Abstract
Although rheumatoid arthritis (RA) is the most common chronic inflammatory autoimmune disease, diagnosis of RA is currently based on clinical manifestations, and there is no simple, practical assessment tool in the clinical field to assess disease activity and severity. Recently, there has been increasing interest in the discovery of new diagnostic RA biomarkers that can assist in evaluating disease activity, severity, and treatment response. Proteomics, the large-scale study of the proteome, has emerged as a powerful technique for protein identification and characterization. For the past 10 years, proteomic techniques have been applied to different biological samples (synovial tissue/fluid, blood, and urine) from RA patients and experimental animal models. In this review, we summarize the current state of the application of proteomics in RA and its importance in identifying biomarkers and treatment targets.
Keywords: Rheumatoid arthritis, Proteomics, Biomarker
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by joint destruction, functional impairment, disability, and premature mortality (1,2,3). The bone and cartilage destruction rarely heals, the damage accumulating over time (4,5,6,7). With regard to inflammation, interfering with the inflammatory cascade before it is fully established is most effective. Therefore, it is evident that therapeutic intervention will have greater effect on the outcome if started early, and ideally, if commenced even before damage has occurred. Presently, RA is defined by the presence of four of the seven criteria developed by the American College of Rheumatology (ACR) in 1987 (8), or a total score of six or greater (of a possible 10) from the individual scores in the four domains in the 2010 Rheumatoid Arthritis Classification Criteria of the American College of Rheumatology/European League Against Rheumatism (EULAR) collaborative initiative (9,10). However, the current classification criteria do not allow early diagnosis (11,12).
Treatment and prevention of the joint destructive process are possible, mainly with the use of steroids, disease-modifying antirheumatic drugs (DMARDs), biologics, or combinations thereof (13,14,15,16). Unfortunately, the use of drug combinations may rely on recommendations and expert opinions rather than on algorithms or criteria derived from clinical studies (17,18). Moreover, no precise universal and/or easy-to-use assessment methods exist that allow for the evaluation of disease activity and the prediction of disease severity. The disease activity score 28 (DAS28) (19) and the Sharp/van der Heijde scoring systems (20) are used to guide treatment decisions, but these assessment tools cannot be easily applied in daily practice. Thus, there is an unmet need for novel biomarkers that can complement conventional measures and that allow precise monitoring of the disease activity and severity of RA.
The proteome, the entire set of proteins produced by a cell or organism (21), varies with time and the distinct requirements, or stresses, that the particular cell or organism undergoes. Proteomics is the large-scale study of proteomes (22,23). It is an emerging area that includes such technical disciplines as light and electron microscopy, array and chip experiments, yeast two-hybrid assay, and mass spectrometry (MS). Because proteomics investigates the overall picture of intracellular protein composition, structure, and activity, it is capable of identifying biomarkers and improving the understanding of pathogenesis. Therefore, this useful tool meets the needs of RA research. During the last 10 years, proteomic techniques have led to numerous advances in the analysis of different types of biological samples collected from RA patients, including synovial tissue/fluid, blood, and urine (Table I). In this review, we summarize the status of the applications of proteomics for RA and their importance in identifying potential biomarkers and treatment targets.
Table I. Various proteomic approaches in rheumatoid arthritis (RA) research.
