Abstract
Leukotoxin (LtxA) is a protein secreted from the oral bacterium Aggregatibacter actinomycetemcomitans. LtxA binds to the β2 integrin lymphocyte-associated function antigen-1 (LFA-1) on human white blood cells (WBCs), resulting in cell death. LtxA is currently under investigation as a novel therapy (Leukothera®) for treating hematologic malignancies and autoimmune diseases. We show here that LtxA has potent in vivo anti-lymphoma activity in mice. LtxA caused complete regression of B-cell tumors and promoted long-term survival of mice. The mechanism of LtxA-mediated killing of malignant lymphocytes was further examined. We found that LtxA kills malignant lymphocytes by a novel mechanism requiring the death receptor Fas and caspase-8, but not Fas ligand (FasL) or caspase-9. We also determined that LFA-1 and Fas are closely associated on the cell surface and this proximity of LFA-1 and Fas could explain how signaling through an integrin can lead to cell death. In addition to LFA-1, this work reveals a second surface protein, Fas, that is critical for LtxA-mediated cell death. Knowledge of the mechanism of cell death induced by LtxA will facilitate the development and understanding of this potent experimental therapeutic agent.
Keywords: integrin, inflammation, lymphocytes, lymphoma, autoimmune disease, cell death
1. Introduction
Leukotoxin (LtxA; Leukothera®) is a bacterial protein produced by the oral bacterium Aggregatibacter actinomycetemcomitans (reviewed in [1]). LtxA kills specifically WBCs by binding to the β2 integrin lymphocyte function associated antigen-1 (LFA-1), which is composed of α (CD11a) and β (CD18) subunits. Several studies have shown that expression of LFA-1 is required for LtxA-mediated cell death [2–4]. LFA-1 is expressed exclusively on WBCs and functions in leukocyte migration and cellular activation through its interaction with the intercellular adhesion molecules (ICAMs) (reviewed in [5]). In its resting (low affinity) state, LFA-1 is unable to bind ICAM-1, but when a cell becomes activated, LFA-1 changes conformation to an active (high affinity) state and can then interact with ICAM-1 [6–8]. Interaction between integrins and their ligands typically lead to enhanced cell survival and numerous immunological events [9, 10]. Thus, it is intriguing that interaction between LFA-1 and LtxA instead leads to rapid cell death.
We have shown that LtxA preferentially targets WBCs expressing the active form of LFA-1 [3, 11, 12]. In monocytes, LtxA induces a novel lysosomal-mediated cell death mechanism that also activates other events [11], including a secondary apoptotic pathway, the activation of caspase-1, phosphorylation of p38, and secretion of IL-1β and IL-18 [13, 14]. After binding to LFA-1, LtxA induces the uptake of the LFA-1/LtxA complex and delivery to the lysosome, where the lysosomal membrane is disrupted and cathepsin D is released [11]. This mechanism of cell death in monocytes is very rapid and irreversible also resulting in cofilin dephosphorylation and actin depolymerization [15]. However, we found that lymphocytes do not undergo a similar mechanism of cell death since inhibitors of the lysosomal pathway do not affect LtxA-mediated cell death in these cells [11]. Fong et al. [16] has shown that LtxA binding to the plasma membrane of lymphocytes leads to an increase in intracellular Ca2+ levels. This rise in calcium then activates the protease calpain and cleaves the cytoskeletal protein talin, which releases LFA-1 into lipid rafts. LtxA binds to LFA-1 that is clustered into lipid rafts where it initiates a signal transduction cascade leading to cytochrome c release from the mitochondria and activation of caspase-9 and -7 [17]. While several important events have been described for LtxA-mediated killing of lymphocytes, the mechanism of how interaction between LFA-1 and LtxA actually leads to cell death is not known.
Because of LtxA’s specificity for activated WBCs and its ability to eliminate these cells so efficiently, the protein is being investigated as a first-in-class therapeutic agent (under trade name Leukothera®) for treating diseases of the immune system, including hematologic malignancies and autoimmune diseases. Indeed we have shown that LtxA has potent anti-leukemia activity in vitro and in vivo [3, 18], is highly effective at treating psoriasis [19] and allergic asthma [20] in mouse models, and is specific, active, and well-tolerated in rodents [21] and nonhuman primates [3].
We sought here to determine if LtxA also has anti-lymphoma activity in vivo and decipher the cell death signaling pathway that is activated by LtxA in malignant lymphocytes. We report here that LtxA acts as a potent anti-lymphoma agent and kills malignant lymphocytes via a mechanism that, in addition to LFA-1, requires Fas (CD95) and caspase-8 activation.
2. Materials and methods
2.1. Cell culture
Human cell lines [RL, CEM, Jurkat E6.1, Jurkat A3, Jurkat I 9.2 (caspase-8 mutant)] were purchased from ATCC and maintained in RPMI 1640 medium (Life technologies) supplemented with 10% FBS (Life Technologies) at 37°C, 5% CO2. RL cells B-lymphoblasts originally isolated from a patient with non-Hodgkin’s lymphoma, CEM cells are T-lymphoblasts originally isolated from a patient with acute lymphoblastic leukemia, and Jurkat cells are T-lymphocytes originally isolated from a patient with acute T-cell leukemia.
