Abstract
Natural Killer T (NKT) cells are αβ T cells that express a semi-invariant T cell receptor (TCR) along with Natural Killer (NK) cell markers, and have an innate cell-like ability to produce a myriad of cytokines very quickly upon antigen exposure and subsequent activation. These cells are diverted from conventional single positive (SP) T cell fate at the double positive (DP) stage where TCR-mediated recognition of a lipid antigen presented on a CD1d molecule promotes their selection into the NKT lineage. Although many key regulatory molecules have been shown to play important roles in the development of NKT cells, the mechanism of lineage specification and acquisition of effector functions in these cells still remain to be fully addressed. In this review we specifically discuss the role of a family of class I Helix Loop Helix proteins known as E proteins, and of their antagonists Id proteins in NKT cell development. Recent works have shown that these proteins play key roles in iNKT development, from the invariant TCR rearrangement to terminal differentiation and maturation. Elucidating these roles provides an opportunity to uncover the transcriptional network that separates NKT cells from the concurrently developed conventional αβ T cells.
Keywords: iNKT, E proteins, Id proteins, development
I. INTRODUCTION
A distinct population of αβ T cells possessing NK (Natural Killer) cell markers and the innate-like ability to mount a potent immune response within hours of exposure to antigens, is referred to as NKT (Natural Killer T) cells. A unique feature that distinguishes these cells from most conventional αβ T cells is the ability to recognize microbial and self-lipids presented on the non-canonical CD1d molecule, which is MHC (Major Histocompatibility Complex) Class-I like in structure. It has been found that NKT cells can be activated directly by antigen recognition, or indirectly by APCs (Antigen presenting cells),1, 2 to produce a wide range of cytokines. Further, unlike the diverse TCR (T cell receptor) repertoire represented by conventional αβ T cells, most of these cells express a semi-invariant TCR. The most well characterized subset of these are the type I NKT cells, or iNKTs (invariant Natural Killer T cells) that express an invariant Vα14-Jα18 TCRα chain paired with primarily Vβ8.2, Vβ7 or Vβ2 chain in mice, or an invariant Vα24-Jα18 Vβ11 TCR in humans.3, 4 This semi-invariant TCR allows these cells to recognize α-GalCer (a marine sponge derived α-galactosylceramide) among other closely related lipids, which is also utilized for their tetramer-based identification across mice, humans and non-human primates.5 The type II NKT cells have more diverse TCR pairings and recognize other CD1d-presented lipids, but will not be focused upon in this review and the term NKT will be used exclusively for iNKT cells. iNKT cells are known to play a contextual role in diseases, as they are found to be protective in infectious diseases, tumors and certain autoimmune diseases but harmful in asthma and allergy.6–8 Their ability to cross-activate dendritic cells (DCs) and other immune effectors through cytokines and chemokines has also garnered a lot of attention to their potential as vaccine adjuvants.9
Although iNKT cells constitute only a small fraction of T cells in the thymus and periphery of mice and humans, their invariant TCR and recognized ligands have been evolutionarily highly conserved across species, indicating a critical role in the immune system. It is currently accepted that these cells arise from conventional αβ T cell progenitors and follow the same developmental program until the DP (CD4+CD8+ double positive) stage, where the stochastic expression of the semi-invariant Vα14-Jα18 TCR (henceforth referred to as iNKT TCR) allows CD1d-mediated selection, bifurcating them from conventional SP (CD4+ or CD8+ single positive) fate.10–12 This developmental pathway is known to be regulated at different stages by several transcription factors including PLZF (Promyelocytic leukaemia zinc finger protein)13 and EGR2 (Early growth response 2),14 but the exact developmental regulatory programs in iNKT cells are far from fully elucidated.
A family of Class I bHLH (basic Helix Loop Helix) proteins known as E proteins can regulate transcription by binding to E-box (CANNTG) domains, and are known to play key roles in both B and T cell development.15 E protein family members (E2A, HEB and E2-2) dimerize with each other in order to bind DNA. This DNA binding and regulation can be prevented by the formation of heterodimers with members of another HLH (Helix Loop Helix) family known as Id (Inhibitor of DNA-binding) proteins.15 Id2 and Id3 are the key Id family members that are known to inhibit E protein activity in lymphocytes.16 In conventional T cell development it is known that E proteins promote lineage commitment and early development, but their activity must be down regulated upon pre-TCR signaling to allow DN to DP transition, and subsequently must be further repressed by Id proteins upon TCR signaling to allow DP to SP transition.17–19 E and Id proteins have been previously demonstrated to play roles in NKT development20–22 such as the critical role of HEB in iNKT TCR rearrangement23 and Id2 in hepatic NKT homeostasis.24 Recently, more substantial evidence has been published to indicate various roles of these proteins in NKT lineage commitment, development and function.25–28
NKT cell development is a very broad subject and has been covered in depth in other reviews.6, 29–44 Several comprehensive reviews have also been written on NKT antigen recognition,45–48 transcriptional regulation,29, 49–52 and function.7, 53, 54 This review will be limited to discussing key publications on the role of E proteins in NKT development as well as highlighting unanswered questions in the field.
II. iNKT DEVELOPMENT AND REGULATION BY E PROTEINS
Ever since their discovery, the lineage history of NKT cells has been a topic of debate in the field, with researchers supporting either the “pre-commitment” or the “TCR-instructive” model of NKT development.43 The first model suggests that NKT lineage fate is preprogrammed, i.e. an intrinsic NKT-specific program exists in precursors even prior to CD1d-mediated selection, which separates them from conventional T cells. These pre-committed NKT cells then undergo NKT lineage specification upon signaling through the NKT TCR and CD1d-mediated selection.55 In support of this theory, Vα14Jα18 rearrangements have been detected prior to development of conventional αβ T cells during fetal life indicating a possibly distinct NKT developmental pathway.56 Also, NKT “precursor” DN4 cells with Vα14Jα18 transcripts (but no surface TCR expression) have been detected in CD1d-deficient mice, and shown to give rise to NKT cells when cultured with normal thymocytes.57 This supports the existence of early precursors with a NKT-directed program, and specifically directed NKT TCR rearrangement prior to selection. Recent data from our lab also hints towards the role of E proteins in promoting NKT TCR rearrangement before the DP stage.25 In addition, it has been proposed that the distinct transcriptional machinery required for development of conventional T and NKT cells supports the idea of distinct precursors.40, 58 In line with this, it has been found that certain mutations selectively impair NKT development without any impact on conventional T cell development.58, 59 It has also been suggested that innate-like αβ and γδ T cells follow a different developmental program from conventional T cells, such that the TCR expression of the opposite isotype is not selected against in these cells.60 The complete block in conventional T cell development in TCRα−/− animals with a Vα14 transgene again indicates that the NKT TCR may primarily be expressed only in the NKT precursor cells.61 Thus, data in support of the pre-commitment theory is isolated but substantial.