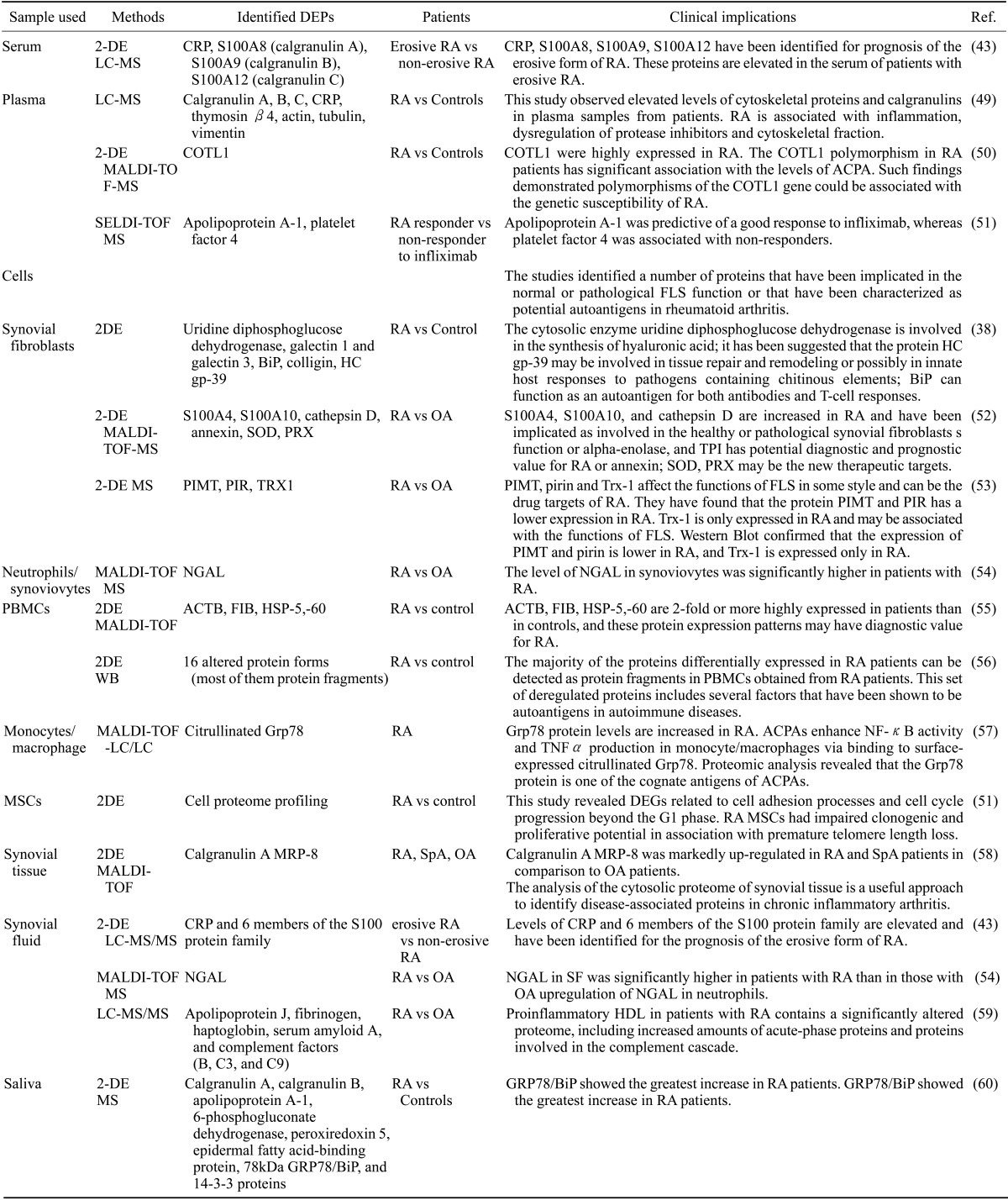
DEPs, Differentially expressed proteins; Ref, references; 2DE, 2-dimensional gel electrophoresis; LC-MS, liquid chromatography-coupled tandem mass spectrometry; CRP, C-reactive protein; MALDI-TOF-MS, matrix-assisted laser desorption ionization mass spectrometry; COTL1, coactosin-like1; SELDI-TOF-MS, surface enhanced laser desorption/ionisation time-of-flight mass spectrometry; OA, osteoarthritis; PIMT, protein isoaspartyl methyltransferase; PIR, pirin iron-binding nuclear protein; Trx-1, thioredoxin 1; NGAL, neutrophil gelatinase-associated lipocalin; PBMCs, peripheral blood mononuclear cells; WB, western blot; GRP78/BiP, glucose-regulated protein precursor; ACPA, anti-citrullinated peptide/protein antibodies; TNFα, tumor necrosis factor-α; MSCs, mesenchymal stem cells; DEGs, differential expression genes; MRP-8, myeloid related protein-8; SpA, spondyloarthropathy.
PROTEOMICS
Methods of studying proteins
Proteomics is the large-scale study of the expression, structure, function, modifications, and interactions of proteins as well as how these aspects of the proteins change in different environments and conditions. Transformational new technologies of MS and liquid chromatography (LC) have enabled rapid advances in proteomics. A typical MS-based proteomic experiment consists of six steps: protein extraction, protein fractionation, peptide fractionation, LC-MS/MS analysis, peptide/protein identification, and protein quantification (24). In step 1, a body fluid or biopsy specimen is obtained for the extraction of proteins. In step 2, the proteins to be analyzed are isolated from the cell lysate or tissue by biochemical fractionation tools, such as oneor two-dimensional gel electrophoresis, capillary electrophoresis, or affinity selection including affinity depletion and immunoprecipitation. In step 3, the proteins from the sample are digested enzymatically, usually with trypsin, into peptides. Step 4 requires that the peptides be separated based on their hydrophobicity using techniques including reversed-phase high-performance liquid chromatography (RP-HPLC) and isoelectric focusing (IEF). The fractionated peptides are ionized and analyzed by the mass spectrometer, which measures mass-to-charge (m/z) ratios of the peptides and their intensities (abundances). After the preliminary scans, those peptides with relatively high intensities are isolated in a data-dependent manner and fragmented by collision-induced dissociation (CID) (25), followed by tandem mass spectrometry (MS/MS) experiments (26). In step 5, peptide/protein identification is performed by various methods including database searching, de novo sequencing, peptide mass fingerprinting (PMF), and accurate mass and time tag (AMT). Finally, in step 6, protein quantification is performed using various labeling methods including isobaric tags for relative and absolute quantitation (iTRAQ), stable isoptope labeling with amino acid in cell culture (SILAC), 15N or chemical protein labeling isotope-coded protein label (ICPL), as well as label-free methods involving the identification of peptides and alignments of the peptides (27).
