Abstract
The present study aimed to evaluate antioxidant and acetylcholinesterase (AChE) inhibitory activities of the ethanolic extracts of six selected Thymus species growing in Croatia (T. longicaulis, T. praecox subsp. polytrichus, T. pulegioides, T. serpyllum subsp. serpyllum, T. striatus, and T. vulgaris). Antioxidant effectiveness was assessed using six different assays, in comparison with rosmarinic acid, luteolin, and reference antioxidants. All tested Thymus extracts possessed DPPH (IC50 = 3–6 μg/mL) and nitric oxide (IC50 = 70–177 μg/mL) free radical scavenging activities, strong reducing properties (IC50 = 11–15 μg/mL), ferrous ion chelating activity (IC50 = 126–389 μg/mL), ability to inhibit lipid peroxidation (IC50 = 34–80 μg/mL), and high total antioxidant capacities (238–294 mg AAE/g). AChE inhibitory activity was examined using Ellman's colorimetric method and all tested extracts showed anti-AChE activity in a dose dependent manner. The values of 10–28%, 23–39%, and 64–86% were obtained for tested concentrations of 0.25, 0.5, and 1 mg/mL, respectively. Additionally, the contents of total hydroxycinnamic derivatives, flavonoids, and tannins in dried plant samples were determined spectrophotometrically. Our results highlighted Thymus species as a rich source of natural antioxidants and AChE inhibitors that could be useful in preventing and treating Alzheimer's disease and other neurodegenerative disorders.
1. Introduction
In recent years, natural antioxidants from medicinal plants have been intensively investigated in order to find compounds capable of protecting against a number of diseases related to oxidative stress and free radical-induced damage. Oxidative stress, as an imbalance between production and removal of free radicals and other reactive species, is well known to cause the oxidation of important biomolecules leading to cellular damage and death. Therefore, oxidative stress plays critical role in the pathogenesis of various diseases, including cardiovascular, neurodegenerative, and inflammatory diseases, cancer, and diabetes, as well as aging processes. Aiming to neutralize the deleterious effects of free radicals and reactive oxygen and nitrogen species, different antioxidant strategies have involved enhancement of enzymatic or nonenzymatic defense mechanisms through dietary or pharmacological means [1]. Plant polyphenols, as being widely distributed secondary metabolites in plant kingdom and abundant in our diet, are considered to be one of the most important classes of phytochemicals. Nowadays, polyphenols have gained a lot of importance because of their potential use as prophylactic and therapeutic agents in many diseases, and much work has been presented by the scientific community which focuses on their antioxidant effects [2]. Polyphenols can prevent that oxidative damage acting as free radical scavengers, reducing agents, chelating transition metals to suppress the initiation of radical formation, inhibiting lipid peroxidation, and regulating defense enzymes, as well as modulating cell signaling pathways and gene expression [3].
Alzheimer's disease (AD) as the most common cause of dementia is a progressive age-related neurodegenerative disorder that is characterized by loss of memory and cognitive abilities which significantly interfere with person's daily life. Characteristic neuropathological findings include selective neuronal and synaptic losses, extracellular neuritic plaques containing the amyloid β-peptide, and intracellular neurofibrillary tangles composed of hyperphosphorylated forms of the tau protein, as well as accumulation of transition metals such as copper, zinc, and iron [4–6]. Despite decades of research and advances in understanding of AD pathogenesis, current pharmacotherapeutic options are still very limited and represent an area of significant unmet need. One of the most used therapeutic strategies in the AD treatment, based on the cholinergic hypothesis, is the use of inhibitors of acetylcholinesterase (AChE), the principal enzyme involved in the hydrolysis of acetylcholine in central cholinergic synapses. Inhibition of AChE also serves as a strategy for the treatment of senile dementia, ataxia, myasthenia gravis, and Parkinson's disease [7]. The present drugs with AChE inhibitory activity are effective only against mild to moderate type of AD, provide only temporary symptomatic relief, and possess some considerable side effects related to cholinergic stimulation in brain and peripheral tissues [8]. Hence, the search for new sources of antiacetylcholinesterase agents which are effective, selective, and with less negative effects has increased greatly in recent years. Previous studies have shown that various plant extracts and their bioactive compounds provide promising alternatives to current therapies for neurodegenerative disorders [9]. Increased oxidative stress and impaired cellular energy metabolism are characteristics of many important age-related diseases, and AD is no exception. Cells in the brains of AD patients exhibit abnormally high amounts of oxidatively modified proteins, lipids, and DNA. Such free radical-mediated molecular damage is particularly prominent in the environment of plaques and in neurofibrillary tangle-bearing neurons suggesting roles for oxidative stress in AD [6]. In this regard, it is important for any drug candidate, which can be used for AD treatment, to possess cholinesterase inhibitory potential and antioxidant activity.
Among the aromatic plants belonging to the Lamiaceae family, the genus Thymus is noteworthy for the numerous species and varieties of wild growing plants. Thymus species are perennial, aromatic herbs, and subshrubs native to Europe, North Africa, and Asia. They are commonly used as culinary herbs and flavouring agents. Because of their antimicrobial, spasmolytic, and antioxidant effects, they are also used for medicinal purposes [10]. Also, anti-inflammatory, antinociceptive, and antitumor activities of Thymus species have been reported [11, 12]. The important representatives of genus Thymus wild growing in Croatia, studied in this paper, are T. longicaulis C. Presl, T. praecox Opiz subsp. polytrichus (A. Kerner Ex Borbás) Jalas, T. pulegioides L., T. serpyllum L. subsp. Serpyllum, and T. striatus Vahl. They are known as a traditional medicine for the treatment of cold, flu, cough, headache, neurosis, stomach diseases, nephritis, and high cholesterol [13–15]. Most of the previous studies of these plants were focused on volatile compounds while other bioactive components and their biological effects have not yet been fully investigated. Due to the lack of scientific evidence, the present study aimed to evaluate antioxidant and anti-AChE potential of the ethanolic extracts of selected wild growing Croatian Thymus species in comparison with T. vulgaris. Additionally, the contents of different polyphenolic components, hydroxycinnamic acids, flavonoids, and tannins were determined in all studied plants.
2. Materials and Methods
2.1. Plant Material
Aerial parts of five investigated Thymus species were collected at the full flowering stage in June and July 2011 from different locations in Croatia: Thymus longicaulis C. Presl (Jasenice, 130 m a.s.l.), Thymus praecox Opiz subsp. polytrichus (A. Kerner Ex Borbás) Jalas (Veliki Alan, 1340 m a.s.l.), Thymus pulegioides L. (Ivanščica, 900 m a.s.l.), Thymus serpyllum L. subsp. serpyllum (Đurđevački pjesci, 110 m a.s.l.), and Thymus striatus Vahl (Buljma, 1450 m a.s.l.). The commercial sample of Thymi herba (Thymus vulgaris L.) was obtained from Jan Spider (Pitomača, Croatia). All plant samples were identified at the Department of Pharmacognosy, Faculty of Pharmacy and Biochemistry and Department of Botany and Botanical Garden, Faculty of Science (University of Zagreb, Croatia) where the voucher specimens have been deposited under the genus number 819.
2.2. Preparation of the Extracts
Air-dried and pulverized plant material (10.00 g) was extracted with 70% ethanol (100 mL) using an ultrasonic bath for 30 min. The extract was filtered using a Büchner funnel and the residue was then reextracted with the same solvent (100 mL) as described above. Obtained extracts were combined and then concentrated to dryness under reduced pressure at 50°C using a rotary evaporator. The extraction yields (w/w) are given in Table 1.