The TCR-instructive model postulates that DP cells which have stochastically rearranged a Vα14-Jα18 TCR, undergo CD1d-mediated selection and subsequent SLAM/SAP (Signaling lymphocytic activation molecule/ SLAM associated protein) signaling,62 that drives their NKT lineage commitment. In favor of this model, there is strong evidence to suggest that the NKT TCR is generated from the same random rearrangement process as conventional T cells, rather than being uniquely regulated as suggested by the pre-commitment model. For instance, the TCR repertoire in NKT cells reflects the same stochastic patterns as conventional TCRα rearrangement.3, 63 Furthermore, it has been found that the NKT-specific Vα14-Jα18 rearrangement is also subject to the same rearrangement bias of pairing with proximal Vα and Jα segments.64 This would imply that only secondary rearrangements can give rise to the distally rearranged Vα14-Jα18 TCR. It has been shown that mice deficient in RORγt (RAR-related orphan receptor gamma t), an important survival factor for DP cells which prolongs the window of secondary rearrangements,65 lack NKT cells due to impaired Vα14-Jα18 rearrangement.10, 11 It was further shown that NKT development could be rescued by expression of a Bcl-xL (B-cell lymphoma – extra large) transgene, which increased DP cell survival and hence promoted distal rearrangements.11 NKT cell development has also been shown to consist of a DP intermediate12, and to proceed through the DP stage using fate mapping techniques.11 It has been proposed that similar to γδ T cells, strong TCR signaling is critical for selection into the NKT lineage,66 which has also been demonstrated in a reporter mouse.67 A role of strong TCR signaling in PLZF expression, a key transcription factor in NKT development, has also been shown.14, 68 These observations have garnered great support in favor of the TCR-instructive model. However, it is important to note that both models heavily stress upon the timing of Vα14-Jα18 rearrangement. While it is clear that CD1d-mediated TCR engagement provides the decisive signal for NKT lineage commitment, it still needs to be determined whether all or just a fraction of DP cells are competent to become NKT cells. Re-evaluating available data in this context may serve as a better approach to uncovering the transcription program that distinguishes iNKT TCR from other TCR signals during NKT commitment.
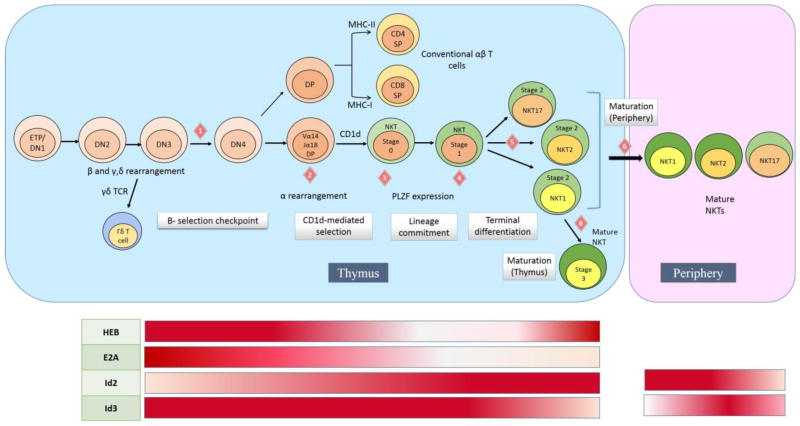
NKT cells are either CD4SP or DN cells that proceed through 4 broad stages of development (figure 1) - commitment (stage 0, CD24hi), expansion (stage 1, CD24lo CD44lo), differentiation (stage 2, CD44hi) and maturation (CD44+ NK1.1+).30 In this figure we have also marked several “steps” in their development, which may be regulated by transcription factors, particularly E proteins, and can therefore impair or support NKT development. These steps include NKT TCR rearrangement, CD1d-mediated selection and subsequent transitions between stages. For example, EGR2 is found to be important for PLZF expression,14 while NF-kB69 (nuclear factor kappa-light-chain-enhancer of activated B cells) and c-Myc70 are important for the proliferative burst at stage 1. Although the roles of E or Id proteins in NKT development have so far been limited to that of HEB23 and Id2,24 several key roles have been revealed in more recent papers which are discussed in subsequent sections. Previously, it was thought that stage 1 iNKTs secrete only IL-4 on stimulation, stage 2 cells produce both IL-4 and IFN-γ and stage 3 cells produce mostly IFN-γ, but it has now been shown that 3 terminally differentiated iNKT subsets (iNKT1, iNKT2, iNKT17) similar to the conventional T helper cell subsets exist within these linearly ordered stages of iNKT development, each with distinct cytokine profiles.71–74 The role of E and Id proteins in influencing subset differentiation and in other steps (figure 1) of NKT development are summarized (table 1) and discussed in detail in the following sections.
FIGURE 1. Stages of NKT cell development and their regulation by E and Id proteins.
According to the currently accepted model, NKT cell development is concurrent with conventional αβ T cell development up to the DP stage. There are several important “steps” (depicted as numbered diamonds) in NKT development that are critical for their appropriate lineage specification and functional maturation, and are regulated by E and Id proteins to influence their development. A functional TCRβ rearrangement allows DN3 cells to proceed beyond pre-TCR selection (step 1) and reach the DP stage where α rearrangement takes place. The DP cells that then stochastically express a Vα14Jα18TCR (step 2) typically diverge from conventional αβ T cells upon CD1d-mediated selection and undergo iNKT lineage commitment marked by PLZF expression at stage 0 and 1 (step 3). This is followed by an expansion phase at stage 1 (step 4) and terminal differentiation into iNKT1, iNKT2 or iNKT17 stage 2 subsets (step 5). These subsets with varying cytokine profiles, then migrate to different parts of the periphery to mature (step 6). The iNKT cells that mature within the thymus were previously characterized as stage3 iNKTs (step 6), but have now been identified to be iNKT1 cells. The regulation of these critical NKT developmental steps by E and Id proteins is summarized in Table1. E and Id protein expression during NKT cell development (beyond DN3 stage) has also been graphically represented below the corresponding stages (dark red represents highest expression, white represents little to no expression).