2.2. Purification of LtxA
LtxA was purified from culture supernatants of A. actinomycetemcomitans strain NJ4500 as described previously [22, 23].
2.3. In vivo lymphoma studies
NOD-SCID mice (10–12 weeks) were purchased from Taconic Farms Inc. and housed in microisolar cages at the Rutgers Cancer Institute of New Jersey vivarium. RL cells (5 million) were injected subcutaneously into the mice (7 mice per group) and when the resulting tumor reached ~100 mm3, LtxA (1 mg/kg) or buffer vehicle was injected intraperitoneally daily for six days. Animal studies were approved by the Rutgers Cancer Institute of New Jersey IACUC committee.
2.4. Inhibitors, antibodies and reagents
The inhibitors used were pancaspase inhibitor z-VAD-FMK, caspase-8 inhibitor z-IETD-FMK, caspase-9 inhibitor z-LEHD-FMK (caspase inhibitors from R&D Systems), and RIPK1 inhibitor necrostatin-1 (Calbiochem). Neutralizing antibodies were anti-human Fas (clone ZB4, Enzo Life Sciences), anti-human TNFα (clone Mab1, Biolegend), anti-human TRAIL (clone RIK-2, Biolegend), anti-human FasL (clone NOK-1, Biolegend). Cell death was measured using annexin V-FITC and 7-aminoactinomycin (7-AAD) (Biolegend). When appropriate, soluble SuperFasLigand (Enzo Life Sciences) or staurosporine (Santa Cruz Biotechnology) were used a positive control for cell death. Anti-human DR4-PE (clone DJR1 from Biolegend), anti-human DR5-PE (clone DJR2-2 from Biolegend), and anti-human Fas-PE (clone DX2 from Biolegend) were used for flow cytometric analysis of surface expression of death receptors. Anti-human Fas-PE, anti-human CD71-PE (clone CY1G4 from Biolegend), anti-human CD18-PE (clone TS1/18 from Biolegend) and anti-human CD11a-FITC (clone HI111 from Biolegend) were used for co-localization experiments. Anti-human cleaved caspase-8 (clone 11G10), anti-human caspase-8 (clone IC12) (both from Cell Signaling), anti-human caspase-9 (clone LAP6, R&D Systems), and anti-GAPDH (cloneFF26A/F9 from BioLegend) were the primary antibodies used for immunoblot analysis or flow cytometry. Primary antibodies were used at 1:1000 dilutions. The secondary antibody (1:200) used to detect cleaved caspase-8 was Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen). Monoclonal antibody to full-length purified LtxA was generated by Promab Biotechnologies and used at 1:500 dilution.
2.5. Cell death assays
For evaluating cell death, 5×105 cells were plated and either inhibitors (75 μM z-VAD-FMK, 50 μM z-IETD-FMK, 50 μM z-LEHD-FMK, 100 μM necrostatin-1) or neutralizing antibodies (20 μg/ml anti-Fas, anti TNFα, anti-TRAIL, or anti-FasL) were added to cells for 1 hour prior to LtxA treatment. Cell death was measured by staining with annexin V-FITC and 7-AAD and analyzed using a FACSCaliber flow cytometry (BD Biosciences). Ten thousand events were recorded per sample. In some experiments, 10 ng/ml soluble FasL was added as a positive control.
2.6. Western blot analysis
Cells (2 × 106) were lysed in MPER Mammalian Protein Extraction Reagent supplemented with Halt Protease and Phosphatase inhibitor cocktail (Thermo Scientific). Lysates were resuspended in SDS dye and equal amounts of protein were loaded (200 ng/sample). Immunoblotting was performed as previously described. Primary antibodies (1:1000) used were anti-human caspase-8 (clone IC12 from Cell Signaling), anti-human caspase-9 (clone LAP6 from R&D Systems), and anti-human GAPDH. HRP-conjugated secondary antibodies at a dilution of 1:5000 (ThermoFisher) were used for detection of the primary antibodies.
2.7. Analysis of caspase-8 activation and death receptor upregulation
To detect activated caspase-8, Jurkat E6.1 cells were treated with LtxA for up to 6 hours. Cells were fixed with 0.1% formaldehyde and permeabilized with Tween 20 (0.5% in PBS). Cells were then washed and treated with anti-human activated caspase-8 (11G10) for 30 minutes followed by Alexa Fluor 488 goat-anti rabbit secondary antibody for 25 minutes. All samples were analyzed using a FACSCalibur flow cytometer and mean fluorescence intensity (MFI) was determined using FlowJo software (Tree Star).
2.8. Co-localization studies with LFA-1
Jurkat E6.1 cells were treated with 0.2 μg/ml LtxA for 15, 45, and 90 minutes, washed with PBS and stained with both anti-CD11a-FITC and anti-CD95-PE for 30 minutes on ice. Cells were washed with PBS two times and fixed with 1% formaldehyde. Untreated Jurkat cells were also stained with anti-human CD11a-FITC and anti-human CD71-PE or anti-human CD11a-FITC and anti-human CD18-PE. Imaging flow cytometry was carried out using the Amnis ImageStream Analyzer (Amnis Corp.), and data were analyzed using the Amnis IDEAS software. Cells with a co-localization value >2.5 were determined to have colocalized receptors. Five thousand images were analyzed.