MS is at the heart of all proteomic studies because it plays a key role in the analysis of proteins. A mass spectrometer consists of three parts: an ion source for the ionization of the peptides, a mass analyzer to measure the m/z of the ionized peptides, and a detector to detect the number of ions at each m/z value. For the ionization of the peptides, electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) are the two most frequently used techniques. As for the second part of the mass spectrometer, mass analyzers fall into four basic types: ion trap, time of flight (TOF), quadrupole, and Fourier transform ion cyclotron (FT-MS). The combination of the ion source and mass analyzer determines the type of mass spectrometry, for example, ESI-ion trap and MALDI-TOF. Liquid chromatography-ESI-tandem mass spectrometry (LC-ESI-MS/MS) and MALDI-MS/MS (MALDI-TOF/TOF) are still commonly used methods because of their simplicity and excellent accuracy (26).
Application of proteomics to protein profiling and protein interactions
Thousands of proteins can be identified from the complex protein mixtures in each study using the methods described above. However, to achieve biologically useful data to guide a comprehensive understanding of cellular functions, it is necessary to link the quantitative proteomic data to genomic sequences, gene expression profiles, and phenotypic data as well. Such efforts generate comprehensive proteome maps in various types of samples including cells and tissues, as well as bio-fluids such as blood (plasma/serum), ascites, cerebrospinal fluid, urine, saliva, and tears. Currently, major efforts such as the Human Proteome Project (HPP) are under way to identify the products of human genes on a large scale (28). Moreover, to support the discovery of non-invasive diagnostic biomarkers, the Human Plasma Proteome Project (HPPP) was carried out, providing a comprehensive serum proteome that can be used to identify secreted biomarker candidates (29).
Most proteins do not exert their function in isolation, but do so rather in the form of protein-protein interactions. Thus, to understand functions of proteins, MS-based methods have been used to identify interaction partners of the proteins. These methods include tandem affinity purification (TAP)-tagging (30) and immunoprecipitation (IP)-MS methods (30). The official method involves the fusion of the TAP tag to the C-terminus of the protein of interest. The tag comprises calmodulin binding peptide (CBP), followed by the tobacco etch virus protease (TEV protease) cleavage site and Protein A, which binds tightly to IgG. Protein A is at the end of the fusion protein such that the entire complex can be isolated using an IgG matrix. The latter method involves immunoprecipitation of a protein of interest to isolate the interactors of the protein using LC-MS/MS analysis. Identifying the interactors of the protein with no interaction data available can incorporate it into the known cellular networks defined by protein-protein interactions. In addition to the global profiling and identification of interactors, MS-based methods have been also applied to measure cellular locations, post-translational modifications, structures, and enzymatic activities of the proteins, thereby providing the entire spectrum of information needed to understand the functions of the proteins (31,32,33,34,35,36).
EXPLORATION OF NOVEL BIOMARKERS USING A PROTEOMIC APPROACH
Biomarkers for diagnosis
The pathogenesis of RA is complex and multifactorial. ACR/EULAR developed a set of criteria for the diagnosis of RA (8,9,10). Although these criteria are designated as diagnostic criteria, more precisely they are less a diagnostic tool than a set of classification criteria intended to facilitate comparisons between RA and other diseases. The criteria were based on the experience of doctors, and it is thus evident that novel biomarkers are needed to facilitate the diagnosis of RA. A substantive effort is being made to identify biomarkers, including combinations of genetic and serologic information or protein profiling using proteomic approaches.