TABLE 1. Role of E and Id proteins in iNKT development.
E and Id proteins have recently been shown to play several roles in NKT TCR rearrangement, NKT lineage commitment, expansion, differentiation and maturation. Thus E and Id proteins regulate this lineage at several key NKT developmental steps which are marked in figure 1. Their functions at the different stages of NKT development are summarized in this table.
| No. | Developmental step | E or Id protein role |
|---|---|---|
| 1 | Pre-TCR / β selection : DN3b stage |
|
| 2 | iNKT TCR rearrangement/ iNKT selection : DP stage | |
| 3 | iNKT program initiation : iNKT Stage 0 | |
| 4 | iNKT proliferation : mainly Stage 1 | |
| 5 | iNKT subset differentiation: Stage 2 | |
| 6 | iNKT Stage 3 maturation |
A. iNKT TCR expression and selection into the NKT lineage
After pre-TCR selection at the DN3 stage, the expression of a hallmark Vα14-Jα18 TCR in DP cells and selection by CD1d-presented antigen on other DP cells, allows commitment to the NKT lineage (step 2, figure 1). This process of “selection” may be randomly governed by TCR expression, or may be pre-determined by an NKT precursor, depending on which model of development is being considered. In this section below, we will primarily discuss the role of E proteins in influencing NKT TCR rearrangement, but it is worthy of mentioning that consistently high levels of E proteins can impair the pre-TCR checkpoint and limit selection into the αβ lineage, which would also take a toll on selection into the NKT lineage.25
It has been demonstrated in the past that Id2 deficiency allows normal NKT development, but impairs NKT survival and localization to the liver.24 However, the first concrete evidence of E proteins playing a role in NKT development was the lack of the E protein HEB leading to a severe reduction in NKT cell number at all stages in the thymus and periphery.23 Such a phenotype could be explained by the associated reduction of RORγt expression, a direct target of E proteins,75, 76 and the downregulation in RORγt target Bcl-xL, an important survival factor for DP cells.77 As mentioned before, a similar phenotype is also observed in RORγt−/− mice due to reduced secondary TCR rearrangements, particularly Vα14-Jα18.11 Another interesting observation made by this group was that even an NKT TCR transgene could not fully rescue development past stage 1, suggesting that HEB may play other roles in influencing NKT differentiation and maturation. It is important to note that E2A and HEB have different expression levels, and hence most likely play different roles during NKT development.23
In a recent paper, Barbara Kee’s group demonstrated that Id3 deficiency led to increased selection into the NKT pathway partially because it allowed continued expression of E protein targets RORγt, RAG1 (Recombination activating gene 1) and RAG2 (Recombination activating gene 2).27 Thus, even after positive selection, a fraction of Id3−/− DP thymocytes continued rearranging their TCR, resulting in a larger fraction of cells with the distal Vα14-Jα18 rearrangement. Also, in our recent publication, we have demonstrated that combined deletion of both Id2 and Id3 at the DN3 stage using Lck (lymphocyte-specific protein tyrosine kinase) Cre led to a dramatic expansion of both CD4+ and DN iNKT cells.25 Using high-throughput sequencing of the TCR repertoire of pre-selection CD69− DP cells from these mice, we observed that there was random pairing of Jα chains in cells with the common Vα8 rearrangement but a positively biased pairing of the Jα18 chain in cells with Vα14 rearrangements, as compared to WT mice. This suggests that the lack of Id proteins or very high E protein levels can perhaps promote a skewed TCR repertoire in favor of selection into the NKT lineage.25
It is important to mention that Id and E protein dosage can influence lineage choice and differentiation in NKT cells. For instance, the NKT population is quite small in WT mice suggesting that low E protein activity after the pre-TCR checkpoint in WT mice is not sufficient to promote selection into the NKT lineage. However it seems that deletion of Id2 and Id3 can enhance the E protein levels sufficiently to allow them to specifically promote the NKT rearrangement and hence promote the NKT lineage fate choice.25 The mechanism by which E proteins promote this rearrangement is however unclear, and may be through binding to Vα14 or Jα18 promoter or enhancer regions. It is also possible that Id proteins suppress this rearrangement instead of E proteins promoting it. There is also potential for exploring the role of HEB in influencing NKT development beyond cell survival and secondary rearrangements, such as through the dysregulation of metabolic genes and cytokines like IL17Rβ and IL12Rβ, which can also be expected to play important roles in this lineage.23
Thus, E proteins are critical for NKT development in order to allow more secondary rearrangements at DP stage, and Id proteins limit selection by preventing the same. However, it still remains to be determined if E proteins are also capable of biasing the lineage decision by specifically regulating NKT TCR expression, and if so, how.
B. Initiation of the iNKT developmental program
Selection into any lineage is coupled with induction of the appropriate transcriptional program, marking “commitment” into the lineage. This program must regulate the appropriate expression of transcription factors needed for NKT development and maturation, and is usually distinctly recognizable by the expression of a “master regulator”. After an intensive screening in search of a unique NKT-specific transcription factor, PLZF was identified as a key regulator of NKT development.13, 78 Later it was discovered that NKT cells shared PLZF expression with other innate-like cells, including Vγ1.1Vδ6.3 γδ T cells68 and MAIT (Mucosal Associated invariant T) cells.13 PLZF is found to be upregulated soon after TCR signaling in stage 0 and stage 1 NKTs (step 3, figure 1).13 PLZF expression is critical for NKT development and function, as demonstrated by the decrease in NKT numbers, disturbed cytokine production and NKT localization in lymph nodes in PLZF-deficient mice.13 Further, expression of a PLZF transgene leads to production of IL-4, IFN-γ, as well as IL-17 by NKT cells suggesting that it promotes a broad NKT effector program in these cells prior to any subset differentiation.13, 28, 71, 79
A recent study has shown that strong TCR signaling, as is observed in case of NKT selection, immediately causes EGR2 upregulation followed by PLZF expression.14 The presence of an EGR2 binding site in the PLZF promoter region and the failure to upregulate PLZF in EGR1/2 double deficient mice indicates that TCR signaling mediated EGR2 upregulation leads to PLZF expression in stage 0 NKT cells. On the other hand, another group’s work has demonstrated that E proteins bind to two E-box sites in the PLZF promoter region to directly regulate PLZF expression.26 Sustained E protein expression has also been shown to cause increase in PLZF expression.28 In contrast however, Id2/Id3 double deficient mice have also been found to have a 2 fold reduction in PLZF expression, as well as a naïve NKT phenotype similar to PLZF−/− mice.27 Further, the authors found an increase in EGR2 levels in these NKT cells, implying that Id2 and Id3 are important for PLZF expression in an EGR2-independent manner.