Table 1.
Yield percentages of the ethanolic extracts and contents of total hydroxycinnamic acids, flavonoids, tannins, and procyanidins in aerial parts of selected Thymus species.
| Plant species | Extract yield∗ | Content (%) | |||
|---|---|---|---|---|---|
| Hydroxycinnamic acids | Flavonoids | Tannins | Procyanidins | ||
| T. longicaulis | 21.34 | 5.41 ± 0.09a | 0.40 ± 0.004a | 1.33 ± 0.02a | 0.70 ± 0.015a |
| T. praecox subsp. polytrichus | 20.06 | 4.39 ± 0.01b | 0.24 ± 0.010b | 0.93 ± 0.05b | 0.29 ± 0.002b |
| T. pulegioides | 22.54 | 6.17 ± 0.07c | 0.42 ± 0.003c | 1.59 ± 0.04c | 0.41 ± 0.002c |
| T. serpyllum subsp. serpyllum | 19.22 | 4.36 ± 0.11b | 0.40 ± 0.006a | 1.06 ± 0.08d | 0.45 ± 0.004d |
| T. striatus | 15.54 | 3.35 ± 0.10d | 0.15 ± 0.003d | 0.77 ± 0.07e | 0.26 ± 0.003e |
| T. vulgaris | 18.28 | 3.58 ± 0.03e | 0.24 ± 0.006b | 0.98 ± 0.03b,d | 0.21 ± 0.002f |
∗Extract yield expressed as percentage weight of air-dried plant material.
Mean values in the same column followed by different letters are significantly different from each other (P < 0.05).
2.3. Chemicals
Acetylcholinesterase (AChE) from electric eel (Type VI-S, EC 3.1.17.), acetylthiocholine iodide (ATChI), bovine brain extract (Folch type VII), 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′′-disulfonic acid (ferrozine), 2,2-diphenyl-1-picrylhydrazyl (DPPH•), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), galantamine hydrobromide, sulfanilamide, and rosmarinic acid (96%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Aluminium chloride, ethylenediaminetetraacetic acid (EDTA), and hexamethylenetetramine were purchased from Kemika (Zagreb, Croatia). Butylhydroxytoluene (BHT, ≥99%), luteolin (≥97%), N-(1-naphthyl)ethylenediamine, sodium nitroprusside, and trolox (≥98%) were purchased from Fluka (Buchs, Switzerland). Folin-Ciocalteu's phenol reagent and potassium ferricyanide were obtained from Merck (Darmstadt, Germany). All other chemicals and solvents used were of analytical grade.
2.4. Phytochemical Analyses of Polyphenols
2.4.1. TLC Analysis
Thin-layer chromatographic (TLC) analysis of phenolic acids and flavonoids was performed on precoated silica gel 60 F254 TLC plate (Merck, Germany) using diisopropyl ether–acetone–formic acid–water (50 : 30 : 10 : 10) as mobile phase. Polyphenolic compounds were detected under UV light at 365 nm after spraying plates with natural products-polyethylene glycol reagent (1% methanolic solution of 2-aminoethyl diphenylborinate and 5% ethanolic solution of PEG 4000) [16].
2.4.2. Assay for Total Hydroxycinnamic Acids
Determination of total hydroxycinnamic acids was performed using method previously described by Štefan et al. [17]. The content of total hydroxycinnamic acids, expressed as rosmarinic acid, was calculated from the expression: (%) = A × 2.5/m, where A is the absorbance of the test solution at 505 nm and m is the mass of the sample, in grams.
2.4.3. Assay for Total Flavonoids
The total flavonoid contents of six Thymus species were determined using aluminum chloride colorimetric method described in European Pharmacopoeia [18]. The percentage content of flavonoids, expressed as isoquercitrin, was calculated from the equation: (%) = A × 1.25/m, where A is the absorbance of the test solution at 425 nm and m is the mass of the sample, in grams.
2.4.4. Assay for Total Tannins
Determination of total tannins was performed following the Folin-Ciocalteu method described in European Pharmacopoeia [18]. The percentage content of tannins, expressed as pyrogallol, was calculated from the following equation: (%) = 3.125 × (A 1 − A 2)/(A 3 × m), where A 1 is the absorbance of the solution containing total polyphenols, A 2 is the absorbance of the solution containing polyphenols not adsorbed by hide powder, A 3 is the absorbance of the test solution containing 0.05 mg of pyrogallol, and m is the mass of the sample, in grams.
2.4.5. Assay for Procyanidins
Determination of procyanidin contents of selected Thymus species was done using European Pharmacopoeia method [18]. The percentage content of procyanidins was calculated as cyanidin chloride from the expression: (%) = A × 6.7/m, where A is the absorbance at 545 nm and m is the mass of the sample, in grams.
2.5. Evaluation of Antioxidant Activity
2.5.1. DPPH Radical Scavenging Assay
The free radical scavenging activities of the samples were measured using the stable DPPH radical, according to the previously reported method [19]. The samples were assayed in the range of 0.2–100 μg/mL. Trolox was used as a positive control. The capability to scavenge the DPPH radicals was calculated using the following equation: (%) = (1 − A 1/A 0) × 100, where A 0 is the absorbance of the control reaction and A 1 is the absorbance in the presence of the sample, corrected for the absorbance of sample itself.
2.5.2. Nitric Oxide Radical Scavenging Assay
The nitric oxide (NO•) scavenging activity was determined according to the method described by Razali et al. [20] with a slight modification. Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitrite ions that can be estimated using Griess reagent. Equal volumes of 10 mmol/L sodium nitroprusside in phosphate buffered-saline (pH 7.4) were mixed with different concentrations of the sample (6.25–400 μg/mL) and incubated at room temperature for 120 min. After the incubation, 0.5 mL of the reaction mixture was mixed with 1.0 mL of 1% sulfanilamide in 5% phosphoric acid. After 5 min, 0.1% naphthylethylenediamine (1.0 mL) was added, the solution was mixed, and the absorbance was measured at 540 nm against the corresponding blank solution. Trolox was used as a standard. The NO• scavenging activity was expressed as the percentage of inhibition according to the following equation: (%) = (1 − A 1/A 0) × 100, where A 0 is the absorbance of the control without a sample and A 1 is the absorbance in the presence of the sample.
2.5.3. Reducing Power Assay
The reducing power of the studied Thymus plant extracts and their polyphenolic constituents were evaluated by the potassium ferricyanide reduction method previously described by Vladimir-Knežević et al. [21]. The effective concentrations of the samples in the reaction mixture were in the range of 0.2–100 μg/mL. Trolox was used as a reference antioxidant compound. The sample concentration providing absorbance of 0.5 (IC50) was calculated from the graph of absorbance at 700 nm against sample concentration.
2.5.4. Metal Ion Chelating Assay
The ability of samples to chelate iron(II) ions at different concentrations (37.5–600 μg/mL) was estimated using the previously reported method [21]. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated using the formula given below: (%) = (1 − A 1/A 0) × 100, where A 0 is the absorbance of the control and A 1 is the absorbance in the presence of sample, corrected for the absorbance of sample itself.