Proteomic studies in RA are largely focused around the identification of autoantigens and protein targets by the differential screening of serum/synovial fluid or synovial/cartilage tissue (37). Kumar and colleagues separated a number of proteins from fibroblast-like synovial (FLS) cells by two-dimensional polyacrylamide gel electrophoresis and analyzed the in-gel digested proteins (38). The identified proteins included uridine diphosphoglucose dehydrogenase, galectin 1, galectin 3, BiP, colligin, and HC gp-39, all of which have been implicated in FLS function or as potential autoantigens (38). Li and colleagues reported that differentially expressed proteins (DEPs) identified in RA-FLS could be candidates for promising diagnostic indicators of RA (39). These proteins included enzymatic and structural proteins (e.g., PKM1/M2, α-enolase, ERp60, and lamin-A/C), signal transduction proteins (e.g., annexin 11, peroxiredoxin 1, and TrpRS), and heat-shock/chaperone proteins (e.g. TCP-1, GRP75, HspB5, and Bip) (39). Using data derived from microarray studies, our group demonstrated that Bip is crucial for synoviocyte proliferation and angiogenesis (40). This approach to analyzing FLS proteins was based on the fact that the synovial membrane becomes the target of a persistent inflammatory process and immune cell accumulation, leading to fundamental changes in the phenotype and function of FLS cells. Thus, the investigation of DEPs in FLS is a promising method to identify novel diagnostic biomarkers for RA.
Biomarkers for monitoring disease activity and disease severity
Disease activity is a central component in the assessment of patients with RA. It comprises the signs and symptoms of the disease and is fundamentally responsible for joint destruction (disease severity). The most frequently used tool for assessing disease activity is the DAS28, based on tender joint counts, swollen joint counts, and the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) (8). However, this instrument has practical the limitations of preventing immediate assessment, requiring specialized expertise, and having poor transparency for patients. For the assessment of disease severity, radiography is widely used. Although the structural damage visible on radiographs is a reflection of the cumulative disease severity and a strong predictor of disability, there is often no visible manifestation for 1~3 years after disease initiation. Moreover, there is no early biomarker to predict a high risk of joint destruction and disability (41).
Previously, Kang et al. performed quantitative urinary proteome profiling of urine samples from RA and osteoarthritis (OA) patients using a label-free LC-MS/MS analysis (42). Using these urinary protein profiles, they identified 134 DEPs between RA and OA urine samples. Through the integration of the analysis of the 134 DEPs with the analysis of mRNA expression profiles in joints and mononuclear cells, they discovered that urinary soluble CD14 (sCD14) had a comparable diagnostic value to that of conventional serum measures (ESR or CRP). They further identified an even higher predictive power for disease activity when combined with serum CRP. Other groups have also searched for biomarkers through 2-dimensional liquid chromatography-coupled tandem mass spectrometry. Liao and colleagues (43) reported that levels of CRP, S100A8 (calgranulin A), S100A9 (calgranulin B), and S100A12 (calgranulin C) proteins identified through screening the synovial fluid proteome profile were also elevated in the serum of patients with erosive disease compared with those levels in patients with nonerosive RA and in healthy individuals. They used the 2-step proteomic approach in which biomarker discovery using semiquantitative protein profiling of diseased tissues was followed by candidate verification using quantitative multiple reaction monitoring (MRM) analysis in peripheral blood. In these processes, at least 33 biomarker candidates for RA were identified, and Liao et al. were able to certify a subset of promising biomarkers for disease severity. Although the sample size was very small (first step: n=5 and second step: n=15), this study demonstrated that proteomic techniques can be used to discover novel biomarkers in RA. As more efficient sample enrichment/separation techniques and more accurate mass spectrometers become available in the future, proteomic methods will have greater efficiency.
Biomarkers for assessing treatment response
The treatment of RA is primarily based on the use of DMARDs (44,45). The term "conventional DMARDs" will be used to include chemical agents such as methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide, whereas tofacitinib, a new synthetic DMARD specifically designed to target janus kinases (JAKs), will be designated as a "targeted synthetic DMARD" (45). Biologics (or biological DMARDs) such as tumor necrosis factor (TNF) inhibitors, T cell costimulation inhibitor (abatacept), anti-B cell agents (rituximab), and the interleukin-6 receptor (IL-6R)-blocking monoclonal antibody (tocilizumab) have revolutionized the treatment of RA. Despite the availability of these therapeutic options, treatment decisions in clinical practice are based more on the physician's experience or expert opinion than on experimental evidence.
A variety of studies have attempted to identify biomarkers of therapeutic responses to various drugs (46,47,48). Inhibitors of TNF are the most widely used of the biological therapies in RA. Although anti-TNFα therapy has revolutionized the treatment of advanced RA, approximately one-third of patients have suboptimal responses or no response (46). Moreover, these agents are expensive compared with conventional DMARDs. Assessing the treatment responses to anti-TNFα agents based on biomarker profiling has the potential to improve the overall disease control and to reduce costs for healthcare providers.