There are several possibilities that can explain these contrasting observations. It is possible that Egr2 positively regulates either E or Id proteins. The fact that TCR signaling promotes Egr2, which lies upstream of Id3 in γδ T cells,80 suggests that Egr2 may downregulate E proteins in these cells. E proteins and Egr2 may also coordinate to promote PLZF, which would explain why loss of either E proteins or Egr2 leads to a similar phenotype. Furthermore, Egr2 deficiency has also been demonstrated to reduce Id2 expression in NKT cells.14 This could imply that Egr2, Id and E proteins can also regulate PLZF expression at different stages during NKT development. For instance, initially Egr2 and E proteins can promote PLZF together, until Egr2-mediated Id2 expression suppresses E protein to prevent further PLZF upregulation. Alternatively, Id2 and Id3 may positively regulate PLZF expression in the early stages of NKT development. This would be supported by the observation that ET-2 mice with sustained E protein levels throughout NKT development, showed low PLZF in stage 1 but high PLZF in stages 2 and 3.28.
While the exact mechanism of PLZF regulation in NKT development is unclear, it is certain that E and Id proteins are critical for appropriate expression of PLZF, and hence, are important for NKT-specific program initiation. It is important to note that PLZF is also considered important for the development of innate-like Vγ1.1Vδ6.3 γδ T cells,68 and so E and Id proteins can promote both iNKTs and these γδ T cells. Indeed, our data has shown that reducing E protein levels to 50% lower (Id2f/f Id3f/f E2A+/f HEB+/f LckCre+) than Id2f/f Id3f/f LckCre+ mice, led to a drastic change in phenotype from predominantly iNKT cells to mostly Vγ1.1Vδ6.3 γδ T cells, suggesting that E protein levels can influence lineage choices between these two closely linked cell types.25 The downstream E protein targets that assist in this lineage choice are yet to be determined.
C. iNKT population expansion
In comparison to conventional T cells that undergo antigen-driven clonal expansion in the periphery,81 stage 1 iNKT cells start undergoing a major population expansion in the thymus immediately following positive selection and iNKT lineage commitment (step 4, figure 1).82 Several transcription factors, including NF-kB34, 69 and c-Myc,70, 83 have been shown to be critical for proliferation of stage 1 and/or stage 2 iNKT cells. It should be noted that although PLZF expression is required for proper development of stage 1 and stage 2 NKTs, it doesn’t seem to influence the proliferation capacity of these cells.78 Our lab has demonstrated that high E protein levels achieved by deletion of both Id2 and Id3 led to a 3 fold increase in proliferation (and hence number) of stage 1 CD4+ iNKTs and DN TCRβlo type II NKTs.25 As mentioned in the previous section, this increase in NKT cells is dependent on E protein levels as deletion of one copy each of E2A and HEB in Id2–Id3 deficient mice led to an expansion of γδ T cells instead.25 A similar increase in total NKTs has also been reported upon deletion of 3 out of 4 Id alleles.25, 26 Further, a mouse model with E2A and HEB deletion at the DP stage along with a Vα14 TCR transgene (to study roles of E proteins in an NKT selection-independent context) showed a 50% reduction in NKT numbers and a block beyond stage 0 partly due to defective proliferation in stage 1 cells.26 It was also noted that there was an expansion of Id3 deficient NKT cells at stages 2 and 3 in Id3 deficient/wild type bone marrow chimeras, suggesting that Id3 limits iNKT expansion of not just stage 1, but at all stages of iNKT development. This increase in iNKT numbers was explained by increased proliferation in Id3 deficient cells, particularly at stage 2. Loss of Id2 however led to no change in iNKT proliferation.26
The NKT expansion phase allows the lineage to make up for the limited initial selection and generate high copy numbers, which are important for quick response to antigens. It is interesting to note that both E protein and c-Myc have been shown to play a role in NKT proliferation.26 However, it is unclear whether E proteins upregulate c-Myc or if they act in a concerted manner as observed in Burkitt’s lymphoma.26, 84 Further, it has been suggested that E proteins may upregulate Id3 during this phase to keep a check on the proliferation.26 Given that Id3 limits NKT expansion, it is yet to be determined if its expression needs to be reduced upon selection to increase NKT numbers,26 and if so, how it is suppressed post TCR signaling. It has been suggested that the induction of the ITK/RAS/ERK (interleukin-2-inducible T-cell kinase/ Ras/ Extracellular-signal-regulated kinases) pathway, SLAM/SAP signaling or β-catenin may play a role in this.27 It has also previously been demonstrated that Id2 can negatively regulate Id3 expression.26, 85 Thus, it is also possible that Id2 upregulation through cytokine signaling85 then suppresses Id3 expression to allow proliferation.
Therefore, high E protein levels promote NKT population expansion during stages 1 and 2 of NKT development, while Id3 limits NKT cell numbers.