2.9. Fas/LtxA competition studies
LtxA (0.5 μg/ml) was added to Jurkat cells (106 cells/ml) and the mixture was incubated on ice for 30 minutes. Unbound LtxA was removed by washing in PBS. Anti-Fas blocking antibody (clone ZB4) (final dilution 1:100) was added to the mixture and it was incubated for 30 minutes on ice. Unbound antibody was removed by washing in PBS and then a 1:100 dilution of fluorescent secondary antibody was added, incubated 20 minutes on ice, and then washed in PBS. Flow cytometry was performed as described above. In the corollary experiment, anti-Fas antibody was first added to cells, washed, and then incubated with LtxA. LtxA was detected using FITC-labeled monoclonal anti-LtxA antibody.
2.10. Statistical analysis
For statistical analyses, data were subjected to an unpaired Student’s t-test, with p<0.05 considered to be statistically significant. Error bars represent the standard error of the mean (SEM).
3. Results
3.1. In vivo anti-lymphoma activity of LtxA
Given the ability for LtxA to efficiently kill malignant lymphocytes [3, 16, 24, 25] and its potent anti-leukemia activity [3], we evaluated the use of LtxA in a humanized mouse model for B-cell lymphoma. Human RL cells (5 million) were injected subcutaneously into NOD-SCID mice and when the resulting tumor reached ~100 mm3, LtxA (1 mg/kg) or buffer vehicle was injected intraperitoneally daily for six days. Tumors in the vehicle-treated mice continued to grow (Fig. 1A) and these mice had to be sacrificed when tumor volumes reached 1000 mm3 (Fig. 1B). In contrast, the tumors in the LtxA-treated mice regressed completely (Fig. 1A and Fig. 1B) and the mice survived for the full duration of the study of 75 days (Fig. 1C). In addition, no observable adverse reactions (such as weight loss, loss of fur, or change in behavior) were detected during the experiment in the LtxA-injected animals.
Figure 1.
In vivo activity of LtxA in a B-cell lymphoma mouse model. NOD-SCID mice (n=7 per group) were injected with RL cells and then treated with vehicle buffer or LtxA (i.p) once daily for six days after the tumors reached 100 mm3. A) Tumor volume was measured using a digital caliper. B) Representative image of mice that were treated with either buffer or LtxA. C) Kaplan-Meier survival plot indicating that LtxA-treated mice remained alive for the duration of the experiment-75 days. When tumors reached 100 mm3, the mice were sacrificed.
3.2. LtxA kills malignant lymphocytes by a caspase-dependent pathway
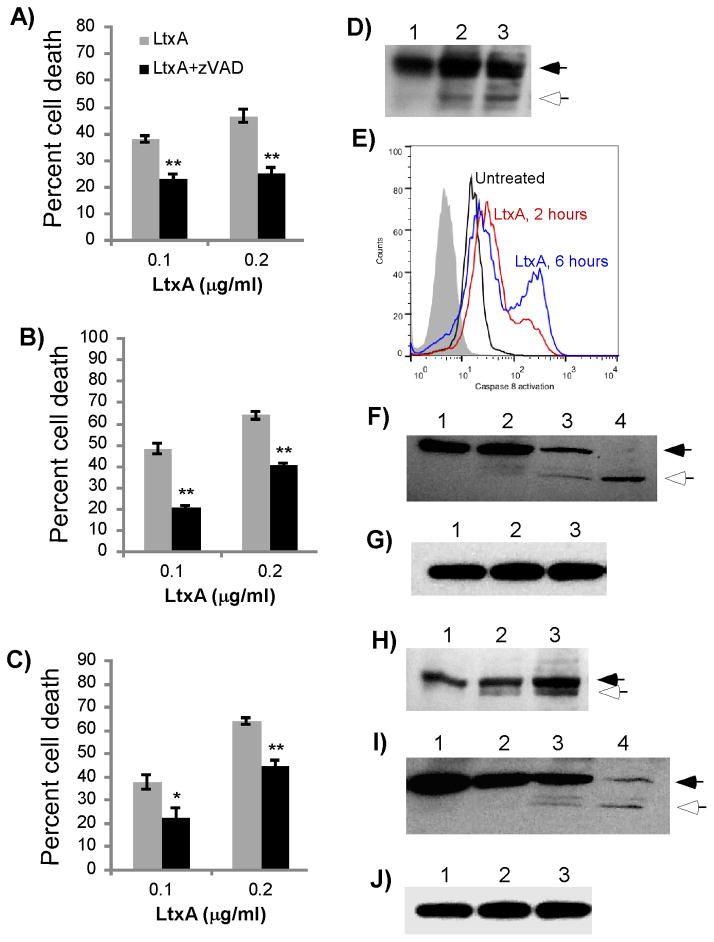
We previously reported that while LtxA kills malignant monocytes utilizing a novel lysosomal-mediated pathway, the killing of malignant lymphocytes by LtxA does not involve a similar mechanism [11]. Others have reported that caspases play an important role in LtxA-mediated death of lymphocytes [11, 14, 15, 17, 26]. Therefore, to begin to decipher the mechanism of LtxA-mediated death of malignant lymphocytes, we examined the role of caspases. Several malignant lymphocytes (CEM T-cells from acute lymphoblastic leukemia, Jurkat T-cells from acute T-cell leukemia and RL B-cells from non-Hodgkin’s lymphoma) were pretreated for 1 hour with the pancaspase inhibitor z-VAD-FMK. Various concentrations of LtxA were then added, and after 24 hours, cell death was measured by flow cytometry using viability stains annexin V and 7-AAD. The percentage of cell death was defined as the total number of annexin V positive/7-AAD negative and annexin V positive/7-AAD positive cells. In all three malignant lymphocytes, cell death was significantly inhibited by z-VAD, indicating that these cells die by a caspase-dependent pathway after LtxA treatment (Figure 2A–C). From this data, we conclude that caspases are necessary for LtxA-mediated cell death in both B- and T-cells.
