Abstract
Obesity is associated with an increase in chronic, low-grade inflammation which has been implicated in the development of type 2 diabetes mellitus and cardiovascular disease. The purpose of this study was to determine whether obesity was associated with an elevation of whole blood lipopolysaccharide (LPS)-stimulated tumor necrosis factor-α (TNF-α) production. African-American women were recruited from a larger study and assigned to one of five groups based on BMI: normal weight (NORM; BMI 20–25, n = 7), overweight (OVER; BMI 25–30, n = 12), class 1 obese (OB1; BMI 30–35, n = 19), class 2 obese (OB2; BMI 35–40, n = 10), or class 3 obese (OB3; BMI >40, n = 17). Body composition was determined via a whole body dual-energy X-ray absorptiometry (DXA) scan. Venous blood samples were collected following an overnight fast (>8 h), and stimulated with five doses of LPS (Salmonella enteriditis): 80, 40, 20, 10, and 5 µg/ml for 24 h in a 37 °C, 5% CO2 incubator. Following stimulation, TNF-α was measured using enzyme-linked immunosorbent assay. OB3 produced 365% more TNF-α than NORM at an LPS dose of 20 µg/ml (P < 0.05). When maximal TNF-α production was assessed regardless of LPS dose, OB3 produced 230% more than NORM and OVER produced 190% more than NW (P = 0.001). Total and trunk fat mass and BMI were significantly correlated with maximal TNF-α production and LPS = 20 µg/ml. Our findings are consistent with previous reports suggesting a relationship between increased adiposity and inflammatory marker production. This is one of the first studies to focus on African-American women, who have higher rates of obesity.
INTRODUCTION
Both physical inactivity and obesity are associated with an increase in systemic inflammation, which has been implicated in the development of type 2 diabetes mellitus, cardiovascular disease, and fatty liver disease (1–4). Non-Hispanic Black women are particularly vulnerable to overweight/obesity (BMI >25.0 kg/m2) in comparison to non-Hispanic white and Hispanic women (4,5). Despite their increased vulnerability, non-Hispanic black women remain understudied with respect to obesity, chronic inflammation, and secondary disease progression.
Visceral adipose tissue is believed to be primarily responsible for the increase in systemic inflammation common in obese individuals (6–8). It has been speculated that resident adipose tissue macrophages (ATMs) produce more proinflammatory cytokines (tumor necrosis factor-α, TNF-α) than visceral adipocytes (8,9) and accumulation of adiposity is associated with a transmigration of monocytes from the blood to adipose tissue compartments, where they mature into macrophages (6–8). Visceral ATMs can be readily assessed in animal models; however, this assessment produces high participant burden in human models. Monocytes may be a suitable alternative because they share a similar lineage (8,10,11). Using another proinflammatory state (physical inactivity), our laboratory and others have demonstrated that stimulated blood monocyte inflammatory cytokine production is a suitable index of whole body inflammatory capacity (12–14).
The key objective of the present investigation was to determine whether lipopolysaccharide (LPS)-stimulated blood TNF-α production could differentiate groups with respect to BMI. This study focused on non-Hispanic, black women because they may be most vulnerable to overweight and/or obese than other ethnic groups and have been understudied. We hypothesized that an increase in BMI would be associated with an increase in the LPS-stimulated TNF-α production in whole blood cultures. The purpose of the present study was to investigate the nature of the relationship between BMI classification (15) and LPS-stimulated TNF-α production in adult African-American women.
METHODS AND PROCEDURES
Participants
All procedures were approved by the Committee for the Protection of Human Subjects at the University of Houston. Participants read and signed an informed consent prior to formal testing. Once consent was given, participants completed a detailed medical history questionnaire. All participants in this study were self-identified African-American women (N = 65) who were part of a larger ongoing research project at the University of Houston (Principal Investigator: Rebecca Lee). Consented participants were grouped according to BMI (15): normal weight (NORM, 18.5–24.9 kg/m2, n = 7), overweight (OVER, 25.0–29.9 kg/m2, n = 12), class 1 obese (OB1, 30.0–34.9 kg/m2, n = 19), class 2 obese (OB2, 35.0–39.9 kg/m2, n = 10), and class 3 obese (OB3, >40.0 kg/m2, n = 17). Class 3 obesity has previously been classified as severe or morbid obesity (16). Participant characteristics are presented in Table 1.