D. NKT differentiation and maturation
Similar to the T helper subsets, three NKT cell subsets NKT1, NKT2 and NKT17, have recently been characterized.71 The definition of conventional stage 2 NKTs has accordingly been revised to that of a heterogeneous population comprising of terminally differentiated NKT2 and NKT17 cells, and NKT1 progenitor cells72 (step 5, figure 1). These subsets later migrate to the periphery or stay in the thymus to mature (step 6, figure 1), and have varying transcriptional programs to direct distinct cytokine production in the periphery. While NKT1 cells are known for their IFN-γ production with high T-Bet (or T-box transcription factor, TBX21) and low PLZF expression, NKT2 cells are IL-4 and IL-13 producing with high GATA3 (GATA Binding Protein 3) and PLZF and moderate IL-17Rβ expression, and NKT17 cells are identified as IL-17 producing RORγt+ cells with low expression of PLZF and T-Bet.71 Similar to skewing of T helper responses in different mouse strains, the distribution of NKT subsets also varies widely between mouse strains.72 Due to the prevalence of NKT1 cells in C57BL/6J mice,86, 87 this subset has been extensively studied and is shown to mature in the thymus. Based on this, some consider mature NKT1 cells to be same as the conventional mature stage 3 NKTs27 (NK1.1+ CD44+), but others believe that stage 3 can consist of all 3 mature subsets.26 All three subsets localize to different locations in the periphery - NKT1 cells are found in the liver and spleen, NKT2 cells in the lung and NKT17 cells are found mostly in lymph nodes.71 The emergence of these subsets has not only changed the paradigm of a linear NKT developmental program, but has also enabled study of subset-specific roles of NKTs in disease contexts and expanded the potential applications of NKT cells as adjuvants to prime other cell types. Therefore, E and Id proteins play distinct roles in influencing the linear part of NKT development until stage 1, which includes lineage selection, commitment and expansion. Beyond stage 2, E and Id proteins play subset specific roles as differentiation into distinct NKT subsets requires unique transcriptional programs. The more recently discovered regulatory NKT (or NKT10) cells are not yet well characterized, and hence are not discussed here.88, 89
For NKT1 development, both Id2 and Id3 have been demonstrated to be important as there is a significant reduction in the frequency of Id3-deficient T-bet+ NKT1 cells in Id3+/+/Id3−/− bone marrow chimeras, as well as reduced frequency and numbers of Id2-deficient NKT1 cells in Id2+/+/Id2−/− bone marrow chimeras.26 This suggests that wild-type level of E protein activity (with both Id2 and Id3 present) supports NKT1 development, as observed in B6 mice. Further, the expression of T-bet, a critical transcription factor for NKT1 development as well as IFN-γ production and IL-2Rβ expression for stage 3 maturation,71, 90, 91 has also been deemed a critical checkpoint in NKT development.27 It has been found that stage 2 iNKTs in ET-2 mice with sustained high E protein levels throughout NKT development failed to upregulate T-bet, causing a block in stage 3 maturation with impaired IFN-γ production.28 These data suggest that E proteins can directly or indirectly suppress T-bet expression to influence NKT maturation and effector functions. Id2 or Id3 deficiency in the BM chimeras described above also led to increased PLZF, but impaired T-bet expression, explaining the decrease in the NKT1 population.26 Id3−/− mice also have reduced numbers of stage 3 cells and IFN-γ producing iNKTs, along with significantly aberrant gene expression from as early as stages 1 and 2.27 Based on the expression profile of Id3 (high in stages 1, 2 and NKT2 cells, but low in stage 3) and its requirement for the development of stage 3 NKT1 cells, the authors suggest that Id3 is important at earlier stages for initiation of the stage 3 maturation program. Deficiency of both Id2 and Id3 also leads to fewer IFN-γ only producing (i.e. NKT1) cells,27 but it is still unclear whether Id2 and Id3 play distinct roles in the development of the subset, or the observations are merely artifacts of functional compensation by Id2 and Id3. Moreover, one of the early pieces of evidence suggesting that of Id or E proteins play a role in NKT development was the role of Id2 in maintaining the homeostasis of hepatic NKTs,24 which are now known to be mostly NKT1 cells. These NKT1 cells were drastically reduced in Id2 deficient mice due to greater apoptosis caused by a reduction in CXCR6 (chemokine (C-X-C motif) receptor 6), Bcl-2 (B-cell lymphoma 2), Bcl-xL and the inability to counter the pro-apoptotic protein BIM, which is also an E protein target.
The role of Id3 in NKT2 development is ambiguous as Id3 deficiency led to a larger population of PLZFhi NKT2 cells, but these cells also had the highest Id3 expression.26 It is possible that the high level of Id3 is essential for selection into the NKT2 subset, but limits the numbers after selection by inhibiting E proteins that would otherwise drive increased differentiation into this subset. In support of this idea, ET-2 mice with sustained E protein expression had greater number of NKT2 cells.28 Along similar lines, NKT17 cells are also promoted by high E protein levels, as reported by increase in this subset in ET-2 mice.28 It is interesting to note that this subset has low to medium expression of both Id2 and Id3, as opposed to a dominant expression of one Id protein as seen in the other subsets.26 Also, this increase in NKT2 and NKT17 cells was accompanied by a reduction in the NKT1 population, indicating that higher than normal E protein levels promote NKT2 and NKT17 subset differentiation at the expense of the otherwise predominant NKT1 lineage.28 Moreover, retroviral overexpression of T-bet in immature NKTs from these mice rescued IFN-γ production and some other features of NKT1 cells, hinting at the plasticity of these subsets.
The discovery of these terminally differentiated subsets of NKT cells has altered our understanding of the development of this innate-like lineage. Although their key transcription factors and cytokine profiles have been well characterized, their differentiation from either a common or distinct NKT precursor(s) is not yet known. Further, it has been suggested that a separate checkpoint may exist for directing NKT cells towards NKT1, NKT2 or NKT17 fate, which is regulated by either transcription factors or TCR signal strength.28 Since all three subsets localize to distinct locations in the periphery, it has been hypothesized that the cytokine milieu in the microenvironment of the NKT subset may also play a major role in their homeostasis.26 Further, the plasticity of the subsets makes it even more difficult to uncouple the intrinsic transcriptional requirements from external stimuli. However, TCR signals and signal strength are unlikely to be the only external stimuli affecting the transcriptional program. The role of cytokines, chemokines and growth factors in this setting are still waiting to be explored. It is interesting to note that besides influencing NKT differentiation, high E protein levels were also shown to impair thymic exit, causing a reduction in peripheral NKT cells.28
All the above data suggest that E and Id protein levels can differentially influence the maturation and development of NKT subsets. High E protein levels were found to promote NKT2 and NKT17 development, whereas Id proteins were necessary for NKT1 development. The implications of these findings can be better understood by bearing in mind their resemblance to T helper subsets. For instance, E proteins are capable of directly regulating GATA3 expression,92 and Id2-deficiency in mice causes a Th2-dominant phenotype.93 Id3 and E proteins have also been demonstrated to be critical for Th17 development by promoting RORγt expression and blocking IL-4 production.94 Overall, these results indicate that E and Id proteins can regulate key transcription factors to bias NKT subset differentiation and their effector function.
