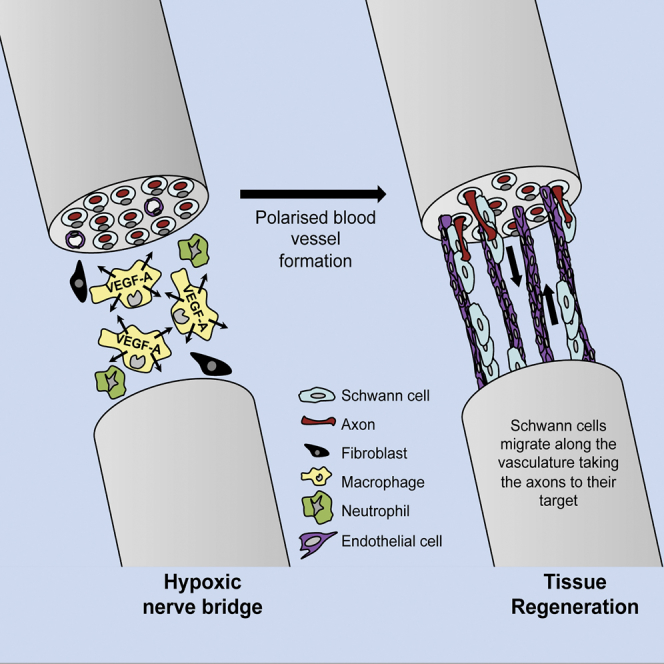
Summary
The peripheral nervous system has remarkable regenerative capacities in that it can repair a fully cut nerve. This requires Schwann cells to migrate collectively to guide regrowing axons across a ‘bridge’ of new tissue, which forms to reconnect a severed nerve. Here we show that blood vessels direct the migrating cords of Schwann cells. This multicellular process is initiated by hypoxia, selectively sensed by macrophages within the bridge, which via VEGF-A secretion induce a polarized vasculature that relieves the hypoxia. Schwann cells then use the blood vessels as “tracks” to cross the bridge taking regrowing axons with them. Importantly, disrupting the organization of the newly formed blood vessels in vivo, either by inhibiting the angiogenic signal or by re-orienting them, compromises Schwann cell directionality resulting in defective nerve repair. This study provides important insights into how the choreography of multiple cell-types is required for the regeneration of an adult tissue.
Graphical Abstract
Highlights
-
•
Hypoxia within the nerve bridge is selectively sensed by macrophages
-
•
Macrophage-derived VEGF-A induces a polarized vasculature within the bridge
-
•
Blood vessels are used as tracks to direct Schwann cell migration across the wound
-
•
Macrophage-induced blood vessels are essential for nerve regeneration
Repairing a cut nerve requires collective migration of Schwann cells guided by a polarized vasculature that is induced by macrophages within the hypoxic bridge.
Introduction
The creation of tissues during development requires the temporal coordination of multiple cell types by a combination of intrinsic and extrinsic developmental signals that control the number and movement of cells (Bryant and Mostov, 2008; Martin and Parkhurst, 2004). Few tissues in the adult mammal are able to recapitulate these processes to regenerate following injury; in some cases, this is due to the absence in the adult of the stem cells that originally gave rise to the tissue, however, the absence of extrinsic developmental, morphogenic and guidance cues present in the developing organism is also likely to play a major role (Poss, 2010). The peripheral nervous system (PNS) is one tissue able to regenerate in the adult mammal. This is all the more remarkable because of the complex structure of nerves and that regeneration requires the regrowth and coordination of multiple cell types over long distances within the architecture of the adult tissue (Zochodne, 2008).
Peripheral nerves consist of bundles of axons, with each axon associated and enveloped by Schwann cells (SCs), the main glial cell of the PNS. SCs either exist in a 1:1 ratio with larger diameter axons, which they myelinate, or group together smaller axons in structures known as Remak bundles. Groups of these axons are further organized into a fascicle, enclosed by the perineurium, which is made-up of layers of specialized, fibroblast-like cells. Several fascicles can be further enclosed within the epineurial sheath that surrounds each nerve. The axons exist in a specialized, privileged compartment, known as the endoneurium, protected by the blood/nerve barrier, which is maintained by both the perineurium and by specialized blood vessels that run throughout the nerve. Fibroblasts and macrophages also reside within the matrix of this compartment (Zochodne, 2008).
Remarkably, in contrast to nerves in the CNS, peripheral nerves can regenerate even following a complete transection. Following a transection, the stumps retract and in the distal part of the nerve, the axons, separated from their cell bodies, rapidly degenerate by an active process known as Wallerian degeneration (Zochodne, 2008). The major aim of the regeneration process is for the axons to regrow back to their targets, which requires guidance signals distinct from those that originally directed the axons during development (Dudanova and Klein, 2013). Following an injury, the SCs in both the proximal stump and throughout the nerve downstream of the cut dedifferentiate to a progenitor-like cell, which proliferate, orchestrate an inflammatory response that clears the debris and remodels the environment (Napoli et al., 2012). In the distal stump, these cells form tube-like structures within their original basement membranes, known as bands of Büngner, which can act as “tunnels” to direct the regrowing axons back to their original targets. However, following a transection, the basement membranes are broken and distinct mechanisms are required to direct the regrowing axons into the bands of Büngner in the distal stump (Fawcett and Keynes, 1990; Nguyen et al., 2002).
By an unknown mechanism, following transection, the two stumps are rejoined by a poorly characterized structure known as “the bridge” that can be several millimeters in length and is composed of a mixture of inflammatory cells and matrix (Jurecka et al., 1975) and thus seemingly a hostile and non-directional environment for axonal regrowth. We recently showed that SCs are responsible for guiding the axons across this bridge region (Parrinello et al., 2010). This contrasts to during development, when axons are guided to their targets by a combination of extrinsic attractive and repulsive signals (Dudanova and Klein, 2013), with the SCs following behind the axons on this journey (Heermann and Schwab, 2013). We showed that cords of SCs migrate out of both distal and proximal stumps until they extend across the bridge, with SC cords from the proximal stump taking the regrowing axons with them. This organized migration is directed by fibroblasts at the wound site that, via EphrinB/EphB2 signaling, convert normally repulsive SCs to an adhesive behavior necessary for their collective migration. Importantly, loss of this signal results in disruption of the SC cords and loss of the directional movement that directs the axons toward the distal stump (Parrinello et al., 2010). However, while these studies showed that the cords of SCs were critical for the directional growth of the axons, it remained unclear how the SCs themselves were finding their way across the bridge.
In this study, we identify critical roles for both chemotactic cues from within the bridge, as well as the topography of the bridge in directing SC migration during PNS regeneration. We find that hypoxia within the bridge is selectively responded to by macrophages, which via VEGF-A secretion triggers the polarized vascularization of the bridge region. These newly formed blood vessels are subsequently used as a guiding path by SCs to invade and cross the bridge, taking the regrowing axons with them. Importantly, we show that the blood vessels are necessary and sufficient to guide the migration of SCs, as misdirection of the blood vessels leads the SCs into surrounding tissues. This work describes how mechanisms distinct from those used during development can regenerate a tissue in the adult. Moreover, it identifies an unanticipated role for macrophage-induced blood vessels in this process that has implications for improving the regenerative process following injury and provides insights into the way cells may spread in pathologies such as cancer.
Results
Polarized Blood Vessels Form within the Bridge prior to Schwann Cell Migration
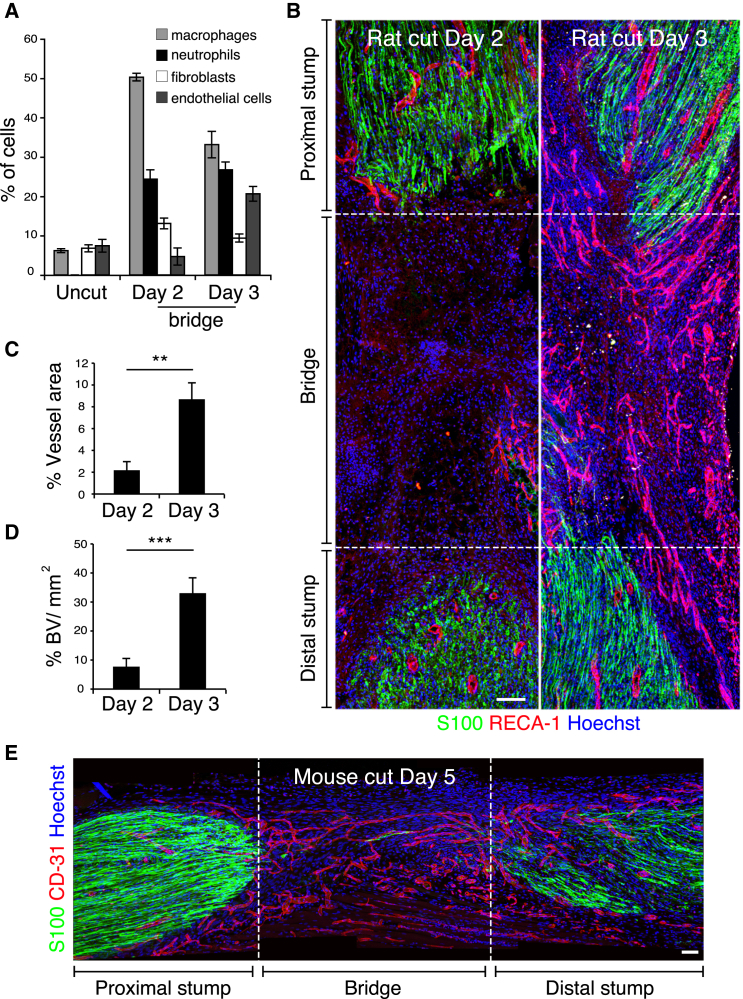
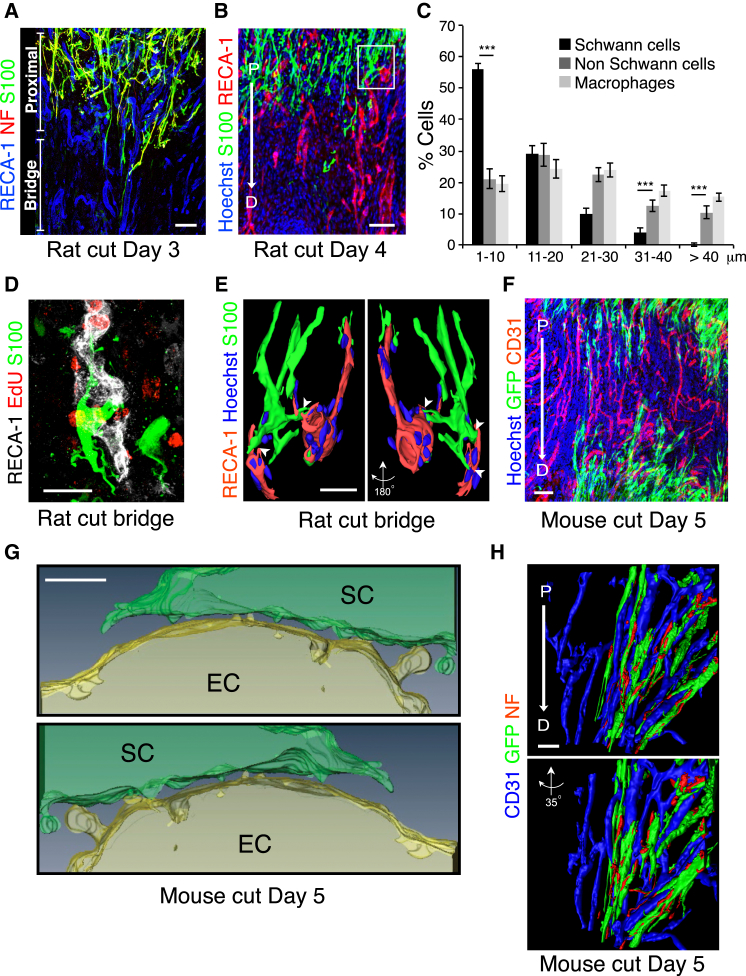
In order to determine the mechanism by which SC cords are directed across the bridge, we first determined the cell composition of the bridge, prior to SC migration. In the vast majority of rats (>95%), a bridge between the distal and proximal nerve stumps was formed 2 days following transection (day 2). In agreement with previous observations (Avellino et al., 1995; Jurecka et al., 1975), we found that the bridge was mostly composed of macrophages (50%) and neutrophils (24%) with fibroblasts (13%) and endothelial cells (ECs) (5%) as minor components (Figures 1A and S1A). At day 3, however, we noted a significant increase in the number of ECs indicating that the bridge may have become vascularized (Figure 1A). Consistent with this, vascularization could be observed macroscopically (Figure S1B) and confocal analysis of the entire bridge region confirmed this to be the case; at day 2, the bridge contained very few blood vessels whereas by day 3, there was a dramatic influx of blood vessels, which permeated the entire bridge (Figure 1B, quantified in Figures 1C and 1D). Analysis of nerves in which the vascularization was at a slightly earlier stage showed that the blood vessels appeared to emanate from both proximal and distal stumps (Figure S1C) but importantly, the nerve was fully vascularized by the time the SC cords entered into the bridge from either stump (Figures 1B and S1D). In mice, the regeneration process is similar but takes place more slowly, but as observed in rats, we found that the bridge was fully vascularized prior to SC migration into the bridge (Figures 1E and S1D). Thus in both rats and mice, ECs cross the bridge prior to the migration of the SC cords.
Figure 1.
Blood Vessels Permeate the Bridge prior to SC Migration
(A) Graph shows the proportion of macrophages (Iba1+), fibroblasts (prolylhydroxylase+/ Iba1−), ECs (RECA-1+), and neutrophils (lipocallin-2+) within the bridge of transected rat sciatic nerves and in contralateral intact nerves (Uncut), Day 2, and Day 3 after transection (n = 4, graph shows mean value ± SEM).
(B) Rat sciatic nerve longitudinal sections immunostained for ECs (RECA-1+, red) and SCs (S100+, green), Day 2 and Day 3 after transection. Nuclei were counterstained with Hoechst (blue). Scale bar, 100 μm.
