Abstract
BACKGROUND
All patients with cirrhosis are at risk of developing hepatocellular carcinoma (HCC). This risk is not uniform because other patient-related factors influence the risk of HCC. The objective of the current study was to develop an HCC risk prediction model to estimate the 1-year probability of HCC to assist with patient counseling.
METHODS
Between 2002 and 2011, a cohort of 34,932 patients with cirrhosis was identified from a national liver transplantation waitlist database from the United States. Cox proportional hazards regression methods were used to develop and validate a risk prediction model for incident HCC. In the validation cohort, discrimination and calibration of the model was examined. External validation was conducted using patients with cirrhosis who were enrolled in the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) study.
RESULTS
HCC developed in 1960 patients (5.6%) during a median follow-up of 1.3 years (interquartile range, 0.47 years-2.83 years). Six baseline clinical variables, including age, diabetes, race, etiology of cirrhosis, sex, and severity (ADRESS) of liver dysfunction were independently associated with HCC and were used to develop the ADRESS-HCC risk model. C-indices in the derivation and internal validation cohorts were 0.704 and 0.691, respectively. In the validation cohort, the predicted cumulative incidence of HCC by the ADRESS-HCC model closely matched the observed data. In patients with cirrhosis in the HALT-C cohort, the model stratified patients correctly according to the risk of developing HCC within 5 years.
CONCLUSIONS
The ADRESS-HCC risk model is a useful tool for predicting the 1-year risk of HCC among patients with cirrhosis.
Keywords: hepatocellular carcinoma, cirrhosis, risk model, risk factors, liver cancer, Scientific Registry of Transplant Recipients (SRTR)
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) has been rising in the United States over the past 2 decades,1,2 increasing by 3.6% and 3.0% per year from 2004 to 2008, in men and women, respectively.3 Mortality from HCC ranks ninth among all cancers in the United States and, in contrast to most other malignancies, which have experienced a decrease in mortality over the past decade, mortality related to HCC has increased.4
Cirrhosis is a well-established risk factor for HCC, with > 90% of patients with HCC having underlying cirrhosis. However, the risk of HCC is not uniform because different etiologies of cirrhosis are associated with variably reported risks of HCC. The incidence of HCC has been most widely studied in patients with hepatitis B virus (HBV) and hepatitis C virus (HCV), with most estimates ranging between 3% and 5% per year.5,6 The risk of HCC in other populations has been poorly characterized, especially in the United States. In addition, factors other than etiology such as age, sex, race, severity of cirrhosis, smoking status, and diabetes may influence the risk of HCC.5 Estimation of HCC risk in an individual patient incorporating all these factors would be useful for clinicians to counsel patients about their prognosis and risk factors for HCC that are potentially modifiable.
Not only is it important to understand an individual’s risk of HCC for improved patient education and counseling, but also to raise awareness of the risk groups for HCC and ultimately to increase the enrolment of patients with cirrhosis into surveillance programs. HCC is a cancer well suited for screening given its near-exclusive occurrence in a highly identifiable population (patients with cirrhosis), early detection with abdominal imaging, and the availability of potentially curable therapy for patients with early-stage disease.7 Surveillance for HCC in patients with cirrhosis is recommended by the American7, European,8 and Asian9 societies for liver disease, the World Gastroenterology Organisation,10 and the National Comprehensive Cancer Network.11 Despite this, the uptake of HCC surveillance by the medical community has been poor.12,13 One potential explanation for this is the lack of a tangible indicator for clinicians to quantify the risk of HCC. Better assessment of an individual’s risk of HCC could help motivate clinicians toward better adherence to screening programs.
The objective of the current study was to develop and validate a risk model for HCC in a large diverse US cohort of patients with cirrhosis that would provide a useful guide for patient counseling and supports HCC surveillance practice.
MATERIALS AND METHODS
Patients
The current study is a cohort study of patients with cirrhosis who were waitlisted for liver transplantation at 127 US liver transplant centers. This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes prospective data on all candidates who were waitlisted in the United States submitted by the members of the Organ Procurement and Transplantation Network, and has been described elsewhere.14 Information regarding the diagnosis of an incident HCC while the patient was on the waitlist was prospectively entered into the database in the form of a model of end-stage liver disease (MELD)-HCC exception application. As of March 1, 2002, patients with cirrhosis on the waitlist were eligible to receive MELD exception points (ie, gain higher priority on the transplant list) if they had a diagnosis of HCC. The criteria for being eligible for this MELD-HCC exception was based on standard diagnostic criteria7 of either 1) histological confirmation and/or 2) typical imaging characteristics of a new or enlarging hepatic lesion with arterial enhancement and portal venous washout on cross-sectional imaging (computed tomography or magnetic resonance imaging) confirmed by a radiologist at the transplant center.