III. DISCUSSION
In this review we have discussed the regulation of innate-like NKT cell development by E and Id proteins in detail. As mentioned before, there is evidence to suggest that E proteins can bind to and control PLZF expression, which is also a key transcription factor for other innate-like cells.26 High E protein levels can promote both innate-like Vγ1.1Vδ6.3 γδT cells and iNKT cells.25 Besides their common signature transcription factor PLZF, the innate-like V6.3 γδ T cells and iNKT cells also have semi-invariant TCRs and very similar RNA expression profiles.95 These observations suggest that these cells may independently diverge from mainstream αβ/γδ lineages by acquiring an innate regulatory program. Alternatively, there may be a shared evolutionary history between these two innate-like lineages, which is distinct from conventional T cell development. A similar model of unique developmental pathways for innate-like and adaptive T cells has been proposed recently.60 The authors found that unlike conventional αβ and γδ T cells, the development of innate-like γδ and innate-like αβ (NKT) cells is independent of the rearrangement status and expression of the TCRβ and TCRγ chains respectively, suggesting that the innate-like T cell developmental program may diverge from conventional T cell development earlier than what is currently understood. In line with this, our evidence suggests that early deletion of Id proteins prior to selection at the DP stage may turn on an early NKT-specific program, causing a larger expansion of the NKT population.25 Further, we found that the level of E proteins tightly regulate the lineage outcome between innate-like Vγ1.1Vδ6.3 and NKT cells. These data raise an interesting possibility that these innate-like cells may be evolutionarily ancient lineages with a shared developmental program that must be repressed by Id proteins to allow appropriate development and expansion of conventional αβ T cells. It is important to note that this proposition doesn’t challenge the role of CD1d-mediated selection in iNKT lineage specification at the DP stage, but rather speculates whether all DP cells have equal competence to become NKT cells or not.
Another challenge in this field is distinguishing between the unique and mutually compensatory roles of Id2 and Id3 in NKT development. Since Id2 deficiency doesn’t lead to a noticeable phenotype but Id3 deficiency does,25, 27 it can be argued that Id3 can compensate for Id2 but not vice versa. At the same time there is a more dramatic expansion in the NKT population upon deleting both Id2 and Id3.25, 27 While this phenotype can be explained solely based on higher E protein levels in Id2/Id3 double deficient mice than in Id3−/− mice, it may also be mediated by a combined effect of distinct roles of Id2 and Id3 in NKT development. Based on the similarity between innate-like NKT cells and innate lymphoid cells (ILCs), which depend on Id2 for their development, it can be proposed that NKT cells also depend on Id2 but functional compensation by Id3 disguises any phenotype in Id2 deficient mice.27 It is likely that Id proteins play compensatory or distinct roles depending on the stage of development and location. For example, it seems that Id3 is a key driver in the thymus whereas Id2 plays a more important role in the periphery.27 Id2 and Id3 also seem to independently and uniquely regulate the development of different NKT subsets.26 Moreover, we have found that Id2 is upregulated upon Id3 deletion in DN3 cells, but not in DP cells (unpublished data). Another group found, however, that Id2-deficient NKT cells possess higher Id3 expression while Id2 expression was reduced in Id3-deficient NKT cells.26 This data suggests that Id2 attenuates Id3 expression, whereas Id3 supports Id2. These contradicting observations highlight the significance of the method (germline vs conditional knockout vs mixed bone marrow chimera) and stage of Id protein deletion in influencing NKT development. These factors should therefore be considered with care while inferring their role in NKT cells. Clearly, more work on the temporal and cross-regulatory aspects of E and Id proteins is needed to decode their myriad functions in NKT development.
To conclude, in this review we have highlighted several roles that E and Id proteins play at key “steps” in iNKT cell development. There has been some contradictory evidence regarding PLZF regulation and Id protein compensation which need to be investigated further. The early differentiation of NKT cells into the different subsets, and their regulation by E and Id proteins has also not been fully characterized. Due to the broad impact of these innate-like NKT cells on the immune response and disease outcomes, a better understanding of their development and regulatory mechanisms will facilitate manipulation of these cells in a clinical context.
LIST OF ABBREVIATIONS
- APC
Antigen Presenting Cell
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- bHLH
Basic Helix Loop Helix
- CD
Cluster of Differentiation
- CXCR6
chemokine (C-X-C motif) receptor 6
- DC
Dendritic Cell
- DN
Double Negative
- DP
Double Positive
- EGR2
Early growth response 2
- ERK
Extracellular-signal-regulated kinases
- GATA3
GATA Binding Protein 3
- HLH
Helix Loop Helix
- Id
Inhibitor of DNA-binding
- IFN
Interferon
- IL
Interleukin
- iNKT
invariant Natural Killer T
- ILC
Innate lymphoid cells
- ITK
interleukin-2-inducible T-cell kinase
- Lck
lymphocyte-specific protein tyrosine kinase
- MAIT
Mucosal Associated invariant T
- MHC
Major Histocompatibility Complex
- NK
Natural Killer
- NKT
Natural Killer T
- PLZF
Promyelocytic leukaemia zinc finger protein
- RAG1/ RAG2
Recombination activating gene 1/2
- RORγt
RAR-related orphan receptor gamma T
- SAP
signaling lymphocytic activation molecule (SLAM)-associated protein
- SLAM
Signaling lymphocytic activation molecule
- SP
Single Positive
- TCR
T cell Receptor
References
- 1.Holzapfel KL, Tyznik AJ, Kronenberg M, Hogquist KA. Antigen-dependent versus -independent activation of invariant NKT cells during infection. Journal of immunology. 2014 Jun 15;192(12):5490–8. doi: 10.4049/jimmunol.1400722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. The Journal of experimental medicine. 2011 Jun 6;208(6):1163–77. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. The Journal of experimental medicine. 1994 Sep 1;180(3):1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. The Journal of experimental medicine. 1997 Jul 7;186(1):109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. The Journal of experimental medicine. 1998 Oct 19;188(8):1521–8. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annual review of immunology. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Current opinion in immunology. 2008 Jun;20(3):358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, DeKruyff RH, Savage PB, Umetsu DT. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nature medicine. 2013 Oct;19(10):1297–304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nature reviews Immunology. 2009 Jan;9(1):28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 10.Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proceedings of the National Academy of Sciences of the United States of America. 2005 Apr 5;102(14):5114–9. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005 Jun;22(6):705–16. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nature immunology. 2001 Oct;2(10):971–8. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 13.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008 Sep 19;29(3):391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Barr K, Meng F, Singh H, Bendelac A. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nature immunology. 2012 Mar;13(3):264–71. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel I, Murre C. The function of E- and Id proteins in lymphocyte development. Nature reviews Immunology. 2001 Dec;1(3):193–9. doi: 10.1038/35105060. [DOI] [PubMed] [Google Scholar]