Table 1.
Participant characteristics
| NORM (n = 7) | OVER (n = 12) | OB1 (n = 19) | OB2 (n = 10) | OB3 (n = 17) | |
|---|---|---|---|---|---|
| Age (years) | 42 ± 5 | 50 ± 3 | 49 ± 2 | 47 ± 2 | 47 ± 2 |
| Height (m) | 1.63 ± 0.01 | 1.63 ± 0.02 | 1.61 ± 0.02 | 1.65 ± 0.02 | 1.61 ± 0.02 |
| Body mass (kg) | 59.1 ± 1.84 | 72.6 ± 2.03 | 84.2 ± 2.21a | 101.3 ± 1.86b | 124.8 ± 4.35c |
| BMI (kg/m2) | 22.3 ± 0.9 | 27.3 ± 0.5 | 32.2 ± 0.3b | 37.1 ± 2.2c | 47.4 ± 2.3d |
| Percent fat | 33.3 ± 2.4 | 38.6 ± 1.2 | 41.5 ± 1.1a | 44.6 ± 1.6b | 48.8 ± 1.0c |
| Lean mass (kg) | 36.25 ± 0.7 | 41.76 ± 1.5 | 46.3 ± 1.3a | 53.4 ± 1.3c | 61.2 ± 1.9d |
| Total fat (kg) | 18.4 ± 1.8 | 26.2 ± 1.1 | 33.1 ± 1.5a | 43.5 ± 2.4c | 59.1 ± 2.9d |
| Trunk fat (kg) | 8.3 ± 1.0 | 12.4 ± 0.7 | 16.5 ± 0.7a | 22.9 ± 1.7c | 30.0 ± 1.8d |
| Total cholesterol (mg/dl) | 179.0 ± 22.5 | 192.3 ± 17.0 | 182.0 ± 18.2 | 181.9 ± 16.4 | 165.6 ± 13.7 |
| HDL (mg/dl) | 70.9 ± 6.3 | 64.1 ± 7.6 | 73.0 ± 7.96 | 66.4 ± 7.9 | 67.9 ± 7.2 |
| LDL (mg/dl) | 92.2 ± 14.3 | 112.6 ± 15.4 | 91.9 ± 22.4 | 99.8 ± 12.4 | 75.4 ± 14.9 |
| Triglycerides (mg/dl) | 83.2 ± 30.2 | 78.4 ± 15.0 | 85.9 ± 21.3 | 78.2 ± 11.3 | 86.4 ± 11.2 |
| WBC (109/l) | 6.40 ± 0.60 | 6.09 ± 0.44 | 5.95 ± 0.42 | 6.08 ± 0.40 | 6.32 ± 0.46 |
All values represent mean ± s.e.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; NORM, normal weight; OB1, class 1 obese; OB2, class 2 obese; OB3, class 3 obese; OVER, overweight; WBC, white blood cell.
Significantly greater than NORM.
Significantly greater than NORM and OVER.
Significantly greater than NORM, OVER, and OB1.
Significantly greater than NORM, OVER, OB1, and OB2.
Venous blood collection
Participants reported to the University of Houston between 0600 h and 0830 h, Tuesday through Thursday, following an overnight fast (>8 h) and sat quietly in the laboratory for 20 min. During the 24 h preceding the blood draw, the participants were instructed to refrain from taking any unnecessary medications and abstain from physical activity. Compliance was determined by asking the participant a series of questions and noncompliant subjects were rescheduled for another blood collection appointment on a different day. Venous blood samples (15 ml) were collected from a peripheral arm vein into Vacutainers pretreated with either sodium heparin or K2 EDTA (Vacutainer; Becton-Dickinson, Franklin Lakes, NJ). All blood samples were held at room temperature on a rocker and processed within 3 h.
Dual X-ray absorptiometry
Whole body dual-energy X-ray absorptiometry (DXA) scans (Discovery-W; Hologic, Bedford, MA) were used to determine whole body fat mass, lean mass, bone mass, percent body fat, and trunk fat (determined using a sub-region placed to exclude the arms and legs). Prior to DXA scanning, each participant completed a urine pregnancy test to confirm that she was not pregnant.
Complete blood counts
Total and differential leukocyte (lymphocyte, monocyte, and granulocyte), erythrocyte, hematocrit, and hemoglobin counts were determined using an automated hematology analyzer (MDII-16 hematology analyzer; Beckman-Coulter, Miami, FL). Interassay coefficients of variation were <5%.
