Abstract
Inflammation can lead to malabsorption of important micronutrients such as iron. Malabsorption and nutritional deficiency can be caused by a variety of pathological and environmental factors causing a range of other symptoms commonly caused by both H. pylori infection and coeliac disease (CD). National guidelines suggest the routine taking of duodenal biopsies to exclude CD when investigating patients for iron deficiency anemia (IDA). Studies suggest that in absence of positive antibodies, IDA is rarely caused by CD. Recent British Society of Gastroenterology guidelines discourage the routine duodenal biopsies in low risk cases but despite this guidance, taking duodenal biopsies for IDA is a common practice. Many studies have reported that H. pylori infection is associated with IDA even in patients with CD. In countries with low H. pylori prevalence we still detect more H. pylori than CD standing behind IDA. Despite the strong association between IDA and H. pylori, taking biopsies to diagnose H. pylori infection is not usually a routine part of the diagnostic workup to identify the etiology of IDA. In this review we will discuss the impact of H. pylori in IDA and highlight the possible gaps in identifying the IDA etiology.
Key Words: Helicobacter pylori, Iron deficiency anaemia, Coeliac disease
Introduction
Iron deficiency anaemia (IDA) is a common cause of referral to gastroenterologists (4%-13% of referrals) (1) and both coeliac disease (CD) and H pylori are associated with IDA. A significant number of patients with IDA (5-10%) present with idiopathic form of IDA with no clear aetiology established, even after extensive examination (2, 3) (Table 1). The effect of H. pylori infection on iron absorption is likely to be multifactorial. Several mechanisms may lead to a decrease in iron absorption including hypochlorhydria and decreased ascorbic acid secondary to chronic gastritis (4-8). Another potentially important mechanism is increased hepcidin production from hepatocytes in response to IL-6 production, secondary to H. pylori infection, IBD, CD or other inflammatory conditions, leading to reduced iron absorption (9).
Table 1.
Frequency investigated etiology of iron deficiency anaemia
| Etiology | Frequency (%) |
|---|---|
| Colonic carcinoma | 5-10 |
| Gastric carcinoma | 5 |
| Benign gastric ulceration | 5 |
| Angiodysplasia | 5 |
| Coeliac disease | 4-6 |
| Excessive blood loss during menstruation | 20-30 |
| Gastric antral vascular ectasia | 1-2 |
| NSAIDs related | 10-15 |
A microscopic enteropathy, with an increased numbers of intestinal intraepithelial lymphocytes (IELs), might be associated with IDA. Increased IELs has been reported in disorders such as H. pylori infection, CD, giardia infection, IgA deficiency, and Crohn’s disease (10-12).
Several studies have reported that numbers of intraepithelial lymphocytes in the duodenal mucosa are more likely increased in patients with H pylori infection. This subtle small bowel inflammation might be implicated in reduced iron absorption and potentially treated by the H. pylori eradication.
H. pylori infection and CD can both be present. Although epidemiological investigations have not confirmed an association between gastritis and CD (13-16), other studies have reported a H. pylori related lymphocytic gastritis and anaemia in patients with CD (17).
Taking duodenal biopsies in every patient with negative coeliac serology presenting with IDA may not be indicated. In this review article we discuss some weaknesses in current policy assessing patients with IDA.