Figure 2.
Role of caspases in LtxA-mediated cell death. CEM (A), Jurkat (B) and RL (C) cells were pretreated with the pancaspase inhibitor z-VAD-FMK (50 μM) for 1 hour and subsequently treated with LtxA for 24 hours. Cytotoxity was determined by flow cytometry, and the percent cell death is expressed as the sum of annexin V+/7-AAD− and annexin V+/7-AAD+ cells. Data represent the average of three independent experiments. Error bars show SEM. The significance of the differences was determined using a Student’s t-test. *p≤0.05; ** p≤0.01. (D–G) Caspase-8 and -9 activation in Jurkat cells. Jurkat cells were treated with 0.2 μg/ml LtxA for various times and cleavage of caspase-8 (D, E) caspase-9 (F) or total GAPDH levels (G) was evaluated. Solid arrows represent the procaspase form and open arrows indicate the cleavage product. D) Western blot analysis of caspase-8. Lane 1, Untreated Jurkat cells; lane 2, 2 hours LtxA; lane 3, 6 hours LtxA. E) Flow cytometric analaysis of Jurkat cells treated with 0.2 μg/ml LtxA, permeabilized, and stained for caspase-8 activation. The gray peak represents unstained cells; black, untreated cells; red, 2 hours LtxA; blue, 6 hours LtxA. F) Western blot analysis of caspase-9. First lane, untreated cells; second lane, 2 hours LtxA; third lane, 6 hours LtxA; fourth lane, 4 hours staurosporine treated cells (1 μg/ml). G) Western blot analysis of GAPDH. First lane, untreated cells; second lane, 2 hours LtxA; third lane, 6 hours LtxA. (H–J) Caspase-8 and -9 activation in RL cells. RL cells were treated with 0.2 μg/ml LtxA for various times and cleavage of caspase-8 (H) or caspase-9 (I) was evaluated by western blot analysis. Solid arrows represent the procaspase form and open arrows indicate the cleavage product. H) Western blot analysis of caspase-8. First lane, untreated cells; second lane, 3 hours LtxA; third lane, 6 hours LtxA. I) Western blot analysis of caspase-9. First lane, untreated cells; second lane, 3 hours LtxA; third lane 6 hours LtxA; fourth lane, 4 hours staurosporine treated cells (1 μg/ml). J) Western blot analysis of GAPDH. First lane, untreated cells; second lane, 3 hours LtxA; third lane, 6 hours LtxA.
3.3. LtxA causes activation of caspase-8 and -9, but only caspase-8 is necessary for cell death
To determine the primary apoptotic pathway that is utilized by lymphocytes, the activation of caspases-8 and -9 was assessed by western blot analysis. Caspase-8 is the initiator of the extrinsic pathway while caspase-9 is the initiator of the intrinsic pathway. In Jurkat cells, cleavage of caspase-8 was seen as early as 2 hours (Figure 2D). Caspase-8 cleavage was further verified by permeabilizing Jurkat cells after LtxA treatment and probing with a primary antibody that only recognizes the cleaved form of caspase-8. Flow cytometry showed time-dependent activation of caspase-8 starting at 2 hours and thus confirmed the data observed by western blot analysis (Figure 2E). Caspase-9 cleavage was detectable after 2 hours of LtxA treatment, although caspase-9 cleavage after 6 hours of LtxA treatment was more apparent (Figure 2F). GAPDH served as the loading control to confirm equal amounts of protein were loaded (Figure 2G). In RL B-cells, western blot analysis also showed cleavage of caspase-8 as early as 3 hours and cleavage of caspase-9 beginning at 6 hours (Figure 2H–I). GAPDH was used as a loading control (Figure 2J).
Although we showed that LtxA induces cleavage of both caspases-8 and -9, activation of these caspases does not necessarily mean that they are indispensable for cell death. To determine if either of these caspases was necessary for LtxA-mediated cell death, cells were pretreated with peptide inhibitors of caspases-8 and -9 for 1 hour before LtxA was added (Figure 3A–B). The caspase-8 inhibitor, z-IETD-FMK, significantly inhibited cell death in both Jurkat and RL cells treated with 0.1 and 0.2 μg/ml LtxA. With both concentrations of LtxA, caspase-9 inhibitor z-LEHD-FMK failed to prevent cell death in either RL or Jurkat cells. To further confirm the importance of caspase-8, a caspase-8 Jurkat mutant cell line [27], and the parent cell line A3 [28], were treated with LtxA and cell death was evaluated after 24 hours (Figure 3C). The caspase-8 mutant demonstrated significant resistance to cell death at the both doses, again reaffirming that caspase-8 was necessary for LtxA-mediated cell death. These data suggest that while both caspases-8 and -9 are activated, lymphoma cells die via a caspase-8-dependent pathway after LtxA treatment.