All patients aged ≥ 18 years with cirrhosis between March 1, 2002 and December 1, 2011 were identified. Patients were included if the underlying cause of cirrhosis based on their primary listing diagnosis (based on SRTR diagnostic codes or manually entered text) was HCV, HBV, alcohol, nonalcoholic steatohepatitis (NASH), cryptogenic cirrhosis, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, alpha-1 antitrypsin deficiency, hereditary hemochromatosis, or Wilson disease. Patients with cryptogenic cirrhosis were grouped into the NASH category.15,16 Patients with a dual diagnosis of HCV and any other cause were classified as having HCV etiology. Patients were excluded if they lacked a unique identifier, were without a listing diagnosis, had a listing diagnosis other than defined in the inclusion criteria, had received a previous liver transplantation, or if their follow-up time was incomplete or < 180 days. Finally, to exclude prevalent cases of HCC in the cohort, all patients with a primary or secondary listing diagnosis of HCC or MELD exception application for HCC within 180 days of initial listing were excluded. The Institutional Review Board at the University of California at San Francisco approved the study protocol (IRB study #11–08062).
Data Elements
The date of the incident HCC was defined as the date of the first application for MELD-HCC exception. The time period of risk began 180 days after listing and ended at the earliest of 1) MELD-HCC exception application, 2) transplantation, 3) waitlist dropout/death, or 4) administrative censoring on December 1, 2011. Demographic and clinical data were obtained from the SRTR database at the time of initial waitlisting. The Child-Turcotte-Pugh (CTP) score was calculated for each individual at listing based on the individual values for total bilirubin, international normalized ratio, and albumin in addition to the documentation of ascites (none, slight, or moderate) and hepatic encephalopathy (none, grade 1/2, or grade 2/3) as previously described.17
Statistical Analyses
The data set was divided into model derivation and validation sets. Instead of a random split, we used the center volume as the criterion for the division. All centers were sorted by the number of transplants performed and patients at high-volume centers (top 50th percentile) were assigned into the derivation set and those at low-volume centers were assigned into the validation set (Fig. 1).
Figure 1.
Development of the derivation and validation cohorts from the Scientific Registry of Transplant Recipients database is shown. HCC indicates hepatocellular carcinoma.
In preliminary analysis, we identified candidate predictors based on face validity and previously reported associations with HCC. These included age, sex, race, etiology of cirrhosis, diabetes, body mass index (BMI), laboratory values, CTP score, MELD score, presence of a transhepatic portosystemic shunt, and history of variceal hemorrhage. We grouped cirrhosis etiology into 3 categories based on HCC risk; HBV or HCV infection was classified as viral group; autoimmune and cholestatic diseases classified as autoimmune and all the others classifed into the metabolic/ alcohol group. Next, we eliminated predictors with P values > .05 in either single-predictor or multivariable Cox models found by manual forward and backward selection. All remaining predictors were considered for inclusion in the prediction model.
In selecting the initial model within the derivation set, an exhaustive sequence of models, including all possible combinations of the predictors remaining after preliminary analysis as well as 2-way interactions, were ranked by the Harrell C-index, a measure of discrimination (an assessment of how well the model is able to separate individuals into those who will and will not develop HCC).19 To avoid overfitting, we estimated the C-index using 10-fold cross-validation and calculated its 95% confidence interval (95% CI) using bootstrapping. The initial model was chosen to maximize the cross-validated C-index.
In the validation data set, the baseline survival function and hazards ratio (HR) for all included predictors, based solely on the derivation set, were used to calculate predicted HCC risk for each patient in the validation cohort. Predicted risks were estimated using the formula F(t, X) = 1-S0(t)HR(X), in which F(t, X) is the predicted risk at time t for a patient with covariates X = (X1, X2, … Xp), S0(t) is the baseline survival function at t for a patient with X = 0, and HR(X) is the overall HR corresponding to X. To assess calibration (a measure of how well the predicted risk based on the ADRESS-HCC model matches with the observed risk), we compared nonparametric Kaplan-Meier HCC risk estimates to average model-based estimates at 1 year after the beginning of follow-up for HCC, stratified into 5 different risk groups between 0% to 6% and increasing by intervals of 1.5%. The external C-index was estimated using a Cox model in which the sole predictor for each patient in the validation set was the log of HR(X), again based on coefficients from the derivation set and a 95% CI was developed using bootstrapping.