Recently identified biomarkers of responses to biological treatments for RA are described below. Segigawa and colleagues (47), using 2D LC-MS/MS analysis, investigated serum or plasma proteins differentially expressed after anti-TNFα therapy. They identified FAM62A/MBC2 proteins related to the TNF-α-mediated pathway for nuclear factor kappa B (NF-κB) activation and/or CTGF protein related to the metabolism (including regeneration) of articular cartilage. Sellam and colleagues (48), using whole-blood transcriptomic profiling, identified molecular signatures that could be predictive of clinical responses to rituximab in patients with RA. The protein signature for the EULAR responder group featured upregulation of the inflammatory pathway, NF-κB, IL33, and STAT5A, and downregulation of the interferon pathway (48). If these approaches are successful and a useful biomarker has been discovered, it could open new perspectives for clinical RA management.
CONCLUSION
Proteomics-based analysis of RA patients over the past 10 years has provided promising data. DEPs may be helpful for better understanding the pathobiology of RA, and those identified by several studies may be essential for the identification of new targets and to monitor current and new treatments. However, most studies are inadequate in allowing reliable conclusions. The analysis of RA may be more complicated than other inflammatory diseases because of its combination of inflammatory processes, including synovial inflammation and angiogenesis. Data obtained from proteomic analysis of studies including a larger number of patients must be considered a fundamental requirement for more targeted progress. In addition, strategies including specialized proteomic technologies such as an isobaric tag for relative and absolute quantitation (iTRAQ), isotope-coded affinity tag (ICAT), and cleavable ICAT (cICAT), which significantly reduce sample-to-sample variation and time-point variation, can drive basic scientific findings closer to clinical practice. Although RA research still has a long way to go, proteomics has helped shorten the distance.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014R1A2A1A11049812) and the Institute for Basic Science (IBS-R013-G1-2015-a00) funded by the Korean government (MSIP).
Abbreviations
- RA
rheumatoid arthritis
- ACR
American College of Rheumatology
- EULAR
European League Against Rheumatism
- DMARDs
disease-modifying antirheumatic drugs
- DAS28
disease activity score 28
- MS
mass spectrometry
- LC
liquid chromatography
- RP-HPLC
reversed-phase high-performance liquid chromatography
- IEF
isoelectric focusing
- CID
collision-induced dissociation
- PMF
peptide mass fingerprinting
- AMT
accurate mass and time tag
- iTRAQ
isobaric tags for relative and absolute quantitation
- SILAC
stable isoptope labeling with amino acid in cell culture
- ICPL
isotope-coded protein label
- MALDI
matrix-assisted laser desorption/ionization
- ESI
electrospray ionization
- TOF
time of flight
- FT-MS
Fourier transform ion cyclotron
- LC-ESI-MS/MS
liquid chromatography-ESI-tandem mass spectrometry
- HPP
human Proteome Project
- HPPP
human Plasma Proteome Project
- TAP
tandem affinity purification
- IP
immunoprecipitation
- CBP
calmodulin binding peptide
- TEV
tobacco etch virus
- FLS
fibroblast-like synovial
- DEPs
differentially expressed proteins
- ESR
erythrocyte sedimentation rate
- CRP
C-reactive protein
- OA
osteoarthritis
- sCD14
soluble CD14
- MRM
multiple reaction monitoring
- IL-6R
interleukin-6 receptor
- iTRAQ
isobaric tag for relative and absolute quantitation
- ICAT
isotope-coded affinity tag
- cICAT
cleavable ICAT
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Smolen JS, Aletaha D. Patients with rheumatoid arthritis in clinical care. Ann Rheum Dis. 2004;63:221–225. doi: 10.1136/ard.2003.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1530–1542. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 3.Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–872. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 4.Sokka T, Hannonen P. Healing of erosions in rheumatoid arthritis. Ann Rheum Dis. 2000;59:647–649. doi: 10.1136/ard.59.8.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rau R, Wassenberg S, Herborn G, Perschel WT, Freitag G. Identification of radiologic healing phenomena in patients with rheumatoid arthritis. J Rheumatol. 2001;28:2608–2615. [PubMed] [Google Scholar]