Discussion
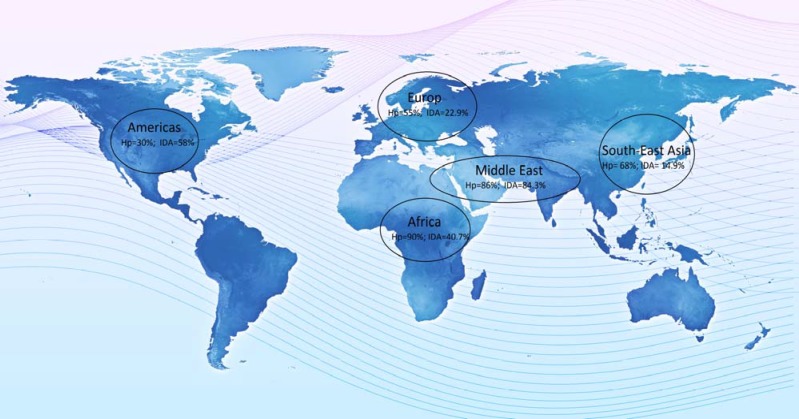
Currently, many studies demonstrate a relationship between IDA and CD and H. pylori infection. A number of studies have suggested that the frequency of H. pylori is high in different parts of the world (24). Around one-third of adults in North American and north European residents are still infected. The prevalence of H. pylori infection is often more than 50% in South and Eastern Europe, South America, and Asia (figure 1). In 2005, Hershko et al. prospectively studied 150 IDA patients to confirm the role of H. pylori and CD in refractory or unexplained IDA (18). In this study, Hershko et al. aimed to confirm the role of H pylori and CD in refractory or unexplained IDA. The authors reported that H. pylori infection was found in 19% of patients and CD was identified in 15% adult patients. According to this study, H. pylori is a major etiological factor for refractory IDA with a higher association rate compared to CD (table 2).
Figure 1.
Global map of H. pylori (Hp) infection and Iron Deficiency Anemiea (IDA).
In countries where little meat is in the diet, IDA is more prevalent than in North Americas and Europe.
Table 2.
Other controverted common etiology of iron deficiency anaemia, less frequently investigated
| H pylori infection |
| Chronic kidney disease |
| Gastrointestinal or systemic |
| Inflammations/autoimmune disorders |
| Non-coeliac gluten sensitivity |
Failing to test for H. pylori infection could lead to a failure to identify a treatable cause of anaemia and could lead to additional and potentially unnecessary investigations.
The study suggests that routine testing for H. pylori in IDA would be appropriate. Cuoco et al. examined the relationship between H. pylori and IDA in 362 patients with CD (19). H. pylori infection was present in 21% (77) of cases. Among the remaining H. pylori-negative subjects, 28% (81 cases) showed anaemia (P< 0.001). The results of this study showed a significant association between H. pylori infection and IDA in patients with CD. This study confirms that H. pylori might be another major etiology of IDA even in patients with CD. It might be entirely appropriate and cost effective to incorporate H. pylori investigations in patients with IDA and those patients who have a diagnosis of CD with persistent anaemia in addition to a gluten free diet. The evidence behind the H. pylori associated IDA in these studies (16-18) strongly suggest that the order of investigation in clinical practice needs to be revised, with the introduction of routine early testing for H. pylori infection into standard practice.
H.pylori and CD frequently are found in association with refractory IDA (20). Refractory IDA may be due to clinically asymptomatic H. pylori infection. Treating CD and eradication of H. pylori with additional iron therapy may assist with the correction of anaemia (21). The association between CD and H. pylori is controversial. However in the presence of IDA the association between these 2 conditions has become more significant according to Demir et al (22).
The result of their study showed that 42% of patients with CD were infected with H. pylori and 7 (47%) of them had iron deficiency anemia. They suggested that CD itself plays a major role in the development of IDA. Contrary to the majority of studies Simondi et al demonstrated that the frequency of H. pylori infection was not significantly different in CD patients with or without IDA (23).