(C and D) Quantification of the vascularization of the bridge as shown in (B). (C) Graph shows the percentage of RECA-1 positive area at the indicated times (n = 6). (D) Graph shows the average number of blood vessels/mm2 of bridge at the indicated times (n = 6). Graphs show mean value ± SEM.
(E) Longitudinal section of a mouse sciatic nerve immunostained for ECs (CD31+, red) and SCs (S100+, green), Day 5 after transection. Scale bar, 100 μm. For reconstruction of longitudinal sections shown in (B) and (E), multiple images from the same sample were acquired using the same microscope settings.
See also Figure S1.
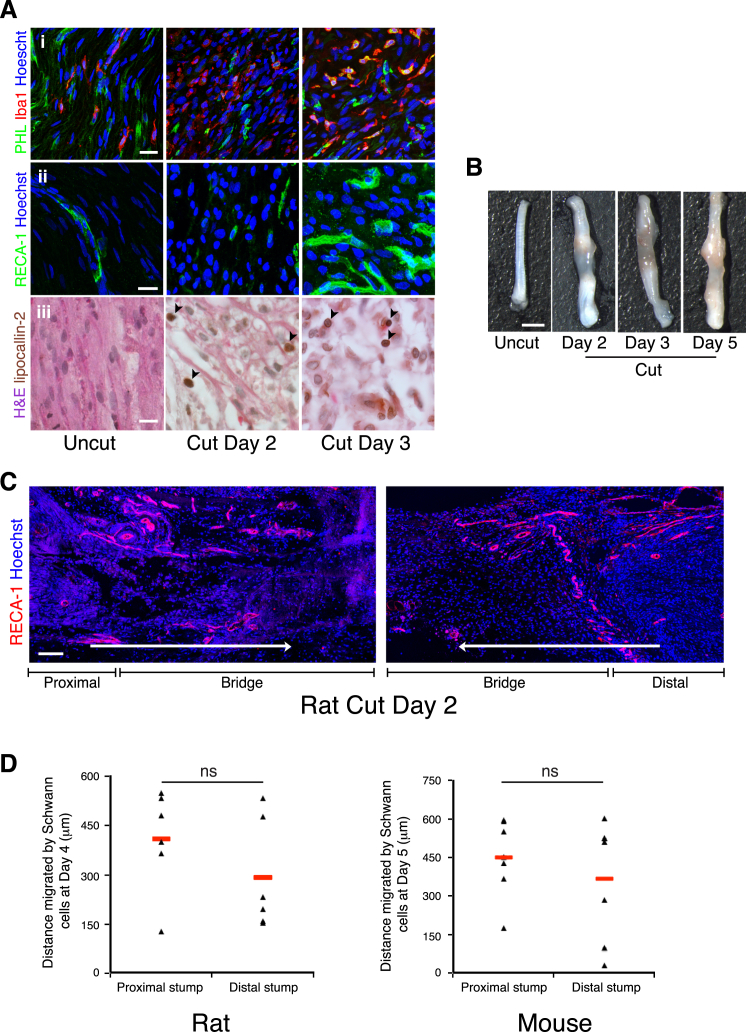
Figure S1.
The Major Cellular Components of the Bridge Are Macrophages, Neutrophils, Fibroblasts, and Endothelial Cells, Related to Figure 1
(A) Representative images of longitudinal sections of rat uninjured and injured sciatic nerve bridges at Day 2 and 3. Sections were immunostained for (i) macrophages (Iba1+, red) and fibroblasts (prolylhydroxylase (PHL)+/ Iba1-, green). Scale bar = 25 μm. (ii) endothelial cells (RECA-1+, green). Scale bar = 10 μm. Nuclei were counterstained with Hoechst (blue). (iii) neutrophils - immunohistochemistry to detect lipocallin-2 (brown). Nuclei and cytoplasm were counterstained with Hematoxylin (violet) and Eosin (pink) respectively. Scale bar = 10 μm. Black arrowheads indicate lipocallin-2+ neutrophils. Quantification of the proportion of each cell-type within the bridge is shown in Figure 1A.
(B) Representative images of uninjured and injured rat sciatic nerves at Day 2, Day 3 and Day 5. Blood vessels can be observed within the bridge at Day 3 but not at Day 2.
(C) Representative immunofluorescence images of longitudinal sections of injured rat sciatic nerve bridges at Day 2 following transection, immunostained to detect blood vessels (RECA-1+, red) with nuclei stained with Hoechst. The images show the blood vessels entering the bridge from both the proximal stump (P) (left panel) and the distal stump (D) (right panel). Scale bar = 100 μm. Arrows indicates the direction of cell movement from the proximal or the distal stump into the bridge. For reconstruction of longitudinal sections, multiple images from the same sample were acquired using the same microscope settings.
(D) Schwann cells migrate from both the proximal and distal stumps. Graphs show the distance migrated by Schwann cells from the proximal and distal stumps in rats (LHS) and mice (RHS). Each point represents an individual nerve; red lines indicate the mean.
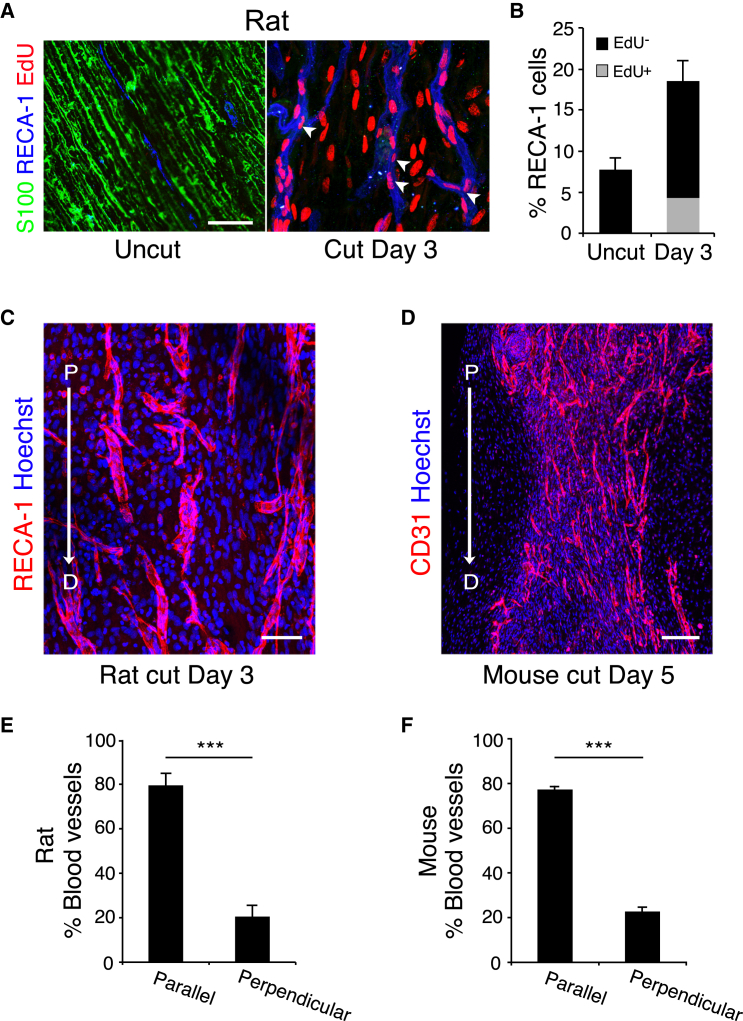
Analysis of the incorporation of EdU found that all the blood vessels within the bridge contained EdU-positive ECs, confirming that they were newly formed (Figures 2A, 2B, and S2A). In contrast, EdU-positive ECs were not found within uncut nerve (Figures 2A and 2B) or in the proximal or distal stumps (Figure S2B). Moreover, erythrocyte staining and the detection of injected immunolabeled-lectin showed that the majority of the newly formed blood vessels within the bridge were functional (Figures S2C and S2D).
Figure 2.
Newly Formed Blood Vessels in the Bridge Are Polarized in the Direction of SC Migration
(A) Representative longitudinal sections of a rat sciatic nerve bridge and the contralateral uninjured nerve, day 3 after transection, and 12 hr after EdU injection. EdU+ cells (red) were co-labeled to detect ECs (blue) and S100 to detect SCs (green). Scale bar, 25 μm. White arrowheads indicate EdU+ ECs.
(B) Quantification of the proportion of EdU+ ECs in the bridge, day 3 after transection compared to uncut (n = 4).
(C) Representative confocal image of a longitudinal section of a rat nerve bridge immunostained for ECs (RECA-1+) at day 3 after transection. Scale bar, 50 μm. Arrow indicates the direction of axonal growth from the proximal (P) to the distal (D) stump.
(D) Representative confocal image of a longitudinal section of a mouse nerve bridge immunostained for ECs (CD31+) at Day 5 after transection. Scale bar, 100 μm. For reconstruction of longitudinal sections shown in (C) and (D), multiple images from the same sample were acquired using the same microscope settings.
(E and F) Quantification of the proportion of blood vessels parallel or perpendicular to the direction of SC migration in the rat bridge (E) or the mouse bridge (F) (n = 4).
Graphs show mean value ± SEM. See also Figure S2.
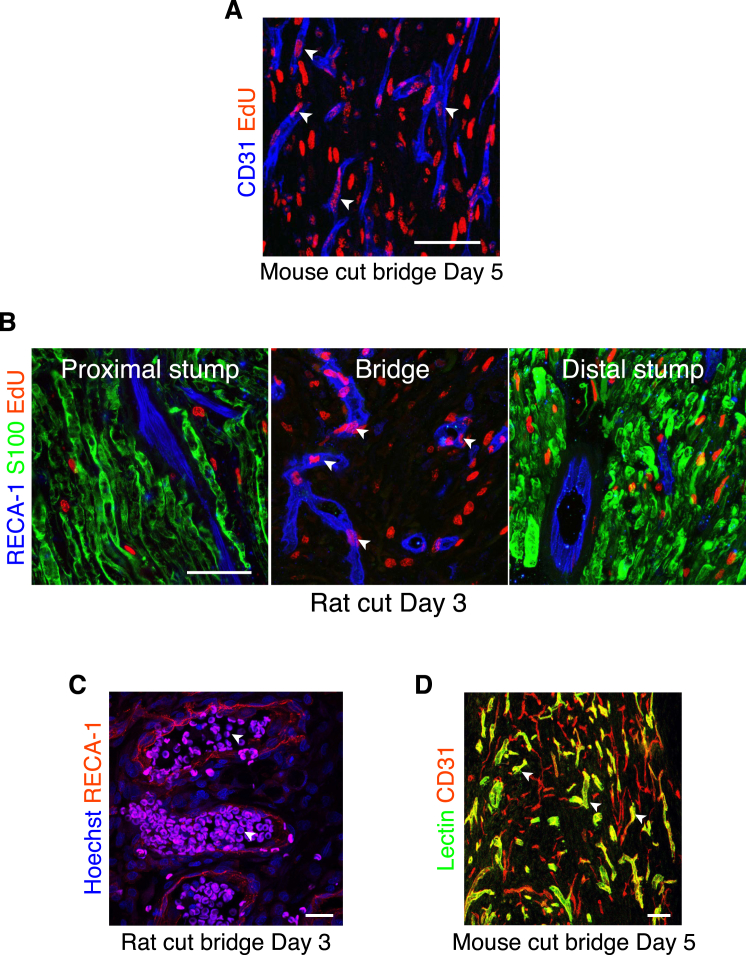
Figure S2.
The Newly Formed Blood Vessels in the Bridge Are Functional, Related to Figure 2
(A) Representative image of a longitudinal section of a mouse sciatic nerve bridge, Day 5 after transection and 12 hr after EdU injection, immunostained to detect EdU (red), endothelial cells (CD31+, blue) to identify the presence of newly-formed blood vessels. Scale bar = 50 μm. White arrowheads indicate EdU+ endothelial cells.
(B) Representative longitudinal cryosections of the nerve stumps and the bridge of rat sciatic nerves, 12 hr after EdU injection and Day 3 after transection, immunostained for endothelial cells (RECA-1+, blue), Schwann cells (S100+, green) and EdU (red). Scale bar = 50 μm. White arrowheads indicate EdU+ endothelial cells.
(C) Representative immunofluorescence image of the bridge of injured rat sciatic nerves at Day 3 showing blood vessels immunostained for RECA-1 and autofluorescent erythrocytes, present in the vast majority of blood vessels. Scale bar = 25 μm. White arrowheads indicate erythrocytes within the lumen of the bridge vasculature.
(D) Representative longitudinal section of the bridge of an injured mouse sciatic nerve at Day 5 and 10 min after lectin-FITC injection and co-labeled to detect endothelial cells (CD31+, red). Scale bar = 50 μm. White arrowheads indicate functional blood vessels.
Remarkably, the blood vessels within the bridge of both rats and mice appeared to be similarly organized, in that the majority seemed aligned to the direction that the SC cords would subsequently travel across the bridge (Figures 2C and 2D). Quantification of longitudinal sections of the bridges established that ∼80% of the blood vessels were orientated in the direction of subsequent SC migration (Figures 2E and 2F). This polarized growth of blood vessels prior to SC migration raised the possibility that blood vessels provide directional signals to the cords of SCs to help them find their way across the bridge.