2.5.5. Lipid Peroxidation Assay
The ability of the extracts to inhibit lipid peroxidation was evaluated by the thiobarbituric acid reactive substances assay using Fe(II)–H2O2 system in bovine brain liposomes [19]. The extracts were tested in the range of 10–1000 μg/mL, while rosmarinic acid and luteolin were tested at concentrations from 1.56 μg/mL to 100 μg/mL. Inhibition of lipid peroxidation in percent was calculated using the following equation: (%) = (1 − A 1/A 0) × 100, where A 0 is the absorbance of the negative control reaction and A 1 is the absorbance in the presence of the tested sample.
2.5.6. Total Antioxidant Capacity Assay
The total antioxidant capacities of the tested extracts, rosmarinic acid, luteolin, and trolox (25–100 μg/mL) were evaluated by the phosphomolybdenum method [22]. The antioxidant capacity of the sample was expressed as equivalents of ascorbic acid (AAE), utilizing a calibration curve of ascorbic acid in the concentration range 0.8–100 μg/mL.
2.6. Acetylcholinesterase Inhibition Assay
The AChE inhibitory activities of Thymus extracts were determined using modified Ellman's colorimetric method described by Conforti et al. [23]. In brief, 1900 μL of 50 mM Tris-HCl buffer (pH 8.0), 40 μL of 0.02 U/mL AChE, and 20 μL of tested solution (0.25–1 mg/mL) were mixed and preincubated for 30 min at 4°C. The reaction was then initiated with the addition of 20 μL of 10 mM DTNB and 20 μL of 12 mM ATChI. AChE activity was determined spectrophotometrically through measuring the change in absorbance of an assay solution at 412 nm over a period of 10 min. Galantamine (0.03–1 μg/mL) was used as a positive control. Percentage enzyme inhibition was calculated by comparing the enzymatic activity with and without inhibitor using the formula (E − S)/E × 100, where E is the activity of enzyme without a test sample and S is the activity of enzyme in the presence of the sample.
2.7. Statistical Analysis
All experiments were carried out in triplicate, and the results are expressed as mean ± standard deviation (SD). The concentrations of the samples that provide 50% inhibition (IC50) were obtained by interpolation from linear regression analysis. The results were tested for normal distribution by Kolmogorov-Smirnov test. Correlations were assessed using Pearson's correlation coefficient (r). One way analysis of variance (ANOVA) coupled with the Holm-Sidak post hoc tests was used to compare the data and to identify means with significant differences. Differences with P < 0.05 were considered statistically significant. The data analysis was performed using Microsoft Excel 2007 software and SigmaPlot for Windows (version 12.0).
3. Results and Discussion
3.1. Phytochemical Analysis of Polyphenolic Compounds
Plant polyphenols have gained increasing scientific interest due to their potent antioxidant properties and their remarkable effects in the prevention of various oxidative stress-associated diseases [24]. Hence, qualitative and quantitative analyses of polyphenolic compounds in plants are important in understanding their medicinal value.
The presence of phenolic acids and flavonoids in Thymus extracts was detected by thin-layer chromatography (TLC) in comparison with related standards. Rosmarinic acid with R f value of 0.81 was identified in all tested extracts while luteolin-7-O-glucoside (R f = 0.43) was detected in the extracts of T. longicaulis and T. vulgaris. The chromatogram also showed several other yellowish fluorescent zones having R f values between 0.13 and 0.71. According to the previously reported data, these compounds are most likely luteolin derivatives [10, 25, 26].
The contents of different polyphenolic subclasses in aerial plant parts were determined spectrophotometrically and the results are presented in Table 1. The most abundant compounds were hydroxycinnamic acids (3.35–6.17%), followed by tannins (0.77–1.59%) and flavonoids (0.15–0.42%). The percentage of procyanidin group of tannins varied between 0.21% (T. vulgaris) and 0.70% (T. longicaulis). Aerial parts of T. pulegioides and T. longicaulis contained the highest amount of hydroxycinnamic acids, 6.17% and 5.41%, respectively. These two Thymus species also had the highest contents of tannins, 1.59% and 1.33%, respectively. The richest species regarding the flavonoid content were found to be T. pulegioides (0.42%), T. longicaulis (0.40%), and T. serpyllum subsp. serpyllum (0.40%).
3.2. Antioxidant Activities of Thymus Ethanolic Extracts
The antioxidant activity may be due to different mechanisms, such as prevention of chain initiation, decomposition of peroxides, prevention of continued hydrogen abstraction, free radical scavenging, reducing capacity, and binding of prooxidant metal ions [27]. Therefore, in the present study, six different assays were employed in order to evaluate the antioxidant properties of the ethanolic extracts of selected Thymus species as well as elucidate its mode of action.
DPPH assay has been widely used to evaluate the free radical scavenging ability of the plant extracts and polyphenolic compounds. The tested Thymus extracts and pure compounds at different concentrations (0.4–25 μg/mL) significantly inhibited DPPH• in a concentration dependent manner (Table 2). The activities of plant extracts were 11–28%, 23–52%, and 52–85% at 1.56 μg/mL, 3.13 μg/mL, and 6.25 μg/mL, respectively. At the mentioned concentrations, T. serpyllum subsp. serpyllum as well as commercial sample of T. vulgaris were the least effective. Rosmarinic acid and luteolin at concentrations up to 3.13 μg/mL showed the highest radical scavenging effectiveness (56% and 50% at 0.8 μg/mL, resp.). Interestingly, at concentrations ≥ 12.5 μg/mL, activities of most Thymus species were comparable to that of luteolin. DPPH radical scavenging activities of the tested samples were assessed using IC50 values (Table 7) which are inversely related to their antioxidant abilities. The obtained IC50 values of studied Thymus extracts were in the range 3.01–6.01 μg/mL. The scavenging effects of the extracts decreased in order of T. longicaulis > T. praecox subsp. polytrichus > T. pulegioides, T. striatus > T. vulgaris > T. serpyllum subsp. serpyllum. Although there have been several reports on DPPH radical scavenging activity of polar extracts of these Thymus species [26–33], the lack of standardization of the results makes it difficult to compare their antioxidant strength [34]. The activities of rosmarinic acid and luteolin did not differ significantly (IC50 = 0.66 μg/mL and IC50 = 0.73 μg/mL, resp., P = 0.612) and were found to be higher than that of trolox (IC50 = 1.67 μg/mL), indicating their high antioxidant potency in terms of electron or hydrogen atom-donating capacity.
Table 2.