Blood cholesterol profile
Plasma total cholesterol, high-density lipoprotein, and triglycerides concentration were determined using separate enzymatic assays in triplicate as described by the manufacture (Pointe Scientific, Canton, MI). Low-density lipoprotein was calculated as described previously (17).
In vitro cytokine stimulation and measurement
Sodium heparin-treated whole blood was diluted 1:20 in RPMI 1640 (supplemented with l-glutamine (2 mmol/l) and penicillin (100 U/ml)/ streptomycin (100 µg/ml)) and dispensed into a 96-well plate (200 µl per well). LPS (from Salmonella enteriditis; Sigma-Aldrich, St Louis, MO) was added to yield a final in-well concentration of 5, 10, 20, 40, and 80 µg/ml. A control well (no LPS) was included with each assay. The whole blood cultures were incubated 24 h in a 37 °C, 5% CO2 environment; after which cell-free supernatants were collected and frozen (−80 °C) until further analysis. Stimulated TNF-α concentration was determined using enzyme-linked immunosorbent assay (Ready-Set-Go ELISA; eBioscience, San Diego, CA). To minimize variation, an automated robot pipetting system (Precision XS Workstation; BioTek, Winooski, VT) was used to dilute supernatants (1:40 in assay diluent) and load samples into enzyme-linked immunosorbent assay plates. Coefficients of variation (inter- and intra-assay) were <10% for all analyses.
Statistical analyses
Prior to formal statistical testing, all data were assessed to ensure that assumptions of normality and constant error variance were met. Non-normal data were log transformed (noted in the results section where appropriate). Statistical analyses were conducted using SPSS v.14 (Chicago, IL). DXA measurements and select blood variables (CBC, cholesterol profile, and maximal TNF-α production) were analyzed using a one-factor analysis of variance (ANOVA) with five levels of BMI. Stimulated TNF-α production was analyzed using a 5 (BMI: NORM, OVER, OB1, OB2, and OB3) × 6 (LPS dose: 0, 5, 10, 20, 40, and 80) ANOVA with repeated measures on the second factor. Significance was set at P < 0.05. Significance for the repeated measures model was adjusted using the Huynh–Feldt method. After formal ANOVA testing was complete, all dependent variables were assessed using a Pearson bivariate correlation. Values are reported as the mean ± s.e.
RESULTS
Participant characteristics
Body weight was significantly greater with increasing BMI group assignment except between NORM and OVER (F = 10.93, P < 0.001). No significant differences were found for age, height, or blood cholesterol profile. These data are summarized in Table 1.
DXA measurements
A main effect was found for percent body fat (F = 16.86, P < 0.001) where nonadjacent groups were significantly different from each other (i.e., NORM was less than OB1 and OVER was less than OB2). Total fat (F = 48.04, P < 0.001), trunk fat (F = 38.66, P < 0.001), and lean (F = 34.52, P < 0.001) mass were significantly different for all groups except between NORM and OVER. DXA measurements are summarized in Table 1.
Complete blood count
No significant differences were found for total leukocyte (Table 1), lymphocyte, monocyte, granulocyte, erythrocyte, hemoglobin, or hematocrit. The later finding for erythrocyte, hemoglobin, and hematocrit suggests that the participants had a similar hydration status at the time when blood samples were collected.