Schwann Cells Interact Directly with Polarized Blood Vessels
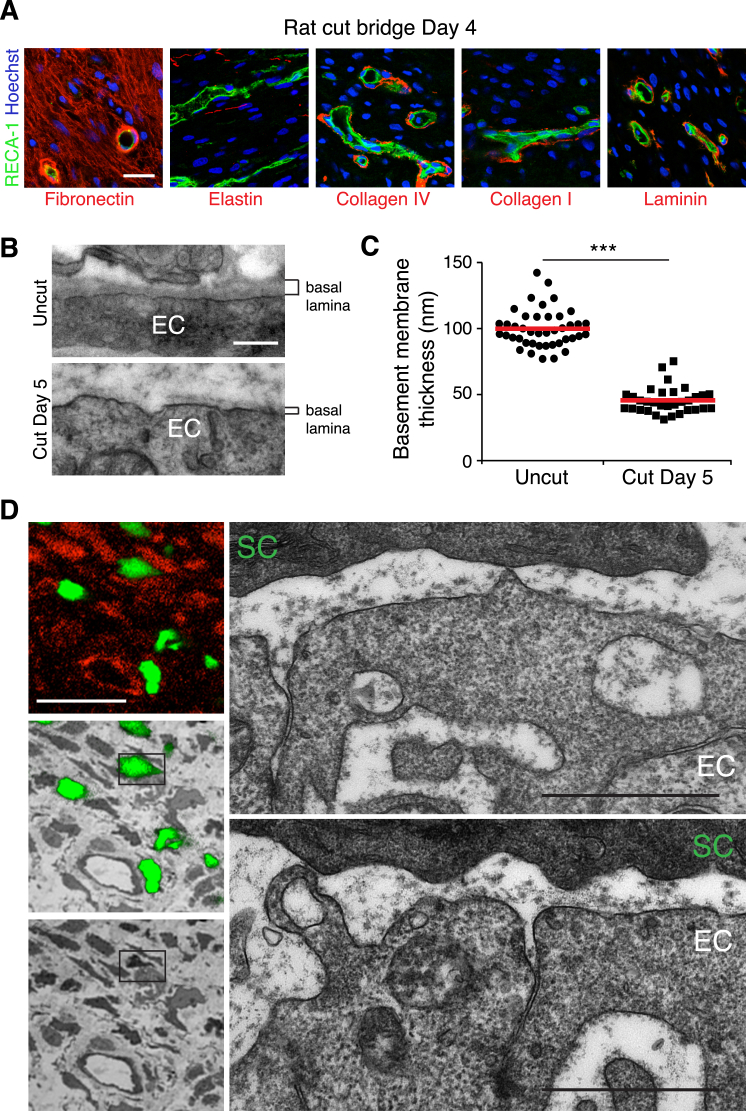
Confocal microscopy analysis of the SCs entering into the bridge demonstrated a close association of the migrating SC cords and the polarized blood vessels (Figure 3A). Moreover, at later time points when the SCs had migrated further into the bridge, these interactions were maintained (Figure 3B). Analysis of matrix components of the bridge showed that fibronectin filled the space between the cells throughout the bridge and that strands of elastin also permeated the bridge region. In contrast, laminin and collagen I and IV could be detected only around the blood vessels (Figure S3A). To quantify the degree and specificity of the interactions between the SCs and the blood vessels, we measured the shortest distance between the nuclei of SCs at the leading edge and their closest blood vessel and compared it to the distance of other cell types present in the bridge. We found that the majority of SCs were extremely close (<10 μm) to blood vessels with the population showing a strong distribution toward the blood vessels, whereas the other cell types had a more random distribution within the bridge (Figure 3C). Moreover, the degree of interaction between SCs and blood vessels was probably underestimated, as we frequently observed SCs interacting with blood vessels via long protrusions while the nuclei were further away.
Figure 3.
Migrating SCs Interact with the Vasculature of the Bridge
(A) Representative confocal image of a longitudinal section of a rat sciatic nerve bridge, Day 3 after transection, immunostained to detect axons (neurofilament (NF), red), SCs (S100+, green), and ECs (RECA-1+, blue) and shows cords of SCs and associated regrowing axons interacting with the vasculature as they emerge from the proximal stump and enter the bridge. Scale bar, 50 μm.
(B) Rat sciatic nerve longitudinal sections immunostained to detect SCs (S100+, green) and ECs (RECA-1+, red), Day 4 after transection. Scale bar, 100 μm. White rectangle indicates the region used to build the 3D model shown in (E). For reconstruction of longitudinal sections shown in (A) and (B), multiple images from the same sample were acquired using the same microscope settings.
(C) Frequency distribution graph showing the distance of the nuclei of SCs (S100+), non SCs (S100-/RECA-), or macrophages (Iba1+) to the closest blood vessel, Day 4 after transection (n = 4, graph shows mean value ± SEM).
(D) 3D-projection of a rat nerve bridge showing a S100-positive SC (green) interacting with a newly formed EdU-positive (red) blood vessel (RECA-1+, white). Scale bar, 20 μm. See also Movie S1.
(E) Snapshots of a 3D-image obtained by the surface rendering of a z-stack projection of confocal images of the rat nerve bridge, immunostained to detect SCs (S100+, green) and ECs (RECA-1+, red). A SC can be seen to interact with two different blood vessels through cytoplasmic protrusions. Scale bar, 20 μm. Arrowheads indicate points of contact between a SC and blood vessels.
(F) Representative confocal image of a longitudinal section of a sciatic nerve bridge from PLP-EGFP mice, Day 5 after transection, immunostained to detect ECs (CD31+, red). Scale bar, 50 μm.
(G) Snapshots from Movie S2 showing blebs and protrusions mediating the contacts between SCs and ECs within the bridge.
(H) Snapshots from Movie S1 of a 3D model obtained by surface rendering of a z stack projection of a longitudinal section of a bridge region from the sciatic nerve of PLP-EGFP mice, Day 5 after transection. The sections were immunostained to detect ECs (CD-31+, blue) and axons (NF+, red). Scale bar, 20 μm.
See also Figure S3.
Figure S3.
Blood Vessels in the Bridge Have Thin Basal Lamina, Allowing Direct Points of Contact with Schwann Cells, Related to Figure 3
(A) Representative confocal images of the bridges of rat sciatic nerves, Day 4 after transection, immunostained to detect the indicated matrix proteins (red) and endothelial cells (green). Scale bar = 25 μm.
(B) Representative TEM images of blood vessels from the bridge region and the contralateral nerve, Day 5 after transection. Note the basal lamina is thinner, less dense and/or absent around blood vessels within the bridge. Scale bar = 250nm.
(C) Quantification of the average thickness of the basal lamina of the blood vessels as described in (B), each point represents a separate blood vessel from 3 independent animals. The red lines represent the mean.
(D) Correlative light and electron microscopy of a 100μm thick vibrating microtome cross section of GFP-expressing Schwann cells (green) from a lectin (red) injected mouse sciatic nerve, Day 5 after transection. Panels on the left show a confocal image of GFP–expressing Schwann cells, either alone (top), overlayed on the correlated TEM image (middle) and TEM image alone (bottom). Outlined box highlights the GFP-expressing Schwann cell (SC) interacting with endothelial cells (EC), enlarged in the panels on the left, reconstructed in Figure 3G, and Movie S2. Note the points of direct contact between the Schwann cell and the blood vessel and sporadic/absent basal lamina of both cell types. Scale bars = 20 μm (white), 1 μm (black).
Higher resolution, 3D-projection views of the bridge indicated that SCs were making direct physical contacts with the EdU-labeled vasculature (Figure 3D; Movie S1). Moreover, 3D surface rendering of high-resolution confocal z-stacks of the bridge confirmed the presence of multiple physical contacts between SCs and the surrounding blood vessels (Figure 3E).
To analyze the interactions between the migrating SC cords and the blood vessels in the mouse, we used a transgenic mouse in which EGFP is specifically expressed in SCs (PLP-EGFP) (Mallon et al., 2002). Co-immunostaining of the vasculature showed GFP-positive migrating SCs closely associated with the blood vessels in the bridge (Figure 3F). EM analysis of the blood vessels showed that compared to established vessels within the contralateral nerve, the vessels of the bridge had an extremely thin basal lamina with regions where there appeared to be little or no matrix that could allow direct contact between the cells (Figures S3B and S3C). Moreover, using correlative light and electron microscopy (CLEM) of blood vessels and SCs within the bridge, we could observe multiple points of direct contact between the two cell types (Figures 3G and S3D). Interestingly, 3D reconstruction of serial sections along the blood vessel showed that the interactions occurred between blebs and protrusions emanating from both cell types, providing a discontinuous surface that could potentially generate traction for movement (Figure 3G; Movie S2) (Bergert et al., 2015; Liu et al., 2015; Tozluoğlu et al., 2013).
3D surface rendering of high-resolution confocal z-stacks of the bridge co-stained with an axonal marker confirmed the direct interaction between the cords of SCs and the blood vessels and showed the SC cords guiding the axons along them (Figure 3H; Movie S1). Thus, in both rats and mice, SCs physically interact with polarized blood vessels as they migrate and guide axons across the bridge.
Schwann Cells Migrate along Capillary-like Endothelial Cells In Vitro
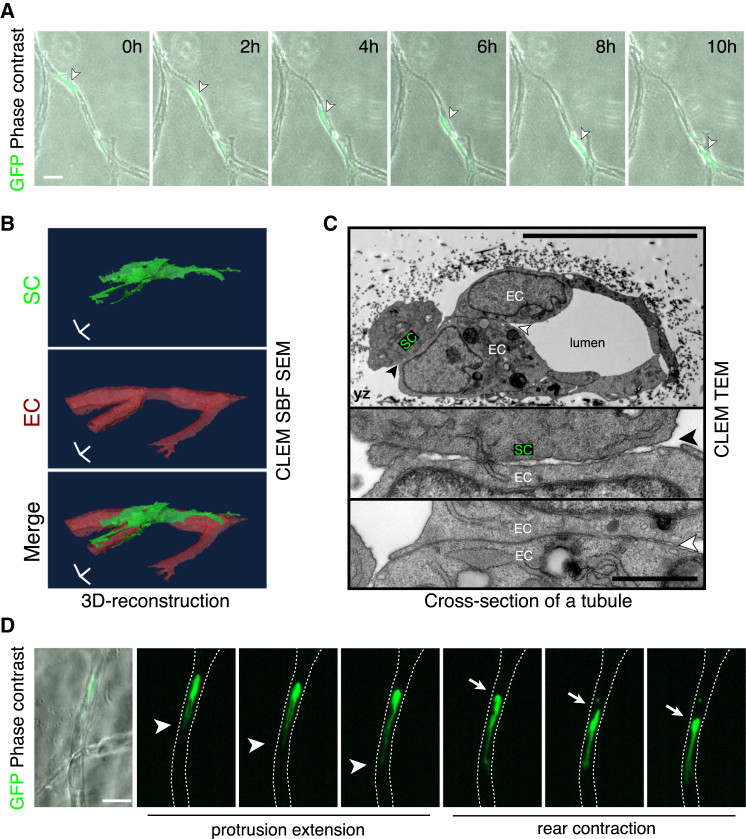
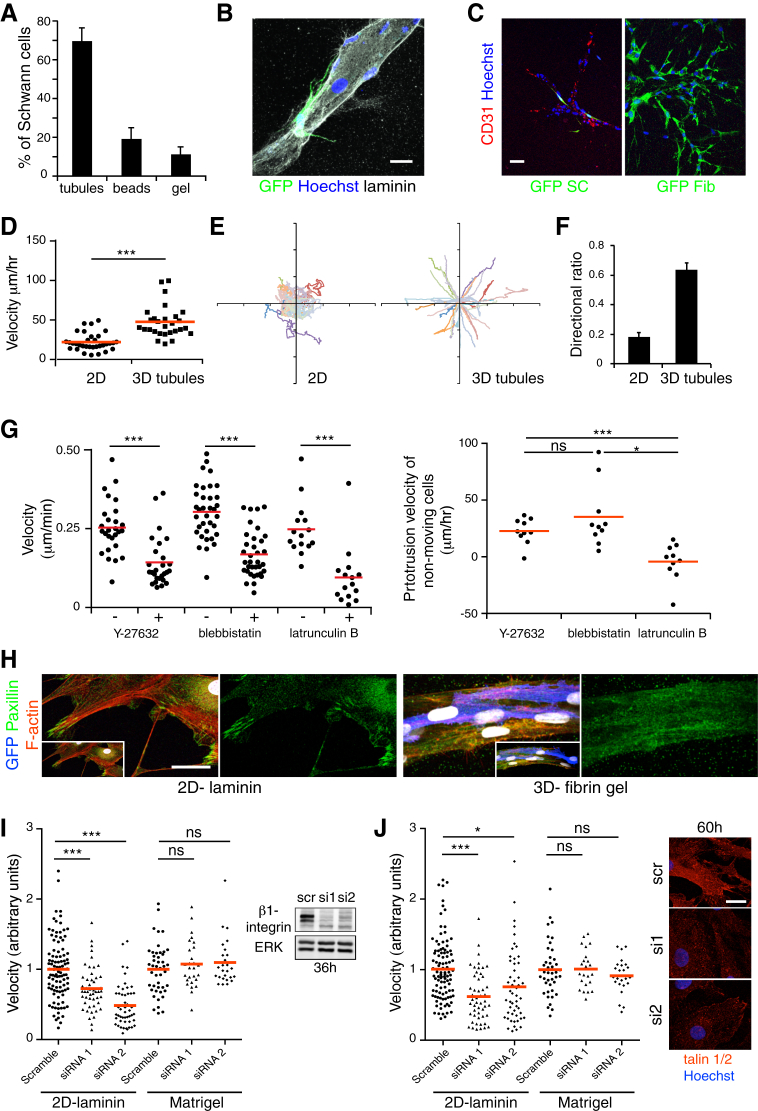
To test whether SCs could interact directly with blood vessels in a simplified system, we co-cultured GFP-positive rat SCs with human umbilical vein endothelial cells (HUVECs), which had been coated onto beads and then placed into a fibrin matrix to form capillary-like structures (Nakatsu et al., 2003). Time-lapse microscopy showed that the vast majority of SCs interacted with the endothelial cell tubules and migrated along them (Figures 4A and S4A; Movie S3). Importantly, confocal microscopy images confirmed that the migrating SCs made direct physical contacts with the ECs as observed in vivo (Figure S4B). A small proportion of the SCs remained within the matrix (<15%) (Figure S4A), yet while these cells were able to form protrusions they were unable to migrate efficiently (Movie S3). In contrast, and consistent with other studies (Hakkinen et al., 2011), we found that fibroblasts, when added to the matrix, did not interact specifically with the tubules but instead spread and migrated within the matrix (Figure S4C). We confirmed that SCs directly migrated along blood vessels by generating tubules of HUVECs in a second matrix, Matrigel and found that SCs migrated efficiently along them (Movie S3). This demonstrates that SCs, unlike fibroblasts, are unable to migrate efficiently within a 3D matrix unless they associate with a scaffold of EC tubules.