DPPH radical scavenging effects of Thymus ethanolic extracts, polyphenolic compounds, and reference antioxidant.
| Samples | DPPH∙ scavenging activity (%) ± SD | ||||||
|---|---|---|---|---|---|---|---|
| 0.39 μg/mL | 0.78 μg/mL | 1.56 μg/mL | 3.13 μg/mL | 6.25 μg/mL | 12.5 μg/mL | 25 μg/mL | |
| T. longicaulis | 12.82 ± 3.02a | 17.74 ± 1.51a | 27.78 ± 0.60a | 51.71 ± 0.03a | 84.62 ± 3.02a | 87.18 ± 0.09a | 87.82 ± 1.51a |
| T. praecox subsp. polytrichus | 10.84 ± 2.73a,b | 13.52 ± 2.12b | 27.68 ± 2.12a | 47.00 ± 2.73b | 83.69 ± 0.61a | 86.70 ± 0.58a | 86.70 ± 0.61a,b |
| T. pulegioides | 8.61 ± 1.87a,c | 12.25 ± 0.05b | 20.20 ± 0.94b | 38.58 ± 0.23c | 73.18 ± 0.94b | 83.94 ± 0.23a,b | 87.25 ± 1.64a |
| T. serpyllum subsp. serpyllum | 2.05 ± 0.48d | 8.39 ± 2.18c | 11.13 ± 0.24c | 22.95 ± 4.36d | 52.23 ± 4.12c | 82.02 ± 1.70b,c | 84.42 ± 0.73b |
| T. striatus | 7.05 ± 1.25b,c,d | 9.91 ± 0.93b,c | 19.82 ± 1.87b | 40.53 ± 0.62c | 75.11 ± 0.31b | 86.34 ± 2.49a | 87.67 ± 0.03a |
| T. vulgaris | 4.75 ± 0.65c,d | 6.58 ± 1.42c | 14.90 ± 1.01d | 29.56 ± 0.26e | 55.27 ± 0.69c | 79.73 ± 0.27c | 80.95 ± 0.13c |
| Rosmarinic acid | 37.45 ± 2.07e | 56.49 ± 1.78d | 89.12 ± 0.02e | 93.10 ± 0.30f | 94.98 ± 0.02d | 94.98 ± 0.09d | 95.19 ± 0.30d |
| Luteolin | 25.00 ± 1.61f | 50.19 ± 1.87e | 82.01 ± 0.80f | 84.47 ± 1.07g | 84.85 ± 0.14a | 85.80 ± 1.87a | 87.12 ± 1.61a |
| Trolox | 18.98 ± 2.82g | 24.44 ± 0.13f | 47.19 ± 2.64g | 87.22 ± 0.60g | 93.98 ± 0.03d | 95.68 ± 0.29d | 96.62 ± 0.02d |
Mean values in the same column followed by different letters are significantly different from each other (P < 0.05).
Table 7.
Comparative overview of IC50 values as well as total antioxidant capacities of Thymus ethanolic extracts, polyphenolic compounds, and reference antioxidants.
| Samples | IC50 (μg/mL) | Total antioxidant capacity (mg AAE/g)∗ | ||||
|---|---|---|---|---|---|---|
| DPPH radical scavenging activity | NO radical scavenging activity | Reducing power | Iron chelating activity | Inhibition of lipid peroxidation | ||
| T. longicaulis | 3.01 ± 0.02a | 71.57 ± 4.85a | 11.84 ± 0.11a | 271.85 ± 7.94a | 34.30 ± 7.37a | 293.82 ± 11.64a,b |
| T. praecox subsp. polytrichus | 3.41 ± 0.16b | 139.04 ± 5.69b | 15.10 ± 0.64b | 388.77 ± 3.28b | 78.74 ± 7.08b | 287.72 ± 12.52b,c |
| T. pulegioides | 4.18 ± 0.02c | 69.77 ± 4.38a | 11.39 ± 0.07a | 277.10 ± 1.03a | 34.83 ± 7.36a | 251.26 ± 0.75d,e |
| T. serpyllum subsp. serpyllum | 6.01 ± 0.44d | 176.59 ± 8.09c | 14.55 ± 0.57b | 342.04 ± 6.89c | 80.00 ± 9.93b | 238.16 ± 2.50d |
| T. striatus | 4.06 ± 0.04c | 91.09 ± 5.34d | 14.68 ± 0.38b | 333.14 ± 4.39c | 63.01 ± 7.44b | 276.04 ± 3.38c |
| T. vulgaris | 5.60 ± 0.07e | 97.93 ± 2.93d | 14.41 ± 0.19b | 125.91 ± 2.50d | 69.60 ± 5.14b | 257.87 ± 5.76e |
| Rosmarinic acid | 0.66 ± 0.03f | 15.67 ± 0.44e | 2.67 ± 0.10c | na | 21.07 ± 1.63a | 598.34 ± 8.26f |
| Luteolin | 0.73 ± 0.11f | 18.31 ± 1.02e | 4.51 ± 0.07d | na | 2.03 ± 0.23c | 67.72 ± 3.00g |
| Trolox | 1.67 ± 0.09g | 53.91 ± 0.13f | 6.64 ± 0.36e | na | — | 307.89 ± 2.00a |
| EDTA | — | — | — | 5.34 ± 0.10e | — | — |
∗Results are calculated for sample concentration of 100 μg/mL.
—: not tested; na: no activity. Mean values in the same column followed by different letters are significantly different from each other (P < 0.05).
NO is an important signaling molecule involved in the regulation of various physiological processes including neurotransmission, vascular homeostasis, antimicrobial defense, and antitumor activities. However, it has been demonstrated that increased NO production may also contribute to the pathogenesis of neurodegenerative human diseases and inflammatory tissue injury. The contribution of NO to the oxidative damage is increasingly becoming evident due to the fact that NO can react with superoxide to form highly reactive nitrogen species [35]. As can be seen in Table 3, all tested Thymus extracts inhibited nitrite formation in a concentration dependent manner. They scavenged NO• by 16–45%, 37–58%, and 54–72% at 50 μg/mL, 100 μg/mL, and 200 μg/mL, respectively. At these concentrations, T. longicaulis and T. pulegioides showed the highest activity, while T. serpyllum subsp. serpyllum demonstrated the weakest effect. Rosmarinic acid and luteolin inhibited the formation of NO• by 58% already at 25 μg/mL. Comparing obtained IC50 values (Table 7), the effectiveness of plant extracts as NO• scavengers was in the following descending order: T. longicaulis, T. pulegioides > T. striatus, and T. vulgaris > T. praecox subsp. polytrichus > T. serpyllum subsp. serpyllum. The IC50 values of the most potent T. longicaulis and T. pulegioides were 71.57 μg/mL and 69.77 μg/mL, respectively. Rosmarinic acid (IC50 = 15.67 μg/mL) and luteolin (IC50 = 18.31 μg/mL) demonstrated the greatest NO• scavenging activity, even significantly higher (P < 0.001) than trolox (IC50 = 53.91 μg/mL), and these results were in accordance with the findings obtained by DPPH assay.
Table 3.
NO radical scavenging effects of Thymus ethanolic extracts, polyphenolic compounds, and reference antioxidant.
