Dear Editor:
Basophils and mast cells are principal cells in the immediate hypersensitivity reaction due to their expression of FceRI and secretion of mediators in response to IgE-mediated stimulation. Although the basophil is one of the least frequent types of leukocyte in circulation, in humans, it is more accessible to study than the tissue bound mast cell. As such a variety of assays have been developed over the last 5 decades to study its behavior and in recent years there has been considerable interest in cell surface molecules that change their expression upon activation of the basophil by any number of stimuli [1]. The use of flow cytometry to detect changes in cell surface expression in impure or whole blood preparations has made it possible to study expression levels on cells that have only experienced removal by venipuncture with no other processing and the ability to do this has revealed some interesting “resting” states for basophils. Two cell surface proteins that have received the most attention are CD63 and CD203c [1-3]. In some individuals, there appears to be an elevated “resting” expression of these (and other related) proteins and the increase in “resting” expression appears to have a relationship to some clinical disease states [4-6]. It is not known if the elevation in the “resting” condition reflects some activation events in vivo.
This study began with a simple question, if the “resting” state is elevated, is it a manifestation of activation in vivo or simply proportional cell surface expression in a cell that is expressing greater levels internally. It would be useful to resolve this question because the underlying mechanisms for these two possible explanations of the increased surface expression might be very different.
Enriched basophils were prepared by methods previously published and the details can be found in the online supplementary material. The protocol for obtaining blood from the subjects that were examined in this study was approved by the Johns Hopkins IRB and the donors provided a written approval of a consent approved by the same IRB. Written consents are saved as part of the standard record keeping for IRB-approved studies. Basophils were enriched on a two-step Percoll gradient [7]. Reactions were performed in either PIPES buffered isotonic saline with Ca and Mg (final volumes of 0.1 ml) or when longer incubations were needed, in RPMI-1640 media supplemented to contain 1 mM Ca++ (final volume of 0.1 ml). The reactions were stopped by adding an equal volume of a 2 or 4% paraformaldehyde solution in PBS (for PAGCM or media, respectively), the cell suspension incubated for 10 minutes at 37°C prior to adding 1.5 ml of a solution of 4% BSA in PBS for storage at 4°C until the day of flow cytometry (usually 24 hours later). Flow cytometry was performed as previously described [7]. Cells were labeled with an anti-FceRI Ab and basophils were gated on the basis of forward/side scatter parameters and the presence of FceRI (figure E1 in the online supplement). Total CD203c was determined by permeabilization (Fix and Perm by Caltag, see online supplement) and surface CD203c determined without permeabilization. These antibodies were detected by streptavidin-alexa647 staining.
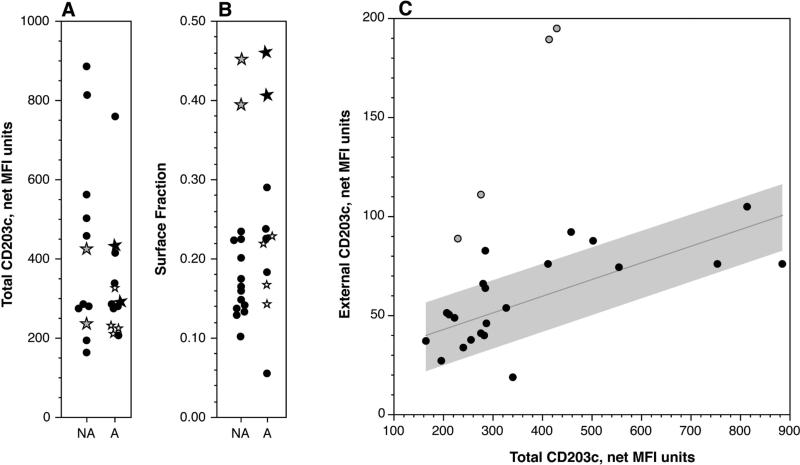
Internal CD203c could be detected by a standard permeabilization method and with this method a range for the measurement of total CD203c was observed. Figure 1A shows a ≈6-fold range and figure 1B shows the fraction that is on the cell surface of un-stimulated cells. There is a tendency for the distribution of the fraction on the surface to compress to a constant of approximately 17%, but there remain outliers. Using a hierarchical clustering algorithm, the top four fractional values in figure 1B group separately and this grouping was excluded from the linear fit shown in figure 1C. Figure 1C shows that the majority of samples lie near a line that represents constant fractional expression throughout a large range of total expression. However, there are also samples that lie far off this line (open symbols). There were 19 unique individuals examined that are shown in figure 1 but to assess the variability in the results, replicate measurements were made across a period of months for three of the subjects; these were plotted with distinct symbols (see figure legend). On the basis of these repeat measurements, the coefficient of variation for total CD203c was approximately 25%. Although the study was not designed and powered to examine the relationship of this metric with clinical condition, the subjects were asked whether they had a documented history of allergies. The two categories are separated in figures 1A and 1B. There is no apparent linkage of the either the total CD203c or its surface fraction to atopy but a more rigorous assessment might be needed.