- 6.van der Heijde D, Landewe R. Imaging: do erosions heal? Ann Rheum Dis. 2003;62(Suppl 2):ii10–ii12. doi: 10.1136/ard.62.suppl_2.ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ideguchi H, Ohno S, Hattori H, Senuma A, Ishigatsubo Y. Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arthritis Res Ther. 2006;8:R76. doi: 10.1186/ar1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 9.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 10.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 11.Harrison BJ, Symmons DP, Barrett EM, Silman AJ American Rheumatism Association. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis. J Rheumatol. 1998;25:2324–2330. [PubMed] [Google Scholar]
- 12.Machold KP, Stamm TA, Eberl GJ, Nell VK, Dunky A, Uffmann M, Smolen JS. Very recent onset arthritis--clinical, laboratory, and radiological findings during the first year of disease. J Rheumatol. 2002;29:2278–2287. [PubMed] [Google Scholar]
- 13.Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, van Denderen JC, van Zeben D, Dijkmans BA, Peeters AJ, Jacobs P, van den Brink HR, Schouten HJ, van der Heijde DM, Boonen A, van der Linden S. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309–318. doi: 10.1016/S0140-6736(97)01300-7. [DOI] [PubMed] [Google Scholar]
- 14.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, Zwinderman AH, Ronday HK, Han KH, Westedt ML, Gerards AH, van Groenendael JH, Lems WF, van Krugten MV, Breedveld FC, Dijkmans BA. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–3390. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 15.Pincus T, Ferraccioli G, Sokka T, Larsen A, Rau R, Kushner I, Wolfe F. Evidence from clinical trials and long-term observational studies that disease-modifying anti-rheumatic drugs slow radiographic progression in rheumatoid arthritis: updating a 1983 review. Rheumatology (Oxford) 2002;41:1346–1356. doi: 10.1093/rheumatology/41.12.1346. [DOI] [PubMed] [Google Scholar]
- 16.Aletaha D, Smolen JS. The rheumatoid arthritis patient in the clinic: comparing more than 1,300 consecutive DMARD courses. Rheumatology (Oxford) 2002;41:1367–1374. doi: 10.1093/rheumatology/41.12.1367. [DOI] [PubMed] [Google Scholar]
- 17.Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, Emery P, Ferraccioli G, Hazes JM, Klareskog L, Machold K, Martin-Mola E, Nielsen H, Silman A, Smolen J, Yazici H. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2007;66:34–45. doi: 10.1136/ard.2005.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis. 2002;61:290–297. doi: 10.1136/ard.61.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–263. [PubMed] [Google Scholar]
- 21.Wilkins MR, Pasquali C, Appel RD, Ou K, Golaz O, Sanchez JC, Yan JX, Gooley AA, Hughes G, Humphery-Smith I, Williams KL, Hochstrasser DF. From proteins to proteomes: large scale protein identification by two-dimensional electrophoresis and amino acid analysis. Biotechnology(N.Y.) 1996;14:61–65. doi: 10.1038/nbt0196-61. [DOI] [PubMed] [Google Scholar]
- 22.Anderson NL, Anderson NG. Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis. 1998;19:1853–1861. doi: 10.1002/elps.1150191103. [DOI] [PubMed] [Google Scholar]
- 23.Blackstock WP, Weir MP. Proteomics: quantitative and physical mapping of cellular proteins. Trends Biotechnol. 1999;17:121–127. doi: 10.1016/s0167-7799(98)01245-1. [DOI] [PubMed] [Google Scholar]
- 24.Mallick P, Kuster B. Proteomics: a pragmatic perspective. Nat Biotechnol. 2010;28:695–709. doi: 10.1038/nbt.1658. [DOI] [PubMed] [Google Scholar]
- 25.Ryu SY. Bioinformatics tools to identify and quantify proteins using mass spectrometry data. Adv Protein Chem Struct Biol. 2014;94:1–17. doi: 10.1016/B978-0-12-800168-4.00001-9. [DOI] [PubMed] [Google Scholar]