| Samples | NO∙ scavenging activity (%) ± SD | ||||||
|---|---|---|---|---|---|---|---|
| 6.25 μg/mL | 12.5 μg/mL | 25 μg/mL | 50 μg/mL | 100 μg/mL | 200 μg/mL | 400 μg/mL | |
| T. longicaulis | 7.29 ± 1.05a | 15.47 ± 1.28a | 29.62 ± 2.75a | 44.17 ± 1.47a | 57.64 ± 1.10a | 72.25 ± 0.11a | 75.01 ± 0.95a |
| T. praecox subsp. polytrichus | na | 0.23 ± 0.03b | 10.16 ± 0.46b | 26.03 ± 1.52b | 43.02 ± 1.72b | 60.77 ± 0.13b | 68.22 ± 0.03b |
| T. pulegioides | 0.37 ± 0.05b | 11.64 ± 0.90c | 29.40 ± 1.10a | 44.58 ± 2.19a,g | 58.01 ± 0.37a | 70.40 ± 0.46a | 70.89 ± 1.54c |
| T. serpyllum subsp. serpyllum | na | na | 2.73 ± 1.46c | 16.38 ± 1.20c | 37.08 ± 1.66c | 53.92 ± 1.26c | 62.38 ± 0.80d |
| T. striatus | 7.73 ± 0.78a,c | 21.19 ± 0.98d | 28.73 ± 2.16a | 39.76 ± 2.04d | 52.17 ± 1.17d | 63.24 ± 2.19b | 66.12 ± 1.26e |
| T. vulgaris | 2.16 ± 0.82b | 14.96 ± 1.23a,c | 24.42 ± 0.35d | 39.02 ± 0.72d | 50.52 ± 0.74d | 60.96 ± 1.17b | 63.73 ± 0.55d |
| Rosmarinic acid | 10.86 ± 3.09c | 47.20 ± 0.23e | 58.40 ± 2.23e | 68.19 ± 0.27e | 73.75 ± 0.33e | 76.23 ± 0.76d | 77.22 ± 0.21f |
| Luteolin | 1.57 ± 0.21b | 43.10 ± 3.95f | 57.64 ± 2.75e | 64.34 ± 0.62f | 70.95 ± 1.92f | 76.25 ± 1.06d | 80.31 ± 0.30g |
| Trolox | na | 5.87 ± 1.20g | 20.06 ± 0.02f | 47.39 ± 0.14g | 80.70 ± 0.54g | 83.62 ± 0.62e | 84.86 ± 1.23h |
na: no activity. Mean values in the same column followed by different letters are significantly different from each other (P < 0.05).
The reducing capacity of a compound is an important mechanism of antioxidant action and may serve as a significant indicator of its potential antioxidant activity. Many studies have indicated that the antioxidant effect is related to the presence of reductones which were reported to be terminators of free radical chain reactions by donating hydrogen atom. Furthermore, reductones can also react with certain precursors of peroxide, thus preventing peroxide formation [36]. Table 4 shows the reducing power for different concentrations (0.8–50 μg/mL) of Thymus extracts, rosmarinic acid, luteolin, and trolox as a positive control. Our results demonstrated that all plant extracts possessed strong ability to reduce iron(III) ions (IC50 = 11.39–15.10 μg/mL) and the reducing power increased with a concentration in a strongly linear manner (R 2 = 0.9840–0.9962). In this assay, T. pulegioides (IC50 = 11.39 μg/mL) and T. longicaulis (IC50 = 11.84 μg/mL) showed stronger effect than other tested extracts (IC50 = 14.41–15.10 μg/mL) which did not differ significantly from one another (Table 7). These results were not consistent with previous research on reducing activities of the ethanolic and methanolic extracts of T. longicaulis subsp. longicaulis var. longicaulis and two varieties of T. praecox growing in Turkey [27, 32, 33] which showed much weaker ability to reduce iron(III) ions. Rosmarinic acid and luteolin with IC50 values of 2.67 μg/mL and 4.51 μg/mL, respectively, demonstrated the strongest reducing properties, even significantly stronger (P < 0.001) than trolox (IC50 = 6.64 μg/mL).
Table 4.
Ferric reducing power of Thymus ethanolic extracts, polyphenolic compounds, and reference antioxidant.
| Samples | Absorbance at 700 nm ± SD | ||||||
|---|---|---|---|---|---|---|---|
| 0.78 μg/mL | 1.56 μg/mL | 3.13 μg/mL | 6.25 μg/mL | 12.5 μg/mL | 25 μg/mL | 50 μg/mL | |
| T. longicaulis | 0.065 ± 0.001a,b | 0.105 ± 0.001a,b | 0.172 ± 0.003a,b | 0.301 ± 0.001a | 0.534 ± 0.008a | 0.946 ± 0.011a | 1.619 ± 0.008a |
| T. praecox subsp. polytrichus | 0.060 ± 0.004a,c | 0.090 ± 0.004a,c | 0.147 ± 0.015a | 0.242 ± 0.019b | 0.432 ± 0.018b | 0.782 ± 0.019b | 1.340 ± 0.014b |
| T. pulegioides | 0.070 ± 0.001b | 0.109 ± 0.001b | 0.179 ± 0.002b | 0.319 ± 0.005c | 0.547 ± 0.004a | 0.961 ± 0.023a | 1.541 ± 0.029c |
| T. serpyllum subsp. serpyllum | 0.060 ± 0.006a,c | 0.090 ± 0.007a,c | 0.147 ± 0.015a | 0.254 ± 0.019b | 0.442 ± 0.023b | 0.809 ± 0.008b | 1.359 ± 0.064b |
| T. striatus | 0.055 ± 0.001c | 0.085 ± 0.004c | 0.143 ± 0.006a | 0.249 ± 0.006d | 0.447 ± 0.010b | 0.795 ± 0.017b | 1.439 ± 0.017d |
| T. vulgaris | 0.062 ± 0.001a,b,c | 0.094 ± 0.003a,b,c | 0.149 ± 0.005a,b | 0.254 ± 0.001b | 0.464 ± 0.014b | 0.797 ± 0.001b | 1.376 ± 0.005b,d |
| Rosmarinic acid | 0.173 ± 0.005d | 0.321 ± 0.013d | 0.581 ± 0.015c | 1.055 ± 0.028e | 1.777 ± 0.065c | 3.251 ± 0.086c | 3.584 ± 0.010e |
| Luteolin | 0.142 ± 0.006e | 0.228 ± 0.008e | 0.390 ± 0.012d | 0.641 ± 0.037f | 1.132 ± 0.026d | 1.782 ± 0.045d | 3.568 ± 0.038e |
| Trolox | 0.089 ± 0.002f | 0.148 ± 0.007f | 0.240 ± 0.016e | 0.432 ± 0.018g | 0.903 ± 0.025e | 1.496 ± 0.021e | 2.941 ± 0.001f |
Mean values in the same column followed by different letters are significantly different from each other (P < 0.05).
An imbalance of metal homeostasis in the brain is thought to play an important role in the pathogenesis of AD. Amyloid β-peptide, as a pathological hallmark of AD, is considered a strong redox active agent that can efficiently generate reactive oxygen species in the presence of the transition metals such as copper and iron [5]. Additionally, iron is known as the most important lipid oxidation prooxidant due to its high reactivity. The ferrous state of iron accelerates lipid oxidation by breaking down hydrogen peroxide and lipid peroxides to reactive free radicals via the Fenton reaction [32]. Therefore, metal-chelating properties of the polyphenols indicate their antioxidant activity. The ability of tested Thymus extracts to compete with ferrozine for iron(II) ions was studied, and the obtained results are presented in Table 5, while the corresponding IC50 values are given in Table 7. All Thymus extracts displayed ferrous ion chelating properties at concentrations higher than 150 μg/mL, apart from T. vulgaris extract which exhibited its effect already at 37.5 μg/mL. At all tested concentrations, T. vulgaris demonstrated the strongest chelating effect (7–96%). The activities of other plant extracts at 150 μg/mL, 300 μg/mL, and 600 μg/mL were 1–15%, 39–58%, and 74–79%, respectively. Among these five extracts, only T. longicaulis and T. pulegioides showed over 50% chelating activity at concentration of 300 μg/mL. The effectiveness of plant extracts decreased in the following order: T. vulgaris > T. longicaulis, T. pulegioides > T. serpyllum subsp. serpyllum, T. striatus > T. praecox subsp. polytrichus, with IC50 values ranging between 125.91 μg/mL and 388.77 μg/mL. The chelating abilities of tested Thymus extracts were much lower compared to reference EDTA (IC50 = 5.34 μg/mL). Presented results also confirmed previous findings on polar extracts of T. longicaulis subsp. longicaulis var. longicaulis and two varieties of T. praecox reported to have ferrous ion chelating activity [27, 32, 33]. Rosmarinic acid and luteolin showed no activity at tested concentrations. These findings indicate that chelating activities of Thymus ethanolic extracts could be attributed to the other phenolic constituents.