LPS-stimulated TNF-α production
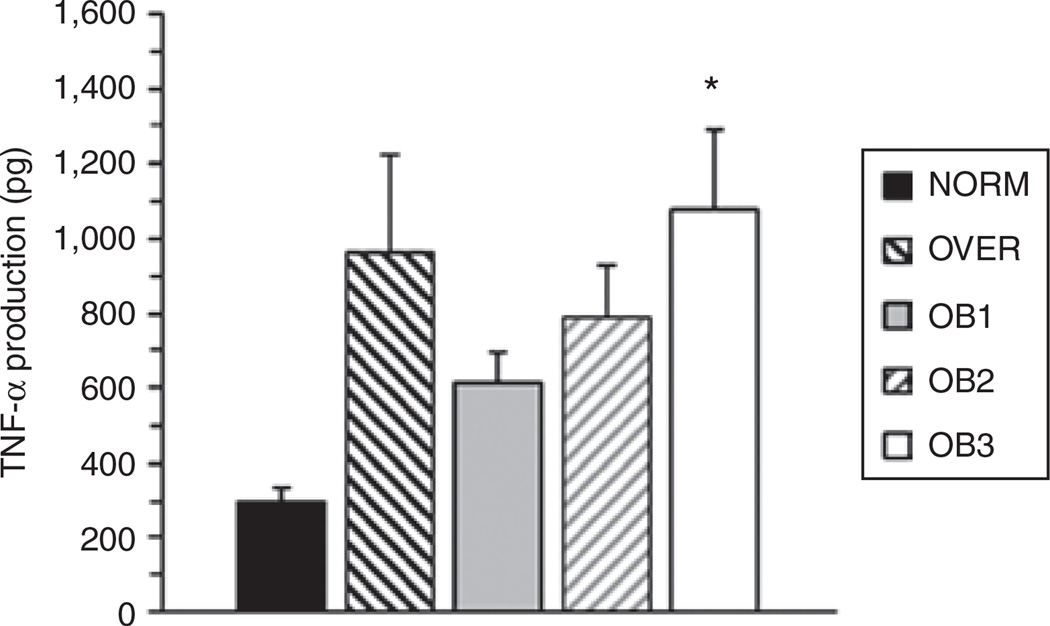
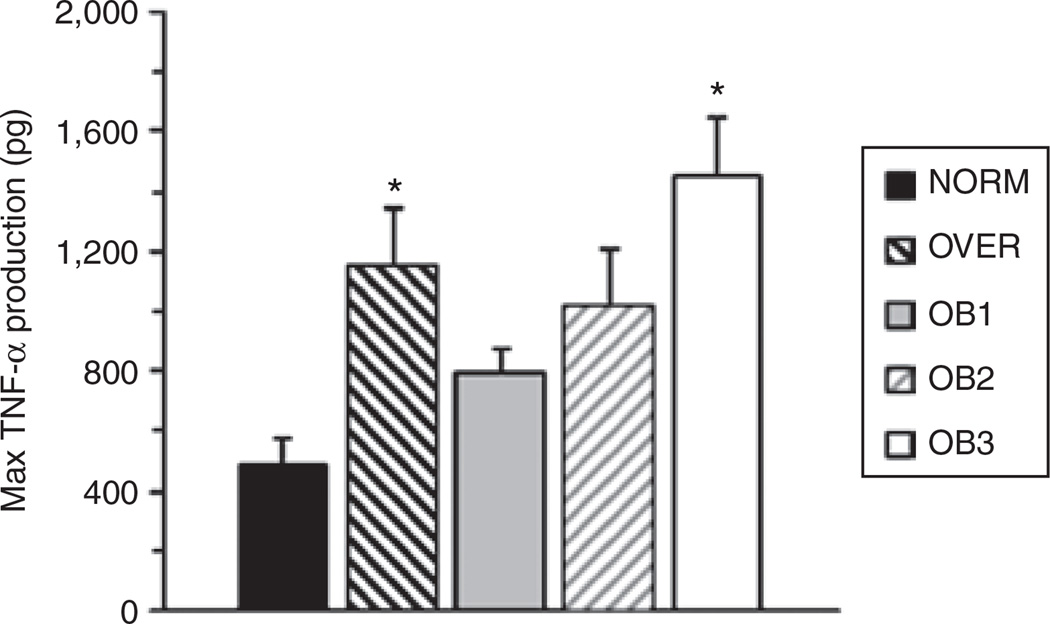
A significant BMI Group × LPS dose interaction (F = 3.022, P = 0.025) was found where OB3 produced 3.65-fold more TNF-α than NORM at an LPS dose of 20 µg/ml (Figure 1). Group comparisons between NORM, OVER, OB1, and OB2 had very low statistical power associated with them. Post hoc sample size analysis indicated that we would need to triple our present sample size to achieve a statistical power of 0.8 for comparisons between NORM, OVER, OB1, and OB2. When maximal TNF-α production was assessed regardless of LPS dose, we found a significant main effect for BMI (F = 5.49, P = 0.001), where OVER (2.9-fold) and OB3 (3.3-fold) produced significantly more TNF-α than NORM (Figure 2). Similar statistical power and sample size issues were found for maximal TNF-α production. Our data are expressed as absolute values rather than a per monocyte basis because we did not find a significant group difference in blood monocyte concentration.