Figure 4.
SCs Migrate along Endothelial Tubules In Vitro
(A) Representative time-lapse microscopy images showing a GFP-positive rat SC migrating along a tubule of HUVECs within a 3D fibrin gel (Movie S3). Scale bar, 40 μm. White arrowheads indicate the cell body of the SC.
(B) Images from Movie S4 of a tilted 3D view of a GFP-positive SC (green) interacting with an EC tubule (red). Scale bar, 10 μm.
(C) Top: a representative EM image of a cross-section of an EC tubule in contact with a GFP-positive SC within a fibrin gel. Scale bar, 10 μm. Middle: a higher magnification view of the SC/EC contact (black arrowhead). Bottom: a higher magnification view of the EC/EC contact (white arrowhead). Scale bar, 1 μm.
(D) Snapshots of Movie S5, showing the amoeboid-like mode of migration observed by the SCs in 3D. White arrowheads and arrows show the leading protrusion and the rear of the cell respectively. Scale bar, 50 μm.
See also Figure S4.
Figure S4.
Primary Schwann Cells Migrate In Vitro along Tubules of Endothelial Cells, Related to Figure 4
(A) Quantification of the proportion of GFP-positive Schwann cells associated with the tubules of HUVECs, the beads or retained within the fibrin gel (n = 5 gels from separate experiments. 100 cells were counted per gel, graph shows mean value ± SEM).
(B) Representative confocal image of a GFP-positive Schwann cell (green) physically interacting with a laminin+ (white) tubule of endothelial cells, while migrating. Nuclei were counterstained with Hoechst (blue). Scale bar = 20 μm.
(C) Representative images of GFP-positive Schwann cells (left panel) or GFP-positive fibroblasts (right panel) co-cultured with endothelial tubules in fibrin gels. Scale bar = 50 μm. Schwann cells associate with the CD31+ endothelial cells whereas fibroblasts migrate within the matrix.
(D) Velocities of tracked single Schwann cells (dots) migrating on 2D laminin-coated surfaces or along tubules of HUVECs within fibrin gels. The red lines represent the mean.
(E) Single Schwann cell tracks migrating on 2D laminin-coated surfaces (left) or along tubules of HUVECs within fibrin gels (right). See also Movies S3 and S5.
(F) Directionality ratio of Schwann cells migrating on 2D laminin-coated surfaces or along tubules of HUVECs within fibrin gels. 30 cells were quantified in each condition from 3 separate experiments; graph shows mean value ± SEM.
(G) Left panel: Rear velocities of tracked single Schwann cells (dots) migrating along tubules of HUVECs upon inhibition with the inhibitors Y27632 (50 μM), blebbistatin (2 μM) or latrunculin B (0.2 μM). The red lines represent the mean. Right panel: Measurements of single Schwann cell protrusion velocities upon inhibition with the inhibitors Y-27632, blebbistatin or latrunculin B. 10 cells were quantified from 2 separate experiments. The red lines represent the mean. One-way ANOVA test was used for statistical analysis. See also Movie S6. Note the movement of the rear of the cells is blocked by all three inhibitors whereas protrusions continue to form in the presence of Y-27632 and blebbistatin but not in the presence of latrunculin B.
(H) Representative confocal images of GFP-positive Schwann cells on a 2D-laminin surface or interacting with a tubule of HUVECs within a fibrin gel, immunostained for the focal adhesion complex marker paxillin (green) and labeled with phalloidin to visualize the cortical actin (red). Note that focal adhesion complexes are not detectable in Schwann cells migrating in 3D. Nuclei were counterstained with Hoechst (white). Scale bar = 25 μm.
(I) Left panel: Velocities of tracked single siRNA-treated Schwann cells (dots) migrating on 2D laminin-coated surfaces or along tubules of HUVECs in Matrigel. The red lines represent the mean. See also Movie S7. Right panel: Western blot analysis of total protein lysates from siRNA-treated Schwann cells showing the efficiency of beta1 integrin knockdown with two independent oligos compared to scrambled control, at 36 hr. Total ERK levels were used as a loading control.
(J) Left panel: Velocities of tracked single siRNA-treated Schwann cells (dots) migrating on 2D laminin-coated surfaces or along tubules of HUVECs in Matrigel. The red lines represent the mean. Right panel: Representative confocal images of talin 1 and 2 siRNA-treated Schwann cells immunostained for talin (red) showing the efficiency of talin 1 and 2 knockdown with two independent oligos at 60 hr. Scale bar = 50 μm.
To analyze the nature of the interaction between SCs and ECs in vitro, we performed CLEM on GFP-positive SCs interacting with ECs within the fibrin gel. We observed that SCs made direct contact with the ECs, with serial block face imaging demonstrating contacts along the length of the migrating SC (Figure 4B; Movie S4). Higher resolution TEM analysis revealed that the interactions were distinct from those between neighboring ECs, which formed a tight uniform junction, consistent with a stable interaction between the cells. In contrast, the contacts between the SCs and ECs although direct, occurred at multiple discrete sites, a morphology consistent with the dynamic movement of the SC along the EC tubules and consistent with our in vivo findings (Figure 4C).
To study further the mode of migration, we analyzed multiple time-lapse movies and compared to SC migration in 2D. The SCs moved faster in 3D and showed a greater persistence of direction as they migrated along the blood vessels (Figures S4D–S4F). In 2D, SCs move in a classical adhesion-dependent manner involving large lamellipodia-like structures (Movie S5). In contrast in 3D, the SCs exhibited a more ameboid-like mode of migration with the extension of protrusions followed by a contraction of the rear of the cell, a movement characteristic of conditions of lower adhesion and higher levels of confinement (Figure 4D; Movie S5) (Lämmermann and Sixt, 2009; Liu et al., 2015). Consistent with this, we found that the rear-contraction of the cell rather than the forward extensions were inhibited by the addition of blebbistatin or the Rho-kinase inhibitor Y27632 demonstrating that actomyosin contractility is required for this mode of migration (Lämmermann et al., 2008) (Figure S4G; Movie S6). In contrast, latrunculin B also inhibited the forward protrusions showing actin structures were required for both cell movements (Figure S4G; Movie S6). Compared to cells migrating in 2D, focal adhesions were absent or extremely small in the 3D cultures (Figure S4H) and consistent with low levels of adhesion, whereas knockdown of beta1 integrin or talins severely inhibited SC migration in 2D, it had no effect on the ability of the same cells to migrate along the EC tubules (Figures S4I and S4J; Movie S7) (Bergert et al., 2015; Lämmermann et al., 2008).
Together with the EM analysis, these results indicate that while SCs are unable to generate sufficient force to migrate within the confinements of the 3D matrix, blood vessels provide a distinct confined environment and a sufficiently frictional or discontinuous surface that allows an actomyosin-driven, amoeboid-like mode of migration in the desired direction of travel (Bergert et al., 2015; Liu et al., 2015; Tozluoğlu et al., 2013).
Macrophages Are the Sensors of Hypoxia within the Bridge
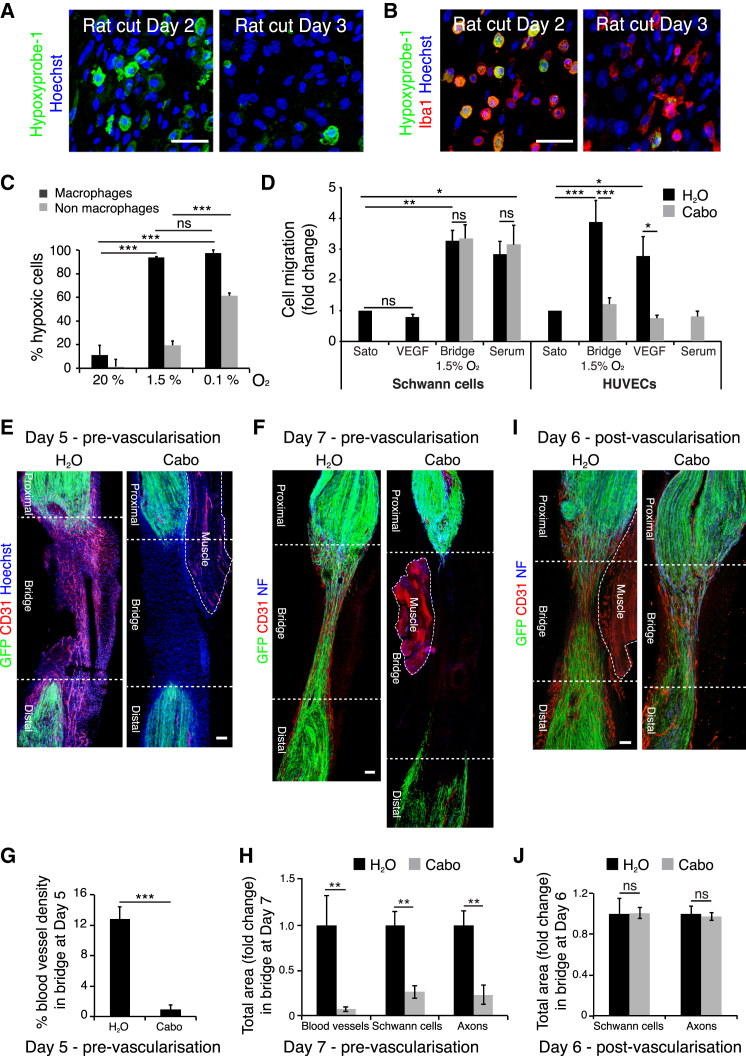
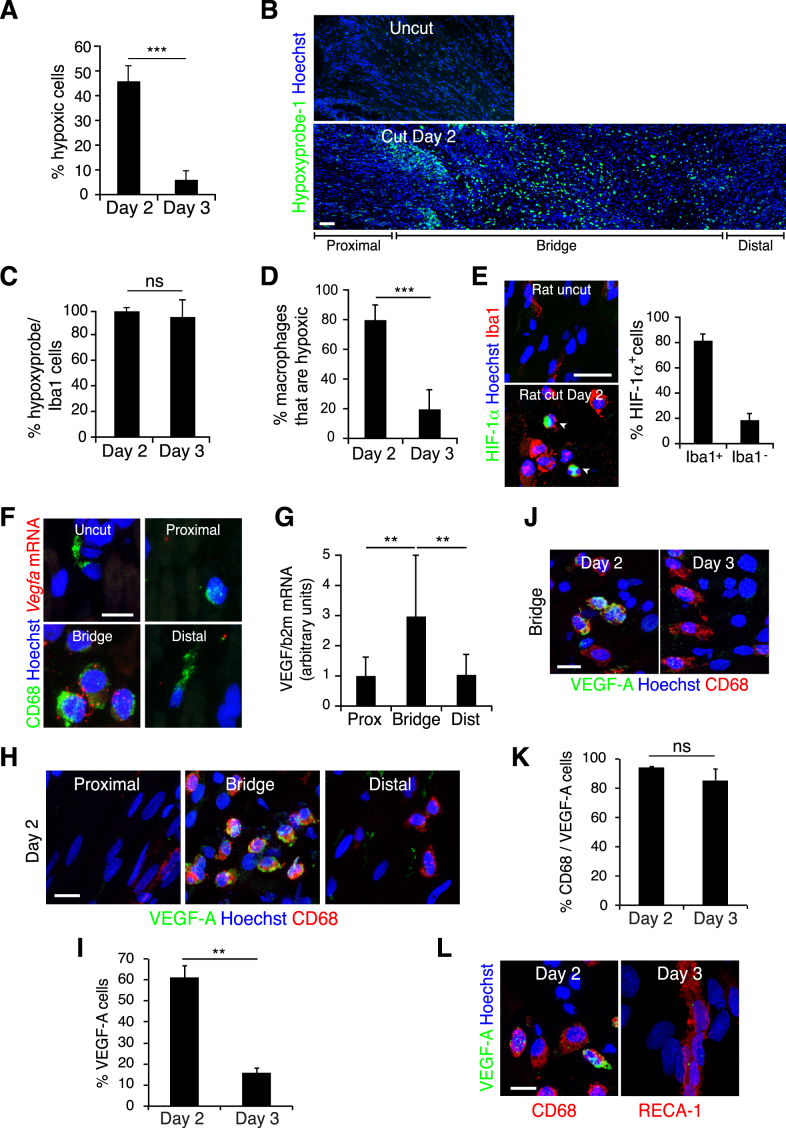
New blood vessels normally form in response to decreased oxygen levels (hypoxia) within a tissue. Upon hypoxia, the transcription factor HIF-1α is stabilized and initiates a transcriptional response that induces angiogenesis by upregulating pro-angiogenic factors such as VEGF (Krock et al., 2011; Pugh and Ratcliffe, 2003). To test whether the nerve bridge was hypoxic, we injected rats with hypoxyprobe-1 (pimonidazole hydrochloride) that forms immunofluorescent detectable protein adducts in hypoxic conditions (pO2 < 10 mm Hg) (Young and Möller, 2010). Immunostaining of day 2 nerve bridges revealed the presence of large numbers of hypoxic cells prior to its vascularization (Figures 5A and S5A). Hypoxic cells were found only in the bridge and at the tips of both the distal and proximal stumps but not further along the stumps or in the uncut nerve (Figure S5B). The proportion of hypoxic cells decreased substantially by day 3, when the bridge had become vascularized (Figures 5A and S5A), consistent with the new blood vessels resolving the hypoxic environment of this new tissue.
Figure 5.
Hypoxia Drives Angiogenesis by a Macrophage-Generated Gradient of VEGF-A
(A) Representative images of sections of a rat sciatic nerve bridge, Day 2 and 3 after transection and 30 min after injection of hypoxyprobe-1, immunolabeled to detect hypoxyprobe-1 (green). Scale bar, 25 μm.
