CRP in Patients with Oral Cavity Cancer. Pathol Oncol Res. 1997;3:126–129. doi: 10.1007/BF02907807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.