Figure 1.
The present study compared in vitro, whole blood lipopolysaccharide (LPS)-stimulated tumor necrosis factor-α (TNF-α) production in African-American women of differing BMI groups. TNF-α production was determined using an LPS dose curve. The results presented are for a final LPS dose of 20 µg/ml. Values represent group means ± s.e. *Significantly greater than NORM (P < 0.05).
Figure 2.
The present study compared in vitro, whole blood lipopolysaccharide (LPS)-stimulated tumor necrosis factor-α (TNF-α) production in African-American women of differing BMI groups. TNF-α production was determined using an LPS dose curve. The results presented are for the maximal TNF-α production, regardless of LPS dose. Values represent group means ± s.e. *Significantly greater than NORM (P < 0.05).
Correlations
The findings of our correlation analysis were consistent with results from our ANOVA testing. Log TNF-α production (at 20 µg/ml LPS dose) was significantly correlated with BMI (r = 0.27; P < 0.05), total body fat mass (r = 0.27; P < 0.05), trunk fat mass (r = 0.30; P < 0.01), lean mass (r = 0.33, P < 0.01), and body weight (r = 0.28, P < 0.05). Similar correlations were found among log max TNF-α production and measures of body plan. Age was significantly correlated with trunk fat (r = 0.31, P < 0.01).
DISCUSSION
Severely obese African-American women had a greater LPS-stimulated TNF-α production than normal weight African-American women. This finding was partially supported by positive correlations that we found between BMI, total fat mass, trunk fat mass, and stimulated TNF-α production. The stimulated TNF-α production which we found in severely obese women was similar to what our laboratory and others have found with physical inactivity (10,12,13), which is another proinflammatory state.
We originally hypothesized that for each group increase in BMI, we would find a similar, significant increase in whole blood LPS-stimulated TNF-α production. Contrary to this hypothesis, we only found a difference in TNF-α production between normal weight, overweight, and severely obese (OB3) women. While the difference found between the normal and overweight groups is consistent with our original hypothesis, it is interesting because there were not statistically significant differences in total body fat or trunk fat mass between these two groups. Despite a statistically significant difference in total fat and trunk fat mass between normal vs. OB1 and OB2, we did not detect a significant difference for stimulated TNF-α production. It is possible that estrogen status may have influenced our measured outcomes; however, we have not previously found this to be a significant confounder (13). Others have reported in animals that slight differences in fat mass alter stimulated TNF-α production in ATMs (6–8). We did not use ATMs and thus it is possible that our present findings do not agree for this reason. It is also possible that it may take larger differences (clinical vs. statistical significance) in adiposity to create measurable changes in monocyte TNF-α production capacity. Thus, it is possible that given the link between disease progression and TNF-α, similar alterations in adiposity (i.e., normal vs. morbid obesity) are needed to significantly advance disease risk. The present study was not designed to measure disease progression, but rather a marker of a potential mechanism, which may underlie disease processes. It is also possible that group assignment based on visceral adiposity may have provided differing results for TNF-α production.
One objective of our present investigation was to utilize blood monocytes as a proxy measure for ATMs, which are known to enhance their inflammatory capacity in response to weight gain in mice (18). Limited data are available concerning macrophage activity in obese humans because of the invasive nature of the tissue collection. The studies which have been completed only compared participants on the extremes of the range of BMI (i.e., normal vs. severely obese) and did not characterize BMI groups that would be in between (19–22). While the present study did not evaluate macrophages, we did examine monocytes which are a naive form. From the data that we collected, it appears that large differences in adiposity may be needed to alter the inflammatory capacity of blood monocytes. Given their similar lineage, we speculate that a similar response may be present for ATMs.
The present study completed a homogenous population of African-American women, who are a traditionally understudied group (23,24). Lee et al. (23) recently reported ethnic variation in the accumulation of systemic inflammation. Specifically, they reported that when diagnosed with the metabolic syndrome, white but not African-American children had elevated biomarkers of systemic inflammation (IL-6, e-selectin, and ICAM-1) (23). Ranjit et al. (24) reported similar findings in adults, but added another dimension to the analysis. They reported that certain social economic status and maximal education level tended to cluster in certain ethnic groups, which may bias the reporting of race/ethnic differences related to inflammatory status (24). Another factor contributing to race/ethnic differences is a known different in absolute adiposity (% body fat) at a given BMI in individuals of differing race/ethnicity (25). With respect to the present study, at a BMI of 30, African Americans would have significantly less percent body fat than either whites or Hispanics. Others have reported elevations of systemic inflammation in obese whites (BM: 30–40); however, we did not find a significant elevation of systemic inflammation until a subject’s BMI exceeded 45. It is possible that if we had included another measure of systemic inflammation that we would have found different results. It is reasonable to speculate that many factors contribute to race/ethnic variation in the accumulation of systemic inflammation. Future research using heterogeneous subject populations should carefully consider the potential implications of race/ethnicity in their experimental model.