(B) As in (A) but immunolabeled to detect macrophages (Iba1+, red) and hypoxic cells (hypoxyprobe-1+, green). Scale bar, 25 μm.
(C) Graph showing percentage of hypoxic cells (hypoxyprobe-1+) in macrophage (Iba1+) and non-macrophage (Iba1−) populations from rat sciatic nerve bridges cultured at indicated oxygen conditions (n = 3).
(D) HUVECs or SCs were placed in the upper compartment of Boyden chambers and allowed to migrate into the lower chamber containing media with no factors (SATO), VEGF-A165, serum, or conditioned medium from bridge cells cultured at 1.5% O2 (n = 5). For (C) and (D) one-way ANOVA test was used for statistical analysis.
(E–H) Confocal images of longitudinal cryosections of injured sciatic nerves from PLP-EGFP mice, Day 5 or Day 7 after transection, following gavage of cabozantinib or control solvent on Day 4 (pre-vascularization), immunostained to detect ECs (CD31+, red) and axons (NF+, blue) Scale bar, 50 μm, quantified in (G) and (H) (n = 3).
(I) As for (F) but cabozantinib was administered on Day 5 (post-vascularization) and harvested on Day 6, quantified in (J) (n = 3). For reconstruction of longitudinal sections shown in (E), (F), and (I), multiple images from the same sample were acquired using the same microscope settings.
Graphs show mean value ± SEM. See also Figure S5.
Figure S5.
Hypoxia within the Bridge Leads to HIF-1α Stabilization and Expression of the Pro-angiogenic Target Gene, Vegfa, Related to Figure 5
(A) Quantification of Figure 5A to show the proportion of hypoxyprobe-1+ cells within the rat bridge (n = 4 animals per group; graph shows mean value ± SEM).
(B) Representative longitudinal sections of a rat sciatic nerve bridge and the contralateral uninjured nerve, Day 2 after transection and 30 min after injection of hypoxyprobe-1 (pimonidazole chloride), immunostained to detect hypoxyprobe-1 (green). Nuclei were counterstained with Hoechst (blue). Scale bar = 100 μm. To reconstruct the longitudinal section of the injured nerve (bottom), multiple images from the same sample were acquired using the same microscope settings.
(C) Quantification of Figure 5B to show the proportion of hypoxic cells that are macrophages at Day 2 and Day 3 (n = 4 animals per group; graph shows mean value ± SEM).
(D) Quantification of the proportion of macrophages (Iba1+) that are hypoxic (hypoxyprobe-1+), Day 2 and Day 3 after injury (n = 4 animals per group, graph shows mean value ± SEM). Note the significant decrease of hypoxic macrophages at Day 3 compared to Day 2.
(E) Representative images of a bridge region of a rat sciatic nerve, Day 2 after transection and the contralateral nerve (uncut), immunolabelled to detect macrophages (Iba1+, red) and HIF-1α expression (green). Scale bar = 20 μm. White arrowheads indicate HIF-1α+/Iba-1+ cells. Graph shows quantification of the proportion of HIF-1α+ cells that are macrophages (Iba1+) within the bridge (n = 3 animals, graph shows mean value ± SEM).
(F) Representative images of sections of rat sciatic nerve, Day 2 after transection and the contralateral uninjured rat sciatic nerve following in situ hybridization of rat Vegfa mRNA (red) and subsequent immunostaining for macrophages (CD68+, green). Scale bar = 10 μm.
(G) Quantitative RT-PCR analysis of Vegfa mRNA isolated from the bridge, the proximal and the distal stump of transected sciatic nerves, Day 2 after injury. Graph shows the Vegfa transcript levels relative to the levels in the proximal stump (n = 8 animals, graph shows mean value ± SEM).
(H–K) Representative images of cryosections of rat sciatic nerve, after transection, immunolabelled to detect VEGF-A (green) and macrophages (CD68+, red) to show proximal, bridge and distal regions at Day 2 (H) and the bridge region at Day 2 and Day 3 (J). The proportion of VEGFA+ cells (I) and VEGFA+ macrophages (CD68+) (K) are quantified at Day 2 and Day 3 in the bridge region (n = 3, graphs show mean value ± SEM).
(L) Representative images of a bridge region of a rat sciatic nerve, Day 2 or Day 3 after transection, immunostained to detect VEGF-A (green) and macrophages (CD68+, red) or blood vessels (RECA-1+, red) as indicated. Note that VEGF-A is undetectable in the blood vessels. Scale bars = 15 μm.
Remarkably, not all cells in the bridge were positive for the hypoxyprobe-1 at day 2, suggesting that certain cell types may be more sensitive to the hypoxia. As macrophages have been shown to promote angiogenesis during wound healing and within tumors (Murdoch et al., 2008; Rodero and Khosrotehrani, 2010) and comprise more than half of the cells in the bridge, we tested whether the hypoxic cells were macrophages. Co-labeling of hypoxic cells and macrophages showed that the vast majority (>98%) of hypoxic cells were macrophages (Figures 5B and S5C) and that most macrophages (∼80%) were hypoxic on day 2 (Figure S5D). These observations indicate that macrophages are selectively sensing the hypoxic environment in the nerve bridge. To test whether this was an intrinsic property of the cells, we purified cells from the bridge and exposed them to varying oxygen concentrations in vitro. Consistent with our in vivo findings, we found that macrophages became hypoxic at higher oxygen concentrations (1.5%) than the other bridge cells (Figure 5C).
The hypoxia at day 2 was associated with increased HIF-1α levels in the bridge macrophages (Figure S5E). Moreover, in situ hybridization (Figure S5F), RT-qPCR (Figure S5G), and antibody staining (Figures S5H–S5L) demonstrated increased expression of VEGF-A in macrophages within the bridge consistent with a role for bridge-derived VEGF in stimulating EC proliferation and migration from the nerve stumps into the bridge.
While VEGF is a potent chemoattractant for ECs, we tested whether it also attracts SCs. Using a transwell assay, we found that ECs but not SCs migrated in response to VEGF-A, a response inhibited by the VEGFR2 inhibitor, cabozantinib (Figure 5D). We also found that conditioned medium from hypoxic purified bridge cells attracted ECs in a cabozantinib-dependent manner, whereas SC migration was independent of VEGF signaling but still responded to other factors secreted by the bridge cells or serum (Figure 5D). Together, these data suggest that after injury, ECs are specifically recruited from both nerve stumps in response to VEGF-A secretion by macrophages within the bridge. To test this in vivo, we treated mice with cabozantinib prior to blood vessel formation and found the inhibitor was able to block both blood vessel entry into the bridge and the subsequent entry of SCs and axons (Figures 5E–5H). Importantly however, the same inhibitor, added just after blood vessel formation, did not impair either SC or axonal entry (Figures 5I and 5J). These results indicate that VEGF-A is required for ECs to cross the bridge but that SCs and axons migrate independently of VEGF signaling once the blood vessels are formed.
Macrophages Drive Angiogenesis within the Bridge
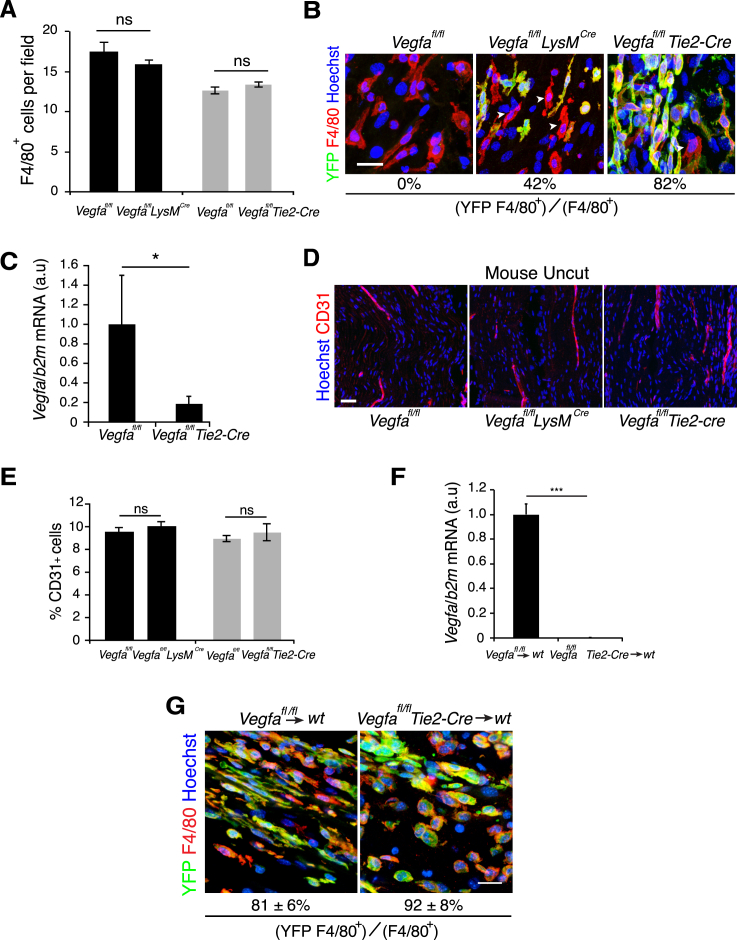
To test the in vivo importance of macrophage-derived VEGF-A in promoting the vascularization of the bridge, we performed nerve transections in two distinct complementary mouse models in which Vegfa was inactivated in macrophages. We crossed floxed Vegfa conditional null mice (Gerber et al., 1999) with mice expressing CRE-recombinase under the control of the Lysm promoter to generate mice lacking Vegfa in most macrophages and granulocytes (Vegfafl/fl LysmCre) (Clausen et al., 1999) and to mice expressing CRE from the Tie2 promoter to generate mice lacking Vegfa in hematopoietic cells and ECs (Vegfafl/fl Tie2-Cre) (Fantin et al., 2010). The mice also contained a floxed YFP-reporter gene in the Rosa26 locus to monitor the efficiency of CRE-mediated recombination (Srinivas et al., 2001).
VEGF-A has been described to play a role in the recruitment of macrophages from the bloodstream (Cursiefen et al., 2004). We therefore tested whether their recruitment was inhibited within the bridge of the knockout animals. We quantified the number of macrophages within the bridges from control and mutant Vegfafl/fl LysmCre and Vegfafl/fl Tie2-Cre animals at day 5 and found no differences (Figure S6A) showing that loss of Vegfa does not impair macrophage recruitment during the early phase of nerve regeneration. We determined the efficiency of recombination and found that 82% of macrophages had been targeted within the bridge of Vegfafl/fl Tie2-Cre animals (Figure S6B). Consistent with this, we observed an ∼80% decrease in Vegfa mRNA levels (Figure S6C). In contrast, the Vegfafl/fl LysmCre mutants showed a lower rate of recombination (42%) (Figure S6B).
Figure S6.
Inactivation of VEGF in Macrophages Inhibits Vascularization of the Bridge, Related to Figure 6
(A) Quantification of immunostained macrophages (F4/80+) within the bridge regions of Vegfafl/flLysmCre mice, Vegfafl/flTie2-Cre mice and their control littermates Vegfafl/fl, Day 5 after transection shows a similar recruitment of macrophages within control and mutant nerve bridges (n = 4 animals per group, graph shows mean value ± SEM).
(B) Representative images of bridges regions from Vegfafl/fl (control), Vegfafl/flLysmCre and Vegfafl/flTie2-Cre mouse sciatic nerves immunostained to detect macrophages (F4/80+, red) and YFP (green), Day 5 after injury, to show the efficiency of recombination in macrophages. Scale bar = 20 μm. White arrowheads indicate examples of YFP-negative macrophages.
(C) Quantitative RT-PCR analysis of Vegfa mRNA levels in the bridge of Vegfafl/flTie2-Cre mice relative to Vegfafl/fl controls, Day 5 after injury (n = 4 for each group, graph shows the mean ± SEM).
(D) Representative images of longitudinal sections of uninjured sciatic nerves from Vegfafl/fl (control), Vegfafl/flLysmCre and Vegfafl/flTie2-Cre mice, immunostained to detect endothelial cells (CD31+, red). Scale bar = 40 μm.
(E) Quantification of (D) showing the number of CD31+ endothelial cells in uninjured nerves of Vegfafl/flLysmCre and Vegfafl/flTie2-Cre animals as compared to their control littermates (n = 3 animals for each group, graph shows the mean ± SEM).
(F) Quantification of the levels of Vegfa mRNA in the bone marrow of wild-type mice transplanted with bone-marrow from Vegfafl/fl (control) and Vegfafl/flTie2-Cre mice. Bone marrow was extracted following the harvesting of the nerves, Day 5 following transection (n = 3); graph shows the mean ± SEM. The loss of Vegfa expression confirms both the efficiency of the bone marrow transplant (for additional information see Supplemental Experimental Procedures) and the efficiency of the recombination of the Vegfa locus in the cells derived from the Vegfafl/flTie2-Cre mice.
(G) Representative images of bridge regions from mouse sciatic nerves Day 5 following transection from wild-type mice transplanted with bone-marrow from Vegfafl/fl (control) and Vegfafl/flTie2-Cre mice immunostained to detect macrophages (F4/80+, red) and YFP (green), to determine the percentage of macrophages in the bridge derived from the transplanted cells. These results confirm the efficiency of the bone marrow transplant (for additional information see Supplemental Experimental Procedures) and demonstrate that the vast majority of macrophages in the bridge are derived from the transplanted stem cells. Scale bar = 20 μm.