Conclusion
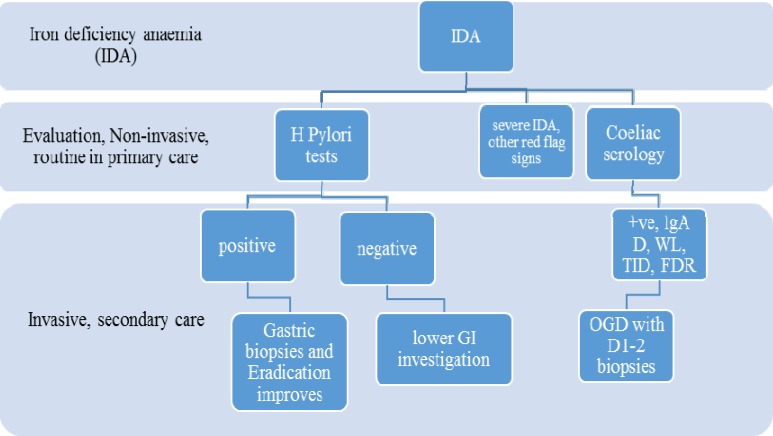
H. pylori infection has a greater prevalence in North America, south/east Europe Asian and Middle Eastern countries. The global prevalence of CD has less variation, with exception of those countries that have traditionally a lower dietary gluten intake. A proportion of patients with IDA might have anaemia secondary to H.pylori infection, especially in countries where H. pylori is very common. There are many studies that report H. pylori infection as a strong etiological factor for IDA, even among patients with CD. The importance of H. pylori infection as a common and readily reversible cause of IDA is not reflected appropriately in current guidelines and routine clinical practice. Investigation and eradication of H.pylori should be incorporated into IDA diagnostic workup, especially in populations where infection is endemic. Achlorhydric gastric atrophy is a common evidence based etiology for IDA. Therefore gastric biopsies should be taken in patients with no other explanation for anaemia (25) (Figure 2).
Figure 2.
Proposed Algorithm
IDA: Iron deficiency anemia; IgA D; IgA deficiency, WL; weight loss, TID; type I diabetes, FDR; first degree relative of coeliac patients
References
- 1.McIntyre AS, Long RG. Prospective survey of investigations in outpatients referred with iron deficiency anaemia. Gut. 1993;34:1102–107. doi: 10.1136/gut.34.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardwick RH, Armstrong CP. Synchronous upper and lower gastrointestinal endoscopy is an effective method of investigating iron-deficiency anaemia. Br J Surg. 1997;84:1725–28. [PubMed] [Google Scholar]
- 3.Zamani F, Mohamadnejad M, Shakeri R, Amiri A, Najafi Ghavamzadeh A, Malekzadeh R. Gluten sensitive enteropathy in patients with iron deficiency anemia of unknown origin. World J Gastroenterol. 2008;14:7381–85. doi: 10.3748/wjg.14.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JW. Does Helicobacter pylori infection relate to iron deficiency anaemia in prepubescent children under 12 years of age? Acta Paediatr. 2003;92:970–72. [PubMed] [Google Scholar]
- 5.Lombard M, Chua E, O’Toole P. Regulation of intestinal nonhaem iron absorption. Gut. 1997;40:435–93. doi: 10.1136/gut.40.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capurso G, Lahner E, Marcheggiano A, Caruana P, Carnuccio A, Bordi C, et al. Involvement of the corporal mucosa and related changes in gastric acid secretion characterize patients with iron deficiency anaemia associated with Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:1753–61. doi: 10.1046/j.1365-2036.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- 7.Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, et al. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut. 2003;52:496–501. doi: 10.1136/gut.52.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciacci C, Sabbatini F, Cavallaro R, Castiglione F, Si Bella S, Iovino P, et al. Helicobacter pylori impairs iron absorption in infected individuals. Dig Liver Dis. 2004;36:455–60. doi: 10.1016/j.dld.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Pellicano R, Rizzetto M. Is hepcidin the bridge linking Helicobacter pylori and anemia of chronic infection? A research proposal. Panminerva Medica. 2004;46:165–59. [PubMed] [Google Scholar]
- 10.Rostami K, Aldulaimi D, Holmes G, Johnson MW, Robert M, Srivastava A, et al. Microscopic enteritis: Bucharest consensus. World J Gastroenterology. 2015;21:2593–604. doi: 10.3748/wjg.v21.i9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pallav K, Leffler DA, Tariq S, Kabbani T, Hansen J, Peer A, et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. 2012;35:380–390. doi: 10.1111/j.1365-2036.2011.04938.x. [DOI] [PubMed] [Google Scholar]