External Validation in the HALT-C Cohort
For further investigation of the external validity of the model, we examined the performance of the model in patients with cirrhosis enrolled in the HALT-C cohort. This cohort has been described previously.20 Abdominal ultrasonography was performed at the time of randomization, 6 months after randomization, and every 6 months to 12 months thereafter. HCC was defined by histological confirmation or by a set of clinical criteria. All cases of HCC were adjudicated by panels of investigators.21
Of 1050 patients with HCV enrolled in the HALT-C study, 426 had cirrhosis at the time of enrollment and were included in the analysis. Patients with other concomitant liver diseases or evidence of hepatic decompensation were excluded. Variables such as age, sex, race, presence of diabetes, CTP score, follow-up duration, and incident HCC were extracted from the HALT-C database. The Cox proportional hazard regression analysis was used to examine the performance of the model in predicting the incidence of HCC up to 5 years. Patients without HCC were censored at the time of liver transplantation or death. All analyses were performed using STATA statistical software (release 12; StataCorp LP, College Station, Tex) and R software (survivalROC package in version 3.02; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Characteristics of the Study Subjects
A total of 109,112 individuals were listed for liver transplantation in the SRTR database between March 1, 2002 and December 1, 2011, and 34,932 met the eligibility criteria (Fig. 1). Among the excluded individuals were 5162 patients who were listed with existing HCC. Those patients were older (median age: 57 years vs 54 years; P < .001), and were more likely to be male (76.4% vs 62.1%; P < .001), to be of nonwhite race (43.5% vs 26.2%; P < .001), and to have CTP class A disease (39.6% vs 12.9%; P<.001) compared with those in the final cohort. Table 1 describes the characteristics of the patients included in the study. The most common etiologies of cirrhosis were HCV (45.7%), alcohol (18.3%), and NASH/cryptogenic (17.5%). Approximately one-half of the patients had CTP class B cirrhosis, 78% had ascites, and 65% were diagnosed with hepatic encephalopathy. The median MELD score at listing was 13 (interquartile range [IQR], 10–16 [range, 6–40]).
TABLE 1.
Baseline Characteristics of the Cohort
| Variable | Entire Cohort (n=34,932) |
Derivation Set (n=17,124) |
Validation Set (n=17,808) |
Pa |
|---|---|---|---|---|
| Median age (IQR), y | 54 (48–59) | 54 (48–59) | 54 (48–59) | .90 |
| Male sex, % | 62.1 | 60.8 | 63.3 | <.001 |
| Race. % | ||||
| Non-Hispanic white | 74.0 | 70.5 | 77.3 | <.001 |
| Hispanic/Latino | 16.1 | 19.6 | 12.7 | |
| African American | 6.3 | 5.4 | 7.1 | |
| Asian | 2.9 | 3.7 | 2.2 | |
| Other | 0.7 | 0.8 | 0.7 | |
| Median body mass index, (IQR) | 28 (25–32) | 28 (25–32) | 28 (25–32) | .16 |
| Diabetes % | ||||
| Yes | 17.1 | 16.3 | 17.9 | <.001 |
| No | 73.1 | 73.8 | 72.5 | |
| Missing | 9.8 | 9.9 | 9.7 | |
| Etiology of cirrhosis % | ||||
| HCV | 45.7 | 46.0 | 45.5 | .018 |
| HBV | 2.8 | 3.0 | 2.6 | |
| NASH/cryptogenic | 17.5 | 17.6 | 17.4 | |
| Alcohol | 18.3 | 18.3 | 18.3 | |
| PBC | 4.6 | 4.5 | 4.6 | |
| PSC | 6.0 | 5.7 | 6.4 | |
| AIH | 3.8 | 3.8 | 3.7 | |
| A1AT | 0.7 | 0.6 | 0.8 | |
| HH | 0.5 | 0.5 | 0.5 | |
| Wilson disease | 0.2 | 0.2 | 0.2 | |
| Median MELD score (IQR) | 13 (10–16) | 12 (10–15) | 13 (11–16) | <.001 |
| Child-Turcotte-Pugh class, % | ||||
| A | 12.9 | 14.8 | 11.2 | <.001 |
| B | 54.1 | 54.6 | 53.6 | |
| C | 33 | 30.6 | 35.3 | |
| Hepatic encephalopathy, % | ||||
| None | 34.7 | 36 | 33.4 | <.001 |
| Grade 1 or 2 | 57.1 | 57.4 | 56.8 | |
| Grade 3 or 4 | 8.2 | 6.7 | 9.7 | |
| Ascites, % | ||||
| None | 22.3 | 23.7 | 20.9 | <.001 |
| Slight | 63.1 | 63.4 | 62.7 | |
| Moderate | 14.6 | 12.9 | 16.4 | |
| Variceal hemorrhage, % | ||||
| Yes | 3.4 | 3.5 | 3.4 | .743 |
| No | 96.6 | 96.5 | 96.6 | |
| TIPS, % | ||||
| Yes | 8.6 | 7.6 | 9.5 | <.001 |
| No | 87.3 | 88.5 | 85.8 | |
| Missing | 4.1 | 3.9 | 4.8 | |
| Median bilirubin (IQR), mg/dL | 2 (1.3–3.3) | 1.9 (1.2–3.2) | 2.1 (1.3–3.4) | <.001 |
| Median albumin (IQR), g/dL | 3.1 (2.6–3.5) | 3.1 (2.7–3.6) | 3.0 (2.6–3.5) | <.001 |
| Median INR (IQR) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | <.001 |
| Median creatinine (IQR), mg/dL | 0.91 (0.8–1.2) | 0.9 (0.8–1.2) | 1.0 (0.8–1.2) | <.001 |
| Median sodium (IQR), Meq/L | 137 (135–140) | 137 (135–140) | 137 (134–139) | <.001 |
| HCC during follow-up, % (no.) | 5.6 (1960) | 6.5 (1116) | 4.7 (844) | |
| Median follow-up (IQR), y | 1.26 (0.47–2.83) | 1.51 (0.58–3.2) | 1.07 (0.39–2.5) | |
| Total person-time, y | 67,014 | 36,719 | 30,295 |
Abbreviations: A1AT, alpha-1 antitrypsin; AIH, autoimmune hepatitis; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HH, hereditary hemochromatosis; INR, international normalized ratio; IQR, interquartile range; MELD, model of end-stage liver disease; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; TIPS, transjugular intrahepatic portosystemic shunt.