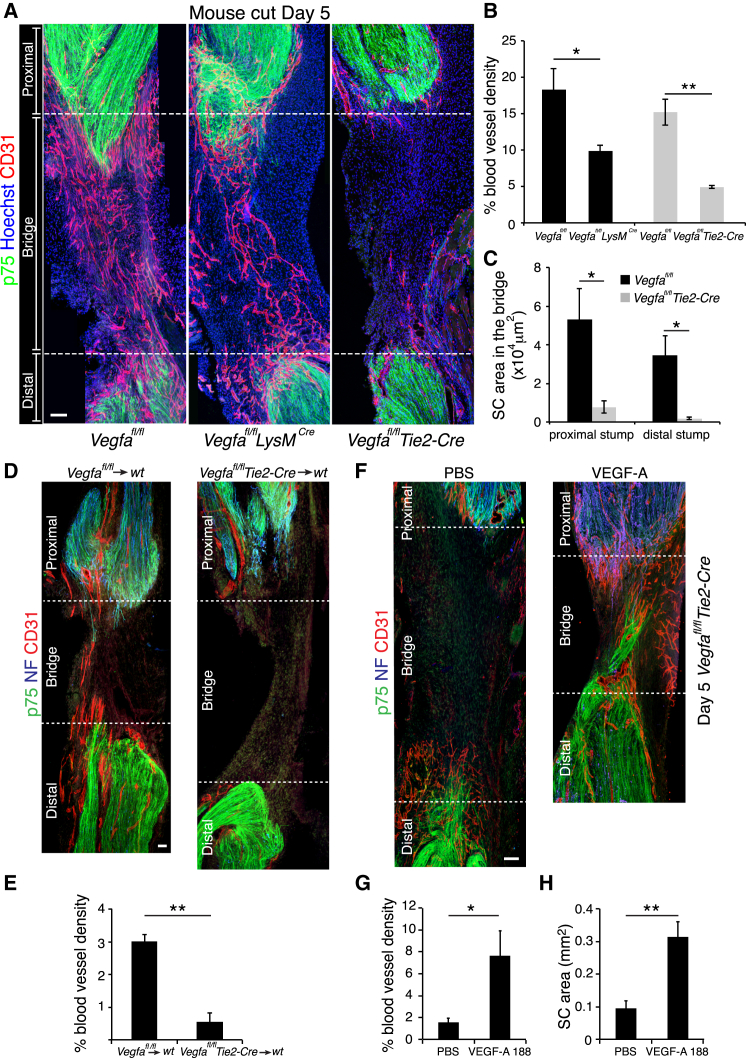
Macrophages have been shown to promote angiogenesis (Fantin et al., 2010; Pollard, 2009) and autocrine VEGF-A signaling helps to maintain the health of ECs (Lee et al., 2007). We therefore analyzed the vasculature of uninjured nerves from all genotypes but found no differences (Figures S6D and S6E). Remarkably however, nerves from both mutant animals showed a reduction in the vascularization of the bridge following injury (Figure 6A). The extent of the inhibition was more dramatic in the Vegfafl/fl Tie2-Cre mice, consistent with the greater degree of recombination in these animals, with very few blood vessels detectable within the bridge (Figure 6B). However, there was also a significant decrease in the Vegfafl/fl LysmCre mice (Figure 6B). Strikingly, SCs remained in the stumps of the Tie2-Cre mutant animals, consistent with a requirement for blood vessels to provide a “track” for the SCs to enter the bridge (Figure 6C). To confirm this was not due to loss of VEGF-A expression in ECs we (1) performed bone marrow transplant experiments from Vegfafl/fl Tie2-Cre and control Vegfafl/fl litter-mates into WT mice and found similar defective entry of blood vessels into the bridges of the mice receiving the mutant bone marrow, confirming that cells derived from hematopoietic-stem cells were responsible for the defect (Figures 6D, 6E, S6F, and S6G); and (2) performed rescue experiments in the Vegfafl/fl Tie2-Cre mice. We injected either VEGF-A or PBS into the bridges of Vegfafl/fl Tie2-Cre mice on day 4 and found that VEGF-A was able to rescue EC migration into the bridge and that SCs and axons migrated along these blood vessels (Figures 6F–6H). These results show that ECs deleted for VEGF-A are able to migrate and survive in the bridge and also provide a substrate for SC migration. Together, these results show that macrophages in the bridge secrete VEGF-A to enable the formation of a polarized endothelial scaffold that can direct SCs out of the nerve stumps and across the bridge.
Figure 6.
Inactivation of Vegfa in Macrophages Inhibits Vascularization of the Nerve Bridge after Nerve Transection
(A) Representative images of longitudinal sections of injured sciatic nerves from Vegfafl/fl (control), Vegfafl/flLysmCre, and Vegfafl/flTie2-Cre mice, Day 5 after transection, immunostained to detect ECs (CD31+, red) and SCs (p75NTR+, green). Scale bar, 50 μm.
(B) Quantification of (A) showing the proportion of CD31-positive area per bridge area and shows that the vascularization of the bridge is significantly reduced in mutants animals (n = 5).
(C) Quantification of (A) showing the area of SC influx from the proximal and distal stumps in Vegfafl/fl versus Vegfafl/flTie2-Cre animals (n = 5).
(D) Representative images of longitudinal sections of injured sciatic nerves from wild-type that have received bone marrow from Vegfafl/fl (control) or Vegfafl/flTie2-Cre mice immunostained to detect ECs (CD31+, red), SCs (p75NTR+, green), and axons (NF+, blue), Day 5 after transection. Scale bar, 100 μm.
(E) Quantification of (D) showing the proportion of CD31-positive area per bridge area (n = 3 for each group).
(F) Representative images of longitudinal sections of injured sciatic nerves of Vegfafl/flTie2-Cre mice, Day 5 after transection following injection of PBS or VEGF-A188 into the bridges at Day 4. Scale bar, 100 μm.
(G and H) Quantification of (F) showing the blood vessel density (G) or area of infiltrating SCs (H) (n = 4). For reconstruction of longitudinal sections shown in (A), (D) and (F), multiple images from the same sample were acquired using the same microscope settings.
Graphs show mean value ± SEM. See also Figure S6.
Schwann Cells Use the Polarized Vasculature as a Scaffold to Guide Regrowing Axons
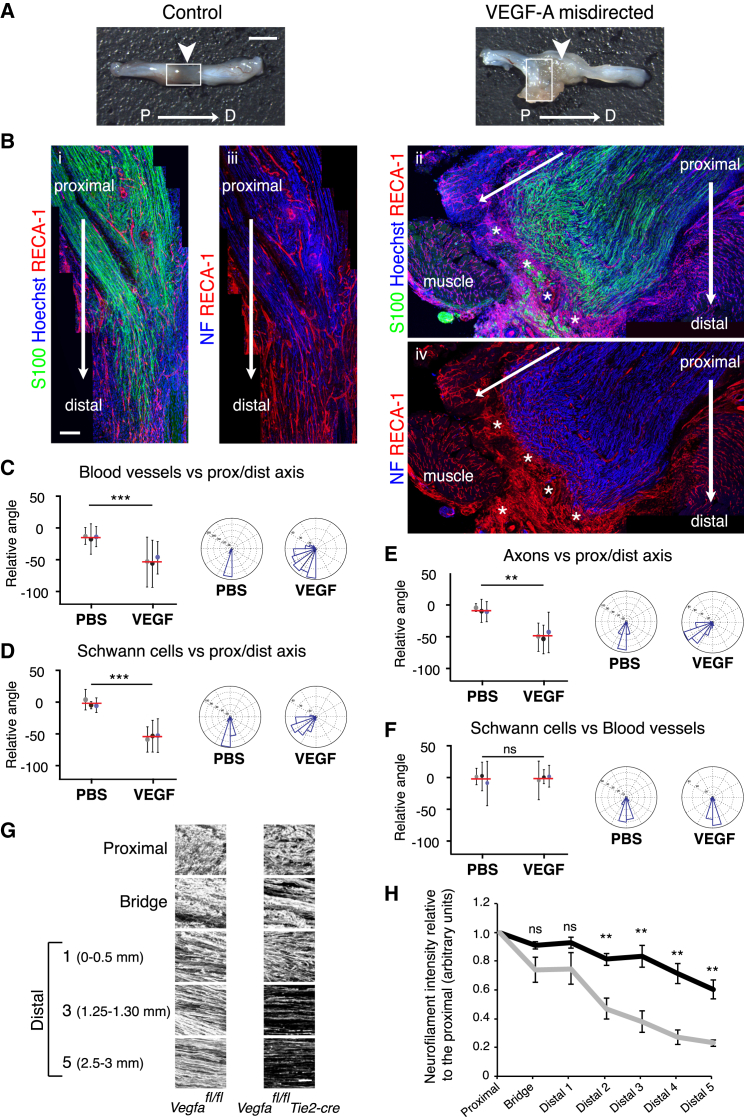
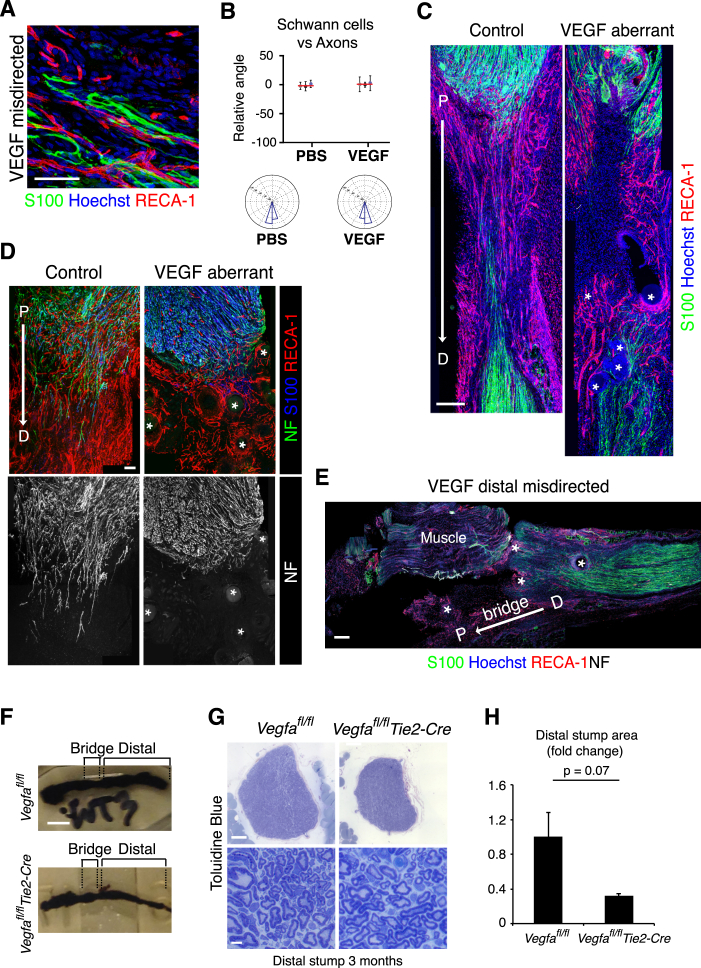
To address whether VEGF-A-induced blood vessels are sufficient to guide cords of SCs, we redirected the blood vessels to test whether the SCs would follow the blood vessels or continue to cross the bridge. To do this, we implanted heparin beads loaded with recombinant human VEGF165 into muscle adjacent to the proximal side of the injury site, immediately after the transection of the rat sciatic nerve. Six days later, the regenerative process was found to be abnormal in 10 out of 13 of the VEGF-treated animals compared to 1 out of 13 PBS-bead-treated controls. In five of the ten VEGF-treated animals in which abnormal regeneration was observed, a complete failure of the regenerative process was associated with misdirection of the blood vessels, SC cords and the accompanying axons, away from the bridge and into surrounding muscle towards the beads (Figures 7A, 7B, and S7A; quantified in Figures 7C–7F and S7B). Analysis of the bridges in a further five cases showed that the beads had moved into the bridge leading to the formation of disorganized blood vessels close to the beads (Figure S7C). In these cases, SCs migrated into the vascularized areas and either appeared “trapped” or deviated from the normal direction of movement, taking the axons along with them (Figure S7D). Moreover, beads implanted adjacent to the distal stump could also redirect blood vessels and SCs (Figure S7E). Together, these results demonstrate that VEGF-induced blood vessels are sufficient to guide SCs and their accompanying axons during peripheral nerve regeneration.
Figure 7.
Redirection of the Blood Vessels Leads to the Misdirection of Migrating SCs
(A) PBS- (control) and VEGF-treated rat sciatic nerve images show that placement of VEGF beads to the side of the injury site, leads to aberrant regeneration. Scale bar, 2 mm. Arrows indicate the bridge region and proximal to distal.
(B) Immunofluorescence images of the regions demarcated by white boxes in (A) of a PBS- (control) and VEGF-treated animal, Day 6 following injury, longitudinal sections were immunostained to detect SCs (S100+, green) and ECs (RECA1+, red). i and ii: show that misdirected blood vessels in the VEGF-treated animals directed the SC cords toward the adjacent muscle. iii and iv: show the axons (NF+) following the SC cords, toward the muscle. Scale bar, 300 μm. White asterisks indicate the beads. For reconstruction of longitudinal sections, multiple images from the same sample were acquired using the same microscope settings.
(C–F) Quantification of (B) to show the direction of blood vessels (C), SCs (D), and axons (E) relative to the proximal/distal axis and the alignment of blood vessels and SCs (F) in the rats treated with PBS or VEGF (n = 3). Graphs show the mean relative angle ± SD for each animal with the mean between animals shown by red lines. Rose plots show the distribution of cells for all animals.
(G) Representative confocal images of axons (NF+) in indicated regions of regenerated nerves in Vegfafl/fl (control) or Vegfafl/flTie2-Cre mice, Day 14 after transection.
(H) Quantification of (G) showing axonal growth in Vegfafl/fl (black line) and Vegfafl/fl Tie2-cre (gray line) mice (n = 5, graph shows mean value ± SEM).
See also Figure S7.
Figure S7.
Disorganization of Blood Vessels Leads to Disrupted Schwann Cell Migration and Axonal Regrowth, Related to Figure 7
(A) A higher magnification of Figure 7B to show the Schwann cell cords (S100+, green), aligned to the blood vessels (RECA-1+, red). Scale bar = 50 μm.
(B) Quantification of Figure 7B to show the alignment between Schwann cells and regrowing axons. Graph shows the mean relative angle ± SD for each animal with the mean between animals shown by the red lines. Rose plots show the distribution of cells for all animals (n = 3 animals for each condition).