- 26.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Chae S, Kim H, Mun DG, Back S, Choi HY, Park KS, Hwang D, Choi SH, Lee SW. A protein profile of visceral adipose tissues linked to early pathogenesis of type 2 diabetes mellitus. Mol Cell Proteomics. 2014;13:811–822. doi: 10.1074/mcp.M113.035501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilhelm M, Schlegl J, Hahne H, Moghaddas Gholami A, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H, Mathieson T, Lemeer S, Schnatbaum K, Reimer U, Wenschuh H, Mollenhauer M, Slotta-Huspenina J, Boese JH, Bantscheff M, Gerstmair A, Faerber F, Kuster B. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509:582–587. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 29.Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, Bletz JA, Mallick P, Katz JE, Malmstrom J, Ossola R, Watts JD, Lin B, Zhang H, Moritz RL, Aebersold R. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics. 2011;10:M110.006353. doi: 10.1074/mcp.M110.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 31.Venne AS, Kollipara L, Zahedi RP. The next level of complexity: crosstalk of posttranslational modifications. Proteomics. 2014;14:513–524. doi: 10.1002/pmic.201300344. [DOI] [PubMed] [Google Scholar]
- 32.Boja ES, Rodriguez H. Mass spectrometry-based targeted quantitative proteomics: achieving sensitive and reproducible detection of proteins. Proteomics. 2012;12:1093–1110. doi: 10.1002/pmic.201100387. [DOI] [PubMed] [Google Scholar]
- 33.Angel TE, Aryal UK, Hengel SM, Baker ES, Kelly RT, Robinson EW, Smith RD. Mass spectrometry-based proteomics: existing capabilities and future directions. Chem Soc Rev. 2012;41:3912–3928. doi: 10.1039/c2cs15331a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson H, Eyers CE. Analysis of post-translational modifications by LC-MS/MS. Methods Mol Biol. 2010;658:93–108. doi: 10.1007/978-1-60761-780-8_5. [DOI] [PubMed] [Google Scholar]
- 35.Konermann L, Pan J, Liu YH. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem Soc Rev. 2011;40:1224–1234. doi: 10.1039/c0cs00113a. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Pan H, Chen X. Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spectrom Rev. 2009;28:147–176. doi: 10.1002/mas.20190. [DOI] [PubMed] [Google Scholar]
- 37.Tilleman K, Deforce D. Proteomics in rheumatology. Expert Rev Proteomics. 2008;5:755–759. doi: 10.1586/14789450.5.6.755. [DOI] [PubMed] [Google Scholar]
- 38.Dasuri K, Antonovici M, Chen K, Wong K, Standing K, Ens W, El-Gabalawy H, Wilkins JA. The synovial proteome: analysis of fibroblast-like synoviocytes. Arthritis Res Ther. 2004;6:R161–R168. doi: 10.1186/ar1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XJ, Xu M, Zhao XQ, Zhao JN, Chen FF, Yu W, Gao DY, Luo B. Proteomic analysis of synovial fibroblast-like synoviocytes from rheumatoid arthritis. Clin Exp Rheumatol. 2013;31:552–558. [PubMed] [Google Scholar]
- 40.Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K, Ahn JH, Hwang D, Lee AS, Kim KJ, Park YJ, Cho CS, Kim WU. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med. 2012;209:871–886. doi: 10.1084/jem.20111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Riel PL, van de Putte LB WM. Evaluation and management of active inflammatory disease. In: Klippel JH DP, editor. Rheumatology. 2nd ed. London: Mosby; 1998. pp. 5.14.11–5.14.13. [Google Scholar]
- 42.Kang MJ, Park YJ, You S, Yoo SA, Choi S, Kim DH, Cho CS, Yi EC, Hwang D, Kim WU. Urinary proteome profile predictive of disease activity in rheumatoid arthritis. J Proteome Res. 2014;13:5206–5217. doi: 10.1021/pr500467d. [DOI] [PubMed] [Google Scholar]
- 43.Liao H, Wu J, Kuhn E, Chin W, Chang B, Jones MD, O'Neil S, Clauser KR, Karl J, Hasler F, Roubenoff R, Zolg W, Guild BC. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:3792–3803. doi: 10.1002/art.20720. [DOI] [PubMed] [Google Scholar]
- 44.Haraoui B, Smolen JS, Aletaha D, Breedveld FC, Burmester G, Codreanu C, Da Silva JP, de Wit M, Dougados M, Durez P, Emery P, Fonseca JE, Gibofsky A, Gomez-Reino J, Graninger W, Hamuryudan V, Jannaut Pena MJ, Kalden J, Kvien TK, Laurindo I, Martin-Mola E, Montecucco C, Santos Moreno P, Pavelka K, Poor G, Cardiel MH, Stanislawska-Biernat E, Takeuchi T, van der Heijde D. Treating Rheumatoid Arthritis to Target: multinational recommendations assessment questionnaire. Ann Rheum Dis. 2011;70:1999–2002. doi: 10.1136/ard.2011.154179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, Emery P, Gaujoux-Viala C, Gossec L, Nam J, Ramiro S, Winthrop K, de Wit M, Aletaha D, Betteridge N, Bijlsma JW, Boers M, Buttgereit F, Combe B, Cutolo M, Damjanov N, Hazes JM, Kouloumas M, Kvien TK, Mariette X, Pavelka K, van Riel PL, Rubbert-Roth A, Scholte-Voshaar M, Scott DL, Sokka-Isler T, Wong JB, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mewar D, Wilson AG. Treatment of rheumatoid arthritis with tumour necrosis factor inhibitors. Br J Pharmacol. 2011;162:785–791. doi: 10.1111/j.1476-5381.2010.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekigawa I, Yanagida M, Iwabuchi K, Kaneda K, Kaneko H, Takasaki Y, Jung G, Sone S, Tanaka Y, Ogawa H, Takamori K. Protein biomarker analysis by mass spectrometry in patients with rheumatoid arthritis receiving anti-tumor necrosis factor-alpha antibody therapy. Clin Exp Rheumatol. 2008;26:261–267. [PubMed] [Google Scholar]