Figure 3.

The role of caspase-8 and caspase-9. RL (A) and Jurkat (B) cells were pretreated with either caspase-8 inhibitor z-IETD-FMK or caspase-9 inhibitor z-LEHD-FMK (50 μM) for 1 hour and subsequently treated with LtxA for 24 hours. Cytotoxity was determined by annexin V/7-AAD staining and flow cytometry. C) A caspase-8 mutant and wild type control A3 cells were treated with various concentrations of LtxA for 24 hours. Cytotoxity was determined by annexin V/7-AAD staining and flow cytometry. Data represent the average of three independent experiments. Error bars show SEM. The significance of the differences was determined using a Student’s t-test. ** p≤0.01; ***p≤0.001.
3.4. LtxA induces a Fas-dependent/FasL-independent mechanism of cell death
Several death receptors and proteins are known to be important in caspase-8-dependent cell death. To determine which components are involved in caspase-8 activation, we pretreated Jurkat cells for 1 hour with either inhibitors or neutralizers to the most likely candidates: RIP1 kinase inhibitor necrostatin-1, anti-TNFα neutralizing antibody, anti-TRAIL neutralizing antibody, anti-Fas ligand (FasL/CD95L) neutralizing antibody and anti-Fas (CD95) blocking antibody. We also treated cells with LtxA alone as a comparison. Out of all possibilities, only Fas blocking antibody had an effect on cell death (Figure 4A). In fact, blocking the receptor rendered cells highly resistant to LtxA treatment at the 0.1 and 0.2 μg/ml doses (Figure 4A). Interestingly, neutralizing FasL showed no difference in cell death. To confirm that a sufficient concentration of anti-FasL neutralizing antibody was used, we performed a control experiment to show that the amount of antibody used was sufficient to prevent FasL-mediated cell death (Figure 4B). This suggests that while interaction with the receptor itself is required, the Fas/FasL interaction is not necessary for LtxA-mediated cell death. Further supporting the importance of Fas in LtxA-mediated cell death is the enhanced LtxA killing of the A3 strain, which are very susceptible to Fas-mediated cell death in relation to E6.1 Jurkat cells [28]. When Jurkat E6.1 and A3 cells were treated with LtxA, there was an approximate doubling in the number of dead A3 cells with both the 0.1 μg/ml and 0.2 μg/ml LtxA doses (Figure 4C).
Figure 4.

Fas is required for LtxA-mediated cell death. A) Jurkat cells were pretreated with RIP1K inhibitor necrostatin (5 μM) or a death receptor/death receptor ligand neutralizing mAb (20 μg/ml each) for 1 hour and subsequently treated with LtxA for 24 hours. B) Jurkat cells were pretreated with 20 μg/ml anti-FasL neutralizing antibody for 1 hr and 10 ng/ml FasL for 24 hours to confirm function of the antibody. C) E6.1 Jurkat cells and A3 Jurkat cells, which are highly sensitive to Fas-mediated apoptosis, were treated with LtxA for 24 hours. Cytotoxity was determined by annexin V/7-AAD staining and flow cytometry. Data represent the average of three independent experiments. Error bars show SEM. The significance of the differences was determined using a Student’s t-test. *p≤0.05; **p≤0.01; *** p≤0.001.
3.5. Co-localization of LFA-1 and Fas
Given that anti-Fas antibody (ZB4) was able to block killing by LtxA, we proposed that LtxA may be interacting with Fas as well. We carried out two different experiments to test this hypothesis. First, Jurkat cells were incubated with buffer or LtxA at 4° C to allow binding, but not cell death [29]. Cells were then washed, stained with anti-Fas antibody and analyzed by flow cytometry. We found that preincubation of cells with LtxA, caused a decrease in the fluorescence signal, suggesting that LtxA was preventing binding by anti-Fas antibody (Figure 5A). In the second experiment, cells were first preincubated with anti-Fas antibody, then washed and incubated with LtxA. Excess LtxA was washed off and cells were stained with anti-LtxA antibody and analyzed by flow cytometry. Figure 5B shows that anti-Fas antibody interfered with binding by LtxA. Taken together, these data indicate that LtxA and anti-Fas antibody bind to overlapping epitopes and suggests the possibility that LtxA associates directly with Fas.
Figure 5.
Interaction studies with Fas and LFA-1. A) Jurkat cells were preincubated with LtxA or buffer and then stained with antibody to Fas (clone ZB4). Fluorescent secondary antibody was used to detect Fas using flow cytometery. B) Jurkat cells were preincubated with anti-Fas antibody or buffer and then treated with LtxA. LtxA was detected using anti-LtxA antibody conjugated to FITC. (C–E) Jurkat cells were treated with LtxA, stained with anti-CD11a-FITC and anti-CD95 (Fas)-PE (C), anti-CD11a-FITC and anti-CD18-PE (D), or anti-CD11a-FITC and anti-CD71-PE (E), and analyzed using Amnis imaging flow cytometry. Six representative images are shown for each sample. BF represents the brightfield images. For CD11a/CD95 staining, both the untreated and LtxA-treated samples gave a similarity score >3.0.