P value for comparison between the derivation and validation sets.
The derivation and validation cohorts consisted of 17,124 patients and 17,808 patients, respectively (Fig. 1). There was no statistically significant difference in age or BMI between the cohorts. There were statistically significant differences noted with regard to sex, diabetes, etiology of cirrhosis, and MELD and its components, although the large sample size may have resulted in P values < .05 even when there were no clinically meaningful differences (Table 1). In general, patients in the derivation cohort were enriched with subjects of Hispanic/ Latino ancestry and patients with less advanced liver disease (CTP class A, without encephalopathy or ascites).
Modeling Incidence of HCC
In the entire cohort, the median follow-up was 1.26 years (IQR, 0.47 years-2.83 years), excluding the first 180 days after waitlist registration, which generated a total of 67,014 person-years of observation. Only 246 individuals (0.002%) in the cohort were lost to follow-up. During the follow-up, 1960 individuals developed incident HCC after a median of 1.71 years (IQR, 0.85 years-3.11 years), with an overall incidence rate of HCC for the cohort of 2.9 per 100 person-years (95% CI, 2.8–3.1). Although the follow-up in derivation cohort was longer than that in the validation cohort, the incidence of HCC in the 2 cohorts was similar: 2.9 per 100 person-years in the derivation cohort and 2.7 per 100 person-years in the validation cohort.
In the derivation cohort, univariate analysis indicated that age, sex, race, BMI, diabetes, etiology of cirrhosis, the CTP score, and the MELD score and its components (bilirubin, international normalized ratio, and creatinine) had statistically significant associations with HCC (Table 2). Of these, the following variables were found to be significant on the subsequent multivariate analysis: age, diabetes, race (non-Hispanic white vs other), etiology of cirrhosis (viral vs metabolic/alcohol vs autoimmune groups), sex, and the CTP score (range, 5–15). A global test of the proportional hazards assumption in the final multivariable model confirmed the proportionality assumption was met (P = .246). Based on the final model, heretofore named the ADRESS-HCC (reflecting the 6 identified predictors-age, diabetes, race, etiology of cirrhosis, sex, and severity of liver dysfunction), a risk score was developed to calculate the 1-year risk of HCC as shown in Table 3. Table 4 shows the clinical application of the ADRESS-HCC risk model in predicting the 1-year risk of HCC in 4 hypothetical patients with cirrhosis. An ADRESS-HCC score of ≥ 4.67 identified those whose risk of HCC was ≥ 1.5% per year. This risk threshold has been suggested to be cost-effective for HCC screening7 and 71% of the cohort (24,940 patients) had an ADRESS-HCC score above this level. No patient with an ADRESS-HCC score of < 2.15 (80 patients) developed HCC during the follow-up period.
TABLE 2.