Table 5.
Ferrous ion chelating capacities of Thymus ethanolic extracts.
| Samples | Iron(II) chelating ability ± SD | ||||
|---|---|---|---|---|---|
| 37.5 μg/mL | 75 μg/mL | 150 μg/mL | 300 μg/mL | 600 μg/mL | |
| T. longicaulis | na | 1.92 ± 0.03a | 15.13 ± 2.71a | 58.05 ± 2.17a | 77.30 ± 0.68a,c |
| T. praecox subsp. polytrichus | na | na | 1.30 ± 0.95b | 39.19 ± 0.48b | 75.72 ± 0.25a,b |
| T. pulegioides | na | na | 9.32 ± 3.06a,c | 57.34 ± 0.93a | 77.40 ± 1.07a,c |
| T. serpyllum subsp. serpyllum | na | na | 2.47 ± 0.44b | 46.04 ± 0.44c | 74.41 ± 1.89b |
| T. striatus | na | na | 3.07 ± 4.34b,c | 46.44 ± 0.42c | 78.61 ± 0.84c |
| T. vulgaris | 6.85 ± 3.07 | 19.61 ± 1.52b | 64.38 ± 1.47d | 92.71 ± 1.38e | 95.70 ± 0.03d |
na: no activity. Mean values in the same column followed by different letters are significantly different from each other (P < 0.05).
The high lipid content of cell membranes makes them one of the main targets of the reactive oxygen species. Lipid peroxidation, as a well-established mechanism of cellular injury, can be used as an indicator of oxidative stress in the cells and tissues. It has been implicated in the pathogenesis of various diseases such as cardiovascular diseases, cancer, and neurodegenerative disorders as well as aging processes [37]. All investigated Thymus extracts inhibited lipid peroxidation in a concentration dependent manner (Table 6). The activities of plant extracts at concentrations of 10 μg/mL and 100 μg/mL were in the ranges 32–40% and 56–76%, respectively. Among investigated species, T. longicaulis (IC50 = 34.30 μg/mL) and T. pulegioides (IC50 = 34.83 μg/mL) exhibited once again the most powerful antioxidant effect, comparable to that of rosmarinic acid (IC50 = 21.07 μg/mL) (Table 7). The IC50 values obtained for other four extracts were in the range 63.01–80.00 μg/mL, without significant difference between them. In contrast to previously presented results which revealed very similar antioxidant activity of rosmarinic acid and luteolin, in this assay, luteolin was found to be the most potent antioxidant against lipid peroxidation with IC50 value of 2.03 μg/mL.
Table 6.
Inhibition of lipid peroxidation by Thymus ethanolic extracts.
| Samples | Inhibition of lipid peroxidation (%) ± SD | |||
|---|---|---|---|---|
| 10 μg/mL | 100 μg/mL | 500 μg/mL | 1000 μg/mL | |
| T. longicaulis | 40.20 ± 3.38a | 76.26 ± 1.80a | 89.75 ± 1.34a | 97.84 ± 1.24a |
| T. praecox subsp. polytrichus | 32.78 ± 1.89b | 55.73 ± 5.12b | 82.56 ± 6.46a | 88.03 ± 3.82b |
| T. pulegioides | 40.03 ± 3.37a | 75.94 ± 1.79a | 89.38 ± 1.33a | 97.43 ± 1.23a |
| T. serpyllum subsp. serpyllum | 32.91 ± 1.90b | 55.45 ± 5.87b | 82.40 ± 7.19a | 88.40 ± 3.84b |
| T. striatus | 31.89 ± 0.08b | 63.68 ± 8.11a,b | 91.29 ± 4.56a | 98.56 ± 1.59a |
| T. vulgaris | 31.76 ± 0.08b | 59.42 ± 2.42b | 90.92 ± 4.54a | 98.15 ± 1.58a |
Mean values in the same column followed by different letters are significantly different from each other (P < 0.05).
Total antioxidant capacities of Thymus ethanolic extracts, rosmarinic acid, luteolin, and trolox were expressed as ascorbic acid equivalents (AAE) and are presented in Figure 1 and Table 7. All investigated plant samples were active in a concentration dependent manner with total antioxidant capacities ranging between 238.16 mg AAE/g and 293.82 mg AAE/g. Their effectiveness decreased in the following order: T. longicaulis, T. praecox subsp. polytrichus ≥ T. striatus > T. pulegioides, T. vulgaris ≥ T. serpyllum subsp. serpyllum. The activity of T. longicaulis was comparable to that of trolox. Rosmarinic acid was found to have much higher total antioxidant capacity (598.34 mg AAE/g) than trolox (307.89 mg AAE/g). Luteolin showed the lowest activity in comparison to all tested samples (67.72 mg AAE/g).
Figure 1.
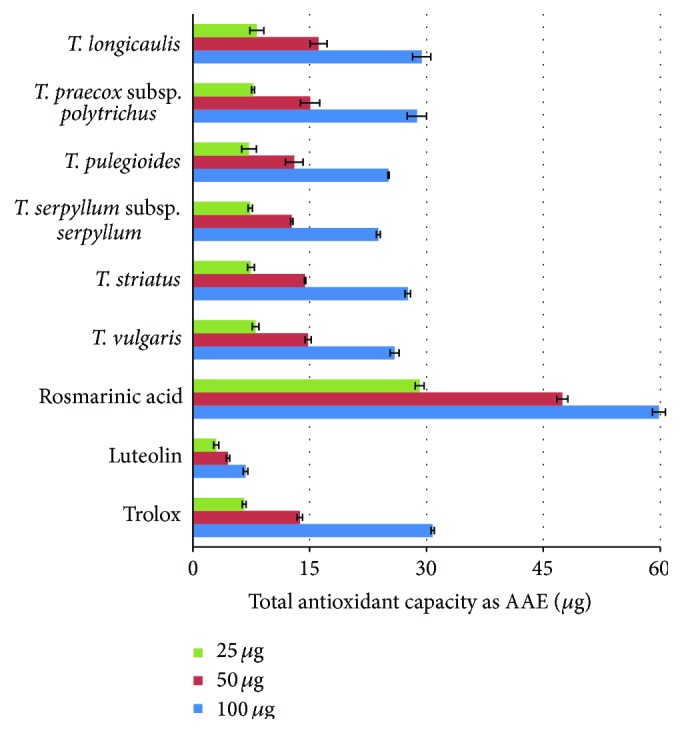
Total antioxidant capacities of Thymus ethanolic extracts, polyphenolic compounds, and reference antioxidant.
For the first time, this paper provides comprehensive comparative data on the antioxidant activities of selected Thymus species growing wild in Croatia. According to our findings, rosmarinic acid was confirmed as important contributor to the overall antioxidant effectiveness of Thymus species, as previously suggested by other researchers. Our results were also consistent with earlier studies which pointed out plants belonging to Thymus genus from different origin as a rich source of antioxidant polyphenols. In these studies, correlations were found between antioxidant effects of Thymus extracts and their flavonoid and phenolic acid contents [25, 38–40].