- 16.Kee BL. E and ID proteins branch out. Nature reviews Immunology. 2009 Mar;9(3):175–84. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 17.Wojciechowski J, Lai A, Kondo M, Zhuang Y. E2A and HEB are required to block thymocyte proliferation prior to pre-TCR expression. Journal of immunology. 2007 May 1;178(9):5717–26. doi: 10.4049/jimmunol.178.9.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones ME, Zhuang Y. Stage-specific functions of E-proteins at the beta-selection and T-cell receptor checkpoints during thymocyte development. Immunologic research. 2011 Apr;49(1–3):202–15. doi: 10.1007/s12026-010-8182-x. [DOI] [PubMed] [Google Scholar]
- 19.Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nature immunology. 2001 Feb;2(2):165–71. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 20.Rankin L, Belz GT. Diverse roles of inhibitor of differentiation 2 in adaptive immunity. Clinical & developmental immunology. 2011;2011:281569. doi: 10.1155/2011/281569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verykokakis M, Zook EC, Kee BL. ID’ing innate and innate-like lymphoid cells. Immunological reviews. 2014 Sep;261(1):177–97. doi: 10.1111/imr.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki K, Miyazaki M, Murre C. The establishment of B versus T cell identity. Trends in immunology. 2014 May;35(5):205–10. doi: 10.1016/j.it.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nature immunology. 2010 Mar;11(3):240–9. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monticelli LA, Yang Y, Knell J, D’Cruz LM, Cannarile MA, Engel I, Kronenberg M, Goldrath AW. Transcriptional regulator Id2 controls survival of hepatic NKT cells. Proceedings of the National Academy of Sciences of the United States of America. 2009 Nov 17;106(46):19461–6. doi: 10.1073/pnas.0908249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Wu D, Jiang N, Zhuang Y. Combined deletion of Id2 and Id3 genes reveals multiple roles for E proteins in invariant NKT cell development and expansion. Journal of immunology. 2013 Nov 15;191(10):5052–64. doi: 10.4049/jimmunol.1301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Cruz LM, Stradner MH, Yang CY, Goldrath AW. E and Id proteins influence invariant NKT cell sublineage differentiation and proliferation. Journal of immunology. 2014 Mar 1;192(5):2227–36. doi: 10.4049/jimmunol.1302904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verykokakis M, Krishnamoorthy V, Iavarone A, Lasorella A, Sigvardsson M, Kee BL. Essential functions for ID proteins at multiple checkpoints in invariant NKT cell development. Journal of immunology. 2013 Dec 15;191(12):5973–83. doi: 10.4049/jimmunol.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu T, Wang H, Simmons A, Bajana S, Zhao Y, Kovats S, Sun XH, Alberola-Ila J. Increased level of E protein activity during invariant NKT development promotes differentiation of invariant NKT2 and invariant NKT17 subsets. Journal of immunology. 2013 Nov 15;191(10):5065–73. doi: 10.4049/jimmunol.1301546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu T, Gimferrer I, Alberola-Ila J. Control of early stages in invariant natural killer T-cell development. Immunology. 2011 Sep;134(1):1–7. doi: 10.1111/j.1365-2567.2011.03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nature reviews Immunology. 2007 Jul;7(7):505–18. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 31.Gapin L. The making of NKT cells. Nature immunology. 2008 Sep;9(9):1009–11. doi: 10.1038/ni0908-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrows PD, Kronenberg M, Taniguchi M. NKT cells turn ten. Nature immunology. 2009 Jul;10(7):669–71. doi: 10.1038/ni0709-669. [DOI] [PubMed] [Google Scholar]
- 33.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Current opinion in immunology. 2007 Apr;19(2):186–93. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nature immunology. 2010 Mar;11(3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 35.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nature reviews Immunology. 2013 Feb;13(2):101–17. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 36.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annual review of immunology. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 37.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nature reviews Immunology. 2004 Mar;4(3):231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 38.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annual review of immunology. 2014;32:323–66. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 39.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. The Journal of experimental medicine. 2005 Aug 15;202(4):485–92. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Current opinion in immunology. 2005 Apr;17(2):122–30. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Berzins SP, Smyth MJ, Godfrey DI. Working with NKT cells--pitfalls and practicalities. Current opinion in immunology. 2005 Aug;17(4):448–54. doi: 10.1016/j.coi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Van Kaer L, Joyce S. Innate immunity: NKT cells in the spotlight. Current biology : CB. 2005 Jun 7;15(11):R429–31. doi: 10.1016/j.cub.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 43.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nature reviews Immunology. 2002 Aug;2(8):557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 44.Engel I, Kronenberg M. Making memory at birth: understanding the differentiation of natural killer T cells. Current opinion in immunology. 2012 Apr;24(2):184–90. doi: 10.1016/j.coi.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girardi E, Zajonc DM. Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunological reviews. 2012 Nov;250(1):167–79. doi: 10.1111/j.1600-065X.2012.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005 Mar 24;434(7032):520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 47.Young DC, Moody DB. T-cell recognition of glycolipids presented by CD1 proteins. Glycobiology. 2006 Jul;16(7):103R–12R. doi: 10.1093/glycob/cwj111. [DOI] [PubMed] [Google Scholar]
- 48.Lawson V. Turned on by danger: activation of CD1d-restricted invariant natural killer T cells. Immunology. 2012 Sep;137(1):20–7. doi: 10.1111/j.1365-2567.2012.03612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das R, Sant’Angelo DB, Nichols KE. Transcriptional control of invariant NKT cell development. Immunological reviews. 2010 Nov;238(1):195–215. doi: 10.1111/j.1600-065X.2010.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan AC, Berzins SP, Godfrey DI. Transcriptional regulation of lymphocyte development. Developing NKT cells need their (E) protein. Immunology and cell biology. 2010 Jul;88(5):507–9. doi: 10.1038/icb.2010.55. [DOI] [PubMed] [Google Scholar]
- 51.D’Cruz LM, Yang CY, Goldrath AW. Transcriptional regulation of NKT cell development and homeostasis. Current opinion in immunology. 2010 Apr;22(2):199–205. doi: 10.1016/j.coi.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engel I, Kronenberg M. Transcriptional control of the development and function of valpha14i NKT cells. Current topics in microbiology and immunology. 2014;381:51–81. doi: 10.1007/82_2014_375. [DOI] [PubMed] [Google Scholar]
- 53.Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells as sensors and managers of inflammation. Trends in immunology. 2013 Feb;34(2):50–8. doi: 10.1016/j.it.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. The Journal of clinical investigation. 2004 Nov;114(10):1379–88. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDonald HR. Development and selection of NKT cells. Current opinion in immunology. 2002 Apr;14(2):250–4. doi: 10.1016/s0952-7915(02)00329-1. [DOI] [PubMed] [Google Scholar]