(C) Images of bridge regions of a control (PBS) and VEGF-treated rat sciatic nerve, Day 6 after injury, immunostained to detect Schwann cells (S100+, green) and endothelial cells (RECA-1+, red). Nuclei were counterstained with Hoechst (blue). Scale bar = 300 μm. The beads are indicated by white asterisks in the VEGF-treated animals. Note the center of the bridge is poorly vascularised in the VEGF-treated mice and the Schwann cell cords fail to enter the bridge.
(D) A further example of aberrant regeneration in a VEGF-treated sciatic nerve. Upper panels show images of a bridge region of control (PBS) and VEGF-treated rat sciatic nerves, Day 6 after injury, immunostained to detect Schwann cells (S100+, blue), endothelial cells (RECA-1+, red) and axons (NF+, green). Scale bar = 100 μm. Lower panels show the same images as in the upper panels, filtered to show only the axons (NF+, white). Note the axons in the VEGF-treated nerves are misdirected, toward the beads, into the adjoining muscle.
(E) Image of a disconnected nerve following treatment with VEGF-treated beads in which the beads redirect Schwann cell cords from the distal stump. Note the blood vessels (RECA-1+, red) and Schwann cells (S100+, green) are directed away from the bridge into the surrounding muscle. Scale bar = 200 μm. For reconstruction of longitudinal sections shown in (C), (D) and (E), multiple images from the same sample were acquired using the same microscope settings.
(F) Images of nerves stained with osmium tetroxide taken from Vegfafl/fl (control) and Vegfafl/flTie2-Cre mice, 6 months following transection. Note the visibly smaller distal stump in the mutant mice. Scale bar = 2mm.
(G) Cross sections of a nerve from Vegfafl/fl (control) and Vegfafl/flTie2-Cre mice 6 months following transection and stained with toluidine blue, at low magnification to show the entire nerve (top panels) and at higher magnification to show the indistinguishable structures of the control and mutant nerves (lower panels). Scale bar = 100 μm (top) and 5 μm (bottom).
(H) Graph to show the difference in area between the Vegfafl/fl (control) and Vegfafl/flTie2-Cre nerves as in (G), n = 3; graph shows the mean ± SEM.
Finally, to address whether disruption of this process leads to long-term defects in the regeneration of a peripheral nerve, we analyzed the nerves of the Vegfafl/fl Tie2-Cre mice at later points following injury. At day 14, while some axons succeeded in crossing the bridge in the mutant mice, a lower number of axons regrew into the distal stump compared to controls (Figures 7G and 7H). This difference is reflected at a later time-point (6 months), when the regenerated distal regions of mutant nerves were visibly smaller (Figures S7F–S7H). As the structure of the nerve within the regenerated regions was indistinguishable from controls (Figure S7G), this most likely reflects a remodeling of the nerve in response to the lower number of axons crossing the bridge and entering into the distal stump. This phenotype shows that the efficient construction of the endothelial bridge structure is essential for the effective regeneration of peripheral nerve.
Discussion
The regeneration of a tissue following an injury requires the repair or replacement of the damaged or lost cellular structures. In some animals, such as newts and salamanders, this process is highly efficient, in that limbs and other organs can be regenerated following their loss after injury. This involves reprogramming of cells at the injury site back to a multipotent progenitor state with encoded positional information that allows the recapitulation of developmental processes to regenerate the lost cellular structures (Poss, 2010). In adult mammals, for reasons that remain unclear, regeneration is limited and seems mostly to take place within the confines of existing tissue structures and involves mechanisms distinct from those used during development. Peripheral nerves, unlike those of the CNS, are one of the few tissues in mammals capable of extensive regeneration, in that even following an injury as severe as a complete transection, the damaged nerves are able to reconnect with their original targets (Nguyen et al., 2002). However, this does not involve regeneration of the entire nerve structure downstream of the cut instead the distal stump remains intact, reconnects with the proximal stump via a “bridge” of new tissue, and is remodeled to provide a suitable environment to guide and support regrowing axons back to their targets. A major hurdle in this process is the guidance and support of the axons across the “bridge” prior to entering into the distal stump of the nerve and it has been unclear how this is achieved.
In rodents, reconnection of the two nerve stumps occurs naturally but involves the formation of a relatively long “bridge” of new tissue, a seemingly hostile environment consisting of inflammatory cells and matrix across which the axons need to find their way, in the absence of the guidance cues that were present during development. In a previous study, we showed that at the wound site, SCs were marshalled into cords following interactions with fibroblasts as the result of ephrinB/EphB2 signaling between the two cell types (Parrinello et al., 2010). The cords of SCs were found to be critical for transporting the axons across the bridge but left open the question of how the cords of SCs manage to find their way. In this study, we have identified two additional processes that are required to direct the SCs. The first involves macrophages within the bridge responding to the hypoxic environment by secreting VEGF-A and thereby stimulating the formation of blood vessels that orientate in the direction of subsequent travel. The second involves the SC cords using the polarized blood vessels as a migratory scaffold to enter and cross the bridge.
Interestingly, while the bridge contains multiple cell types, only macrophages exhibited a detectable hypoxic response, showing that distinct cell types respond differentially to a hypoxic environment and indicating a specific role for macrophages in directing the regeneration process. The macrophages display a classical hypoxic response involving the stabilization of HIF-1α and increased VEGF-A levels, and it remains unclear why only macrophages respond despite being in the same environment as the other cell types. Yet the response appears to be an intrinsic property of the cells as we found it can be reproduced in vitro. The critical role of macrophages in triggering the angiogenic response was confirmed by our findings that loss of Vegfa from myeloid cells blocked both the angiogenic response and subsequent SC entry into the bridge. Other studies have also reported a role for macrophages in inducing blood vessels following injury, suggesting that this may be a general mechanism (Wynn et al., 2013). For example, macrophages promote angiogenesis following a wound to the skin, although it was not reported whether the macrophages responded differentially to the hypoxic environment than other cell types in the wound (Rodero and Khosrotehrani, 2010). Moreover, during tumor development, hypoxia leads to the accumulation of macrophages that, via VEGF secretion, promote the vascularization of tumors (Murdoch et al., 2008; Qian and Pollard, 2010). Macrophages may therefore play a general role as primary sensors of hypoxia to induce neovascularization in the adult. It would be of great interest to characterize the mechanisms by which macrophages specifically sense the hypoxic environment and to determine the generality of this phenomenon following injury and in pathological conditions.
The newly formed blood vessels within the bridge provide a scaffold for the migrating SC cords. The importance of this appears to be twofold. First, SCs appear unable to migrate within the 3D matrix but instead require the physical surface of blood vessels in order to migrate efficiently. The amoeboid-like mode of migration observed by our live-imaging is seen in conditions of low adhesion and higher levels of confinement (Lämmermann and Sixt, 2009; Liu et al., 2015) and consistent with this, we find migration appears independent of the focal adhesions required for migration of SCs in 2D while dependent on actomyosin contractility from the rear of the cell. Moreover, recent studies describe that non-specific blebs coupled to a discontinuous environment are sufficient to provide propelling forces for migration in 3D (Bergert et al., 2015; Liu et al., 2015; Tozluoğlu et al., 2013), and we observe multiple blebs providing points of contact between the migrating SCs and ECs. Together, these findings indicate that SCs, unlike fibroblasts and ECs, cannot generate sufficient force to migrate through a 3D matrix and, while there may be specific molecular interactions between the SCs and ECs, our results are consistent with the blood vessels providing non-specific friction and a surrounding environment that allows actomyosin-driven migration along their surface.
Second, the blood vessels provide directionality to the SC movement—this is clearly demonstrated by our findings that redirection of the blood vessels can lead SC cords out of the nerve and into surrounding tissue. The requirement of a cellular substrate for cells to migrate in vivo may be a more general phenomenon than is broadly appreciated. During development, many cell types travel substantial distances and many appear to use pre-existing cell structures as a substrate to find their way. For example, during development, lymphatic ECs have been shown to migrate along pre-existing arteries (Bussmann et al., 2010) and neuroblasts migrate along radial glia (Nadarajah and Parnavelas, 2002). In the adult, mass migrations are less frequent but are associated with pathological conditions. For example during adult neurogenesis neuroblasts migrate along blood vessels (Bovetti et al., 2007), a process enhanced after a stroke (Kojima et al., 2010). In addition, there are increasing reports that tumor cells use the vasculature as a means to migrate away from the site of the primary tumor. Glioma cells are frequently highly invasive and appear to migrate along pre-existing vasculature to spread within the brain (Farin et al., 2006). Moreover, melanoma cells have also been seen to disseminate using the vasculature as a substrate (Lugassy and Barnhill, 2007) and metastatic cells appear to establish lesions in the brain along the vasculature (Carbonell et al., 2009). Identification of the mechanisms responsible for these processes is therefore likely to be of therapeutic interest.
While many nerve injuries in humans can be aided by surgery, a major therapeutic problem is the frequent loss of segments of nerve at the site of injury (Pfister et al., 2007). To overcome this problem, attempts are made to bridge the gap by the use of nerve grafts or artificial nerve conduits, however, the efficiency of axonal regrowth across the injury site is often poor. Our results suggest that encouraging or mimicking a polarized vasculature within the grafts (Hobson et al., 1997), could increase the efficiency of this process by encouraging SC entry into the bridge to provide a more conducive environment for axonal regrowth.
Experimental Procedures
Animals
Animal work was carried out in accordance to regulations of the UK Home Office. Adult (6- to 8-week-old) Sprague-Dawley male rats and 4- to 6-week-old mice were used for all experiments. To delete Vegfa in macrophages, we crossed floxed Vegfa (Vegfafl/fl) mice (Gerber et al., 1999) with mice carrying the Tie2-Cre transgene (Kisanuki et al., 2001) or the knock-in LysmCre (Clausen et al., 1999). These mice also contained the floxed Rosa26Yfp reporter (Srinivas et al., 2001). PLP-EGFP transgenic mice (Mallon et al., 2002) were used for studies requiring GFP+ SCs.
In Vivo Analysis
Sciatic nerves were exposed under general anesthesia in aseptic conditions and transected at mid-thigh. Nerves were dissected at the indicated days for analysis by immunostaining or EM. For immunostaining, pre- or post-fixed longitudinal sections of the sciatic nerves were immunostained as detailed in the Supplemental Experimental Procedures and analyzed using confocal microscopy. Blood vessel density and the area occupied by SCs and axons within the nerve bridge, was quantified using Fiji. For analysis of cell proliferation, EdU incorporation was measured using the Click-iT cell proliferation assay kit (Invitrogen). For analysis of hypoxia, the hypoxyprobe-1 kit (hypoxyprobe) was used according to the manufacturer’s instructions. To determine the functionality of the blood vessels, fluorescein-conjugated Griffonia Simplicifolia lectin I (Vector Lab) was injected into the tail vein prior to harvesting. For EM analysis, fixed nerves were embedded in Epon and analyzed for TEM or CLEM as detailed in the Supplemental Experimental Procedures. 3D reconstruction of confocal images of the nerve bridge in both rats and mice was performed using Fiji and Imaris software. 3D reconstruction of TEM images was performed using Amira software.
Bone Marrow Transplantation
Bone marrow cells (3 × 106) from donor mice (Vegfafl/fl control and Vegfafl/fl Tie2-Cre mutant) were injected into the tail vein of lethally irradiated recipient WT mice. To check the engraftment efficiency, peripheral blood was collected after 4 weeks and analyzed by FACS. Only mice with higher than 90% engraftment of donor cells were used.
In Vivo Rescue, Inhibitor, and Bead Studies
To inhibit VEGFR, PLP-EGFP mice were orally administrated with indicated doses of 100 mg/kg of Cabozantinib and harvested at the indicated times. To rescue the loss of Vegfa, nerves bridges of Vegfafl/fl Tie2-Cre mice day 4 after injury were re-exposed under general anesthesia and injected with 5 μl of VEGF-A188 or PBS 1 day before harvesting. To redirect the blood vessels, VEGF165- or PBS-coated heparin beads were placed to the side of the injury site within adjacent muscle.
Cells
Rat SCs and fibroblasts were cultured from P7 animals as described (Mathon et al., 2001). HUVECs were cultured in endothelial cell growth medium-2 (ECGM-2, PromoCell). Immortalized human dermal fibroblasts (HDFs) were cultured as primary rat fibroblasts.
In Vitro Migration Assays
The fibrin gel bead assay was performed as described (Nakatsu et al., 2003). GFP-expressing SCs were added to the beads prior to thrombin addition at a final concentration of 1.25 × 104 cells/ml. Time-lapse microscopy or immunofluorescence analysis was performed 10 days later with or without the presence of indicated inhibitors. For Matrigel assays, 150 μl of Matrigel (BD) was added to a 24-well plate and 2.5 × 104 HUVECs were seeded. Fourteen hours later, 5 × 103 GFP SCs were added to the Matrigel and time-lapse microscopy was performed.
In Vitro Bridge Analysis
Rat nerve bridges at day 2 following injury were collected and enzymatically digested. For hypoxia analysis, 6 × 104 cells were seeded in a 24-well plate and incubated overnight. The cells were then incubated at 20%, 1.5%, or 0.1% O2 for 4 hr in the presence of 100 μM pimonidazole HCl. For cell migration analysis, 105 nerve bridge cells were incubated in minimal media in the bottom of transwells for 24 hr. SCs and HUVECs were then added to the fibronectin-coated inserts and allowed to migrate for 4 hr at 20% O2.
Statistical Analysis
All data are represented as mean values ± SEM unless indicated otherwise. Unpaired two-tailed Student’s t tests were used for statistical analysis unless indicated otherwise and p values are indicated by asterisks as follows: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Acknowledgments
This work was supported by a programme grant from CRUK (C378/A17135). I.N. was supported by Worldwide Cancer Research. F.M. and C.R. were supported by the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/J00930X/1) and the Rosetrees Trust (A533). We thank UCL Biological Services for the maintenance of the animals and useful advice, W. Macklin and M. Kerschensteiner for the PLP-EGFP mice, N. Ferrara and Genentech for the Vegfa floxed mice, K. Davidson for help with genotyping, A. Fantin for helpful suggestions, S. Mole for HDFs, T. Nightingale for HUVECs, P. Munro for assistance with SBF/SEM, and E. Paluch, Y. Mao, and M. Tozluoğlu for advice on cell migration. A.C.L. would like to dedicate this work to the memory of Alan Hall and Chris Marshall, who both mentored her throughout her career.