3.3. Antiacetylcholinesterase Activities of Thymus Ethanolic Extracts
A variety of plants and their polyphenolic constituents have been reported to show AChE inhibitory activity and thus could be useful in the prevention and treatment of neurodegenerative disorders such as AD [7]. In this study, Thymus ethanolic extracts were tested for their inhibitory activity against AChE at concentrations of 0.25, 0.5 and 1 mg/mL using Ellman's colorimetric assay. All tested extracts exhibited AChE inhibitory activity in a dose dependent manner (Figure 2). The values of 10−28%, 23−39%, and 64−86% were obtained for tested concentrations of 0.25, 0.5, and 1 mg/mL, respectively. Among tested plant extracts, T. longicaulis, T. pulegioides, and T. vulgaris showed the most potent inhibitory activity against AChE with IC50 values 656.06–667.91 μg/mL, with no significant difference between them. The AChE inhibitory activities of three remaining plant extracts were in the following descending order: T. serpyllum subsp. serpyllum > T. striatus > T. praecox subsp. polytrichus. Obtained results for T. serpyllum subsp. serpyllum (IC50 = 742.11 μg/mL) confirmed previous findings on anti-AChE activity of T. serpyllum ethanolic extract [30]. Studies on AChE inhibitory properties of the ethanolic extracts of T. praecox [41] and T. praecox subsp. caucasicus var. caucasicus [33] showed no activity, which is not in accordance with our evidence on anti-AChE activity of T. praecox subsp. polytrichus (IC50 = 837.96 μg/mL). Rosmarinic acid inhibited 47% and 87% of AChE activity at 0.25 and 0.5 mg/mL, respectively. AChE inhibition by luteolin was not determined because of its insolubility at tested concentrations. Galantamine, as a reference AChE inhibitor used, demonstrated the strongest effect comparing with the tested samples (IC50 = 0.122 ± 0.004 μg/mL).
Figure 2.
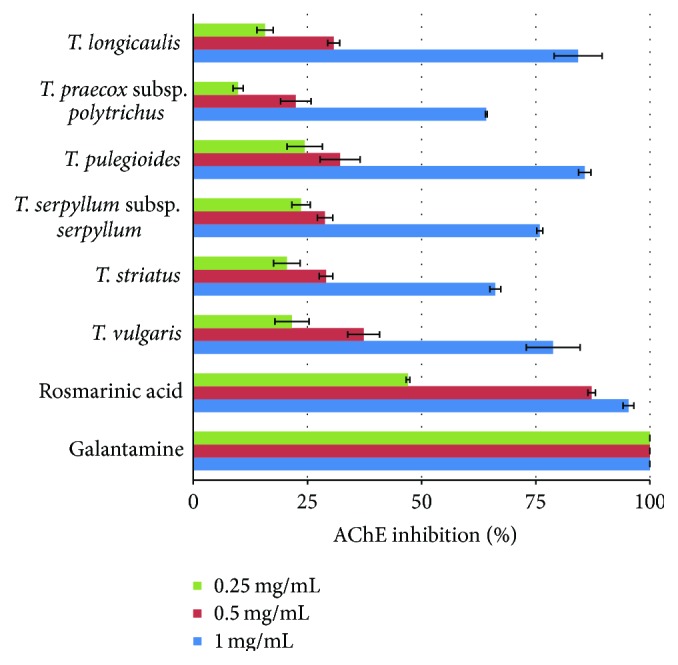
Acetylcholinesterase (AChE) inhibitory activities of Thymus ethanolic extracts, rosmarinic acid, and galantamine as reference drug.
As suggested in our previous work [22], considerable antioxidant abilities and AChE inhibition of most Lamiaceae species could be attributed to the high level of polyphenols, particularly rosmarinic acid. Hence, the influence of polyphenol contents on antioxidant and AChE inhibitory properties of the tested Thymus species was evaluated. A very strong correlation was observed only between the content of polyphenols and reducing power (r = −0.920, P = 0.009), while no significant correlation was found between polyphenol levels and all other effects of tested extracts proved in this study. These findings are probably due to the presence of terpene compounds which are known to possess antioxidant and anti-AChE properties [42].
4. Conclusions
Considering a very large body of evidence supporting the involvement of oxidative stress in neurodegenerative pathologies as well as the fact that AChE inhibition is up to now the most effective therapeutic approach to dementia, this study aimed to evaluate the neuroprotective potential of six Thymus species through investigation of their antioxidant and anti-AChE activities. Our results showed that all tested plant species possess strong antioxidant properties acting as radical scavengers, reducing agents, chelating transition metals, and inhibiting lipid peroxidation. Also, all tested extracts exhibited anti-AChE activity in a dose dependent manner. The obtained results provide evidence that the investigated Thymus species containing both terpenes and polyphenols could be used as promising therapeutic agents for neurodegenerative disorders. These findings have inspired our current study for isolation and structure elucidation of active components of Thymus extracts.
Acknowledgments
This research was funded by the Ministry of Science, Education and Sports of the Republic of Croatia (number of the project: 006-0061117-1238). The authors also want to thank Gordan Lukač, PhD. (NP Paklenica), and Assistant Professor Antun Alegro, PhD. (University of Zagreb, Faculty of Science, Department of Botany and Botanical Garden) for their assistance in collection and identification of plant material.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Ratnam D. V., Ankola D. D., Bhardwaj V., Sahana D. K., Kumar M. N. V. R. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. Journal of Controlled Release. 2006;113(3):189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Vladimir-Knežević S., Blažeković B., Bival Štefan M., Babac M. Plant polyphenols as antioxidants influencing the human health. In: Rao V., editor. Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health. Rijeka, Croatia: InTech; 2012. pp. 155–180. [Google Scholar]
- 3.Mitjavila M. T., Moreno J. J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochemical Pharmacology. 2012;84(9):1113–1122. doi: 10.1016/j.bcp.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Biran Y., Masters C. L., Barnham K. J., Bush A. I., Adlard P. A. Pharmacotherapeutic targets in Alzheimer's disease. Journal of Cellular and Molecular Medicine. 2009;13(1):61–86. doi: 10.1111/j.1582-4934.2008.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenough M. A., Camakaris J., Bush A. I. Metal dyshomeostasis and oxidative stress in Alzheimer's disease. Neurochemistry International. 2013;62(5):540–555. doi: 10.1016/j.neuint.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Mattson M. P. Pathways towards and away from Alzheimer's disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee P. K., Kumar V., Mal M., Houghton P. J. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14(4):289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Massoud F., Gauthier S. Update on the pharmacological treatment of Alzheimer's disease. Current Neuropharmacology. 2010;8(1):69–80. doi: 10.2174/157015910790909520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasool M., Malik A., Qureshi M. S., et al. Recent updates in the treatment of neurodegenerative disorders using natural compounds. Evidence-Based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/979730.979730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl-Biskup E., Sáez F. Thyme, the Genus Thymus. 1st. London, UK: Taylor and Francis; 2002. [Google Scholar]
- 11.Mahmoudi M., Morteza-Semnani K., Mojra E. Anti-inflammatory and antinociceptive activity of Thymus pubescens extract. Fitoterapia. 2008;79(5):361–365. doi: 10.1016/j.fitote.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Nikolić M., Glamočlija J., Ferreira I. C. F. R., et al. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Industrial Crops and Products. 2014;52:183–190. doi: 10.1016/j.indcrop.2013.10.006. [DOI] [Google Scholar]
- 13.Kültür Ş. Medicinal plants used in Kırklareli Province (Turkey) Journal of Ethnopharmacology. 2007;111(2):341–364. doi: 10.1016/j.jep.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Redžić S. S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Collegium Antropologicum. 2007;31(3):869–890. [PubMed] [Google Scholar]