Because our studies revealed that both LFA-1 and Fas are important in LtxA-mediated cell death and given the results obtained above, we proposed that LFA-1 and Fas could be associating due to close proximity. To determine if LtxA is causing LFA-1 and Fas to associate, cells were treated with 0.2 μg/ml LtxA for 45 minutes, stained with anti-CD11a-FITC and anti-Fas (CD95)-PE, fixed in 1% formaldehyde and analyzed with imaging flow cytometry (Figure 5C). If an expression similarity score >2.5 is obtained, the receptors are considered to be co-localized [30]. We found that even without LtxA, cells showed a high baseline co-localization level of the receptors, with a score of 3.6. LtxA treatment did not affect the proximity of these receptors (Figure 5C, right). This co-localization between LFA-1 and Fas was even more substantial than that observed for CD11a and CD18, the two subunits of LFA-1 which are known to physically interact (Figure 5D). Imaging flow cytometry of CD11a and CD18 resulted in a similarity score of 3.4. In contrast, staining of CD11a and CD71 (transferrin, a protein known to not localize to lipid rafts) failed to detect co-localization and resulted in a similarity score of 2.1, serving as our negative control (Figure 5E).
4. Discussion
We demonstrate here that LtxA has potent in vivo anti-lymphoma activity in a murine model and that LtxA kills malignant lymphocytes by a caspase-8/Fas-dependent pathway with secondary activation of caspase-9. The fact that tumors did not re-grow during the 75-day study in the LtxA-treated mice indicates that LtxA exerts a robust and complete effect on the B-cell tumor cells. Similar results were obtained previously in a human xenograft model for leukemia [3]. LtxA (Leukothera®) is currently in preclinical development as a biologic for the treatment of hematologic malignancies and autoimmune/inflammatory diseases. In addition to showing significant efficacy in models for leukemia and lymphoma, LtxA was highly effective at alleviating symptoms of disease in models for psoriasis [19] and allergic asthma [20].
Others have shown that LtxA activates caspases -3, -7, and -9 in B-cells [17]. However, there was no inhibition data to support the necessity of caspase-9 activation in LtxA-mediated cell death. Our results corroborate the activation of caspase-9 in both Jurkat and RL cells, but pretreatment with the caspase-9 inhibitor did not prevent killing. Moreover, when caspase-8 mutant Jurkat cells were treated with LtxA, caspase-9 activation still occurred. This result was very surprising, because Jurkat cells are well documented to undergo type II extrinsic apoptosis where caspase-8 is not just required to activate caspase-9, but caspase-9 is necessary to kill the cell [31]. Neither is observed after Jurkat cells are treated with LtxA, suggesting that these cells undergo a non-canonical caspase-8 pathway and that caspase-9 is activated through a secondary, unrelated pathway.
The necessity of caspase-8 in LtxA-mediated cell death was determined by pretreating Jurkat and RL cells with caspase-8 inhibitor and using a functional caspase-8 mutant. In both the inhibitor-treated and mutant cells, LtxA was unable to kill without caspase-8. Caspase-8 can be activated by a number of different pathways. After screening with several death ligand neutralizers and receptor blockers, we found that blocking Fas significantly inhibited LtxA-mediated cell death. Even more surprising was that blocking FasL did not prevent killing, despite the fact that sufficient concentrations of neutralizing antibody were used. This suggests that while the receptor is required for LtxA-mediated cell death, interaction with its ligand is not. The significance of Fas was further confirmed when comparing cell death of two Jurkat cell derivates: E6.1 and A3. A3 cells are very susceptible to apoptosis induced by agonistic anti-Fas monoclonal antibody [28]. Based on our results, A3 cells are also more susceptible to LtxA. Nearly 40% more A3 cells died than E6.1 cells when treated with LtxA.
We recently showed that that LtxA kills malignant monocytes via a lysosomal pathway [11]. Interestingly, monocytes express little or no Fas on their surface and are highly resistant to Fas-mediated cell death [32], but contain a large abundance of lysosomes due to their phagocytic nature. In turn, lymphocytes are known to express abundant Fas on their surface. Thus, it appears that LtxA has cleverly “evolved” ways of usurping the cell death pathway that is available to it, regardless of the type of WBC.
An important question that these results raise is precisely how LFA-1, Fas, and LtxA are all interacting. Although it has been suspected that death receptors and integrins crosstalk [33], no definitive proof has been offered. Given the close proximity of Fas and LFA-1, that LtxA may associate with Fas, and the fact that LFA-1 in lipid rafts is necessary for LtxA killing [16], it is possible that these two receptors are communicating either extracellularly or intracellulary through the lipid raft-signaling platform. Extracellularly, it is possible that LtxA is physically interacting with both LFA-1 and Fas molecules on the surface of the cell. It is noteworthy that LtxA is post-translationally modified with fatty acyl chains through unique linkages at internal lysine residues [34, 35]. The only other molecules in biology that are known to have this fatty acyl linkage at internal lysine residues are certain cytokines, namely those of the tumor necrosis family (TNF) [36], which includes FasL. Thus, one plausible hypothesis is that this fatty acyl modification plays a role in interaction with Fas; in essence, LtxA may be mimicking FasL to activate Fas.