Cox Models Evaluating Risk Factors for the ADRESS-HCC Model (Derivation Cohort [n=17,124])
| Variable | Univariate Model HR (95% CI) P | Multivariate Model HR (95% CI) P | ||
|---|---|---|---|---|
| Age (per y increase) | 1.04 (1.03–1.04) | <.001 | 1.05 (1.04–1.06) | <.001 |
| Etiology of cirrhosisa | ||||
| Autoimmune | Reference | – | Reference | – |
| Alcohol/metabolic | 2.28 (1.69–3.07) | <.001 | 1.43 (1.04–1.97) | .030 |
| Viral | 4.72 (3.55–6.26) | <.001 | 3.38 (2.50–4.55) | <.001 |
| Diabetes present | 1.31 (1.11–1.54) | .001 | 1.25 (1.06–1.48) | .008 |
| Male sex | 2.03 (1.76–2.33) | <.001 | 1.99 (1.71–2.30) | <.001 |
| CTP score (per unit increase) | 1.17 (1.13–1.21) | <.001 | 1.17 (1.13–1.21) | <.001 |
| Nonwhite race | 1.26 (1.12–1.43) | <.001 | 1.20 (1.06–1.37) | .005 |
| MELD score (per unit increase) | 1.02 (1.01–1.04) | .007 | – | – |
| Body mass index (per 5-unit increase) | 1.01 (0.96–1.07) | .681 | – | – |
| Presence of TIPS | 0.88 (0.69–1.12) | .315 | – | – |
| Previous variceal hemorrhage | 0.93 (0.67–1.29) | .643 | – | – |
| Bilirubin (per unit increase) | 1.03 (1.01–1.05) | <.001 | – | – |
| INR (per unit increase) | 1.36 (1.22–1.52) | <.001 | – | – |
| Creatinine (log) | 1.16 (1.00–1.33) | .040 | – | – |
Abbreviations: 95% CI, 95% confidence interval; ADRESS, age, diabetes, race, etiology cirrhosis, sex, and severity; CTP, Child-Turcotte-Pugh; HCC, hepatocellular carcinoma; HR, hazards ratio; INR, international normalized ratio; MELD, model of end-stage liver disease; TIPS, transjugular intrahepatic portosystemic shunt.
Autoimmune: autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis. Alcohol/metabolic: hereditary hemochromatosis, alpha-1 antitrypsin, nonalcoholic steatohepatitis cryptogenic, and alcohol. Viral: hepatitis B virus and hepatitis C virus.
TABLE 3.
Calculation of 1-Year HCC Risk Using the ADRESS-HCC Model
| Step 1: calculate ADRESS-HCC score = ([age] + [diabetes] + [race] + [etiology] + [sex] + [severity]) in which: |
| Age indicates the age in years × 0.0532 |
| Diabetes indicates 0.2135 if present and 0 if absent |
| Race indicates 0.2058 if nonwhite or Hispanic or 0 if non-Hispanic white |
| Etiology indicates 0 if autoimmune, 0.3509 if alcohol/metabolic, and 1.246 if viral |
| Sex indicates 0.5114 if male and 0 if female |
| Severity indicates a CTP score (5–15) × 0.1170 |
| Step 2: baseline hazard at 1 year: S0(t)=0.99986 |
| Step 3: [100*(1-S0(t)*exp(ADRESS-HCCScore)]=1-year HCC risk (%) |
Abbreviations: ADRESS, age, diabetes, race, etiology of cirrhosis, sex, and severity; CTP, Child-Turcotte-Pugh; HCC, hepatocellular carcinoma.
TABLE 4.
Application of the ADRESS-HCC Risk Model to Hypothetical Patients With Cirrhosis
| Patient | Age | Diabetes | Race | Etiology | Sex | Severity | ADRESS-HCC Score |
1-Year HCC Risk, % |
|---|---|---|---|---|---|---|---|---|
| 1a | 1.957 | 0 | 0 | 0 | 0 | 0.5850 | 2.542 | 0.2 |
| 2b | 1.957 | 0 | 0.2058 | 1.246 | 0 | 0.8190 | 4.228 | 1.0 |
| 3c | 3.029 | 0 | 0 | 0.3509 | 0.5114 | 0.9360 | 4.827 | 1.7 |
| 4d | 2.330 | 0.2135 | 0.2058 | 1.246 | 0.5114 | 1.287 | 5.794 | 4.6 |
Abbreviations: ADRESS, age, diabetes, race, etiology of cirrhosis, sex, and severity; HCC, hepatocellular carcinoma.
Patient 1 is a 42-year-old white woman with autoimmune hepatitis, no diabetes, and a Child-Turcotte-Pugh (CTP) of score of 5.
Patient 2 is a 42-year-old Asian woman with hepatitis C, no diabetes, and a CTP score of 7.
Patient 3 is a 65-year-old white man with alcohol-related cirrhosis, no diabetes, and a CTP score of 8.
Patient 4 is a 50-year-old Asian man with hepatitis B cirrhosis, diabetes, and a CTP score of 11.
We performed a sensitivity analysis examining the HRs of the predictors (age, diabetes, race, etiology, and sex) exclusively in those with CTP class A disease in the waitlist cohort. The point estimates for all predictors were not found to be clinically different from those we obtained using the entire cohort with all CTP classes included (data not shown).