The exact mechanism underlying an obesity-induced increase in systemic, low-grade inflammation is not fully understood; however, it has been speculated that adipocyte necrosis is a key stimulus (8,18,26). Release of adipocyte intracellular contents to the extracellular space is a recruitment signal for blood monocytes (26). Based on our present findings, it is possible that adipocyte necrosis only occurs when a critical level of adiposity has been reached, resulting in maximal activation of blood monocytes in individuals with severe obesity (class 3). This interpretation of our present findings is supported by Ghanim et al. who reported that monocytes from individuals with severe obesity (class 3) exhibited a proinflammatory phenotype compared to normal weight individuals (11). Our present findings provide support for those of Ghanim et al. (11) because they used a completely different method to measure inflammatory capacity (nuclear factor-κB binding assay). Nuclear factor-κB plays a key role as a promoter of proinflammatory gene expression following cellular stimulation with LPS (27). Thus, our present measurement and that of Ghanim et al. (11) are assessing different aspects of the same pathway.
In conclusion, severely obese African-American women had higher LPS-stimulated TNF-α production than normal weight African-American women. We did not find a difference among intermediate groups (i.e., class 1 and 2 obese vs. normal). Our ANOVA results are partially supported by significant positive correlations we found between BMI, total fat mass, trunk fat mass, and stimulated TNF-α production. Our present findings are consistent with what others have reported with respect to obesity and monocyte inflammatory capacity using other methodologies. Future investigations should attempt to provide a clear link between laboratory measurements of inflammatory capacity and clinical markers of disease state.
ACKNOWLEDGMENTS
We thank MegginBaxter, Jorge Banda, and Ashley Medina for their assistance with scheduling subjects and processing blood samples.
Footnotes
Disclosure
The authors declared no conflict of interest.
REFERENCES
- 1.Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- 2.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, Zinman B, Haffner SM, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 10.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–1727. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 11.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 12.Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol. 2003;95:1833–1842. doi: 10.1152/japplphysiol.00359.2003. [DOI] [PubMed] [Google Scholar]
- 13.McFarlin BK, Flynn MG, Campbell WW, et al. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci. 2006;61:388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- 14.McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc. 2004;36:1876–1883. doi: 10.1249/01.mss.0000145465.71269.10. [DOI] [PubMed] [Google Scholar]
- 15.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults— The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 16.National Heart Lung and Blood Institute. NHLBI Education Programs Information Center. Information alert: updates on disease prevention, education, and control from the National Heart, Lung, and Blood Institute. Bethesda, MD: The Institute; [Google Scholar]
- 17.Abudu N, Levinson SS. Calculated low-density lipoprotein cholesterol remains a viable and important test for screening and targeting therapy. Clin Chem Lab Med. 2007;45:1319–1325. doi: 10.1515/CCLM.2007.291. [DOI] [PubMed] [Google Scholar]
- 18.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 19.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 20.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 22.Cancello R, Tordjman J, Poitou C, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Bacha F, Gungor N, Arslanian S. Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatrics. 2008;152:177–184. doi: 10.1016/j.jpeds.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 24.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116:2383–2390. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- 25.Rush EC, Goedecke JH, Jennings C, et al. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes. 2007;31:1232–1239. doi: 10.1038/sj.ijo.0803576. [DOI] [PubMed] [Google Scholar]
- 26.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Meja KK, Seldon PM, Nasuhara Y, et al. p38 MAP kinase and MKK-1 co-operate in the generation of GM-CSF from LPS-stimulated human monocytes by an NF-κB-independent mechanism. Br J Pharmacol. 2000;131:1143–1153. doi: 10.1038/sj.bjp.0703684. [DOI] [PMC free article] [PubMed] [Google Scholar]