The interaction between LxtA and LFA-1/Fas might lead to intracellular communication between LFA-1 and Fas and subsequent recruiting and activation of caspase-8. Canonical activation of caspase-8 occurs via association between Fas and FADD. However, our initial studies suggest that FADD may not be required for LtxA-mediated cell death (unpublished results), and continued work will further clarify the contribution of FADD in this pathway. In addition, a link between the β2 integrins and Fas pathway is the PI3K signaling pathway. In neutrophils, β2 integrins can control the cell’s fate by either turning on the PI3K and Akt pathway for survival or downregulating them to promote apoptosis [37]. There have also been reports that if the PI3K pathway is inhibited, then Fas-mediated apoptosis independent of FasL is promoted [38]. Interestingly, we previously showed that LtxA causes dephosphorylation (inhibition) of Akt, a protein that is part of the PI3K pathway (unpublished results). One possible explanation for how LtxA functions could be that when LtxA binds to LFA-1, the cell recognizes LFA-1 as an incorrectly ligated integrin, similar to the unligated integrins of adherent cells [39]. This interaction between LtxA and LFA-1 would then downregulate the survival signal and promote cell death through Fas/LFA-1 interaction.
While the LtxA-activated Fas/caspase-8 pathway that we reveal here likely plays a role in elimination of the B-cell tumor in vivo, we cannot rule out the possibility that malignant cells are also dying via other mechanisms that are induced by LtxA. Given the unique targeting specificity and potential novel mechanism of cell death and its demonstrated anti-tumor activity, LtxA may be a highly effective new therapy for treating hematologic cancers.
In summary, this work expands our knowledge on the mechanism of LtxA-mediated cell death in malignant lymphocytes and demonstrates the need for caspase-8 and Fas, but not Fas ligand. Along with LFA-1, Fas may be acting as a co-receptor for LtxA on malignant lymphocytes. We also observed that an integrin and death receptor may be co-localized and this result could explain how interaction through integrins can lead to cell death.
Highlights.
Leukotoxin (LtxA; Leukothera®) has potent activity in a B-cell lymphoma mouse model
LtxA requires caspase-8 and Fas, but not FasL, to kill malignant lymphocytes
In addition to LFA-1, LtxA may associate with Fas to mediate cell death
LFA-1 and Fas co-localize on the cell surface
Acknowledgments
Funding: This work was supported by a small business grant from the National Cancer Institute (R41CA173900) to B.A. Belinka and grants from the St. Baldrick’s Foundation and the New Jersey Commission on Cancer Research to S.C. Kachlany.
We thank Amy Le for purification of LtxA, Raymond Birge for critical reading of the manuscript, and Sukhwinder Singh for technical assistance with flow cytometry.
Abbreviations
- LtxA
leukotoxin
- LFA-1
leukocyte associated function antigen-1
- WBC
white blood cell
- FADD
Fas-Associated protein with Death Domain
- FasL
Fas ligand
- MFI
mean fluorescence intensity
Footnotes
Conflict of interest statement
S.C. Kachlany declares competing interests in the form of stock ownership in the company (Actinobac Biomed, Inc.) that has licensed the use of leukotoxin. In addition, S.C. Kachlany has received consulting fees from this company. B.A. Belinka is an employee of Actinobac Biomed, Inc. and owns stock in this company. The other authors declare no conflict of interest.
Author’s contributions: K.M.D., N.J-F, J.R.B., B.A.B., and S.C.K. designed experiments and analyzed data. K.M.D., N.J-F, D.E., and B.A.V. performed experiments. K.M.D., B.A.V. and S.C.K. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res. 2010;89:561–70. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dileepan T, Kachlany SC, Balashova NV, Patel J, Maheswaran SK. Human CD18 is the functional receptor for Aggregatibacter actinomycetemcomitans leukotoxin. Infect Immun. 2007;75:4851–6. doi: 10.1128/IAI.00314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, et al. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leuk Res. 2010;34:777–85. doi: 10.1016/j.leukres.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, et al. RTX toxins recognize a beta2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–9. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 5.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 6.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Current opinion in cell biology. 2003;15:547–56. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Hogg N, Harvey J, Cabanas C, Landis RC. Control of leukocyte integrin activation. The American review of respiratory disease. 1993;148:S55–9. doi: 10.1164/ajrccm/148.6_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 8.Hogg N, Smith A, McDowall A, Giles K, Stanley P, Laschinger M, et al. How T cells use LFA-1 to attach and migrate. Immunology letters. 2004;92:51–4. doi: 10.1016/j.imlet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–67. [PMC free article] [PubMed] [Google Scholar]
- 10.de la Fuente MT, Casanova B, Moyano JV, Garcia-Gila M, Sanz L, Garcia-Marco J, et al. Engagement of alpha4beta1 integrin by fibronectin induces in vitro resistance of B chronic lymphocytic leukemia cells to fludarabine. J Leukoc Biol. 2002;71:495–502. [PubMed] [Google Scholar]