Validation of the Model in Patients on the Liver Transplant Waitlist
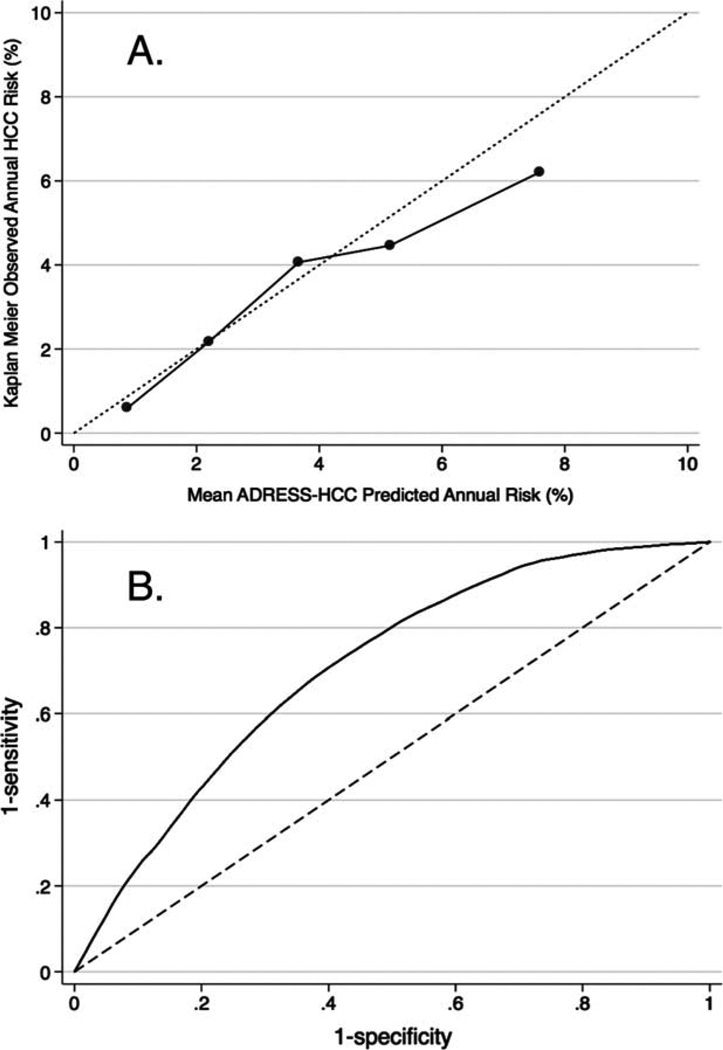
Calibration of the ADRESS-HCC risk model to predict HCC incidence at 1 year in the validation cohort is shown in Figure 2A. The patients were divided into 5 groups according to the ADRESS-HCC score. As the risk score increased, the predicted HCC incidence increased. More importantly, the observed incidence in the validation cohort matched closely with the predicted incidence. We also evaluated the calibration of the model in predicting the risk of HCC out to 8 years and the predicted and observed incidences continued to match closely in all risk groups (data not shown). The C-index of the ADRESS-HCC model was 0.705 (95% CI, 0.688–0.722) in the derivation cohort and 0.691 (95% CI, 0.672–0.712) in the validation cohort with the receiver operating characteristic curve22 of the final model displayed in Figure 2B.
Figure 2.
Calibration of the ADRESS (age, diabetes, race, etiology of cirrhosis, sex, and severity)- a) Calibration plot in the internal validation cohort (n=17,808) and b) receiver operating curve of the entire cohort (n=34,932).
Performance of the Model in Patients Listed With Prevalent, Incident, and Without HCC
Because the demographics of the individuals listed with prevalent HCC were different from the cohort used to derive the ADRESS-HCC model, we wanted to determine whether the model would have predicted a high risk of HCC in this population (5162 patients) compared with those patients who developed incident HCC (1960 patients) and those patients who never developed HCC (32,972 patients). Those listed with prevalent HCC had a median ADRESS-HCC score of 4.74 (IQR, 4.44–5.04), those with incident HCC had a median score of 4.68 (IQR, 4.37–4.97), and those without HCC had a median score of 4.51 (IQR, 4.13–4.87), which corresponded to annual risks of 1.6%, 1.5%, and 1.3%, respectively (P < .001).
Performance of the Model in HALT-C Cohort
Because a high percentage of patients on the liver transplant waitlist have decompensated liver disease, we next evaluated the performance of the ADRESS-HCC model in a cohort of patients with preserved hepatic function using the subgroup of patients with cirrhosis in the HALT-C cohort. The mean age of the cohort was 50.1 years, with 72% of patients being men and 71% being non-Hispanic whites. Diabetes was present in 28% of patients. Only patients with CTP class A disease were included in the HALT-C population and the mean CTP score was 5.3. After a median follow-up of 5 years, 29 patients developed HCC. The median ADRESS-HCC score was 4.96 (IQR, 4.71–5.24 [range, 3.45–6.30]) with a corresponding 1-year median risk of 2.0% (IQR, 1.55%-2.63% [range, 0.44%-7.62%]). Patients were then divided into 3 groups based on the calculated ADRESS-HCC score (quartile 1 indicates low risk, quartiles 2 and 3 indicate intermediate risk, and quartile 4 indicates high risk). Compared with those in the low-risk group, patients with intermediate-risk or high-risk ADRESS-HCC scores had a higher hazard of developing HCC during follow-up (HR, 3.1; 95% CI, 0.8–19.6 [P=.09] and HR, 6.9; 95% CI, 1.9–43.9 [P<.01], respectively) (Fig. 3). When comparing the low-risk group with the intermediate-risk and high-risk groups (ADRESS-HCC score of 4.71), the ADRESS-HCC score was found to have a sensitivity of 93.1% and a specificity of 26.2%.