- 48.Sellam J, Marion-Thore S, Dumont F, Jacques S, Garchon HJ, Rouanet S, Taoufik Y, Hendel-Chavez H, Sibilia J, Tebib J, Le Loet X, Combe B, Dougados M, Mariette X, Chiocchia G. Use of whole-blood transcriptomic profiling to highlight several pathophysiologic pathways associated with response to rituximab in patients with rheumatoid arthritis: data from a randomized, controlled, open-label trial. Arthritis Rheumatol. 2014;66:2015–2025. doi: 10.1002/art.38671. [DOI] [PubMed] [Google Scholar]
- 49.Zheng X, Wu SL, Hincapie M, Hancock WS. Study of the human plasma proteome of rheumatoid arthritis. J Chromatogr A. 2009;1216:3538–3545. doi: 10.1016/j.chroma.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 50.Jin EH, Shim SC, Kim HG, Chae SC, Chung HT. Polymorphisms of COTL1 gene identified by proteomic approach and their association with autoimmune disorders. Exp Mol Med. 2009;41:354–361. doi: 10.3858/emm.2009.41.5.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kastrinaki MC, Sidiropoulos P, Roche S, Ringe J, Lehmann S, Kritikos H, Vlahava VM, Delorme B, Eliopoulos GD, Jorgensen C, Charbord P, Haupl T, Boumpas DT, Papadaki HA. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis. 2008;67:741–749. doi: 10.1136/ard.2007.076174. [DOI] [PubMed] [Google Scholar]
- 52.Bo GP, Zhou LN, He WF, Luo GX, Jia XF, Gan CJ, Chen GX, Fang YF, Larsen PM, Wu J. Analyses of differential proteome of human synovial fibroblasts obtained from arthritis. Clin Rheumatol. 2009;28:191–199. doi: 10.1007/s10067-008-1013-y. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Fan LY, Zong M, Sun LS, Lu L. Proteins related to the functions of fibroblast-like synoviocytes identified by proteomic analysis. Clin Exp Rheumatol. 2012;30:213–221. [PubMed] [Google Scholar]
- 54.Katano M, Okamoto K, Arito M, Kawakami Y, Kurokawa MS, Suematsu N, Shimada S, Nakamura H, Xiang Y, Masuko K, Nishioka K, Yudoh K, Kato T. Implication of granulocyte-macrophage colony-stimulating factor induced neutrophil gelatinase-associated lipocalin in pathogenesis of rheumatoid arthritis revealed by proteome analysis. Arthritis Res Ther. 2009;11:R3. doi: 10.1186/ar2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dotzlaw H, Schulz M, Eggert M, Neeck G. A pattern of protein expression in peripheral blood mononuclear cells distinguishes rheumatoid arthritis patients from healthy individuals. Biochim Biophys Acta. 2004;1696:121–129. doi: 10.1016/j.bbapap.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Schulz M, Dotzlaw H, Mikkat S, Eggert M, Neeck G. Proteomic analysis of peripheral blood mononuclear cells: selective protein processing observed in patients with rheumatoid arthritis. J Proteome Res. 2007;6:3752–3759. doi: 10.1021/pr070285f. [DOI] [PubMed] [Google Scholar]
- 57.Lu MC, Lai NS, Yu HC, Huang HB, Hsieh SC, Yu CL. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010;62:1213–1223. doi: 10.1002/art.27386. [DOI] [PubMed] [Google Scholar]
- 58.Tilleman K, Van Beneden K, Dhondt A, Hoffman I, De Keyser F, Veys E, Elewaut D, Deforce D. Chronically inflamed synovium from spondyloarthropathy and rheumatoid arthritis investigated by protein expression profiling followed by tandem mass spectrometry. Proteomics. 2005;5:2247–2257. doi: 10.1002/pmic.200401109. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, Lee TD, Reddy ST. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012;64:1828–1837. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giusti L, Baldini C, Ciregia F, Giannaccini G, Giacomelli C, De FF, Delle SA, Riente L, Lucacchini A, Bazzichi L, Bombardieri S. Is GRP78/BiP a potential salivary biomarker in patients with rheumatoid arthritis? Proteomics Clin Appl. 2010;4:315–324. doi: 10.1002/prca.200900082. [DOI] [PubMed] [Google Scholar]