- 15.Sekeroglu N. S., Deveci M., Buruk C. K., Gurbuz B., Ipek A. Chemical composition and antimicrobial activity of Anzer tea essential oil. Journal of the Science of Food and Agriculture. 2007;87(7):1424–1426. doi: 10.1002/jsfa.2847. [DOI] [Google Scholar]
- 16.Fecka I., Turek S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chemistry. 2008;108(3):1039–1053. doi: 10.1016/j.foodchem.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Štefan M. B., Rodríguez J. V., Blažeković B., Kindl M., Vladimir-Knežević S. Total hydroxycinnamic acids assay: prevalidation and application on Lamiaceae species. Food Analytical Methods. 2014;7(2):326–336. doi: 10.1007/s12161-013-9630-8. [DOI] [Google Scholar]
- 18.EDQM (European Directorate for the Quality of Medicines & Health Care) European Pharmacopoeia. 7th. Strasbourg, France: Council of Europe; 2011. [Google Scholar]
- 19.Blažeković B., Vladimir-Knežević S., Brantner A., Štefan M. B. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: a comparative study with L. angustifolia Mill. Molecules. 2010;15(9):5971–5987. doi: 10.3390/molecules15095971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razali N., Razab R., Junit S. M., Aziz A. A. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale) Food Chemistry. 2008;111(1):38–44. doi: 10.1016/j.foodchem.2008.03.024. [DOI] [Google Scholar]
- 21.Vladimir-Knežević S., Blažeković B., Štefan M. B., Alegro A., Kőszegi T., Petrik J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules. 2011;16(2):1454–1470. doi: 10.3390/molecules16021454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vladimir-Knezevic S., Blazekovic B., Kindl M., Vladic J., Lower-Nedza A. D., Brantner A. H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the lamiaceae family. Molecules. 2014;19(1):767–782. doi: 10.3390/molecules19010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conforti F., Statti G. A., Tundis R., Loizzo M. R., Menichini F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer's disease. Phytotherapy Research. 2007;21(5):427–433. doi: 10.1002/ptr.2077. [DOI] [PubMed] [Google Scholar]
- 24.Dai J., Mumper R. J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fecka I., Raj D., Krauze-Baranowska M. Quantitative determination of four water-soluble compounds in herbal drugs from Lamiaceae using different chromatographic techniques. Chromatographia. 2007;66(1-2):87–93. doi: 10.1365/s10337-007-0233-7. [DOI] [Google Scholar]
- 26.Galasso S., Pacifico S., Kretschmer N., et al. Influence of seasonal variation on Thymus longicaulis C. Presl chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry. 2014;107:80–90. doi: 10.1016/j.phytochem.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Sarikurkcu C., Sabih Ozer M., Eskici M., Tepe B., Can Ş., Mete E. Essential oil composition and antioxidant activity of Thymus longicaulis C. Presl subsp. longicaulis var. longicaulis . Food and Chemical Toxicology. 2010;48(7):1801–1805. doi: 10.1016/j.fct.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Chizzola R., Michitsch H., Franz C. Antioxidative properties of Thymus vulgaris leaves: comparison of different extracts and essential oil chemotypes. Journal of Agricultural and Food Chemistry. 2008;56(16):6897–6904. doi: 10.1021/jf800617g. [DOI] [PubMed] [Google Scholar]
- 29.Ložiene K., Venskutonis P. R., Šipailiene A., Labokas J. Radical scavenging and antibacterial properties of the extracts from different Thymus pulegioides L. chemotypes. Food Chemistry. 2007;103(2):546–559. doi: 10.1016/j.foodchem.2006.08.027. [DOI] [Google Scholar]
- 30.Mata A. T., Proença C., Ferreira A. R., Serralheiro M. L. M., Nogueira J. M. F., Araújo M. E. M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chemistry. 2007;103(3):778–786. doi: 10.1016/j.foodchem.2006.09.017. [DOI] [Google Scholar]
- 31.Roby M. H. H., Sarhan M. A., Selim K. A.-H., Khalel K. I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Industrial Crops and Products. 2013;43(1):827–831. doi: 10.1016/j.indcrop.2012.08.029. [DOI] [Google Scholar]
- 32.Ozen T., Demirtas I., Aksit H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii . Food Chemistry. 2011;124(1):58–64. doi: 10.1016/j.foodchem.2010.05.103. [DOI] [Google Scholar]
- 33.Orhan I., Şenol F. S., Gülpinar A. R., et al. Acetylcholinesterase inhibitory and antioxidant properties of Cyclotrichium niveum, Thymus praecox subsp. caucasicus var. caucasicus, Echinacea purpurea and E. pallida . Food and Chemical Toxicology. 2009;47(6):1304–1310. doi: 10.1016/j.fct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Scherer R., Godoy H. T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chemistry. 2009;112(3):654–658. doi: 10.1016/j.foodchem.2008.06.026. [DOI] [Google Scholar]
- 35.Jiang Y., Hu W., Han W., Yeo J.-H., Wang M.-H. Antioxidant and nitric oxide production inhibitory activities of scouring rush (Equisetum hyemale L.) Food Science and Biotechnology. 2012;21(4):1037–1044. doi: 10.1007/s10068-012-0135-9. [DOI] [Google Scholar]
- 36.Lee Y.-J., Kim D.-B., Lee J. S., et al. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013;18(10):12937–12950. doi: 10.3390/molecules181012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radical Biology and Medicine. 2009;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 38.Ertas A., Boga M., Yilmaz M. A., et al. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymus nummularius (Anzer tea): rosmarinic acid. Industrial Crops and Products. 2015;67:336–345. doi: 10.1016/j.indcrop.2015.01.064. [DOI] [Google Scholar]
- 39.Méndez-Tovar I., Sponza S., Asensio-S-Manzanera M. C., Novak J. Contribution of the main polyphenols of Thymus mastichina subsp. mastichina to its antioxidant properties. Industrial Crops and Products. 2015;66:291–298. doi: 10.1016/j.indcrop.2014.11.029. [DOI] [Google Scholar]
- 40.Ben El Hadj Ali I., Bahri R., Chaouachi M., Boussaiïd M., Harzallah-Skhiri F. Phenolic content, antioxidant and allelopathic activities of various extracts of Thymus numidicus Poir. organs. Industrial Crops and Products. 2014;62:188–195. doi: 10.1016/j.indcrop.2014.08.021. [DOI] [Google Scholar]
- 41.Sigurdsson S., Gudbjarnason S. Inhibition of acetylcholinesterase by extracts and constituents from Angelica archangelica and Geranium sylvaticum . Zeitschrift für Naturforschung C. 2007;62(9-10):689–693. doi: 10.1515/znc-2007-9-1011. [DOI] [PubMed] [Google Scholar]
- 42.Syad A. N., Shunmugiah K. P., Kasi P. D. Assessment of anticholinesterase activity of Gelidiella acerosa: implications for its therapeutic potential against Alzheimer's disease. Evidence-Based Complementary and Alternative Medicine. 2012;2012:8. doi: 10.1155/2012/497242.497242 [DOI] [PMC free article] [PubMed] [Google Scholar]