Figure 3.
ADRESS (age, diabetes, race, etiology of cirrhosis, sex, and severity)- hepatocellular carcinoma (HCC) risk model score and probability of HCC in the HALT-C (Hepatitis C Antiviral Long-term Treatment against Cirrhosis) cohort is shown. Low indicates first quartile, intermediate, second and third quartiles; high, fourth quartile (P <.01, log-rank test).
DISCUSSION
In the current study, we developed and validated a risk prediction model for HCC based on a large US cohort of patients with cirrhosis of various etiologies and severity of liver disease with excellent calibration across all risk groups. The ADRESS-HCC risk model provides an individualized approach to HCC counseling based on specific patient characteristics. At a policy level, it confirms that the risk of HCC is elevated in patients with cirrhosis from a variety of chronic liver diseases other than viral hepatitis and supports their inclusion into HCC surveillance programs.
There are other HCC predictive models that have been developed in patients with chronic liver diseases23–27; however, these other models have been limited by modest sample sizes, a lack of stringent validation, the inclusion of mainly HBV and HCV disease etiologies, and unknown applicability to a North American population. In what to our knowledge is the only model derived from a US cohort, the HALT-C investigators developed a risk score for HCC from 1005 patients with advanced fibrosis but compensated disease (mean CTP score was 5) due to HCV.24 Similar to the ADRESS-HCC model, predictors of HCC included older age, nonwhite (black) race, and portal hypertension as assessed by the presence of esophageal varices and a low platelet count. They also found that an elevated alkaline phosphatase level and a smoking history were associated with HCC, variables that were not included in the SRTR database and thus were not evaluable in our model. The HALT-C model in HCV was not validated and therefore measures of calibration and discrimination were not obtained and the generalizability of the model is limited by the inclusion of only patients with well-compensated cirrhosis with HCV.
Other models were developed in cohorts outside of North America and primarily among cohorts with viral hepatitis. Japanese investigators used data collected from the Japan Public Health Center-based Prospective Study Group and the national cancer registry to develop an HCC risk model.26 Not surprisingly, HCV and HBV seropositivity were found to be the strongest predictors of developing HCC, however, the presence of cirrhosis in the population was unspecified and the model did not undergo validation. The REVEAL-HBV cohort was used to develop and validate an HCC prediction model in Asian patients with chronic HBV who did not have cirrhosis and was validated in a hospital-based cohort.25 The model demonstrated good discrimination (area under the receiver operating characteristic curve, 0.796 at 5 years) and calibration. However, it is again limited by its unknown applicability to patients with cirrhosis and disease etiologies other than HBV. Finally, the GAG-HCC model (Guide with Age, Gender, HBV DNA, Core promoter mutations and Cirrhosis)23 was derived from a single-center prospective cohort of patients with HBV from China in whom male sex, age, HBV DNA level, core promoter mutations, and the presence of cirrhosis were found to be independently associated with HCC. Although they calculated a sensitivity and specificity of 88% and 79%, respectively, there was no external validation and the cohort consisted only of patients with liver disease of HBV etiology. Thus, the ADRESS-HCC risk score offers significant advantages over these previous risk models, including applicability to North American groups of patients with cirrhosis of diverse etiology and severity of liver dysfunction, the ability to calculate the annual incidence of HCC, a large sample size and statistical power, and a robust performance in both internal and external validation data sets.
Within the transplant waitlist data set, we elected to divide the derivation and validation data sets in a nonrandom fashion. Random splitting of a large data set is used commonly; however, successful internal validation of a model in a patient group dissimilar to the one from which the model is developed is stronger evidence of model validity.18 Furthermore, in our external validation in the HALT-C data, the ADRESS-HCC score was found to be sensitive in predicting HCC, thereby supporting its effectiveness in patients with well-compensated cirrhosis who are typically the group of patients undergoing routine surveillance for HCC. Clearly, the HALT-C cohort only included patients with confirmed HCV infection and the model’s performance in patient cohorts with liver disease from other etiologies may be examined in future studies. However, to our knowledge to date, there are no databases with large cohorts of patients with cirrhosis of etiologies other than HCV available for validation.