- 12.Aziz I, Key T, Goodwin JG, Sanders DS. Predictors for celiac disease in adult cases of duodenal intraepithelial lymphocytosis. J Clin Gastroenterol. 2015;49:477–82. doi: 10.1097/MCG.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 13.Memeo L, Jhang J, Hibshoosh H, Green PH, Rotterdam H, Bhagat G. Duodenal intraepithelial lymphocytosis with normal villous architecture: common occurrence in H. pylori gastritis. Mod Pathol. 2005;18:1134–44. doi: 10.1038/modpathol.3800404. [DOI] [PubMed] [Google Scholar]
- 14.Memeo L, Jhang J, Hibshoosh H, Green PH, Rotterdam H, Bhagat G. Duodenal intraepithelial lymphocytosis with normal villous architecture: Common occurrence in H. pylori gastritis. Mod Pathol. 2005;18:1134–44. doi: 10.1038/modpathol.3800404. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree JE, O'Mahony S, Wyatt JI, Heatley RV, Vestey JP, Howdle PD, et al. Helicobacter pylori serology in patients with coeliac disease and dermatitis herpetiformis. J Clin Pathol. 1992;45:597–600. doi: 10.1136/jcp.45.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massarrat S, Saberi-Firoozi M, Soleimani A, Himmelmann GW, Hitzges M, Keshavarz H. Peptic ulcer disease, irritable bowel syndrome and constipation in two populations in Iran. Eur J Gastroenterol Hepatol. 1995;7:427–33. [PubMed] [Google Scholar]
- 17.Rostami-Nejad M, Villanacci V, Mashayakhi R, Molaei M, Bassotti G, Zojaji H, et al. Celiac disease and Hp infection association in Iran. Rev Esp Enferm Dig. 2009;101:850–54. doi: 10.4321/s1130-01082009001200004. [DOI] [PubMed] [Google Scholar]
- 18.Hershko C, Hoffbrand AV, Keret D, Souroujon M, Maschler I, Monselise Y, et al. Role of autoimmune gastritis, Helicobacter pylori and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica. 2005;90:585–95. [PubMed] [Google Scholar]
- 19.Cuoco L, Cammarota G, Jorizzo RA, Santarelli L, Cianci R, Montalto M, et al. Link between Helicobacter pylori infection and iron-deficiency anaemia in patients with coeliac disease. Scand J Gastroenterol. 2001;36:1284–88. doi: 10.1080/003655201317097137. [DOI] [PubMed] [Google Scholar]
- 20.Corazza GR, Valentini RA, Andreani ML, D'Anchino M, Leva MT, Ginaldi L, et al. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand J Gastroenterol. 1995;30:153–56. doi: 10.3109/00365529509093254. [DOI] [PubMed] [Google Scholar]
- 21.Fayed SB, Aref MI, Fathy HM, Abd El Dayem SM, Emara NA, Maklof A, et al. Prevalence of celiac disease, Helicobacter pylori and gastroesophageal reflux in patients with refractory iron deficiency anemia. J Trop Pediatr. 2008;54:43–53. doi: 10.1093/tropej/fmm080. [DOI] [PubMed] [Google Scholar]
- 22.Demir H, Saltik IN, Yüce A, Ozen H, Gürakan F, Koçak N. Is there any relation between Helicobacter pylori infection and iron deficiency anemia in children with celiac disease? Helicobacter. 2004;9 doi: 10.1111/j.1083-4389.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 23.Simondi D, Ribaldone DG, Bonagura GA, Foi S, Sapone N, Garavagno M, et al. Helicobacter pylori in celiac disease and in duodenal intraepithelial lymphocytosis: Active protagonist or innocent bystander? Clin Res Hepatol Gastroenterol. 2015;pii:S2210–7401. doi: 10.1016/j.clinre.2015.03.005. (15)00079-0. [DOI] [PubMed] [Google Scholar]
- 24.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19:1–5. doi: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 25.Dickey W1, Kenny BD, McMillan SA, Porter KG, McConnell JB. Gastric as well as duodenal biopsies may be useful in the investigation of iron deficiency anaemia. Scand J Gastroenterol. 1997;32:469–72. doi: 10.3109/00365529709025083. [DOI] [PubMed] [Google Scholar]