- 56.Makino Y, Kanno R, Koseki H, Taniguchi M. Development of Valpha4+ NK T cells in the early stages of embryogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1996 Jun 25;93(13):6516–20. doi: 10.1073/pnas.93.13.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dashtsoodol N, Watarai H, Sakata S, Taniguchi M. Identification of CD4(−)CD8(−) double-negative natural killer T cell precursors in the thymus. PloS one. 2008;3(11):e3688. doi: 10.1371/journal.pone.0003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pear WS, Tu L, Stein PL. Lineage choices in the developing thymus: choosing the T and NKT pathways. Current opinion in immunology. 2004 Apr;16(2):167–73. doi: 10.1016/j.coi.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. Journal of immunology. 1999 Oct 15;163(8):4091–4. [PubMed] [Google Scholar]
- 60.Kisielow J, Tortola L, Weber J, Karjalainen K, Kopf M. Evidence for the divergence of innate and adaptive T-cell precursors before commitment to the alphabeta and gammadelta lineages. Blood. 2011 Dec 15;118(25):6591–600. doi: 10.1182/blood-2011-05-352732. [DOI] [PubMed] [Google Scholar]
- 61.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Essential requirement of an invariant V alpha 14 T cell antigen receptor expression in the development of natural killer T cells. Proceedings of the National Academy of Sciences of the United States of America. 1996 Oct 1;93(20):11025–8. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007 Nov;27(5):751–62. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimamura M, Ohteki T, Beutner U, MacDonald HR. Lack of directed V alpha 14-J alpha 281 rearrangements in NK1+ T cells. European journal of immunology. 1997 Jun;27(6):1576–9. doi: 10.1002/eji.1830270638. [DOI] [PubMed] [Google Scholar]
- 64.Hager E, Hawwari A, Matsuda JL, Krangel MS, Gapin L. Multiple constraints at the level of TCRalpha rearrangement impact Valpha14i NKT cell development. Journal of immunology. 2007 Aug 15;179(4):2228–34. doi: 10.4049/jimmunol.179.4.2228. [DOI] [PubMed] [Google Scholar]
- 65.Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, Krangel MS, He YW. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nature immunology. 2002 May;3(5):469–76. doi: 10.1038/ni791. Epub 2002/04/23. eng. [DOI] [PubMed] [Google Scholar]
- 66.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004 Dec 3;306(5702):1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 67.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of experimental medicine. 2011 Jun 6;208(6):1279–89. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jul 28;106(30):12453–8. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nature immunology. 2009 Mar;10(3):306–13. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dose M, Sleckman BP, Han J, Bredemeyer AL, Bendelac A, Gounari F. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proceedings of the National Academy of Sciences of the United States of America. 2009 May 26;106(21):8641–6. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Current opinion in immunology. 2013 Apr;25(2):161–7. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nature immunology. 2013 Nov;14(11):1146–54. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS biology. 2012 Feb;10(2):e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proceedings of the National Academy of Sciences of the United States of America. 2008 Aug 12;105(32):11287–92. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xi H, Schwartz R, Engel I, Murre C, Kersh GJ. Interplay between RORgammat, Egr3, and E proteins controls proliferation in response to pre-TCR signals. Immunity. 2006 Jun;24(6):813–26. doi: 10.1016/j.immuni.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 76.Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007 Dec;27(6):860–70. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000 Jun 30;288(5475):2369–73. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 78.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant’Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nature immunology. 2008 Sep;9(9):1055–64. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant’Angelo DB. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. Journal of immunology. 2010 Jun 15;184(12):6746–55. doi: 10.4049/jimmunol.1000776. [DOI] [PubMed] [Google Scholar]
- 80.Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, Pereira P, Nichols KE, Koretzky GA, Jordan MS, Sant’Angelo DB. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. Journal of immunology. 2010 Feb 1;184(3):1268–79. doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nature immunology. 2001 May;2(5):423–9. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 82.Benlagha K, Bendelac A. CD1d-restricted mouse V alpha 14 and human V alpha 24 T cells: lymphocytes of innate immunity. Seminars in immunology. 2000 Dec;12(6):537–42. doi: 10.1006/smim.2000.0276. [DOI] [PubMed] [Google Scholar]
- 83.Mycko MP, Ferrero I, Wilson A, Jiang W, Bianchi T, Trumpp A, MacDonald HR. Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. Journal of immunology. 2009 Apr 15;182(8):4641–8. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 84.Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, Wright G, Shaffer AL, Hodson DJ, Buras E, Liu X, Powell J, Yang Y, Xu W, Zhao H, Kohlhammer H, Rosenwald A, Kluin P, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Ogwang MD, Reynolds SJ, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pittaluga S, Wilson W, Waldmann TA, Rowe M, Mbulaiteye SM, Rickinson AB, Staudt LM. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012 Oct 4;490(7418):116–20. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D’Cruz LM, Watowich SS, Murre C, Goldrath AW. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nature immunology. 2011 Dec;12(12):1221–9. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, Godfrey DI. The influence of CD1d in postselection NKT cell maturation and homeostasis. Journal of immunology. 2005 Sep 15;175(6):3762–8. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 87.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002 Apr 19;296(5567):553–5. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 88.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. The Journal of clinical investigation. 2014 Sep 2;124(9):3725–40. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, Godfrey DI, Leadbetter EA, Sant’Angelo DB, von Andrian U, Brenner MB. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nature immunology. 2015 Jan;16(1):85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004 Apr;20(4):477–94. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 91.Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006 Apr 1;107(7):2797–805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu W, Kee BL. Growth factor independent 1B (Gfi1b) is an E2A target gene that modulates Gata3 in T-cell lymphomas. Blood. 2007 May 15;109(10):4406–14. doi: 10.1182/blood-2006-08-043331. [DOI] [PubMed] [Google Scholar]
- 93.Kusunoki T, Sugai M, Katakai T, Omatsu Y, Iyoda T, Inaba K, Nakahata T, Shimizu A, Yokota Y. TH2 dominance and defective development of a CD8+ dendritic cell subset in Id2-deficient mice. The Journal of allergy and clinical immunology. 2003 Jan;111(1):136–42. doi: 10.1067/mai.2003.29. [DOI] [PubMed] [Google Scholar]
- 94.Zhang F, Fuss IJ, Yang Z, Strober W. Transcription of RORgammat in developing Th17 cells is regulated by E-proteins. Mucosal immunology. 2014 May;7(3):521–32. doi: 10.1038/mi.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL Immunological Genome Project C. Molecular definition of the identity and activation of natural killer cells. Nature immunology. 2012 Oct;13(10):1000–9. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]