Further analyses including the comparison between the patients with prevalent and incident HCC and excluding patients with decompensated liver disease add to our confidence of the applicability of the ADRESS-HCC model in groups with varying severity of liver dysfunction. A potential concern about our model is the finding that candidates on liver transplant waitlists may represent a selected subgroup of patients with end-stage liver disease because patients with serious comorbidities are excluded and patients with more advanced hepatic decompensation may be overrepresented compared with average patients with cirrhosis seen in clinical practice. We point out that our transplant cohort included a large number of patients with CTP class A disease (1616 patients with a CTP score of 5 and 2903 patients with a CTP score of 6) and that the HALT-C cohort consisted entirely of patients with compensated liver disease. The results of our sensitivity analysis suggest that in those patients, other than hepatic decompensation, all of the components of the ADRESS-HCC model are preserved. In addition, HCC surveillance is to be targeted for patients without significant comorbidities to allow curative resection or transplantation if HCC is detected.7 Therefore, we suggest that the ADRESS-HCC model is appropriate to apply to the entire US population of patients with cirrhosis who are potentially eligible for transplant and to other populations whose etiologies of liver disease are similar to the current study population (ie, high percentages of viral, alcohol, and NASH cirrhosis).
Finally, we recognize that the ability of the ADRESS-HCC model to separate patients with cirrhosis into those who will and those who will not develop HCC is moderate (C-indices in derivation and validation cohorts of approximately 0.7). Therefore, we cannot suggest that clinicians should determine who should or should not enter HCC surveillance programs using this model but believe that the clinical usefulness of the ADRESS-HCC score is the ability to use the information to counsel patients with cirrhosis regarding their individual annual risk of developing HCC. The latter is best evaluated with calibration, which we have shown is excellent and represents an important strength of the model. In the future, discrimination of the ADRESS-HCC model may be improved with the addition of HCC biomarkers that have also been to predict the occurrence of HCC (ie, AFP-L3).28
The current study presents a validated risk model that represents a clinically useful tool in predicting the risk of HCC in patients with cirrhosis of diverse etiologies. For clinicians, the model may be used to help anticipate the future occurrence of HCC in patients with cirrhosis as well as to guide the counseling of patients regarding HCC. It may also be useful to inform future policies regarding HCC surveillance in an evidence-based fashion.
Acknowledgments
FUNDING SUPPORT
Sponsored by the Canadian Association for the Study of the Liver (CASL)/Merck Clinical Hepatology Fellowship (to J.A.F.) and in part by a grant from the National Institutes of Health (1K24 DK092336 [to W.R.K.]). The funding sources had no role in the design, analysis, or reporting of the study.
CONFLICT OF INTEREST DISCLOSURES
Dr. Flemming was supported by the Canadian Association for the Study of the Liver/Merck Clinical Hepatology Fellowship. Dr. Vittinghoff received salary support from the National Institutes of Health for statistical consulting for work related to the current study. Dr. Kim received a grant from the National Institutes of Health for work related to the current study.
Footnotes
The data reported herein have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be viewed as an official policy of or interpretation by the SRTR or the US Government.
REFERENCES
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 4.Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed February 12, 2013];Mortality Files. cdc.gov/Nchs/
- 5.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Gastroenterology Organisation. [Access on February 3, 2013];Global Guidelines. 2009 worldgastroenterology.org/
- 11.National Comprehensive Cancer Network. NCCN Guidelines Version 2.2012: Hepatocellular Carcinoma. Fort Washington, PA: National Comprehensive Cancer Network; 2012. [Google Scholar]
- 12.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 13.Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54:2712–2721. doi: 10.1007/s10620-009-1015-x. [DOI] [PubMed] [Google Scholar]
- 14.Levine GN, McCullough KP, Rodgers AM, et al. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6(5 pt 2):1228–1242. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 16.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32(4 pt 1):689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 17.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok AS, Everhart JE, Wright EC, et al. HALT-C Trial Group. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–849. doi: 10.1053/j.gastro.2010.11.050. quiz e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heagerty PJ, Lumley T, Pepe MS. Time-dependant ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 23.Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang HI, Yuen MF, Chan HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 26.Michikawa T, Inoue M, Sawada N, et al. Japan Public Health Center-based Prospective Study Group. Development of a prediction model for 10-year risk of hepatocellular carcinoma in middle-aged Japanese: The Japan Public Health Center-based Prospective Study Cohort II. Prev Med. 2012;55:137–143. doi: 10.1016/j.ypmed.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Velazquez RF, Rodriguez M, Navascues CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda H, Kumada T, Tada T. Highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein: a new tool for the management of hepatocellular carcinoma. Oncology. 2011;81(suppl 1):61–65. doi: 10.1159/000333263. [DOI] [PubMed] [Google Scholar]