Figure 1.
Distributions of total and fractional surface expression of CD203c. Panel A: Total (internal+external) CD203c (see methods) distribution. The grey-filled plotted points represent the 3 data points shown in panel B that were grouped separately by a hierarchal clustering algorithm. Stars represent repeated measurements of 3 different subjects (different star symbols distinguish the subjects). NA = reported non-atopic, A = reported atopic. Panel B: Fraction of total CD203c expressed on the cell surface (accessible without permeabilization) for the same group of subjects. The symbols follow the description for panel A. Panel C: Relationship between total CD203c and surface CD203c. The least squares fit of the solid data points is shown with a grey region delineating 1 standard deviation above and below the line. The grey-filled data points represent the unique group identified in panel B.
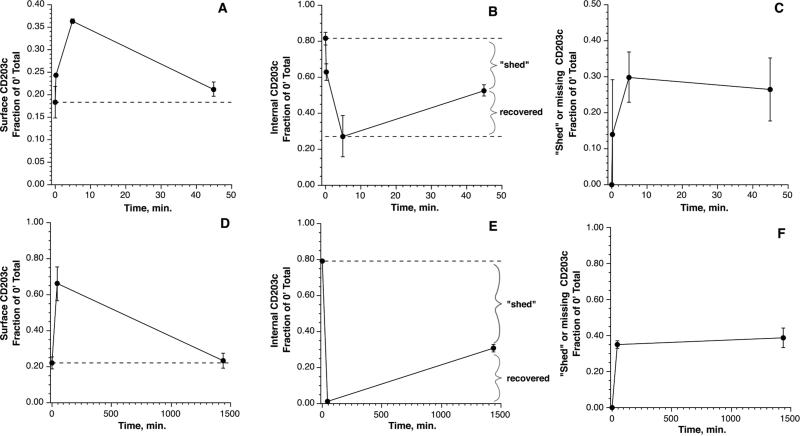
For some of these experiments, the cells were stimulated for 5 minutes with 100 nM FMLP or 0.5 μg/ml anti-IgE Ab in order to determine the relationship between internal CD203c and the known increase in surface CD203c with stimulation. Figure E2 A&B in the online supplement show the relationships for FMLP and anti-IgE Ab, respectively. It was notable that the total CD203c content decreases during stimulation for both stimuli. The cells were then examined for whether externalised CD203c returned to the intracellular compartment. Two stimuli were examined kinetically, one which causes degranulation (FMLP) and one which does not (IL-3). The time frames for each stimulus were different but both showed a similar pattern of behavior, which is shown in figure 2.
Figure 2.
Panels A-C: kinetics of cell surface, internal and missing CD203c during stimulation with 1 μM FMLP. All results are expressed as a fraction of the pre-stimulation total CD203c levels (n=3). Panel A: cell surface CD203c. Panel B: internal CD203c, calculated from the total – surface CD203c at the respective time point (but expressed relative to the 0 minute time point). Panel C: lost or shed CD203c, calculated from the total CD203c at time 0 – total at the respective time. Panels D-F: kinetics of cell surface, internal and lost/shed CD203c during stimulation with 10 ng/ml IL-3. The calculations were similar to those in panels A-C.
This figure shows three ways of analysing the results; surface CD203c, internal CD203c and lost CD203c (see figure legend for the calculation details). For each type of stimulus, it is apparent that the process of loss (or shedding) only occurs early in the reaction and that some cell surface CD203c is returned to the internal compartment later in the reaction. Generally, cell surface CD203c returns to pre-stimulation levels.
Although the surface CD203c returns to pre-stimulation levels on an absolute basis, the loss of some of the initial total CD203c means that a re-calculation of the surface/total CD203c at a late time point would show a higher ratio than pre-stimulation levels. Performing this calculation for the results in figure 2 shows that surface/total ratios transition from approximately 20% to 29% but with a potential upper range of 40% in some of the experiments using anti-IgE Ab for stimulation.