Published: August 13, 2015
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and seven movies and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2015.07.021.
Supplemental Information
Rotating 3D projection showing a S100-positive Schwann cell (green) interacting with an EdU-positive (red) blood vessel (white) as shown in Figure 3D. Z stacks of confocal images were processed using Fiji software.
Rotating 3D reconstruction showing GFP-positive migrating cords of Schwann cells and their associated axons interacting with the vasculature within the nerve bridge as shown in Figure 3H. Z stacks of confocal images of longitudinal sections from an injured sciatic nerve of PLP-EGFP mice stained for CD31 (blue) and neurofilament (NF, red) were processed using the Imaris software to make a 3D surface-rendered reconstruction of the interaction between the vasculature and Schwann cells.
3D reconstruction showing direct contact between a migrating Schwann cell (green) and an endothelial cell (yellow) in vivo as shown in Figures 3G and S3D. Serial 70 nm sections were imaged, aligned, segmented and rendered in Amira to produce a 3D reconstruction of the contact between a Schwann cell (green) and an endothelial cell (yellow) identified by correlative light and electron microscopy of a 100 μm vibrotome section of an injured sciatic nerve from a PLP- EGFP mouse.
Time-lapse microscopy of a GFP-expressing rat Schwann cell migrating along a tubule of HUVECs within a fibrin gel as shown in Figure 4A. Frames were taken every 10 min for 10 hr. GFP fluorescence and phase contrast are shown.
Time-lapse microscopy of a GFP-expressing rat Schwann cells migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 10 min for 15 hr. GFP fluorescence and phase-contrast are shown.
Time-lapse microscopy of a GFP-expressing rat Schwann cell within a fibrin gel. Frames were taken every 10 min for 10 hr. GFP fluorescence and phase-contrast are shown. Time-lapse microscopy of GFP-expressing rat Schwann cells migrating along a tubule of HUVECs in Matrigel. Frames were taken every 10 min for 8 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence, in order to observe more clearly the migratory behavior of the Schwann cell.
3D-reconstruction showing direct contact between a migrating Schwann cell (green) and a tubule of HUVECs (red) within a fibrin gel as shown in Figure 4B. After serial block face imaging of the tubule using a Sigma FEG-SEM coupled to a 3View, images were processed using Amira software to generate a 3D-reconstruction of the contact between the Schwann cell and the endothelial cells.
Time-lapse microscopy of GFP-expressing rat Schwann cells migrating on a 2D laminin-coated surface. Frames were taken every 10 min for 10 hr. Phase-contrast is shown.
Time-lapse microscopy to exemplify the mode of migration of a GFP-expressing rat Schwann cell migrating along a tubule of HUVECs within a 3D fibrin gel. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence, in order to observe more clearly the migratory behavior of the Schwann cell. Frames were taken every 10 min for 10 hr. See snapshots in Figure 4D.
Time-lapse microscopy showing a GFP-expressing rat Schwann cell at a higher magnification migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 10 min for 8 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence, in order to observe more clearly the migratory behavior of the Schwann cell.
Time-lapse microscopy of a GFP-expressing rat Schwann cell migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 7.5 min for 10 hr. The Rho-kinase inhibitor (Y-27632 50 μM) was added after 5 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence. Results are quantified in Figure S4G.
Time-lapse microscopy of a GFP-expressing rat Schwann cells migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 10 min for 7.5 hr, latrunculin B (0.2 μM) was added after 5 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence. Results are quantified in Figure S4G.
Time-lapse microscopy of beta1 integrin knocked down GFP-expressing rat Schwann cells migrating on a 2D laminin-coated surface followed by time-lapse microscopy of a cell from the same experiment migrating along tubules of HUVECs in Matrigel. Frames were taken every 10 min for 4 hr. Phase contrast is shown for the cells migrating on laminin and both GFP fluorescence and phase contrast are shown for the Matrigel experiments. Note the migration of the cells is severely inhibited in 2D but is unaffected in 3D. Results are quantified in Figure S4I.
References
- Avellino A.M., Hart D., Dailey A.T., MacKinnon M., Ellegala D., Kliot M. Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons. Exp. Neurol. 1995;136:183–198. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Bergert M., Erzberger A., Desai R.A., Aspalter I.M., Oates A.C., Charras G., Salbreux G., Paluch E.K. Force transmission during adhesion-independent migration. Nat. Cell Biol. 2015;17:524–529. doi: 10.1038/ncb3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S., Hsieh Y.C., Bovolin P., Perroteau I., Kazunori T., Puche A.C. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D.M., Mostov K.E. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J., Bos F.L., Urasaki A., Kawakami K., Duckers H.J., Schulte-Merker S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development. 2010;137:2653–2657. doi: 10.1242/dev.048207. [DOI] [PubMed] [Google Scholar]
- Carbonell W.S., Ansorge O., Sibson N., Muschel R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE. 2009;4:e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen B.E., Burkhardt C., Reith W., Renkawitz R., Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Cursiefen C., Chen L., Borges L.P., Jackson D., Cao J., Radziejewski C., D’Amore P.A., Dana M.R., Wiegand S.J., Streilein J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudanova I., Klein R. Integration of guidance cues: parallel signaling and crosstalk. Trends Neurosci. 2013;36:295–304. doi: 10.1016/j.tins.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Fantin A., Vieira J.M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S.W., Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin A., Suzuki S.O., Weiker M., Goldman J.E., Bruce J.N., Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- Fawcett J.W., Keynes R.J. Peripheral nerve regeneration. Annu. Rev. Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Gerber H.P., Hillan K.J., Ryan A.M., Kowalski J., Keller G.A., Rangell L., Wright B.D., Radtke F., Aguet M., Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Hakkinen K.M., Harunaga J.S., Doyle A.D., Yamada K.M. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng. Part A. 2011;17:713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heermann S., Schwab M.H. Molecular control of Schwann cell migration along peripheral axons: keep moving! Cell Adhes. Migr. 2013;7:18–22. doi: 10.4161/cam.22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson M.I., Brown R., Green C.J., Terenghi G. Inter-relationships between angiogenesis and nerve regeneration: a histochemical study. Br. J. Plast. Surg. 1997;50:125–131. doi: 10.1016/s0007-1226(97)91325-4. [DOI] [PubMed] [Google Scholar]
- Jurecka W., Ammerer H.P., Lassmann H. Regeneration of a transected peripheral nerve. An autoradiographic and electron microscopic study. Acta Neuropathol. 1975;32:299–312. doi: 10.1007/BF00696792. [DOI] [PubMed] [Google Scholar]
- Kisanuki Y.Y., Hammer R.E., Miyazaki J., Williams S.C., Richardson J.A., Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kojima T., Hirota Y., Ema M., Takahashi S., Miyoshi I., Okano H., Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- Krock B.L., Skuli N., Simon M.C. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T., Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Lämmermann T., Bader B.L., Monkley S.J., Worbs T., Wedlich-Söldner R., Hirsch K., Keller M., Förster R., Critchley D.R., Fässler R., Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Lee S., Chen T.T., Barber C.L., Jordan M.C., Murdock J., Desai S., Ferrara N., Nagy A., Roos K.P., Iruela-Arispe M.L. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Le Berre M., Lautenschlaeger F., Maiuri P., Callan-Jones A., Heuzé M., Takaki T., Voituriez R., Piel M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell. 2015;160:659–672. doi: 10.1016/j.cell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Lugassy C., Barnhill R.L. Angiotropic melanoma and extravascular migratory metastasis: a review. Adv. Anat. Pathol. 2007;14:195–201. doi: 10.1097/PAP.0b013e31805048d9. [DOI] [PubMed] [Google Scholar]
- Mallon B.S., Shick H.E., Kidd G.J., Macklin W.B. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Parkhurst S.M. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Mathon N.F., Malcolm D.S., Harrisingh M.C., Cheng L., Lloyd A.C. Lack of replicative senescence in normal rodent glia. Science. 2001;291:872–875. doi: 10.1126/science.1056782. [DOI] [PubMed] [Google Scholar]
- Murdoch C., Muthana M., Coffelt S.B., Lewis C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Nadarajah B., Parnavelas J.G. Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci. 2002;3:423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nakatsu M.N., Sainson R.C., Aoto J.N., Taylor K.L., Aitkenhead M., Pérez-del-Pulgar S., Carpenter P.M., Hughes C.C. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc. Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Napoli I., Noon L.A., Ribeiro S., Kerai A.P., Parrinello S., Rosenberg L.H., Collins M.J., Harrisingh M.C., White I.J., Woodhoo A., Lloyd A.C. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.T., Sanes J.R., Lichtman J.W. Pre-existing pathways promote precise projection patterns. Nat. Neurosci. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- Parrinello S., Napoli I., Ribeiro S., Wingfield Digby P., Fedorova M., Parkinson D.B., Doddrell R.D., Nakayama M., Adams R.H., Lloyd A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister L.A., Papaloïzos M., Merkle H.P., Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J. Peripher. Nerv. Syst. 2007;12:65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- Pollard J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss K.D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C.W., Ratcliffe P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodero M.P., Khosrotehrani K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010;3:643–653. [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozluoğlu M., Tournier A.L., Jenkins R.P., Hooper S., Bates P.A., Sahai E. Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat. Cell Biol. 2013;15:751–762. doi: 10.1038/ncb2775. [DOI] [PubMed] [Google Scholar]
- Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.J., Möller A. Immunohistochemical detection of tumour hypoxia. Methods Mol. Biol. 2010;611:151–159. doi: 10.1007/978-1-60327-345-9_12. [DOI] [PubMed] [Google Scholar]
- Zochodne D.W., editor. Neurobiology of Peripheral Nerve Regeneration. First Edition. Cambridge University Press; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rotating 3D projection showing a S100-positive Schwann cell (green) interacting with an EdU-positive (red) blood vessel (white) as shown in Figure 3D. Z stacks of confocal images were processed using Fiji software.
Rotating 3D reconstruction showing GFP-positive migrating cords of Schwann cells and their associated axons interacting with the vasculature within the nerve bridge as shown in Figure 3H. Z stacks of confocal images of longitudinal sections from an injured sciatic nerve of PLP-EGFP mice stained for CD31 (blue) and neurofilament (NF, red) were processed using the Imaris software to make a 3D surface-rendered reconstruction of the interaction between the vasculature and Schwann cells.
3D reconstruction showing direct contact between a migrating Schwann cell (green) and an endothelial cell (yellow) in vivo as shown in Figures 3G and S3D. Serial 70 nm sections were imaged, aligned, segmented and rendered in Amira to produce a 3D reconstruction of the contact between a Schwann cell (green) and an endothelial cell (yellow) identified by correlative light and electron microscopy of a 100 μm vibrotome section of an injured sciatic nerve from a PLP- EGFP mouse.
Time-lapse microscopy of a GFP-expressing rat Schwann cell migrating along a tubule of HUVECs within a fibrin gel as shown in Figure 4A. Frames were taken every 10 min for 10 hr. GFP fluorescence and phase contrast are shown.
Time-lapse microscopy of a GFP-expressing rat Schwann cells migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 10 min for 15 hr. GFP fluorescence and phase-contrast are shown.
Time-lapse microscopy of a GFP-expressing rat Schwann cell within a fibrin gel. Frames were taken every 10 min for 10 hr. GFP fluorescence and phase-contrast are shown. Time-lapse microscopy of GFP-expressing rat Schwann cells migrating along a tubule of HUVECs in Matrigel. Frames were taken every 10 min for 8 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence, in order to observe more clearly the migratory behavior of the Schwann cell.
3D-reconstruction showing direct contact between a migrating Schwann cell (green) and a tubule of HUVECs (red) within a fibrin gel as shown in Figure 4B. After serial block face imaging of the tubule using a Sigma FEG-SEM coupled to a 3View, images were processed using Amira software to generate a 3D-reconstruction of the contact between the Schwann cell and the endothelial cells.
Time-lapse microscopy of GFP-expressing rat Schwann cells migrating on a 2D laminin-coated surface. Frames were taken every 10 min for 10 hr. Phase-contrast is shown.
Time-lapse microscopy to exemplify the mode of migration of a GFP-expressing rat Schwann cell migrating along a tubule of HUVECs within a 3D fibrin gel. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence, in order to observe more clearly the migratory behavior of the Schwann cell. Frames were taken every 10 min for 10 hr. See snapshots in Figure 4D.
Time-lapse microscopy showing a GFP-expressing rat Schwann cell at a higher magnification migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 10 min for 8 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence, in order to observe more clearly the migratory behavior of the Schwann cell.
Time-lapse microscopy of a GFP-expressing rat Schwann cell migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 7.5 min for 10 hr. The Rho-kinase inhibitor (Y-27632 50 μM) was added after 5 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence. Results are quantified in Figure S4G.
Time-lapse microscopy of a GFP-expressing rat Schwann cells migrating along a tubule of HUVECs within a fibrin gel. Frames were taken every 10 min for 7.5 hr, latrunculin B (0.2 μM) was added after 5 hr. GFP fluorescence and phase-contrast are shown initially followed by the same movie showing only GFP fluorescence. Results are quantified in Figure S4G.
Time-lapse microscopy of beta1 integrin knocked down GFP-expressing rat Schwann cells migrating on a 2D laminin-coated surface followed by time-lapse microscopy of a cell from the same experiment migrating along tubules of HUVECs in Matrigel. Frames were taken every 10 min for 4 hr. Phase contrast is shown for the cells migrating on laminin and both GFP fluorescence and phase contrast are shown for the Matrigel experiments. Note the migration of the cells is severely inhibited in 2D but is unaffected in 3D. Results are quantified in Figure S4I.