- 11.DiFranco KM, Gupta A, Galusha LE, Perez J, Nguyen TV, Fineza CD, et al. Leukotoxin (Leukothera(R)) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J Biol Chem. 2012;287:17618–27. doi: 10.1074/jbc.M111.314674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hioe CE, Tuen M, Vasiliver-Shamis G, Alvarez Y, Prins KC, Banerjee S, et al. HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA) PLoS ONE. 2011;6:e23202. doi: 10.1371/journal.pone.0023202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelk P, Abd H, Claesson R, Sandstrom G, Sjostedt A, Johansson A. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell death & disease. 2011;2:e126. doi: 10.1038/cddis.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelk P, Johansson A, Claesson R, Hanstrom L, Kalfas S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 2003;71:4448–55. doi: 10.1128/IAI.71.8.4448-4455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur M, Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera) induces cofilin dephosphorylation and actin depolymerization during killing of malignant monocytes. Microbiology. 2014;160:2443–52. doi: 10.1099/mic.0.082347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong KP, Pacheco CM, Otis LL, Baranwal S, Kieba IR, Harrison G, et al. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell Microbiol. 2006;8:1753–67. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi N, Kieba IR, Korostoff J, Howard PS, Shenker BJ, Lally ET. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell Microbiol. 2001;3:811–23. doi: 10.1046/j.1462-5822.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Le A, Belinka BA, Kachlany SC. In vitro synergism between LFA-1 targeting leukotoxin (Leukothera) and standard chemotherapeutic agents in leukemia cells. Leuk Res. 2011;35:1498–505. doi: 10.1016/j.leukres.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Stenderup K, Rosada C, Dam TN, Salerno E, Belinka BA, Kachlany SC. Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. The Journal of investigative dermatology. 2011;131:2033–9. doi: 10.1038/jid.2011.161. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Espinosa V, Galusha LE, Rahimian V, Miro KL, Rivera-Medina A, et al. Expression and targeting of lymphocyte function-associated antigen 1 (LFA-1) on white blood cells for treatment of allergic asthma. J Leukoc Biol. 2014 doi: 10.1189/jlb.5HI0414-196R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiFranco KM, Kaswala RH, Patel C, Kasinathan C, Kachlany SC. Leukotoxin kills rodent WBC by targeting leukocyte function associated antigen 1. Comparative medicine. 2013;63:331–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Balashova NV, Crosby JA, Al Ghofaily L, Kachlany SC. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:2015–21. doi: 10.1128/IAI.74.4.2015-2021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz R, Ghofaily LA, Patel J, Balashova NV, Freitas AC, Labib I, et al. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb Pathog. 2006;40:48–55. doi: 10.1016/j.micpath.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Taichman NS, McArthur WP, Tsai CC, Baehni PC, Shenker BJ, Berthold P, et al. Leukocidal mechanisms of Actinobacillus actinomycetemcomitans. In: Genco RJ, Mergenhagen SE, editors. Host-parasite interactions in periodontal diseases. American Society for Microbiology; Washington, D.C: 1982. pp. 261–9. [Google Scholar]
- 25.Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 26.Korostoff J, Yamaguchi N, Miller M, Kieba I, Lally ET. Perturbation of mitochondrial structure and function plays a central role in Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Microb Pathog. 2000;29:267–78. doi: 10.1006/mpat.2000.0390. [DOI] [PubMed] [Google Scholar]
- 27.Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Current biology : CB. 1998;8:1001–8. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- 28.Juo P, Kuo CJ, Reynolds SE, Konz RF, Raingeaud J, Davis RJ, et al. Fas activation of the p38 mitogen-activated protein kinase signalling pathway requires ICE/CED-3 family proteases. Molecular and cellular biology. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai CC, McArthur WP, Baehni PC, Hammond BF, Taichman NS. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979;25:427–39. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erie AJ, Samsel L, Takaku T, Desierto MJ, Keyvanfar K, McCoy JP, et al. MHC class II upregulation and colocalization with Fas in experimental models of immune-mediated bone marrow failure. Exp Hematol. 2011;39:837–49. doi: 10.1016/j.exphem.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–40. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. Journal of cell science. 2002;115:3729–38. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 34.Balashova NV, Shah C, Patel JK, Megalla S, Kachlany SC. Aggregatibacter actinomycetemcomitans LtxC is required for leukotoxin activity and initial interaction between toxin and host cells. Gene. 2009;443:42–7. doi: 10.1016/j.gene.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Fong KP, Tang HY, Brown AC, Kieba IR, Speicher DW, Boesze-Battaglia K, et al. Aggregatibacter actinomycetemcomitans leukotoxin is post-translationally modified by addition of either saturated or hydroxylated fatty acyl chains. Molecular oral microbiology. 2011;26:262–76. doi: 10.1111/j.2041-1014.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley P, Koronakis V, Hughes C. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol Mol Biol Rev. 1998;62:309–33. doi: 10.1128/mmbr.62.2.309-333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for alpha(M)beta(2) integrin clustering or activation in the control of apoptosis via regulation of akt and ERK survival mechanisms. J Cell Biol. 2000;151:1305–20. doi: 10.1083/jcb.151.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beneteau M, Pizon M, Chaigne-Delalande B, Daburon S, Moreau P, De Giorgi F, et al. Localization of Fas/CD95 into the lipid rafts on down-modulation of the phosphatidylinositol 3-kinase signaling pathway. Molecular cancer research : MCR. 2008;6:604–13. doi: 10.1158/1541-7786.MCR-07-0331. [DOI] [PubMed] [Google Scholar]
- 39.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]