The primary hypothesis was that differences in surface CD203c expression would be related to the total stores of CD203c and specifically that high levels of surface expression would represent the same fractional expression after taking into account the total stores of CD203c. This possibility would suggest a different underlying mechanism for the high surface expression noted in previous studies of this phenomenon. There is a 5.9 fold range for total CD203c expression, a fact not previously appreciated, although this spread may be insufficient to fully explain the 10 fold spread in surface CD203c observed in published studies or this study. It is apparent that majority of subject samples in this study followed a line of constant surface expression but that there were outliers with 2-3 fold differences in the surface/total ratio. . It is notable that there are examples of high surface CD203c that only represent a “normal” fraction of the total CD203c. The implication is that measuring cell surface CD203c alone doesn't sufficiently capture what is unique about a particular individual's level of expression. While the majority of tested subjects lie on the constant ratio line, 15% of the subjects showed an unusual relationship and it is this unique group that might more properly reflect events happening in vivo, at least with respect to stimulation. All subjects were asked about their atopic and asthmatic status and the population studied includes atopics and asthmatics, but there was no relationship in this limited study to the ratio of surface/total CD203c.
These studies also found that any type of stimulation, whether it results in degranulation or not, leads to a loss of total CD203c. As noted previously, it was observed in studies of degranulation by electron microscopy that membrane was shed from a degranulating cell. It would be expected that membrane proteins would be lost during shedding [8] and if some of these membrane proteins had only become associated with the plasma membrane during degranulation, it stands to reason that this would manifested as a loss of the total cellular content of the protein. However, the phenomenon also occurs with IL-3, which does not cause degranulation. If the mechanism of increased cell surface expression of CD203c involves the fusion of vesicles as found for CD11b [9], the additional membrane provided by the vesicular fusion might also lead to shedding or the generation of exosomes. Alternatively, the loss mechanism may involve endocytosis of some of the expressed CD203c and degradation by endosomal mechanisms. Whatever the process, it doesn't provide a means to differentiate the type of stimulus. Despite a return to surface levels that are similar, in absolute terms, to the resting state, the calculation of surface/total will have changed due to the partial loss of total CD203c. In this sense, if a high ratio is observed on freshly isolated cells, i.e., without apparent stimulation, it may indicate that the cell experienced stimulation in vivo and the final stimulated state is what is being observed. However, without measuring both surface and total CD203c, it cannot be known whether a high surface level results from high total expression and is therefore not representative of prior stimulation or a non-normal ratio that may be representative of prior stimulation.
Supplementary Material
Acknowledgements
This study was supported by a grant from NIH, AI100952.
References
- 1.MacGlashan DW., Jr. Basophil activation testing. J Allergy Clin Immunol. 2013;132:777–87. doi: 10.1016/j.jaci.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Chirumbolo S, Vella A, Ortolani R, De Gironcoli M, Solero P, Tridente G, Bellavite P. Differential response of human basophil activation markers: a multi-parameter flow cytometry approach. Clin Mol Allergy. 2008;6:12–25. doi: 10.1186/1476-7961-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy. 2005;3:9–16. doi: 10.1186/1476-7961-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono E, Taniguchi M, Higashi N, Mita H, Kajiwara K, Yamaguchi H, Tatsuno S, Fukutomi Y, Tanimoto H, Sekiya K, Oshikata C, Tsuburai T, Tsurikisawa N, Otomo M, Maeda Y, Hasegawa M, Miyazaki E, Kumamoto T, Akiyama K. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2010;125:483–489. e3. doi: 10.1016/j.jaci.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 5.Gernez Y, Tirouvanziam R, Yu G, Ghosn EE, Reshamwala N, Nguyen T, Tsai M, Galli SJ, Herzenberg LA, Nadeau KC. Basophil CD203c Levels Are Increased at Baseline and Can Be Used to Monitor Omalizumab Treatment in Subjects with Nut Allergy. Int Arch Allergy Immunol. 2010;154:318–327. doi: 10.1159/000321824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasnowsky KM, Dreskin SC, Efaw B, Schoen D, Vedanthan PK, Alam R, Harbeck RJ. Chronic urticaria sera increase basophil CD203c expression. J Allergy Clin Immunol. 2006;117:1430–4. doi: 10.1016/j.jaci.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Ishmael SS, MacGlashan DW., Jr. Syk expression in peripheral blood leukocytes, CD34+ progenitors, and CD34-derived basophils. J Leukoc Biol. 2010;87:291–300. doi: 10.1189/jlb.0509336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorak AM, Warner JA, Kissell S, Lichtenstein LM, MacGlashan DW., Jr. F-met peptide-induced degranulation of human basophils. Lab. Invest. 1991;64:234–253. [PubMed] [Google Scholar]
- 9.MacGlashan D., Jr. Marked Differences in the Signaling Requirements for Expression of CD203c and CD11b versus CD63 Expression and Histamine Release in Human Basophils. Int Arch Allergy Immunol. 2012;159:243–252. doi: 10.1159/000332150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.